Abstract
Enterococcus faecium is an emerging pathogen that causes infections in hospitalized patients with various co-morbid diseases. These underlying diseases are often associated with an acute-phase response that renders patients vulnerable to nosocomial infections. To study the influence of the acute-phase response induced by sterile tissue injury on host defence against E. faecium, mice were injected subcutaneously with either turpentine or casein 1 day before intraperitoneal infection with E. faecium. Control mice were subcutaneously injected with saline or sodium bicarbonate, respectively. Turpentine and casein induced an acute-phase response as reflected by increases in the plasma concentrations of interleukin-6, serum amyloid P and C3. A pre-existent acute-phase response in mice was associated with a strongly reduced capacity to clear E. faecium, resulting in prolonged bacteraemia for several days. The inflammatory response to E. faecium was impaired in mice with an acute-phase response, as shown by reduced capacity to mount a neutrophilic leucocytosis in peripheral blood and by decreased local cytokine concentrations. These data indicate that the acute-phase response impairs host defence against E. faecium, suggesting that this condition may contribute to the increased vulnerability of critically ill patients to enterococcal infections.
Keywords: acute-phase proteins, bacterial infection, Enterococcus faecium, immunosuppression, inflammation, innate immunity
Introduction
Nosocomial infections with multiresistant Enterococcus faecium are a growing problem worldwide. Currently, enterococci, represent the third leading cause of nosocomial bloodstream infections in the USA.1 Patients developing infections with E. faecium are almost invariably hospitalized and severely immune debilitated, suffering from different co-morbid diseases. Knowledge of host defence mechanisms contributing to an effective immune response to E. faecium is limited. Such knowledge is necessary in light of the growing impact of E. faecium on health care and the relative lack of antibiotics that are active against this multiresistant bacterium.
Tissue injury, infection and inflammation are associated with a non-specific systemic response, the so-called acute-phase response (APR).2 This response to tissue damage is often seen in patients suffering from a variety of medical conditions such as trauma, major surgery, burn, tissue infarction, chronic illness or advanced cancer. During the APR, levels of many plasma proteins are increased, e.g. proteinase inhibitors, clotting and complements proteins, C-reactive protein, serum amyloid A and, in mice, serum amyloid P (SAP). The APR has generally been regarded as a beneficial response for the host, e.g. facilitating the elimination of micro-organisms and the repair of injured tissue. In line with this assumption, mice with a pre-existing APR demonstrated survival benefits in models of overwhelming sepsis induced by high-dose administration of Escherichia coli or Klebsiella pneumoniae.3,4 However, our laboratory recently provided evidence that the APR impairs host defence in pneumonia caused by the clinically relevant nosocomial pathogens Acinetobacter baumannii5 and Pseudomonas aeruginosa.6 Clinical studies have further supported the possibility that the APR may have a negative impact on the immune response to infection; i.e. patients with an APR before going into surgery had more and worse infection outcomes.7,8
Considering that patients infected with E. faecium virtually always have underlying diseases that are accompanied by an APR, we here investigated the effect of a pre-existing APR, induced by two well-established models for this response, subcutaneous injections of turpentine5,6,9 or casein,3 on host defence against E. faecium peritonitis.
Materials and methods
Mice
Specific pathogen-free 10-week-old female C57BL/6 mice were purchased from Harlan Sprague-Dawley (Horst, the Netherlands). The animals were housed in rooms with a controlled temperature and a 12-hr/12-hr light/dark cycle. They were acclimatized for 1 week before use, and received standard rodent chow and water ad libitum. The Animal Care and Use Committee of the University of Amsterdam approved all experiments.
Bacterial strain
A vancomycin-resistant E. faecium strain, E155, was used in all experiments. This clinical isolate from the Cook County Hospital, Chicago, IL, belongs to a genetic subset, called clonal complex-17 (CC17) that is responsible for the worldwide emergence of nosocomial multiresistant E. faecium. CC17 is characterized by high-level quinolone resistance, ampicillin resistance and a recently identified pathogenicity island, containing the variant esp gene.10 For all experiments the bacteria were grown overnight on agar sheep blood plates and then grown for approximately 3·5 hr in Todd–Hewitt broth (Difco, Detroit, MI) to mid-logarithmic phase at 37°, while shaking.
Experimental designs
To induce an APR, mice were subcutaneously injected with either 100 μl turpentine (Sigma, St Louis, MO) in both hind limbs, or 0·5 ml of 10% (wt/v) casein (Sigma) in 0·05 m NaHCO3 on the back, as described previously.3,5,6,9 Control mice received subcutaneous saline or 0·05 m NaHCO3 buffer, respectively. Twenty-four hours later, mice were intraperitoneally injected with E. faecium according to methods described previously.11 Bacteria were cultured in Todd–Hewitt broth (Difco) at 37°, harvested at mid-log phase, and washed twice in sterile saline to clear the bacteria of medium. Bacteria were resuspended in sterile isotonic saline and mice were injected intraperitoneally with approximately 108 colony-forming units (CFU) of E. faecium in 200 μl sterile isotonic saline. This bacterial dose is gradually cleared by normal wild-type mice and is not associated with lethality.11 We specifically selected this dose because it allows for investigating the impact of an APR on antibacterial defence mechanisms; higher doses are less clinically relevant because these result in early lethality caused by a toxic effect of the extremely high bacterial load administered.11 The inoculum was plated immediately after inoculation on sheep blood agar plates to determine viable counts. Experiments with turpentine-induced APR were performed on two separate occasions. In the first experiment mice were injected with a final inoculum of 8 × 107 CFU of E. faecium and killed after 2, 6 and 24 hr. In the second experiment the final inoculum was 9 × 107 CFU and mice were killed after 2, 3 and 7 days. Casein-injected mice were inoculated intraperitoneally with 9 × 107 CFU of E. faecium and killed 2 or 48 hr after infection.
Collection of samples
Mice were anaesthetized by isoflurane inhalation (Abbot, Laboratories Ltd., Kent, UK)/O2 (2%/2 litre) and a peritoneal lavage was performed with 5 ml sterile phosphate-buffered saline using an 18-gauge needle; peritoneal lavage fluid was collected in sterile polypropylene tubes (Plastipack; Beckton-Dickinson, Mountain View, CA). After collection of peritoneal fluid, blood was drawn by cardiac puncture, transferred to heparin-gel vacutainer tubes and immediately placed on ice. Next, the abdomen was opened and the liver and lungs were harvested.
Determination of bacterial outgrowth
The number of E. faecium CFU was determined in peritoneal lavage fluid, blood, liver and lung homogenates. To correct for the differences in organ weight, four times the weight (in milligrams) in microlitres of sterile saline was added. The organs were homogenized at 4°C with a tissue homogenizer (Biospect Products, Bartlesville, UK). Next, serial 10-fold dilutions were made of each sample of the homogenates, peritoneal lavage fluid, and blood in sterile saline and 50 μl of each dilution was plated onto blood agar plates. The plates were incubated at 37°C under 5% CO2, and CFU were counted after 20 hr and corrected for the dilution factor.
Cell counts and differentials
Erythrocytes were lysed with ice-cold isotonic NH4Cl solution (155 mm NH4Cl, 10 mm KHCO3, 0·1 mm ethylenediaminetetraacetic acid, pH 7·4) and the remaining cells were washed with phosphate-buffered saline. These cells and cells in the peritoneal lavage samples were counted using a Coulter Counter (Beckman Coulter, Fullerton, CA). Differential cell counts for the determination of neutrophils, macrophages/monocytes and lymphocytes were performed on cytospin preparations, stained with a modified Giemsa stain (Diff-Quick; Dade Behring, Leusden, the Netherlands). Peritoneal fluid supernatant and plasma were stored at −20° until determination of cytokines.
Assays
The SAP was measured by a sandwich enzyme-linked immunsorbent assay (ELISA), as described previously.9 In short, sheep anti-mouse SAP was used as coating antibody, and rabbit anti-mouse SAP as detecting antibody (both Calbiochem-Novabiochem International, San Diego, CA), after which an alkaline phosphatase-conjugated anti-rabbit antibody (Sigma Chemical Co., St Louis, MO) was added. The assay was developed using p-nitrohpenylphosphate; absorption was measured at 405 nm. Complement 3 (C3) was detected by sandwich ELISA as described elsewhere,9 using goat anti-mouse C3 (ICN, Costa Mesa, CA) as coating antibody, and goat anti-mouse C3c (Nordic, Tilburg, the Netherlands) as detecting antibody. The assay was developed using tetramethyl benzidine and measured at 450 nm. In both ELISAs a standard curve was made by serial dilutions of acute-phase mouse serum (Calbiochem-Novabiochem International) with known concentrations of SAP and C3. Macrophage inflammatory protein (MIP-2) and cytokine-induced neutrophil chemoattractant (KC) in peritoneal lavage fluid were measured using ELISAs (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. Tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6), IL-10 and monocyte chemoattractant protein-1 (MCP-1) were measured in peritoneal lavage fluid and plasma using a commercially available cytometric bead array multiplex assay (BD Biosciences, San Jose, CA) in accordance with the manufacturer’s recommendations.
Statistical analysis
All data are expressed as mean ± SEM. Serial data were analysed by two-way analysis of variance (anova) followed by a post hoc Bonferroni test. Two group comparisons were performed by Mann–Whitney U-test. For all analysis graphpad prism version 4 (GraphPad Software, San Diego, CA) was used. A P-value <0·05 was considered statistically significant.
Results
The turpentine-induced APR strongly impairs bacterial clearance
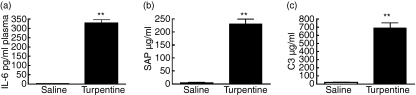
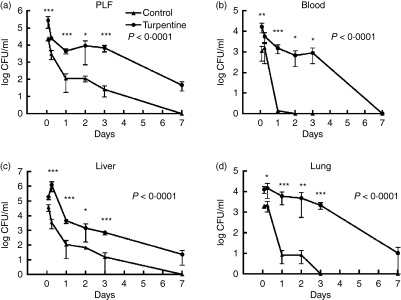
To determine the effect of a pre-existing APR on host defence against E. faecium peritonitis, mice were infected 24 hr after subcutaneous administration of turpentine or saline. As expected,5,6,9 administration of turpentine elicited an APR reflected by marked elevations of the plasma concentrations of IL-6 and the major acute-phase proteins SAP and C3, 24 hr after injection (Fig. 1, all P< 0·01 versus saline controls). Importantly, a pre-existing APR strongly impaired the clearance of E. faecium after intraperitoneal infection (Fig. 2). Indeed, whereas mice injected with saline 24 hr before infection demonstrated a rapid decline in bacterial loads in all body compartments examined, mice with a pre-existing APR had approximately 2 log more E. faecium at the primary site of infection (peritoneal fluid) up to 7 days after infection (Fig. 2a). In addition, in the presence of a pre-existing APR, blood cultures remained positive for at least 3 days, while control mice were no longer bacteraemic after 1 day (Fig. 2b). Differences in bacterial loads in livers more or less followed the pathogen burdens in peritoneal fluid (Fig. 2c), whereas differences in lungs were even more profound between groups (Fig. 2d).
Figure 1.
Turpentine-induced acute-phase response in mice. (a) Interleukin-6 (IL-6) and the acute-phase proteins (b) serum amyloid P (SAP) and (c) complement 3 (C3) concentrations were measured in plasma 24 hr after turpentine (solid bars) or saline (open bars) injection. All values are means ± SEM. n = 6 mice per group at each time-point **P< 0·01 compared to saline-injected mice.
Figure 2.
Impaired clearance of Enterococcus faecium peritonitis during turpentine-induced acute-phase response. Mean (± SEM) E. faecium colony-forming units (CFU) in (a) peritoneal lavage fluid (PLF), (b) blood, (c) liver and (d) lungs 2 and 6 hr and 1, 2, 3 and 7 days after inoculation with 108 CFU E. faecium (at T = 0). Mice were inoculated 24 hr after subcutaneous turpentine (circles) or saline (triangles) injection; n = 8 per group at each time-point. P-values in figure represent the overall difference between groups; asterisks indicate differences between groups at one time point. *P< 0·05, **P< 0·01, ***P< 0·001 compared to saline-injected mice.
The turpentine-induced APR is associated with a reduced peritoneal neutrophil recruitment
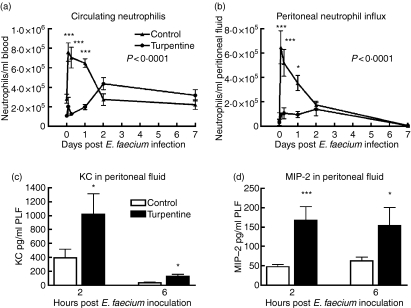
Mice that received turpentine injections had a modestly reduced number of neutrophils in their peripheral blood 24 hr after the injection (i.e. at the time of infection with E. faecium) (Fig. 3a, control mice: 2·8 × 105 ± 4·6 × 104, turpentine-injected mice: 1·1 × 105 ± 1·8 × 104, P< 0·05). Neutrophil counts in peritoneal fluid did not differ between turpentine-injected and saline-injected mice at this time-point (Fig. 3b). In mice without a pre-existing APR, intraperitoneal administration of E. faecium resulted in an increase in peripheral blood neutrophil counts within the first 2 hr after infection, accompanied by a strong and brisk influx of neutrophils into their peritoneal cavity. Remarkably, mice with a turpentine-induced APR displayed a reduced and delayed capacity to mount a peripheral neutrophilia upon intraperitoneal infection with E. faecium; in these animals neutrophil counts in blood only started to increase 2 days after infection (Fig. 3a, P< 0·001 versus saline injected mice). Even more strikingly, turpentine-injected mice had a strongly diminished influx of neutrophils into their peritoneal fluid after infection with E. faecium (Fig. 3b, P< 0·001). Even though circulating neutrophils were increased after 2 days in these mice, a substantial peritoneal neutrophil influx was not observed, although high bacterial loads were found for at least 3 days after infection. Since CXC chemokines are important for the recruitment of neutrophils to sites of infection and inflammation,12 we measured the concentrations of KC and MIP-2 in peritoneal fluid at various time-points after infection with E. faecium. At 2 and 6 hr post-infection, the local levels of both chemokines were higher in mice with a turpentine-induced APR (Fig. 3c,d). At later time-points peritoneal levels of KC and MIP-2 were undetectable in both groups (data not shown).
Figure 3.
Reduced circulating neutrophil numbers and peritoneal neutrophil influx in mice with a turpentine-induced acute-phase response. Mean (± SEM) of (a) circulating and (b) peritoneal neutrophils. Mice were inoculated intraperitoneally with Enterococcus faecium at T = 0, 24 hr after injection of turpentine (circles) or saline (triangles). Peritoneal levels of neutrophil attracting chemokines (c) cytokine-induced neutrophil chemoattractant (KC) and (d) macrophage inflammatory protein-2 (MIP-2) were measured 2 and 6 hr post-infection; n = 6 per group for uninfected control mice (T = 0) and n = 8 mice per group for infected mice at each time-point. P-values in figure represent the overall difference between groups; asterisks indicate differences between groups at one time-point. *P< 0·05, ***P< 0·001.
Effect of the turpentine-induced APR on local and systemic cytokine release
The turpentine-induced APR did not influence cytokine or chemokine concentrations before infection with E. faecium, besides the increased plasma IL-6 levels listed above (Fig. 1a). To obtain insight into the effect of a pre-existing APR on cytokine release during E. faecium peritonitis, we measured the concentrations of TNF-α, IL-6, IL-10 and MCP-1 in peritoneal fluid and plasma at 2 and 6 hr and 1, 2 and 7 days after infection. At 2 hr post-infection TNF-α, IL-6 and MCP-1 concentrations were lower in the peritoneal fluid of turpentine-injected mice (P< 0·05 versus saline-injected mice), whereas IL-10 levels were similarly low in both groups (Table 1). In plasma, besides an increased level of IL-6 in turpentine-injected mice, no differences in cytokine responses were measured (Table 1). At later time-points post-infection, TNF-α, IL-10 and MCP-1 were low or undetectable in all mice. Plasma IL-6 levels were low but higher in turpentine-injected mice throughout the entire experiment (data not shown).
Table 1.
Effect of the turpentine-induced acute-phase response on cytokine concentrations in peritoneal lavage fluid and plasma 2 hr after infection
| Peritoneal lavage fluid |
Plasma |
|||
|---|---|---|---|---|
| Cytokine (pg/ml) | Control | Turpentine | Control | Turpentine |
| TNF-α | 27 ± 5 | 14 ± 3* | 34 ± 7 | 34 ± 5 |
| IL-6 | 956 ± 114 | 494 ± 134* | 379 ± 99 | 666 ± 46* |
| IL-10 | 78 ± 14 | 77 ± 8 | 63 ± 16 | 57 ± 17 |
| MCP-1 | 419 ± 63 | 261 ± 57* | 228 ± 58 | 203 ± 21 |
Mice were inoculated intraperitoneally with 108 colony-fomring units Enterococcus faecium 1 day after subcutaneous turpentine or saline injections. Data are mean ± SEM, n = 8 mice/group. *P< 0·05 compared to saline-injected mice.
IL-6 and IL-10, interleukin-6 and -10; MCP-1, monocyte chemoattractant protein-1; tumour necrosis factor-α (TNF-α).
Casein-induced APR also impairs bacterial clearance
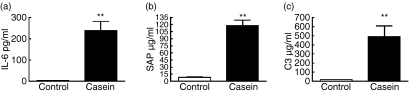
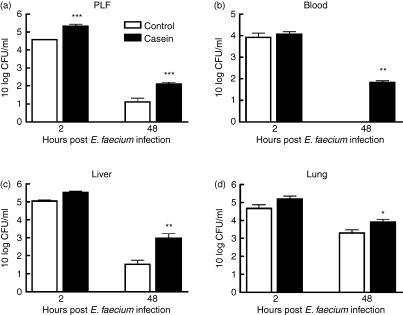
Having established that mice with a pre-existing APR induced by subcutaneous injection of turpentine displayed a diminished host defence against E. faecium peritonitis, we next determined whether an APR induced by casein, another well-established model of induced APR,3 influenced the course of this infection in a similar way. Based on the studies using turpentine to induce an APR, we chose to kill the mice 2 or 48 hr after infection with E. faecium, considering that these time-points were most suitable to study the host inflammatory response (2 hr) and the impact on bacterial clearance (48 hr). As expected,3 subcutaneous injection of casein elicited an APR, as reflected by marked elevations of the plasma concentrations of IL-6, SAP and C3, 24 hr after injection (Fig. 4, all P< 0·01 versus NaHCO3 controls). In line with the experiments using turpentine, casein-injected mice had an impaired E. faecium clearance compared to control mice. Two hours after the start of the infection, almost 1 log more bacteria were cultured from the primary site of the infection (peritoneal cavity) of mice injected with casein (Fig. 5a, P< 0·001 versus NaHCO3 controls). Forty-eight hours after the start of the infection higher loads of E. faecium were cultured from all tested organs, i.e. peritoneal lavage fluid, blood, liver and lungs (Fig. 5). The most striking difference between casein-injected and control mice was found in blood at this time-point: whereas all control mice had cleared E. faecium from their circulation, seven out of eight casein-injected mice were bacteraemic.
Figure 4.
Casein-induced acute-phase response in mice. (a) Interleukin-6 (IL-6) and the acute-phase proteins (b) serum amyloid P (SAP) and (c) complement 3 (C3) concentrations were measured in plasma 24 hr after casein (solid bars) or NaHCO3 (open bars) injection. All values are means ± SEM. n = 5 mice per group at each time-point **P< 0·01 compared to NaHCO3-injected mice.
Figure 5.
Impaired clearance of Enterococcus faecium peritonitis during casein-induced acute-phase response. Mean (± SEM) E. faecium in (a) peritoneal lavage fluid (PLF), (b) blood, (c) liver, (d) lungs 2 and 48 hr after inoculation with 108 colony-forming units (CFU) E. faecium (T = 0). Mice were infected intraperitoneally 24 hr after subcutaneous casein (solid bars) or NaHCO3 (open bars) injection; n = 8 per group at each time-point *P< 0·05, **P< 0·01, ***P< 0·001 compared to NaHCO3-injected mice.
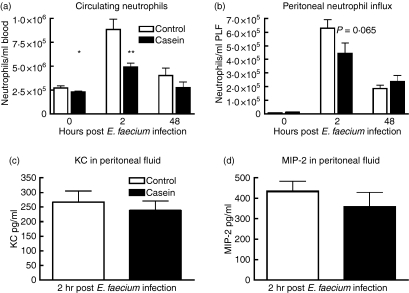
Casein administration was associated with a small but statistically significant decrease in peripheral blood neutrophil counts 24 hr post-injection (i.e. directly before infection with E. faecium; Fig. 6a, P< 0·05); in peritoneal fluid such an effect of casein on neutrophil numbers was not observed (Fig. 6b). Two hours after the infection casein-injected mice were less capable of mounting a neutrophilic response in the circulation (Fig. 6a, P< 0·01), while they tended to recruit fewer neutrophils into their peritoneal cavity (Fig. 6b, P= 0·065). At 48 hr after infection, neutrophil counts did not differ between groups in either blood or peritoneal fluid. Two hours after E. faecium infection cytokine and chemokine levels did not differ between casein-injected and control mice, except for lower peritoneal IL-6 levels in casein-injected mice (377 ± 75 versus 766 ± 107 pg/ml in control mice, P< 0·05; other cytokines not shown). Forty-eight hours after the start of the infection all cytokine and chemokine levels were low or below the detection limit (data not shown).
Figure 6.
Reduced circulating neutrophils during casein-induced acute-phase response. Mean (± SEM) of (a) circulating and (b) peritoneal neutrophils directly before intraperitoneal infection with Enterococcus faecium (T = 0) and 2 and 48 hr thereafter. Mice were inoculated peritoneally with E. faecium 24 hr after injection of casein (solid bars) or NaHCO3 (open bars). Peritoneal levels of neutrophil attracting chemokines (c) cytokine-induced neutrophil chemoattractant (KC) and (d) macrophage inflammatory protein-2 (MIP-2) were measured 2 hr post-infection. n = 5 mice per group for uninfected control mice (T = 0) and n = 8 mice per group for infected mice *P< 0·05, ***P< 0·001.
Discussion
Infections with multiresistant nosocomial pathogens like E. faecium are almost exclusively found in immunocompromised patients. Many patients who are vulnerable to E. faecium infection demonstrate an APR as a consequence of their underlying disease, for example after major trauma, surgery or burns.13,14 Indeed, in one study 74 of 158 (48%) patients with enterococcal bacteraemia had undergone recent major surgery or had sustained full-thickness burns or multiple traumatic injuries.15 Increasing evidence exists that the APR may render patients more susceptible to infections.5,6,16 As a follow-up of our previous studies on the effect of the sterile APR on pulmonary antibacterial host defence in mice,5,6 we here studied the effect of this state on host defence in the peritoneal cavity. By using two well-established murine models for a sterile APR we show that mice with a pre-existing APR are less capable of clearing E. faecium from the primary site of infection (i.e. the peritoneal cavity) and in addition remain bacteraemic for several days, whereas control mice had lower bacterial loads in peritoneal fluid and cleared E. faecium from their circulation within 1 day. These data clearly indicate that a pre-existing APR impairs host defence against E. faecium peritonitis.
To obtain insight into the impact of the APR on the innate immune response to E. faecium peritonitis we used two different models. Both the turpentine-induced and the casein-induced APR were associated with a reduced capacity to mount a rapid neutrophilic leucocytosis in peripheral blood upon infection. In the turpentine model this deficient response was accompanied by a strongly reduced influx of neutrophils into the peritoneal cavity, an effect that was not present to a significant extent in the casein model. It is likely that the attenuated neutrophil response, at least in part, explains the impaired systemic E. faecium clearance in both APR models. It should be noted that the casein-induced APR resulted in higher bacterial loads in the peritoneal cavity in spite of an only modestly and statistically not significant reduction in neutrophil influx, which suggests that additional mechanisms are involved. Indeed, our laboratory previously provided evidence that neutrophils obtained from mice with an APR are less easily activated by bacterial agonists, as reflected by a reduced capacity to upregulate the expression of CD11b/CD18.6 Moreover, we here show that mice with a turpentine-induced APR are not capable of recruiting neutrophils to the primary site of infection in spite of high bacterial loads and – at 2 days post-infection – similar blood neutrophil counts as in mice without a pre-existing APR. Moreover, we found increased levels of neutrophil attracting chemokines MIP-2 and KC in the peritoneal cavity of mice with a turpentine-induced APR, suggesting that their blood neutrophils are less responsive to these chemokines. The current data are in line with earlier investigations from our laboratory demonstrating that the turpentine-induced APR is associated with a reduced neutrophil recruitment to the lungs during pneumonia caused by either Pseudomonas or Acinetobacter.5,6
Acute injury or trauma is associated with a reduced capacity of immune cells to release proinflammatory cytokines upon stimulation with bacterial agonists.16,17 We here show that the peritoneal concentrations of TNF-α, IL-6 and MCP-1 were reduced in mice with a turpentine-induced APR after infection with E. faecium peritonitis. Similarly, the APR elicited by turpentine injection was accompanied by a diminished capacity to release cytokines in the pulmonary compartment during bacterial pneumonia.5,6 The casein-induced APR only resulted in lower peritoneal IL-6 levels. In this respect it should be noted that casein elicited a less strong APR than turpentine, as indicated by SAP and C3 concentrations. Together with the less profound effects of the casein-induced APR on neutrophil responses, these data suggest that in our studies the reduced cytokine and neutrophil responses are proportional to the extent of the APR.
The effects of a sterile APR on host defence against infection in vivo have been investigated in several other studies.3–6 Hochepied et al.4 demonstrated that turpentine injections protected mice against a lethal intraperitoneal challenge with K. pneumoniae, with reduced systemic bacterial counts. In another lethal model, caused by E. coli peritonitis, fewer bacteria were cultured when the APR was induced by subcutaneous casein injection before the infection.3 Additionally, these authors found improved survival when casein-treated mice were infected intraperitoneally or intramuscularly with Streptococcus pyogenes. Both studies used very high bacterial challenges that resulted in overwhelming sepsis that was rapidly fatal in normal mice. In fulminant sepsis models inhibition of inflammation may improve outcome. In accordance, both turpentine and α1-acid glycoprotein (a major acute-phase protein) protected mice against lethal challenges of lipopolysaccharide and TNF-α.18 In our previous studies we investigated respiratory tract infections by the nosocomial pathogens A. baumannii5 and P. aeruginosa6 at infectious doses that were not lethal in previously healthy mice. In these settings, the attenuated local proinflammatory reaction hampered the normally active innate mechanisms in the lung. Similarly, in the model used here E. faecium is rapidly cleared by the healthy host, resembling the clinical scenario where previously healthy humans are unlikely to develop enterococcal infection. As such, the model is suitable to investigate mechanisms involved in the increased susceptibility of hospitalized patients to this opportunistic pathogen. Our current data clearly suggest that an APR is one underlying condition contributing to impaired defence against E. faecium. The clinical importance of a pre-existing APR and the vulnerability for nosocomial infections is supported by a study in which patients who had an APR before surgery were at increased risk of developing infectious complications postoperatively.7 The discrepancy between our current finding that a pre-existing APR impairs host defence against E. faecium peritonitis and an earlier report demonstrating beneficial effects of an APR in other infection models3 may be related to the characteristic of bacterial species and/or infectious doses used.
Severe E. faecium infections are almost exclusively seen in severely immunocompromised patients; many of those patients display an APR. Here we sought to determine whether and, if so, to what extent an existing APR (i.e. in the absence of other predisposing factors) contributes to an enhanced susceptibility to E. faecium infection. We have demonstrated that mice treated with either turpentine or casein to induce an APR are less capable of clearing E. faecium peritonitis, resulting in persistent bacteraemia. Together with our earlier studies on the impact of the APR on pulmonary host defence, these data suggest that the APR that accompanies critical illness renders the host vulnerable to infection by common nosocomial pathogens.
Acknowledgments
The authors would like to thank J. Daalhuisen and M. ten Brink for their expert technical assistance.
References
- 1.Willems RJ, Bonten MJ. Glycopeptide-resistant enterococci: deciphering virulence, resistance and epidemicity. Curr Opin Infect Dis. 2007;20:384–90. doi: 10.1097/QCO.0b013e32818be63d. [DOI] [PubMed] [Google Scholar]
- 2.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 3.Noursadeghi M, Bickerstaff MC, Herbert J, Moyes D, Cohen J, Pepys MB. Production of granulocyte colony-stimulating factor in the nonspecific acute phase response enhances host resistance to bacterial infection. J Immunol. 2002;169:913–9. doi: 10.4049/jimmunol.169.2.913. [DOI] [PubMed] [Google Scholar]
- 4.Hochepied T, Van Molle W, Berger FG, Baumann H, Libert C. Involvement of the acute phase protein alpha 1-acid glycoprotein in nonspecific resistance to a lethal gram-negative infection. J Biol Chem. 2000;275:14903–9. doi: 10.1074/jbc.275.20.14903. [DOI] [PubMed] [Google Scholar]
- 5.Renckens R, Roelofs JJ, Knapp S, de Vos AF, Florquin S, van der Poll T. The acute-phase response and serum amyloid A inhibit the inflammatory response to Acinetobacter baumannii pneumonia. J Infect Dis. 2006;193:187–95. doi: 10.1086/498876. [DOI] [PubMed] [Google Scholar]
- 6.Renckens R, van Westerloo DJ, Roelofs JJ, Pater JM, Schultz MJ, Florquin S, van der Poll T. Acute phase response impairs host defense against Pseudomonas aeruginosa pneumonia in mice. Crit Care Med. 2008;36:580–7. doi: 10.1097/01.CCM.0B013E3181620652. [DOI] [PubMed] [Google Scholar]
- 7.Haupt W, Hohenberger W, Mueller R, Klein P, Christou NV. Association between preoperative acute phase response and postoperative complications. Eur J Surg. 1997;163:39–44. [PubMed] [Google Scholar]
- 8.Haupt W, Zirngibl H, Klein P, Riese J, Hohenberger W. Reduced TNFalpha and IL-6 production in patients who mount a preoperative acute phase response. Langenbecks Arch Surg. 1998;383:71–4. doi: 10.1007/s004230050094. [DOI] [PubMed] [Google Scholar]
- 9.Renckens R, Roelofs JJ, de Waard V, Florquin S, Lijnen HR, Carmeliet P, van der Poll T. The role of plasminogen activator inhibitor type 1 in the inflammatory response to local tissue injury. J Thromb Haemost. 2005;3:1018–25. doi: 10.1111/j.1538-7836.2005.01311.x. [DOI] [PubMed] [Google Scholar]
- 10.Willems RJ, Top J, van Santen M, Robinson DA, Coque TM, Baquero F, Grundmann H, Bonten MJ. Global spread of vancomycin-resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerg Infect Dis. 2005;11:821–8. doi: 10.3201/eid1106.041204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leendertse M, Willems RJ, Giebelen IA, et al. TLR2-dependent MyD88 signaling contributes to early host defense in murine Enterococcus faecium peritonitis. J Immunol. 2008;180:4865–74. doi: 10.4049/jimmunol.180.7.4865. [DOI] [PubMed] [Google Scholar]
- 12.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 13.Caballero-Granado FJ, Becerril B, Cisneros JM, Cuberos L, Moreno I, Pachon J. Case–control study of risk factors for the development of enterococcal bacteremia. Eur J Clin Microbiol Infect Dis. 2001;20:83–90. doi: 10.1007/s100960000429. [DOI] [PubMed] [Google Scholar]
- 14.Noskin GA, Peterson LR, Warren JR. Enterococcus faecium and Enterococcus faecalis bacteremia: acquisition and outcome. Clin Infect Dis. 1995;20:296–301. doi: 10.1093/clinids/20.2.296. [DOI] [PubMed] [Google Scholar]
- 15.Maki DG, Agger WA. Enterococcal bacteremia: clinical features, the risk of endocarditis, and management. Medicine (Baltimore) 1988;67:248–69. [PubMed] [Google Scholar]
- 16.Munford RS, Pugin J. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med. 2001;163:316–21. doi: 10.1164/ajrccm.163.2.2007102. [DOI] [PubMed] [Google Scholar]
- 17.Volk HD, Reinke P, Docke WD. Clinical aspects: from systemic inflammation to ‘immunoparalysis’. Chem Immunol. 2000;74:162–77. doi: 10.1159/000058753. [DOI] [PubMed] [Google Scholar]
- 18.Alcorn JM, Fierer J, Chojkier M. The acute-phase response protects mice from d-galactosamine sensitization to endotoxin and tumor necrosis factor-alpha. Hepatology. 1992;15:122–9. doi: 10.1002/hep.1840150121. [DOI] [PubMed] [Google Scholar]