Abstract
Allergic asthma is a chronic inflammatory disease mediated by T helper (Th)2 cell immune responses. Currently, immunotherapies based on both immune deviation and immune suppression, including the development of recombinant mycobacteria as immunoregulatory vaccines, are attractive treatment strategies for asthma. In our previous studies, we created a genetically recombinant form of bacille Calmette–Guerin (rBCG) that expressed Der p2 of house dust mites and established that it induced a shift from a Th2 response to a Th1 response in naive mice. However, it is unclear whether rBCG could suppress allergic airway inflammation in a mouse model. In this article we report that rBCG dramatically inhibited airway inflammation, eosinophilia, mucus production and mast cell degranulation in allergic mice. Analysis of interferon-γ (IFN-γ) and interleukin-4 (IL-4) levels in bronchoalveolar lavage fluid (BALF) and lung tissue revealed that the suppression was associated with a shift from a Th2 response to a Th1 response. At the same time, rBCG induced a CD4+ CD25+ Foxp3+ T-cell subtype that could suppress the proliferation of Th2 effector cells in vitro in an antigen-specific manner. Moreover, suppression of CD4+ CD25+ T cells could be adoptively transferred. Thus, our results demonstrate that rBCG induces both generic and specific immune responses. The generic immune response is associated with a shift from a Th2 to a Th1 cytokine response, whereas the specific immune response against Der p2 appears to be related to the expansion of transforming growth factor-β (TGF-β)-producing CD4+ CD25+ Foxp3+ regulatory T cells. rBCG can suppress asthmatic airway inflammation through both immune deviation and immune suppression and may be a feasible, efficient immunotherapy for asthma.
Keywords: airway inflammation, asthma, Dermatophagoides protein II group, recombined BCG, regulatory T cell
Introduction
Allergic asthma is a chronic disorder of the airways and is characterized by reversible airflow obstruction and airway eosinophil inflammation. The pathology in asthma occurs as a consequence of the increased production of interleukin (IL)-4, IL-5 and IL-13 by allergen-specific CD4+ T helper (Th)2 cells.1,2 In addition, the risk of developing asthma is directly related to the acquisition of immediate hypersensitivity to environmental allergens.3 Meanwhile, house dust mites are the most important sources of indoor allergens responsible for the development of asthma.
Current asthma therapies, such as inhaled corticosteroids, β2-agonists, M cholinergic receptor antagonists, or anti-leukotrienes, are directed at symptom relief, reduction or neutralization of effector molecules and inflammatory mediators. These therapies are effective for acute disease and for relieving symptoms. However, they have limited long-term salutary effects. Conventional allergen immunotherapy, while having long-term and impressive efficacy, requires multiple injections over several years and is associated with frequent failure and occasional immunoglobulin E (IgE)-mediated adverse events.4 Therefore, an alternative, more effective and long-lasting therapeutic approach for asthma has been focused on the development of vaccine strategies that alter the underlying immune response and convert detrimental allergic responses to protective immune responses, thereby modifying the course of the disease.
We previously modified bacille Calmette–Guerin (BCG) to express Der p2 of house dust mites on the bacterial cell wall.5 Subsequently, we established that the Der p2 rBCG induced a shift from a Th2 response to a Th1 response in naive mice.6 However, the regulatory role of the Der p2 rBCG in an animal model of Th2-dominated illness is still unknown.
The BCG vaccine is the most widely used Th1-inducing vaccine.7 Several studies have argued that BCG may be applied to treatment of allergy by inducing an immune deviation from Th2 to Th1.8–10 However, recently the Th1/Th2 bias theory was challenged by an immune-suppression theory because some data showed the importance of regulatory T cells (Tregs) in the pathogenesis of asthma. Accordingly, several studies suggested that mycobacteria can be used as an adjuvant to induce Tregs. Treatment of mice with mycobacterium-induced allergen-specific Tregs produced IL-10 and transforming growth factor-β (TGF-β), which protected against airway inflammation.11 Killed Mycobacterium vaccae can suppress airway eosinophilia through the induction of allergen-specific Tregs.12 Therefore, whether immune deviation or immune suppression may be responsible for the suppressive effect of BCG/rBCG on allergic airway inflammation remains to be further investigated.
The aim of this study was to investigate whether Der p2 rBCG can regulate allergic eosinophil inflammation in a mouse model of asthma and to explore the possible mechanisms of this regulation.
Materials and methods
Animals
Healthy female C57BL/6 mice (6–8 weeks of age) and green fluorescent protein (GFP) transgenic mice (6–8 weeks of age) were purchased from the experimental animal center of Forth Military Medical University (Xi’an, China). All mice were bred under pathogen-free conditions. All mouse protocols were approved by the Animal Experiment Administration Committee of Fourth Military Medical University.
Der p2 rBCG
Der p2 rBCG was constructed in our own laboratory by professor Shi.5 In brief, the gene fragment encoding the 19 000 molecular weight antigen and the upstream control element (19-ss) was amplified by polymerase chain reaction (PCR) from Mycobacterium tuberculosis H37Rv. The 19 000 molecular weight antigen is a cell wall-associated lipoprotein present in Mycobacterium tuberculosis and in BCG vaccine strains.13,14 After sequencing, the PCR product was cloned into the Escherichia coli–BCG shuttle vector, pOLYG, and then into pCW. The Der p2 gene was also cloned into the pCW vector, and the Der p2–pCW–rBCG construct was induced into BCG by electroporation.
Administration of BCG or rBCG
Mice were vaccinated intraperitoneally (i.p.) with 106 colony-forming units (CFU) of rBCG or BCG on day 1. Control mice were injected with physiological saline (PS). Six weeks later, mice were sensitized (to investigate the effect of rBCG on allergic airway inflammation) or killed (for analysis of spleen cells, proliferation assays and adoptive cell transfer) on day 43.
Allergen sensitization and challenge protocol
Mice were sensitized i.p. with 100 μg of ovalbumin (OVA) (A5503; Sigma-Aldrich, St Louis, MO) or Der p2 (Indoor Biotechnologies Inc., Charlottesville, VA) adsorbed to 9% potassium alum (A1577; Sigma-Aldrich) on days 43 and 50. On days 57 and 64, mice were challenged with 100 μg of OVA or Der p2 in phosphate-buffered saline (PBS) by the intratracheal route. Control mice were treated with PS.15
Bronchoalveolar lavage fluid
Twenty-four hours after the final challenge, mice were killed. Bronchoalveolar lavage fluid (BALF) was obtained by the slow injection of ice-cold saline (0·3 ml) into the trachea three times (total, 0·9 ml). Fluids were centrifuged at 1200 g, and pellets were recovered for cellular analysis. Supernatants were stored at −80° for biochemical analyses. The total number of cells in BALF was counted using a haemocytometer. A differential count was performed using cytomear preparations. The cells were fixed and stained with Wright’s stain. Differential counts of 200 cells were carried out using standard morphological criteria to identify eosinophils.
Histology
For histopathology, lungs were inflated and fixed with 10% buffered formalin after collection of BALF. Samples were embedded in paraffin and then sectioned (5 μm). Sections were stained with haematoxylin and eosin (H & E) for analyzing airway inflammation and pathological changes. Goblet cells were stained with Alcian blue–periodic acid schiff (AB-PAS), and the histological mucus index was quantified for goblet cell hyperplasia.16
Enzyme-linked immunosorbent assay
The concentrations of IL-4, interferon-γ (IFN-γ), IL-5, IL-13, IL-10 and TGF-β in BALF were determined (JingMei Biotech, Shenzhen, China) according to the manufacturer’s protocols.
To detect β-hexosaminidase activity, samples were incubated with 80 μl of substrate solution [1·3 mg/ml of p-nitrophenyl-β-d-2-acetamido-2-eoxyglucopyranozide (Sigma-Aldrich) in 0·1 m citrate, pH 4·5]. The reaction was stopped by the addition of 200 μl of 0·2 m glycine (pH 10·7). βeta-hexosaminidase activity was expressed as the absorbance at 405 nm.
Quantitative reverse transcription–PCR
Total RNA was isolated from lung tissue using TRIzol (Invitrogen, Carlsbad, CA) and quantified. Two micrograms of total RNA was reverse transcribed using reverse transcriptase (Promega, Madison, WI) according to the manufacturer’s instructions. Real-time PCR was performed using a kit (SYBR Premix EX Taq; Takara, Dalian, China) and the ABI PRISM 7300 Real-Time PCR system, with β-actin as a reference control. Primers for β-actin, IL-4 and IFN-γ were as follows: β-actin, 5′-CTAAGGCCAACCGTGAAAAG, 5′-AGCCTGGATGGCTACGTACAT; IL-4, 5′-ACAGGAGAAGGGACGCCAT, 5′-GAAGCCCTACAGACGAGCTCA; and IFN-γ, 5′-TCTTGAACGGCAGCTCTGAG, 5′-TGGCGACAGGTCATTCATCA.
Flow cytometric analysis
Splenocytes were freshly isolated. For surface staining, cells were labeled with anti-CD4–phycoerythrin (PE) (553049; BD Biosciences) or anti-CD25–fluorescein isothiocyanate (FITC) (553071; BD Biosciences, San Jose, CA). For staining intracellular TGF-β, cells were permeabilized in cytofix/cytoperm, washed in perm/wash buffer (BD Biosciences) and stained with anti-TGF-β–biotin (sc-80346; Santa Cruz Biotechnology, Santa Cruz, CA) for 30 min. For staining Foxp3, a kit from eBioscience containing the monoclonal antibody (mAb) FKJ-16s was used according to the manufacturer’s instructions. Labeled cells were analysed using a flow cytometer (BD Calibur, San Jose, CA). The frequency of each cell subset was calculated based on the percentage of positive cells in the CD4 gate.
Isolation of CD4+ CD25+ T cells and CD4+ CD25− T cells
Isolation of mouse CD4+, CD4+ CD25−, and CD4+ CD25+ T cells was performed by using a mouse Treg isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany), according to the manufacturer’s instructions. Briefly, CD4+ T cells were first enriched through negative selection by magnetically removing other types of cells. The CD4+ T cells were incubated with magnetic beads conjugated with an anti-CD25 IgG to separate CD4+ CD25− and CD4+ CD25+ T-cell subpopulations. The purity of the resulting T-cell subpopulations was confirmed to be higher than 95% by flow cytometry.
Reverse transcription–PCR analysis
The CD4+ T cells were isolated and the total RNA was isolated from each sample using TRIzol (Invitrogen) and quantified. Two micrograms of total RNA was reverse transcribed with reverse transcriptase (Promega) according to the manufacturer’s instructions. All PCR experiments were performed with Taq polymerase (Promega) and the primers 5′-AGGAGAAAGCGGATACCA and 5′-TGTGAGGACTACCGAGCC for Foxp3, and 5′-AACCCTAAGGCCAACCGTGAAAAG and 5′-GCAGGATGGCGTGAGGGAGAG for the β-actin control.
Proliferation assays
Cells were cultured in RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin (100 U/ml), streptomycin (100 μg/ml), glutamine (2 mmol/l), non-essential amino acids (0·1 mol/l), sodium pyruvate (1 mmol/l) and 2-mercaptoethanol (2-ME) (55 μmol/l) (all from Invitrogen Life Technologies, Carlsbad, CA). To assay the suppressive activity of rBCG-induced Tregs, 105 CD4+ CD25− T cells from OVA-sensitized or Der p2-sensitized mice, 105 CD4+ CD25+ Tregs from rBCG-vaccinated mice, 105 antigen-presenting cells (APCs) and allergens (OVA or Der p2) were plated in triplicate in 96-well plates for 7 days. APCs were irradiated spleen cells depleted of T cells. Dead cells were excluded by Trypan Blue staining and cell proliferation was assessed by cell counting on day 7. We also assessed proliferation by 5(6)-(N-Succinimidyloxycarbonyl)-3′,6′,0,0′-diacetylfluorescein (CFSE) dilution. CD4+ CD25− T cells were first labeled with 1 μM CFSE. After 7 days of culture, CFSE dilution was assessed and analysed using cell quest (BD Biosciences).
Adoptive cell transfer
CD4+ CD25+ T cells from rBCG-vaccinated mice were isolated on day 42. Approximately 3 × 105 CD4+ CD25+ T cells were transferred into Der p2-sensitized or OVA-sensitized recipient mice. For analysis of the migration of donor CD4+ CD25+ T cells, Der p2-sensitized GFP Tg mice were used as recipients.
Statistical analysis
Data were expressed as mean ± standard error of the mean (SEM). Statistical analyses were performed on the data using single-factor analysis of variance (anova) on the three groups and with a Student’s unpaired t-test for comparisons of two groups. A P-value of < 0·05 was assumed to be significant.
Results
Significantly reduced airway inflammation in rBCG-vaccinated mice
We first studied the effect of rBCG vaccination on the airway environment, having established that no changes in airway cell composition or BALF cytokine secretion in mice occurred as a result of rBCG vaccination per se (H.-F. Ou-Yang, J.-R. Shi, C.-G. Wu, unpublished data). Mice were sensitized twice with allergen Der p2, on days 43 and 50 after BCG or rBCG vaccination (day 1), before airway challenge on days 57 and 64. Recovery of BALF was performed on day 65.
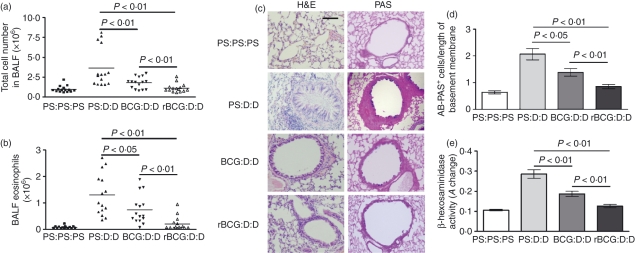
Both BCG and rBCG significantly reduced the amount of airway cellular infiltrates after challenge with Der p2 (BCG, P < 0·01; rBCG, P < 0·01) (Fig. 1a). Differential cell counting revealed a profound reduction of airway eosinophilia in both BCG- and rBCG-vaccinated mice (Fig. 1b; BCG, P < 0·05; rBCG, P < 0·01). Notably, rBCG had a more potently suppressive effect on local airway inflammation than BCG (total cell number, P < 0·01; number of eosinophils, P < 0·01) (Fig. 1a,b).
Figure 1.
Airway cell infiltration and tissue pathology were inhibited in mice vaccinated with bacille Calmette–Guérin (BCG)- and recombinant bacille Calmette–Guérin (rBCG)-. Mice were vaccinated intraperitoneally (i.p.) with 106 colony-forming units (CFU) of BCG or rBCG on day 1. Six weeks later, mice were sensitized i.p. with 100 μg of Der p2 adsorbed to 9% potassium alum on days 43 and 50. On days 57 and 64, mice were challenged with 100 μg of Der p2 in phosphate-buffered solution (PBS) by the intratracheal route. Lavage cells were recovered 24 hr after final airway challenge (day 65), and histological sections of lung tissues were made. Labels indicate treatment/sensitization/challenge. The letter ‘D’ denotes Der p2. PS, physiological saline. (a) Total bronchoalveolar lavage (BAL) cell numbers (n = 15). Horizontal bars denote paired experimental groups for which statistical significance is displayed in the figure. Data represent one of three independent experiments. (b) Eosinophil numbers (n = 15). (c) Formalin-fixed lung 5-μm sections stained with haematoxylin and eosin (H & E) or Alcian blue–periodic acid schiff (AB-PAS). Scale bar, 40 μm. (d) Enumeration of goblet cells. Data represent the mean ± standard deviation (SD) of five mice, with a mean score from three representative lung sections per mouse. (e) βeta-hexosaminidase in bronchoalveolar lavage fluid (BALF) (n = 10). A, absorbance.
Reduced tissue pathology in rBCG-vaccinated mice
To determine whether suppressed airway cellular infiltration represented a more general down-modulation of pathology, lung histological sections were compared in four groups of mice. H & E staining was used to characterize cellular infiltrates, and AB-PAS was used to identify mucus-producing goblet cells in the epithelial border. In addition, mast cell degranulation was estimated by measuring the levels of β-hexosaminidase in BALF.
In asthmatic control mice, airway challenge leads to a dense peribronchiolar inflammatory infiltrate of lymphocytes, and to the production of mononuclear and polymorphonuclear cells with epithelial shedding and extended columnal cells (Fig. 1c). Furthermore, an accumulation of mucin-containing goblet cells line the connecting airways, underpinning the overall increase in mucus production (Fig. 1c). In BCG- and rBCG-vaccinated mice, however, tissue inflammation was greatly reduced, with significantly less peribronchial and perivascular cellular infiltration and mucin staining (Fig. 1c). Goblet cell numbers were substantially reduced in both BCG- and rBCG-vaccinated mice (Fig. 1d; BCG, P < 0·05; rBCG, P < 0·01). In addition, the allergen-induced increase in BALF β-hexosaminidase, indicating mast cell mediator release, was also attenuated in both BCG- and rBCG-vaccinated mice (Fig. 1e; BCG, P < 0·01; rBCG, P < 0·01). rBCG had a more potently suppressive effect on the proliferation of goblet cells (Fig. 1d, P < 0·01) and mast cell mediator release (Fig. 1e, P < 0·01) than BCG.
Thus, both BCG and rBCG can protect mice against a range of allergic airway inflammatory pathologies, including the infiltration of inflammatory cells, mast cell degranulation and goblet cell proliferation. Furthermore, rBCG had a more potent inhibitory effect than BCG. We selected cellular infiltration in BALF as the keynote parameter to dissect the mechanisms leading to this broader diminution of pathology.
Reversion of Th2 bias by rBCG in allergic mice
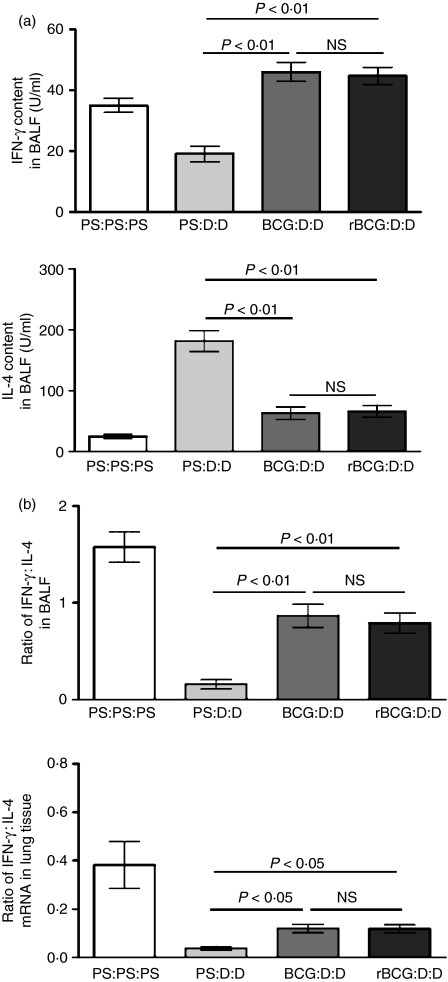
Reportedly, BCG can reduce allergic asthma to OVA in BALB/c mice,17 rats18 and guinea pigs19 through inducing an immune deviation from Th2 to Th1. To examine whether vaccination with rBCG could also reverse the Th2 bias, the levels of IFN-γ and IL-4 in BALF were measured. BCG- and rBCG-vaccinated mice all showed diminished IL-4 responses (Fig. 2a; BCG, P < 0·01; rBCG, P < 0·01), whereas the concentration of the Th1 cell signature cytokine, IFN-γ, was elevated (Fig. 2a; BCG, P < 0·01; rBCG, P < 0·01). The ratio of IFN-γ : IL-4 was higher in BCG- or rBCG-vaccinated mice than in unvaccinated mice (Fig. 2a; BCG, P < 0·01; rBCG, P < 0·01). However, there was no difference between BCG-vaccinated mice and rBCG-vaccinated mice in the content of IFN-γ (Fig. 2a, P > 0·05), IL-4 (Fig. 2a, P > 0·05) and the ratio of IFN-γ : IL-4(Fig. 2a, P > 0·05).
Figure 2.
Reversion of the T helper (Th)1/Th2 bias by bacille Calmette–Guérin (BCG) and recombinant bacille Calmette–Guérin (rBCG) in asthmatic mice. Labels indicate treatment/sensitization/challenge. D, Der p2; NS, not significant; PS, physiological saline. (a) The levels of interferon-γ (IFN-γ) and interleukin-4 (IL-4) in bronchoalveolar lavage fluid (BALF) from four mice per group and the ratio of IFN-γ : IL-4 (n = 10). Data represent one of three independent experiments. (b) The levels of IFN-γ and IL-4 mRNA isolated from lung tissue were quantified using the real-time polymerase chain reaction (PCR) SYBR Green System (n = 10). The results represent the expression of the individual mRNAs with normalization to β-actin, using the cycle threshold (Ct) method. The results represent values from duplicate measurements from one of three independent experiments.
We also analysed the levels of IFN-γ and IL-4 mRNA in lung tissue using real-time PCR, and the ratio of IFN-γ : IL-4 mRNA was established. The data show that this ratio was significantly higher in the BCG- and rBCG-vaccinated groups than in the asthmatic control group (Fig. 2b; BCG, P < 0·05; rBCG, P < 0·05) and there was no significant difference between BCG-vaccinated mice and rBCG-vaccinated mice (Fig. 2b, P > 0·05).
These data indicate that BCG and rBCG had a similar ability to induce a shift from the Th2 to the Th1 response in an animal model of asthma and altered the highly polarized type 2 cytokine environment both in local airway and in whole-lung tissue.
Suppression of local type 2 effector cytokines
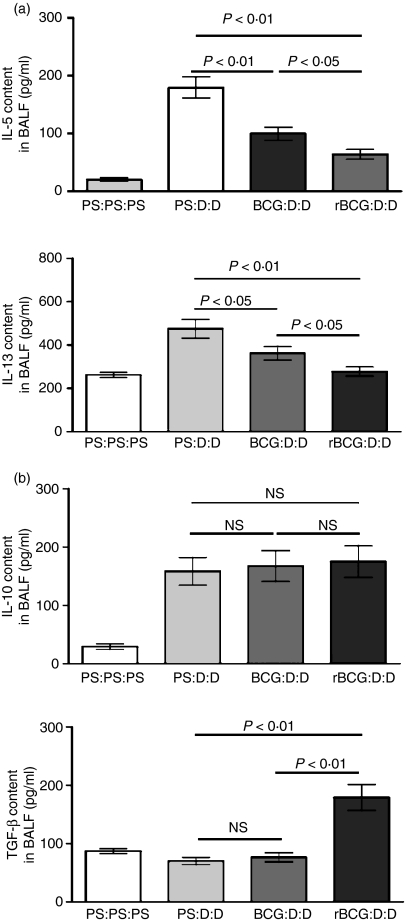
To analyse in greater detail the mechanism of the inhibitory effect of BCG and rBCG, we measured local cytokine IL-5 and IL-13 levels in BALF. After airway challenge the levels of both IL-5 and IL-13 were elevated in the BALF of asthmatic control mice (Fig. 3a). However, IL-5 was significantly diminished by BCG or rBCG vaccination (Fig. 3a; BCG, P < 0·01; rBCG, P < 0·01). Reductions in the key agents in the mobilization and extravasation of eosinophils provided a mechanistic explanation for the dramatically reduced airway eosinophilia in BCG- and rBCG-vaccinated mice. Similarly, the elevation of the IL-13 level was also reversed by BCG or rBCG vaccination (Fig. 3a; BCG, P < 0·05; rBCG, P < 0·01), which may explain the down-regulation of goblet cell number in BCG- and rBCG-vaccinated mice. Notably, rBCG had a more potent inhibitory effect on the level of IL-5 and IL-13 than BCG (Fig. 3a; IL-5, P < 0·05; IL-13, P < 0·05).
Figure 3.
Cytokine responses in bronchoalveolar lavage fluid (BALF). BALF from a group of four mice were assayed for the indicated cytokines (n = 10). Data represent one of three independent experiments. Labels indicate treatment/sensitization/challenge. BCG, bacille Calmette–Guérin; D, Der p2; NS, not significant; PS, physiological saline; rBCG, recombinant bacille Calmette–Guérin. (a) Interleukin (IL)-5 and IL-13. (b) IL-10 and transforming growth factor-β (TGF-β).
We also examined the regulatory cytokines IL-10 and TGF-β (Fig. 3b). There was no difference in the IL-10 level among asthmatic control mice, BCG-vaccinated mice and rBCG-vaccinated mice. However, rBCG, but not BCG, can up-regulate the level of TGF-β in BALF (Fig. 3b; BCG, P > 0·05; rBCG, P < 0·01). This difference suggested that, in addition to inducing an immune deviation from Th2 to Th1, rBCG may down-regulate the allergic immune response through other regulatory mechanisms that may be mediated mainly through TGF-β.
Induction of antigen-specific CD4+ CD25+ Foxp3+ T cells by rBCG
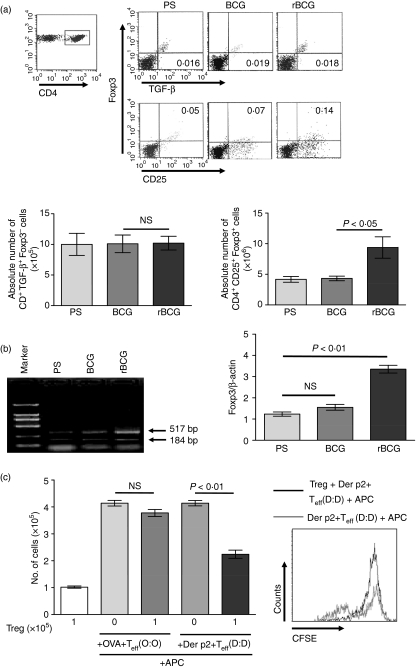
Reportedly, the suppressive function of both adaptive CD4+ CD25+ Foxp3+ T cells and CD4+ TGF-β+ Foxp3− T cells (Th3) was associated with TGF-β. To investigate whether a defined subset of regulatory T cells were generated after vaccination with rBCG, we analysed spleen cells taken from mice that had been vaccinated with rBCG or BCG 42 days previously. As shown in Fig. 4a, the relative proportion and the absolute number of CD4+ CD25+ Foxp3+ T cells increased after vaccination with rBCG (Fig. 4a, P < 0·05), but not with BCG. However, the relative proportion and the absolute number of CD4+ TGF-β+ Foxp3− T cells were not skewed. We also determined Foxp3 expression by reverse transcription–PCR: CD4+ spleen cells from rBCG-vaccinated mice contained a level of Foxp3 mRNA that was more than threefold higher than found in similar cells from naive animals (Fig. 4b; P < 0·01). Therefore, rBCG may induce a CD4+ Foxp3+ T-cell subtype.
Figure 4.
Induction of antigen-specific CD4+ CD25+ Foxp3+ T cells by recombinant bacille Calmette–Guérin (rBCG). (a) Surface expression of CD25 and intracellular expression of Foxp3 and transforming growth factor-β (TGF-β) in spleen CD4+ T cells from groups of three mice. Labels indicate treatment 42 days before mice were killed. Representative flow cytometric analysis and percentages of cells are shown in the indicated squares. Absolute cell numbers were calculated for total splenocytes. Data are the mean ± standard deviation (SD) from three experiments (n = 15). (b) The expression of Foxp3 mRNA in CD4+ T cells from groups of three mice. Data represent the mean ± SD values from three experiments (n = 15). (c) Approximately 105 CD4+ CD25− T cells from ovalbumin (OVA)-sensitized or Der p2-sensitized mice, 105 CD4+ CD25+ regulatory T cells (Tregs) from rBCG-vaccinated mice, 105 antigen-presenting cells (APCs) and allergens (OVA or Der p2) were plated in triplicate in 96-well plates for 7 days. Cell proliferation was assessed by cell counting and flow cytometry on day 7. Data are the mean ± SD from three experiments (n = 15). Labels indicate sensitization/challenge. BCG, bacille Calmette–Guérin; CFSE, 5(6)-(N-Succinimidyloxycarbonyl)-3′,6′,0,0′-diacetylfluorescein; D, Der p2; NS, not significant; O, ovalbumin; PS, physiological saline; Teff, effector T cell.
To determine the suppressive activity of the induced CD4+ CD25+ Foxp3+ T cells and the specificity of the inhibitory response, we performed proliferation assays in vitro. CD4+ CD25+ T cells from rBCG-vaccinated mice were isolated from spleen cells by magnetic antibody cell sorting (MACS). CD4+ CD25− T cells from OVA- or Der p2-sensitized mice were also isolated as responders. CD4+ CD25+ T cells, CD4+ CD25− T cells alone and CD4+ CD25+ T cells plus CD4+ CD25− T cells, were assayed Fig. 4c. Cell proliferation was assessed by cell counting on day 7. The data showed that the proliferation of CD4+ CD25− T cells from Der p2-sensitized mice was significantly inhibited. However, although the proliferation of CD4+ CD25− T cells from OVA-sensitized mice was also down-regulated by CD4+ CD25+ T cells, this did not reach statistical significance. We also determined the suppressive activity of CD4+ CD25+ T cells by CFSE dilution. CD4+ CD25− T cells from Der p2-sensitized mice were labeled with CFSE and cocultured with APCs and Der p2, in the presence or absence of CD4+ CD25+ T cells. CFSE dilution was assessed and analysed by flow cytometry. As shown in Fig. 4c, when CD4+ CD25+ T cells were added, the number of cells with reduced CFSE expression decreased. Hence, CD4+ CD25+ T cells induced by rBCG can suppress the proliferation of Th2 effector cells in vitro in a Der p2-specific manner.
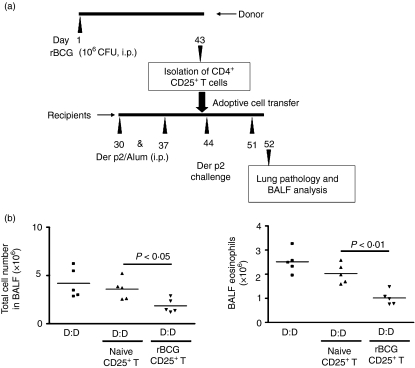
Transfer of protection with CD4+ CD25+ T cells
To investigate the suppressive activity of the CD4+ CD25+ T cells induced by rBCG in vivo, CD4+ CD25+ T cells from rBCG-vaccinated mice were isolated from spleen cells by MACS. Then, CD4+ CD25+ T cells were transferred into allergen-sensitized recipients 1 day before airway challenge (Fig. 5a).The most puissant reduction of airway inflammation occurred in recipient mice (Fig. 5b). The number of total airway cells and eosinophils were all significantly decreased (total cell, P < 0·05; eosinophils, P < 0·01). However, there was no significant difference in OVA-sensitized mice (data not shown). Thus, our data suggest that the CD4+ CD25+ phenotype T-cell population can mediate Der p2-specific suppression of airway allergy in vivo.
Figure 5.
Transfer of the protective effect with recombinant bacille Calmette–Guérin (rBCG)-induced CD4+ CD25+ T cells. (a) Protocol for transfer. Approximately 3 × 105 CD4+ CD25+ T cells were transferred into Der p2-sensitized recipient mice 1 day before airway challenge. (b) Total cell counts and eosinophil numbers in the bronchoalveolar lavage fluid (BALF) from recipients (n = 5). Data represent one of three independent experiments. Labels indicate sensitization/challenge. CFU, colony-forming units; D, Der p2; i.p., intraperitoneal.
Migration of donor CD4+ CD25+ T cells to the lungs and thoracic lymph nodes (TLNs)
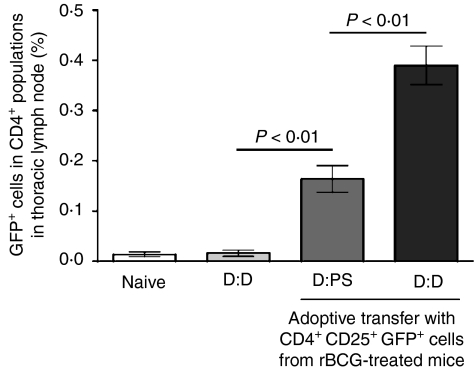
To investigate whether transferred CD4+ CD25+ T cells can relocate to airway-associated tissues in recipient mice, we followed the fate of transferred cells bearing the GFP marker, which permits donor cell identification after transfer into receptor mice. CD4+ CD25+ T cells from rBCG-vaccinated GFP transgenic mice transferred i.v. into sensitized mice 1 day before airway challenge could be found in the local draining lymph nodes, comprising 0·39 ± 0·09% (challenged with Der p2) and 0·16 ± 0·06% (challenged with PS) of the CD4+ population, respectively (Fig. 6). These data suggest that donor cells from rBCG-vaccinated mice migrated to sites of inflammation, which may directly suppress host responses.
Figure 6.
Tracking of donor lymphocytes in recipient mice. Approximately 3 × 105 CD4+ CD25+ T cells from recombinant bacille Calmette–Guérin (rBCG)-vaccinated green fluorescent protein (GFP) transgenic (Tg) mice were transferred into Der p2-sentisized recipient mice 1 day before challenge with physiological saline (PS) or Der p2. After the second airway challenge, thoracic lymph node cells were recovered and stained for CD4 expression. Data are the mean ± standard deviation (SD) of cells from three experiments (n = 15). Labels indicate sensitization/challenge. D, Der p2.
Discussion
In this study we demonstrated, in a murine model of allergic asthma, that Der p2 rBCG very effectively inhibited airway inflammation. These effects were accompanied by both immune deviation from the Th2 to the Th1 response and by the induction of a CD4+ CD25+ Foxp3+ T-cell subtype. Our observations demonstrate that antigen-recombined BCG is a very promising method in the development of allergen immunotherapy.
Asthma is characterized by the overproduction of Th2 cytokines.1 The Th2-driven inflammatory process may be a consequence of a relative insufficiency in IFN-γ production because IFN-γ can inhibit the development of Th2 responses.20 In addition, clinical studies have demonstrated that reduced IFN-γ secretion in neonates is associated with the subsequent development of atopy.21 Furthermore, a predisposition towards the overproduction of Th1 cytokines may protect against atopy. Thus, methods to enhance IFN-γ production might be clinically useful in the treatment of allergic asthma. Indeed, immunotherapies and immune-modulatory approaches that enhance Th1-dominated responses appear to be beneficial in allergic individuals and in animal models of allergic disease. BCG has a long record of safe use in humans and is a potent activator of the Th1 response, as shown by delayed-type hypersensitivity or by IFN-γ production by peripheral blood lymphocytes. Our current report describes that immunotherapy with Der p2 rBCG is highly effective in preventing Der p2-induced allergic airway inflammation. The down-regulation of airway inflammation was associated with a significant increase in IFN-γ and a significant reduction of IL-4. Our results extend earlier work with BCG that demonstrate a reduction in allergic asthma to OVA in BALB/c mice,15 rats16 and guinea pigs.17 These findings can be translated to the human situation, in which exposure to M. tuberculosis and development of allergy were inversely correlated in Japanese schoolchildren in an epidemiological study.9 Notably, because BCG can reduce allergic airway inflammation in both OVA- and Der p2-induced murine models of asthma, we speculate that the shift from a Th2 to a Th1 cytokine response induced by BCG/rBCG may be a generic immune response.
In our study, we also found that rBCG had a more potent inhibitory effect than BCG. The potent capacity of rBCG to reduce airway inflammation might be a result of the fact that rBCG activates multiple immunological mechanisms. The fact that the TGF-β level in BALF was elevated and that spleen cells from rBCG-vaccinated mice contained incremental numbers of CD4+ CD25+ Foxp3+ T cells hinted that the activity of Tregs may also be responsible for the rBCG-induced suppression. The results of the suppression assay in vitro indicated that the CD4+ CD25+ T-cell subtype induced can suppress the proliferation of Th2 effector cells in vitro in a Der p2-specific manner. Using an adoptive cell transfer experiment, we demonstrated that the induced CD4+ CD25+ T cells also had antigen-specific suppressive activity in vivo. Then, rBCG-induced suppression may also be mediated through antigen-specific CD4+ CD25+ Foxp3+ Tregs. Because Tregs can suppress both Th1 and Th2 responses, the elevation of IFN-γ cannot be interpreted with immune suppression. Therefore, our result suggests that rBCG induces both generic and specific immune responses. The generic immune response is associated with a shift from a Th2 to a Th1 cytokine response, whereas the specific immune response against Der p2 appears to be related to the expansion of TGF-β-producing CD4+ CD25+ Foxp3+ Tregs.
In the past few years, antigen-specific adaptive Tregs have been induced by a number of different immunization strategies. Many of these antigen-specific Tregs have been shown to inhibit the development of allergy. Reportedly, repeated low-dose inhaled exposure to antigen induced Foxp3+ CD4+ T cells, which expressed surface TGF-β and suppressed development of airway inflammation in vivo, and expressed antigen-induced T-cell proliferation in vivo, through a contact-dependent mechanism.22,23 Whether Der p2 rBCG induced Tregs through a similar mechanism is currently under active investigation.
Most studies agree that although different types of Tregs are antigen specific, they exert suppressive activity in a non-antigen-specific manner.24 Then, why can suppression by rBCG-induced Tregs not be transferred into OVA-sensitized mice? In this respect, the possible reason may be that the activation of Tregs requires adequate antigen restimulation. As shown in another study, Tregs specific for exogenous antigen suppressed bystander allograft responses that required re-activation in the donor before transfer.25
In conclusion, we demonstrated that one dose of Der p2 rBCG can greatly inhibit allergic airway inflammation. The effect involved two different mechanisms: eliciting an immune deviation from Th2 to Th1; and inducing antigen-specified regulatory T cells. Clinically, allergic asthmatic patients usually appear to be hypersensitive to one major allergen and to several minor allergens. Der p2 rBCG can induce both a specific and a generic immune response to inhibit the detrimental Th2 response across multiple allergens. Therefore, our results suggest that allergen-recombined BCG may be a feasible and highly attractive immunotherapy for the treatment of allergic disease.
Acknowledgments
The work was supported by grants from the National Natural Science Foundation of China (30570804, 30770927).
References
- 1.Umetsu DT, DeKruyff RH. TH1 and TH2 CD4+ cells in human allergic diseases. J Allergy Clin Immunol. 1997;100:1–6. doi: 10.1016/s0091-6749(97)70186-6. [DOI] [PubMed] [Google Scholar]
- 2.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 3.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 4.Cox L. Accelerated immunotherapy schedules: review of efficacy and safety. Ann Allergy Asthma Immunol. 2006;97:126–37. doi: 10.1016/S1081-1206(10)60003-8. [DOI] [PubMed] [Google Scholar]
- 5.Shi JR, Li Y, Qi HW, Li BH, Fan XL. Construction and identification of the E.coli-BCG shuttle vector expressing lipoprotein Der p2 on cell wall of mycobacterium vaccae. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2003;19:132–5. [PubMed] [Google Scholar]
- 6.Shi JR, Zhang XH, Shi CH, Cao YX, Wu CG, Li Y, Fan XL, Qi HW. The effects of rBCG expressing Der p2 in the form of lipoprotein on murine immune response. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2005;21:287–9. [PubMed] [Google Scholar]
- 7.Bloom BR, Fine PEM. The BCG Experience: Implications for Future Vaccines against Tuberculosis, in Tuberculosis, Pathogenesis, Protection and Control. Washington, DC: American Society for Microbiology Press; 1994. pp. 531–57. [Google Scholar]
- 8.Erb KJ, Holloway JW, Sobeck A, Moll H, Le GG. Infection of mice with Mycobacterium bovis-Bacillus Calmette-Guérin (BCG) suppresses allergen-induced airway eosinophilia. J Exp Med. 1998;187:561–9. doi: 10.1084/jem.187.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shirakawa T, Enomoto T, Shimazu S, Hopkin JM. The inverse association between tuberculin responses and atopic disorder. Science. 1997;275:77–9. doi: 10.1126/science.275.5296.77. [DOI] [PubMed] [Google Scholar]
- 10.Janssen R, Kruisselbrink A, Hoogteijling L, Lamb JR, Young DB, Thole JE. Analysis of recombinant mycobacteria as T helper type 1 vaccines in an allergy challenge model. Immunology. 2001;102:441–9. doi: 10.1046/j.1365-2567.2001.01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen G, Yeung VP, Berry G, Umetsu DT, DeKruyff RH. Vaccination with heat-killed Listeria as adjuvant reverses established allergen-induced airway hyperreactivity and inflammation: role of CD8+ T cells and IL-18. J Immunol. 2000;164:223–30. doi: 10.4049/jimmunol.164.1.223. [DOI] [PubMed] [Google Scholar]
- 12.Zuany-Amorim C, Sawicka E, Manlius C, et al. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat Med. 2002;8:625–9. doi: 10.1038/nm0602-625. [DOI] [PubMed] [Google Scholar]
- 13.Yeremeev VV, Lyadova IV, Nikonenko BV, Apt AS, Abou-Zeid C, Inwald J, Young DB. The 19-kD antigen and protective immunity in a murine model of tuberculosis. Clin Exp Immunol. 2000;120:274–9. doi: 10.1046/j.1365-2249.2000.01212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayward CM. Construction and murine immunogenicity of recombinant Bacille Calmette Gürin vaccines expressing the B subunit of Escherichia coli heat labile enterotoxin. Vaccine. 1999;17:1272–81. doi: 10.1016/s0264-410x(98)00350-8. [DOI] [PubMed] [Google Scholar]
- 15.Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR, Maizels RM. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med. 2005;202:1199–212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Fan Y, Wang S, Han X, Yang J, Bilenki L, Chen L. Mycobacterial infection inhibits established allergic inflammatory responses via alteration of cytokine production and vascular cell adhesion molecule-1 expression. Immunology. 2002;105:336–43. doi: 10.1046/j.0019-2805.2002.01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herz U, Gerhold K, Grüber C, Braun A, Wahn U, Renz H, Paul K. BCG infection suppresses allergic sensitization and development of increased airway reactivity in an animal model. J Allergy Clin Immunol. 1998;102:867–74. doi: 10.1016/s0091-6749(98)70030-2. [DOI] [PubMed] [Google Scholar]
- 18.Koh YI, Choi IS, Kim WY. BCG infection in allergen-presensitized rats suppresses Th2 immune response and prevents the development of allergic asthmatic reaction. J Clin Immunol. 2001;21:51–9. doi: 10.1023/a:1006745116360. [DOI] [PubMed] [Google Scholar]
- 19.Su YC, Peng HJ, Wang SR, Han SH, Tsai JJ. Effects of BCG on ovalbumin-induced bronchial hyperreactivity in a guinea pig asthma model. J Microbiol Immunol Infect. 2001;34:25–34. [PubMed] [Google Scholar]
- 20.Coffman RL, Seymour BW, Lebman DA, et al. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev. 1988;102:5–28. doi: 10.1111/j.1600-065x.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 21.Tang ML, Kemp AS, Thorburn J, Hill DJ. Reduced interferon-gamma secretion in neonates and subsequent atopy. Lancet. 1994;344:983–5. doi: 10.1016/s0140-6736(94)91641-1. [DOI] [PubMed] [Google Scholar]
- 22.Hurst SD, Seymour BW, Muchamuel T, Kurup VP, Coffman RL. Modulation of inhaled antigen-induced IgE tolerance by ongoing Th2 responses in the lung. J Immunol. 2001;166:4922–30. doi: 10.4049/jimmunol.166.8.4922. [DOI] [PubMed] [Google Scholar]
- 23.Ostroukhova M, Seguin-Devaux C, Oriss TB, Dixon-McCarthy B, Yang L, Ameredes BT, Corcoran TE, Ray A. Tolerance induced by inhaled antigen involves CD4(+) T cells expressing membrane-bound TGF-beta and FOXP3. J Clin Invest. 2004;114:28–38. doi: 10.1172/JCI20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romagnani S. Regulatory T cells: which role in the pathogenesis and treatment of allergic disorders? Allergy. 2006;61:3–14. doi: 10.1111/j.1398-9995.2006.01005.x. [DOI] [PubMed] [Google Scholar]
- 25.Karim M, Feng G, Wood KJ, Bushell AR. CD25+CD4+ regulatory T cells generated by exposure to a model protein antigen prevent allograft rejection: antigen-specific reactivation in vivo is critical for bystander regulation. Blood. 2005;105:4871–7. doi: 10.1182/blood-2004-10-3888. [DOI] [PubMed] [Google Scholar]