Abstract
Apoptosis of macrophages infected with pathogenic mycobacteria is an alternative host defence capable of removing the environment supporting bacterial growth. In this work the influence of virulence and bacterial load on apoptosis of alveolar macrophages during the initial phase of infection by Mycobacterium bovis was investigated. BALB/c mice were infected intratracheally with high or low doses of the virulent (ATCC19274) or attenuated (bacillus Calmette–Guérin Moreau) strains of M. bovis. The frequency of macrophage apoptosis, the growth of mycobacteria in macrophages, and the in situ levels of the cytokines tumour necrosis factor-α (TNF-α), interleukin-10 (IL-10) and IL-12 and of the anti-apoptotic protein Bcl-2 were measured at day 3 and day 7 post-infection. An increase of macrophage apoptosis was observed after infection with both strains but the virulent strain induced less apoptosis than the attenuated strain. On the 3rd day after infection with the virulent strain macrophage apoptosis was reduced in the high-dose group, while on the 7th day post-infection macrophage apoptosis was reduced in the low-dose group. Inhibition of apoptosis was correlated with increased production of IL-10, reduced production of TNF-α and increased production of Bcl-2. In addition, the production of IL-12 was reduced at points where the lowest levels of macrophage apoptosis were observed. Our results indicate that virulent mycobacteria are able to modulate macrophage apoptosis to an extent dependent on the intracellular bacterial burden, which benefits its intracellular growth and dissemination to adjacent cells.
Keywords: apoptosis, cytokines, infection, macrophages, mycobacteria
Introduction
Tuberculosis (TB) is a disease of importance worldwide responsible for two million deaths and eight million new cases annually.1,2 The causative agents of TB belong to the Mycobacterium tuberculosis complex, which is a group of closely related mycobacteria including M. tuberculosis and Mycobacterium bovis. The tubercle bacilli enter the organism principally through the respiratory tract and phagocytosis of the bacilli by alveolar macrophages is the first event in the host–pathogen interaction.3,4 These bacteria are able to avoid phagosome maturation and fusion with lysosomes and so escape from the major cytotoxic mechanisms of phagocytes.5,6 Surviving in the interior of the phagosome of the macrophage, the bacillus is guaranteed an environment that protects it from the effector mechanisms of the host and allows it to replicate.7
During the initial stages of infection, control of proliferation of intracellular bacteria depends on natural resistance mediated by macrophages.8,9 Besides the innate effector mechanisms used by these cells, it has been suggested that apoptosis of the infected macrophages constitutes an alternative strategy that can contribute in several ways to the host defence. This type of cellular death deprives the pathogen of its intracellular refuge, and obstructs its propagation by sequestering and retaining the mycobacteria within apoptotic bodies, which ultimately are engulfed by recruited phagocytes.10–14
Recent studies have demonstrated that virulent strains of mycobacteria, such as M. tuberculosis H37Rv and M. bovis wild-type, induce significantly less apoptosis of the infected macrophages than the attenuated strains M. tuberculois H37Ra and M. bovis bacillus Calmette–Guérin (BCG), indicating that inhibition of apoptosis of the infected macrophages is a virulence factor associated with mycobacteria.13,15,16 Also it seems that the bacillary load can influence the type of death undergone by the infected macrophages.17
Some studies have highlighted the importance of the cytokines tumour necrosis factor-α (TNF-α) and interleukin-10 (IL-10) and of proteins of the Bcl-2 family in the modulation of apoptosis of infected macrophages.9,18–20 During infection by mycobacteria TNF-α and IL-10 have opposite effects on various functions of the macrophages, including apoptosis induced by the infection.9 It has been suggested that production of TNF-α is critical for the induction of apoptosis of macrophages infected with mycobacteria,11,21,22 although TNF-α-independent apoptosis was shown in macrophages infected at a high multiplicity of infection (MOI) with virulent M. tuberculosis.17 On the other hand, IL-10 could act as an anti-apoptotic mediator.23,24 Anti-apoptotic protein Bcl-2 also seems to act during infection with mycobacteria, extending the survival of the infected cell.15,16,19
Besides its role in innate immunity, apoptosis of the infected macrophages may also contribute to the development of specific immunity against mycobacteria. It has been demonstrated that when entering into apoptosis, infected macrophages release apoptotic bodies containing mycobacterial antigens, which are engulfed by uninfected dendrític cells, processed and subsequently presented via major histocompatibility complex class I, making the activation of CD8+ T cells possible through a mechanism of cross-priming.25,26
The mechanisms involved in modulation of apoptosis of macrophages during infection by Mycobacterium are complex and seem to include both signals of induction and signals of inhibition of apoptosis of the host cell. Despite recent advances in understanding these mechanisms, few studies have sought to analyse the modulation of apoptosis of macrophages in in vivo conditions and to evaluate their relation with multiplication and spread of mycobacteria in macrophages. We, therefore, decided to assess the influence of virulence and of bacterial load on the incidence of apoptosis of macrophages during the initial phase of experimental pulmonary M. bovis infection in mice. We measured the percentage of infected macrophages and the rates of macrophage apoptosis, and next analysed its correlation with the in situ levels of the cytokines TNF-α, IL-10 and IL-12 and of the anti-apoptotic protein Bcl-2.
Materials and methods
Animals
Male BALB/c mice between 8 and 10 weeks of age, obtained from the animal care facilities of the Federal University of Juiz de Fora (UFJF), were housed in microisolator cages receiving chow and water ad libitum. All procedures were in accordance with the principles of the Brazilian Code for the Use of Laboratory Animals and were approved by the Ethics Committee on the use of laboratory animals of UFJF.
Bacteria and infection
Wild-type M. bovis (ATCC 19274) was obtained from the National Institute of Quality Control in Health – Oswaldo Cruz Foundation, Rio de Janeiro. The M. bovis BCG (Moreau substrain) was obtained from Ataupho de Paiva Foundation, Rio de Janeiro. Both strains of M. bovis were cultured in Lowenstein–Jensen medium for 21 days. Colonies were harvested at mid-log phase and stirred vigorously with sterile glass beads for 5 min and resuspended in phosphate-buffered saline (PBS).27 To further disrupt clumps, the bacterial suspensions were sonicated (20 watts for 5 seconds). The viable counts of bacteria were determined by serial dilutions and plating into six-well plates containing Lowenstein–Jensen medium. To monitor the effect of the bacterial burden on apoptosis of macrophages during the initial phase of infection two different inocula were used: a low dose of about 105 organisms or the higher dose of about 107 organisms.28 Mice were anaesthetized intraperitoneally with 0·2 ml of an anaesthetic solution containing 0·9% NaCl, 2% xylazine and 5% ketamine. The attenuated and virulent strains of M. bovis were administered intratracheally in a total volume of 50 μl PBS using a microsyringe. Control mice were injected with 50 μl PBS.
Obtaining lung cell suspensions
At day 3 and day 7 post-infection, mice (n = 6/group) were killed using an anaesthetic overdose. For the isolation of lung cells, the lungs of infected and control mice were perfused by gently infusing 10 ml PBS into the right ventricle of the heart to minimize contamination with blood. Perfused lungs were aseptically removed and cut into small pieces with a scalpel. The dissected tissue was incubated at 37° for 40 min in 5 ml RPMI-1640 medium containing type I collagenase (2·5 mg/ml; Gibco, Grand Island, NY, USA). After incubation the enzymatic activity was stopped by adding 10 ml RPMI-1640 medium containing 10% fetal bovine serum (cRPMI). The digested lungs were further disrupted by gently pushing the tissue through a nylon screen (70 μm). The single-cell suspension was then washed and centrifuged at 300 g for 10 min. To lyse contaminating red blood cells the cellular preparations were resuspended in red blood cell lysis buffer and incubated at room temperature for 5 min. After centrifugation at 300 g for 10 min the pelleted cells were washed, resuspended in cRPMI and kept on ice until further use. Viable cells were counted by Trypan blue exclusion.
Determination of bacterial growth
The cytospin slides of pulmonary cells obtained by enzymatic digestion were stained for acid-fast bacilli using the Ziehl–Neelsen stain. The number of macrophages containing M. bovis in a total of 100 macrophages per sample was recorded and the number of bacilli per infected macrophage was scored at 1–5 bacilli/macrophage, 5–10 bacilli/macrophage or more than 10 bacilli/macrophage. The percentage of alveolar macrophages infected and the percentage of macrophages infected with the scored number of bacteria per cell were determined.
Apoptosis assay
The annexin V assay was used to detect cells in apoptosis.29 Lung cell suspensions obtained by enzymatic digestion were washed with 500 μl annexin binding buffer (10 mm HEPES, 150 mm NaCl, 5 mm KCl, 1 mm MgCl2, 1,8 mm CaCl2). Supernatants were removed and 100 μl annexin binding buffer with fluorescein isothiocyanate (FITC)-conjugated annexin V (1 : 500) was added to each pellet. Cells were stained for 20 min in the dark at room temperature. Immediately before collection, 400 μl of annexin binding buffer and 40 μl (at 100 μg/ml) of propidium iodide (PI) were added to each sample. For flow cytometry analysis, 80 000 events were collected for each condition. Macrophages were gated based on forward and side scatter and identified as large autofluorescent cells.30 Annexin V/PI dot plots of these populations were generated and the percentages of apoptotic macrophages (annexinV+ PI−) were determined.
Measurement of cytokine concentrations in lungs
One hundred milligrams of lung from infected or non-infected mice was homogenized using 1 ml of 0·05% Tween-20–PBS containing antiproteases (0·1 mm phenylmethylsulphonyl fluoride, 0·1 mm benzethonium chloride, 10 mm ethylenediaminetetraacetic acid and 20 kallikrein-inhibitor-units of aprotinin A). The samples were then centrifuged for 10 min at 3000 g and the supernatants were collected and frozen at −70° until further use. The levels of TNF-α, IL-10 and IL-12 p40 in lung homogenate supernatants were measured by enzyme-linked immunosorbent assay (ELISA) using commercially available antibodies and following the instructions supplied by the manufacturer (BD Biosciences Pharmingen, San Diego, CA). The reading was made in a microplate reader (Spectramax 190; Molecular Devices, Sunnyvale, CA) at 450 nm. The amount of cytokines was calculated from the standard curve, for the different concentrations of the recombinant cytokines.
Immunohistochemical detection of anti-apoptotic molecule Bcl-2
For immunohistochemistry analysis, lung sections (4 μm thick) were mounted on silane-covered slides. After deparaffination and rehydration, endogenous peroxidase activity was blocked by incubation with 3% H2O2 for 10 min. Antigens were retrieved by microwave treatment at 730 W in 10 mm sodium citrate buffer pH 6·0 for two cycles of 9 min each. Non-specific binding was blocked by incubation in 2% bovine serum albumin–PBS for 30 min. Lung sections were incubated overnight at 4° in a humidified chamber with 1 : 1000 diluted polyclonal rabbit anti-mouse Bcl-2 antibody (BD Biosciences Pharmingen) or with diluted normal rabbit serum as control. Slides were rinsed three times with PBS before adding 1 : 200 diluted biotinylated goat anti-rabbit immunoglobulin G (BD Biosciences Pharmingen). After incubation at room temperature for 30 min, slides were washed and avidin–horseradish peroxidase was added. After 30 min incubation slides were washed with PBS. Colour development was achieved by application of 3,3’-diaminobenzidine for 3–5 min in the dark. The slides were then washed and counterstained with haematoxylin. Five random fields of each lung were evaluated at 400 × magnification. Positive and negative stained macrophages from alveolar spaces were counted and data were expressed as the percentage of Bcl-2-positive macrophages, as described previously.16
Statistical analysis
Results are expressed as means ± standard error of the mean. The data were analysed by Mann–Whitney test using the graphpad prism 5·00 for Windows (GraphPad Software, San Diego, CA). P< 0·05 was used as the limit of statistical significance. Each experiment was repeated at least twice with comparable results.
Results
Mycobacterial multiplication and spread in alveolar macrophages
As an alternative for evaluating the multiplication and spread of mycobacteria, we carried out acid-fast staining (Ziehl–Nielsen) in the pulmonary cells of BALB/c mice injected intratracheally with either virulent or attenuated M. bovis. The number of macrophages infected with different amounts of mycobacteria was calculated at day 3 and day 7 post-infection.
The percentage of infected macrophages at day 3 post-infection with the virulent strain was similar both in the infection with low dose and in the infection with high dose (Table 1). In both bacterial load conditions most of the infected macrophages contained fewer than five bacilli (Fig. 1a,b), while the percentage of macrophages infected with more than five bacilli was five times larger in the infection with the high dose (Fig. 1b). On the 7th day post-infection with low dose, the percentage of infected macrophages did not alter significantly, rising from 32% to 34% (Table 1). Most of the infected macrophages (84%) contained from 5 to 10 bacilli (Fig. 1a). This result indicates that between the 3rd and 7th days of infection with a low dose only multiplication of mycobacteria took place in the infected cells. In the infection with high dose, however, the percentage of infected macrophages increased from 34% to 50% between the 3rd and the 7th days of infection (Table 1). Besides this, we notice that most of the infected macrophages contained more than 10 bacilli (Fig. 1b), indicating that between the 3rd and 7th days of infection with a high dose intracellular multiplication of the pathogen as well as spread of the infection took place in the alveolar macrophages. In infection with the attenuated strain the percentage of infected macrophages was around 20% in both bacterial load conditions, both on the 3rd and on the 7th day of infection (Table 1), and most of the infected macrophages contained a single bacillus.
Table 1.
Percentage of infected macrophages1
| Attenuated strain |
Virulent strain |
|||
|---|---|---|---|---|
| Time post-infection | Low dose | High dose | Low dose | High dose |
| 3 days | 19 | 20 | 32 | 34 |
| 7 days | 18 | 21 | 34 | 50 |
BALB/c mice were intratracheally infected with 1 × 105 (low dose) or 1 × 107 (high dose) of attenuated (bacillus Calmette–Guérin) or virulent Mycobacterium bovis.
Figure 1.
Number of bacilli in macrophages infected with low dose (a) or high dose (b) of virulent Mycobacterium bovis at day 3 and day 7 post-infection. Cytocentrifuge slides were stained for acid-fast bacilli using the Ziehl–Neelsen stain and the number of bacilli in each macrophage infected was scored as 1–5 bacilli/macrophage (c), 5–10 bacilli/macrophage (d) and > 10 bacilli/macrophage (e). Approximately 100 macrophages per sample were counted. Results are representative of two different experiments. (c, d, e × 1000).
Effect of low and high bacterial load on in vivo apoptosis induction by virulent and attenuated strains of M. bovis
To evaluate in vivo the influence of virulence and bacterial load on the frequency of apoptosis of macrophages, BALB/c mice were intratracheally infected either with the virulent M. bovis wild-type or with the attenuated M. bovis (BCG), using the low (1 × 105) and the high (1 × 107) mycobacteria inocula. We noticed in the groups infected with both strains of M. bovis a significant increase in apoptosis of macrophages when compared with the uninfected controls, both on the 3rd and on the 7th day postinfection (Fig. 2). In general, the rates of apoptotic macrophages were lower in the infection with the virulent strain when compared with the attenuated strain, except on the 3rd day of infection with low dose, where the virulent strain induced more apoptosis than the attenuated strain, 52% and 37% respectively. Interestingly, on the 3rd day of infection with the virulent strain there was a reduction of apoptosis in the infection with high dose, whereas on the 7th day we observed greater reduction in the lower dose (Fig. 2). Together these results suggest that the modulation of apoptosis promoted by the virulent strain is dependent on the intracellular bacterial burden.
Figure 2.
Apoptosis of alveolar macrophages induced by Mycobacterium bovis at day 3 and day 7 post-infection. BALB/c mice were intratracheally infected with low dose (1 × 105) and high dose (1 × 107) of attenuated (bacillus Calmette–Guérin) and virulent (ATCC19274) M. bovis strains. Lung cells obtained by enzymatic digestion were stained with annexin V and propidium iodide for analysis by flow cytometry. Annexin V/PI dot plots of these macrophage populations were generated and the percentage of apoptotic macrophages (Annexin V+/PI−) was determined. (a) Each bar represents the arithmetic mean ± SEM of six mice. Results are representative of two different experiments. *P< 0·05 versus non-infected control; #P< 0·05 versus infected with attenuated strain. (b) Dot plots of representative samples are shown.
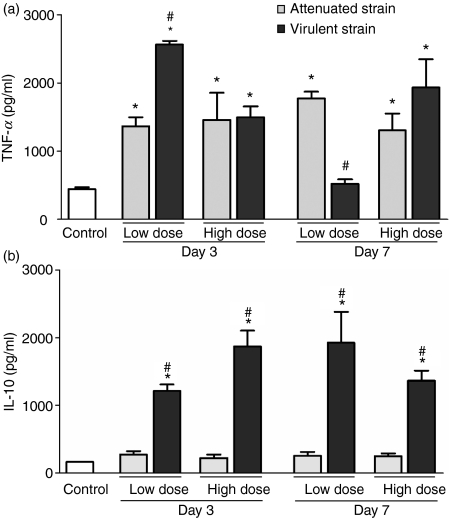
Differential production of TNF-α and IL-10 in lung homogenates after M. bovis infection
Levels of TNF-α and IL-10 in lung homogenates were quantified by ELISA at day 3 and day 7 after infection with the two M. bovis strains, to evaluate any association in vivo between the production of these cytokines and the induction/inhibition of apoptosis induced by mycobacteria. Figure 3(a) shows that the production of TNF-α increased significantly (P< 0·05) after the infection with both virulent and attenuated M. bovis. On the 3rd day of infection with the low dose the virulent strain induced more TNF-α than the attenuated strain (P< 0·05). Inversely on the 7th day of infection with low dose, the virulent strain induced significantly less TNF-α than the attenuated strain (P< 0·05). Regarding production of IL-10, we observed a significant increase in the production of this cytokine only in the infection with the virulent strain, whereas in the infection with the attenuated strain the levels of IL-10 remained near those of the uninfected control (Fig. 3b).
Figure 3.
Production of tumour necrosis factor-α (TNF-α) (a) and interleukin-10 (IL-10) (b) in the lungs of mice infected with either low or high dose of attenuated and virulent Mycobacterium bovis strains at day 3 and day 7 post-infection. Control mice were inoculated with phosphate-buffered saline. Each bar represents the arithmetic mean ± SEM of six mice. Results are representative of two different experiments. *P< 0·05 versus non-infected control; #P< 0·05 versus infected with attenuated strain.
Correlating the production of TNF-α and IL-10 (Fig. 3) with the rates of apoptosis (Fig. 2), we noted in the infection with the attenuated strain a significant increase of TNF-α, but not of IL-10, while high rates of macrophage apoptosis were also observed. On the other hand, in the infection with the virulent strain, though a significant increase in levels of both cytokines took place, we observed a reduction of apoptotic macrophages on the 3rd day (high dose) and on the 7th day (low dose), times at which we registered high levels of IL-10 and lower levels of TNF-α.
Increase of Bcl-2 expression in the infection with the virulent strain of M. bovis
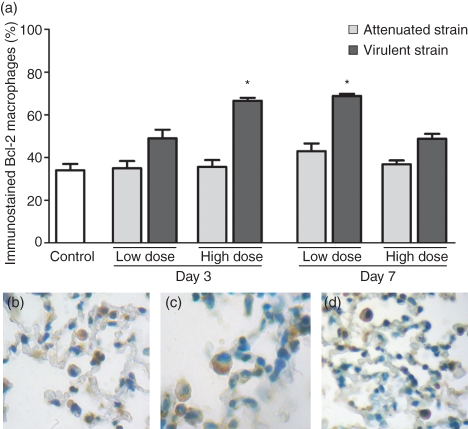
Next we evaluated the expression of Bcl-2, an anti-apoptotic protein, in alveolar macrophages of the infected mice and control mice using immunohistochemistry. We observed that the expression of Bcl-2 was not altered after the infection with the attenuated strain; it increased significantly in the infection with the virulent strain on the 3rd day of infection with the high dose and on the 7th day of infection with the low dose (Fig. 4). Interestingly, the levels of Bcl-2 were higher at the points at which elevated levels of IL-10 were registered.
Figure 4.
Expression of Bcl-2 in alveolar macrophages of mice infected with low or high dose of attenuated and virulent Mycobacterium bovis strains at day 3 and day 7 post-infection. Control mice were inoculated with phosphate-buffered saline. (a) Each bar represents the arithmetic mean ± SEM of six mice. Results are representative of two different experiments. *P< 0·05 versus control. Immunohistochemical staining for Bcl-2 in a paraffin-embedded specimen of control (b) and infected (c, d) mice. Negative cells in blue and positive cells in brown were easily detected in the lung tissue. (b, c, d × 1000).
Production of IL-12 after infection with the virulent and attenuated strains of M. bovis
In vitro studies have shown that apoptotic vesicles of macrophages infected with mycobacteria stimulate primarily the production of IL-12 by dendritic cells.31 Therefore we measured the level of IL-12p40 in homogenized lungs of mice infected with M. bovis and in uninfected controls. Figure 5 shows that infection with the attenuated strain (BCG) did not significantly raise the levels of IL-12. However, infection with the virulent strain induced a significant increase in the production of this cytokine, except on the 7th day of infection with low dose. Interestingly, in the infection with the virulent strain, the production of IL-12 was higher at the points where we observed high rates of apoptosis, and was lower at the points where the apoptotic rates were lower.
Figure 5.
Interleukin-12 (IL-12) production in the lung of mice infected with low or high dose of attenuated and virulent Mycobacterium bovis strains at day 3 and day 7 post-infection. Control mice were inoculated with phosphate-buffered saline. Each bar represents the arithmetic mean ± SEM of six mice. Results are representative of two different experiments. *P< 0·05 versus control.
Discussion
In this work, we investigated the influence of the virulence and of the bacterial load of M. bovis on the induction of apoptosis of alveolar macrophages in vivo and its correlation with the in situ levels of the cytokines TNF-α, IL-10 and IL-12 and of the anti-apoptotic protein Bcl-2. An increase of apoptosis of macrophages in the lungs of the infected animals in relation to the uninfected controls was observed, indicating that the infection with M. bovis constitutes a stimulus for induction of apoptosis of macrophages. The recent discovery of the Ipr1 gene in mice emphasizes the importance of this cell death mechanism in the host response to infection. Expression of Ipr1 in macrophages limits the multiplication of M. tuberculosis and switches a cell death pathway of the infected macrophages from necrosis to apoptosis.32 In this work we found that the virulent strain is able to inhibit apoptosis of the macrophages in vivo, confirming results of in vitro studies, which suggested that the capacity to inhibit apoptosis of infected macrophages is a factor in the virulence associated with mycobacteria.13,15 Additionally, in this work it was observed for the first time in vivo that modulation of apoptosis promoted by the virulent strain appears to be associated with bacterial burden within the infected macrophage.
Different in vitro studies have supplied evidence that bacterial load can influence apoptosis of macrophages induced by mycobacteria. At low MOI (≤ 10) virulent strains induce less apoptosis than attenuated strains.13 At high MOI (≥ 25) the virulent strain presents equal or greater cytotoxicity than the attenuated strain. Additionally, it was seen that apoptosis at high MOI does not reduce the viability of the mycobacteria and progresses quickly to necrosis.17 In this case, it has been suggested that as soon as the infection of macrophages is established and an ideal intracellular growth is reached, the priority of the virulent mycobacteria eventually changes in favour of extracellular growth necessary for transmission of the infection into other cells.17 In accordance, our results suggest that modulation of apoptosis promoted by the virulent strain is dependent on the number of bacteria in the interior of the cell. Therefore, on the 3rd day of infection with a low dose of the virulent strain we did not observe inhibition of apoptosis, possibly because at this initial moment the number of bacilli in the interior of the macrophages was still insufficient to promote this inhibition. By contrast, on the 3rd day of infection with a high dose of the virulent strain, where the percentage of macrophages infected with more bacilli was higher, a reduction of apoptosis of macrophages was already noticed. On the 7th day of infection with low dose a greater inhibition of apoptosis and the predominance of macrophages infected with 5–10 bacilli was observed, suggesting this to be a moment when a large amount of replication is occurring, therefore increasing the need for extending the viability of the host cell. In contrast, in the infection with high dose most of the infected macrophages contained more than 10 bacilli, probably a sufficient number to promote extracellular dissemination of the bacteria. In this case, it is possible that the mechanisms of apoptosis inhibition, active when the bacillary intracellular load is still low, have ceased. This can explain the increase of the apoptotic rates. It is accepted that apoptotic cells occasionally lyse and become necrotic when the load of dying cells exceeds the local capacity for phagocyte-mediated clearance.33 This cell lysis would allow the bacilli to avoid the microbicidal effect of apoptosis, and provide access to the extracellular environment to infect other cells. Therefore, it is possible that the mechanism of macrophage death is similar at both high and low burdens but that at high burden the cells progress from apoptosis to secondary necrosis, allowing the bacilli to escape from the macrophage before they are killed.34
Participation of the cytokines TNF-α and IL-10 in the modulation of apoptosis induced by mycobacteria has been demonstrated in vitro.9,22,35 Human macrophages undergo TNF-α-dependent apoptosis when infected with non-virulent M. tuberculosis at a low MOI, whereas virulent strains inhibit this apoptosis.13 In contrast, murine macrophages infected at a high MOI with the virulent strain of M. tuberculosis undergo TNF-α-independent apoptosis.17,34 In this study, however, we observed that for both low and high doses with either attenuated or virulent strains the induction of apoptosis of macrophages in vivo was associated with higher production of TNF-α, while its inhibition was associated with higher production of IL-10. We have noted that whenever the increase of production of TNF-α was greater than that of IL-10, a greater induction of apoptosis occurred. Inversely, when the production of IL-10 was increased and that of TNF-α was reduced, a tendency to inhibition of apoptosis of macrophages was observed. Consistent with our results, Balcewicz-Sablinska et al.23,36 noted in vitro that the virulent strain of M. tuberculosis H37Rv induced higher production of IL-10 than the attenuated strain H37Ra and that in spite of both strains inducing comparable levels of TNF-α, the bioactivity of TNF-α was reduced in the infection with H37Rv. Subsequently, it was suggested that IL-10 down-regulates apoptosis of macrophages through the inhibition of TNF-α production, and by inducing the liberation of sTNF-R2, which leads to inactivation of the TNF-α. Influence of TNF-α and IL-10 on the modulation of apoptosis of macrophages during infection by mycobacteria is demonstrated also in in vitro studies showing that anti-TNF-α reduces and anti-IL-10 increases apoptosis of macrophages.9,36 These cytokines can even modulate apoptosis in non-infected macrophages.9 However, it remains to be determined whether infected macrophages are more susceptible than non-infected macrophages to apoptosis induced by TNF-α. It is possible that the presence of bacterial infection in the host cell may increase the expression of death receptors, such as TNFR1. By varying inversely with TNF-α, IL-10 may define the virulence of the mycobacterial infection by inhibiting apoptosis induced by TNF-α. Whether this TNF-α-dependent apoptosis or a TNF-α-independent direct mechanism are exclusive or concurrent requires further exploration.
The anti-apoptotic effect of IL-10 might be exerted not only through the inhibition and inactivation of TNF-α, but also through the increased expression of the anti-apoptotic protein Bcl-2. The level of expression of this protein increased in the infection with the virulent strain at the points where production of IL-10 was high. In contrast, in the infection with the attenuated strain the expression of Bcl-2 remained at basal level in a similar manner to the observed level of IL-10. The increase of the expression of Bcl-2 after infection with the virulent strain (H37Rv) was also observed by other authors.15,16 Mogga et al.19 verified positive colocalization between Bcl-2 and MTB antigens on lung macrophages in 80% of tissue sections from mice infected with the H37Rv strain of M. tuberculosis. In addition, it was demonstrated that IL-10 is able to increase the expression of Bcl-2, hence obstructing spontaneous apoptosis of T cells37 and of B cells of the germinative centres.38
Interestingly, in the infection with the virulent strain, the production of IL-12 was lower at the points where the rates of apoptotic macrophages were lower and higher at the points of greater apoptosis, suggesting that apoptosis of the infected macrophages can contribute to the production of IL-12. Recent studies have demonstrated that, in response to infection with mycobacteria, dendritic cells release great quantities of IL-12, whereas in macrophages IL-12 secretion is limited.39,40 Dendritic cells produce IL-12 when in contact with apoptotic vesicles from infected macrophages, the production being directly proportional to the quantity of available vesicles.31 It is possible that the inhibition of apoptosis of macrophages promoted by the virulent strain of M. bovis reduces the cross-presentation of antigens and consequently the production of IL-12, so damaging the activation and differentiation of CD4+ T cells to the T helper type 1 profile.
In conclusion, this study indicates that the pulmonary infection with M. bovis constitutes a stimulus for inducing apoptosis of alveolar macrophages and that the virulent strain may develop strategies that inhibit apoptosis, favouring its intracellular replication by extending the survival of the host cell. The increase of IL-10 production seems to be fundamental for promoting this inhibition, and the reduction of IL-12 production at the points of lower apoptosis of macrophages reinforces the hypothesis that apoptosis of infected cells contributes to the presentation of antigens and to the development of a specific response to infection by mycobacteria. Future mechanistic studies could lead to a clear definition of the role played by these molecules on mycobacteria survival strategies.
Acknowledgments
H.C.T. acknowledges financial support from CNPq (479724/2007-5 and 310912/2006-7) and FAPEMIG (CBB PPM 0247/08) (Brazil).
Disclosures
Authors have have no competing financial interests.
References
- 1.Flynn JL, Chan J. Tuberculosis: latency and reactivation. Infect Immun. 2001;69:4195–201. doi: 10.1128/IAI.69.7.4195-4201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaufmann SH. Tuberculosis: deadly combination. Nature. 2008;453:295–6. doi: 10.1038/453295a. [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann SH. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–63. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 4.Raja A. Imunology of tuberculosis. Indian J Med Res. 2004;120:213–32. [PubMed] [Google Scholar]
- 5.Russell DG. Phagosomes, fatty acids and tuberculosis. Nat Cell Biol. 2003;5:776–8. doi: 10.1038/ncb0903-776. [DOI] [PubMed] [Google Scholar]
- 6.Warner DF, Mizrahi V. The survival kit of Mycobacterium tuberculosis. Nat Med. 2007;13:282–4. doi: 10.1038/nm0307-282. [DOI] [PubMed] [Google Scholar]
- 7.McDonough KA, Kress Y, Bloom BR. Pathogenesis of tuberculosis: interaction of Mycobacterium tuberculosis with macrophages. Infect Immun. 1993;61:2763–73. doi: 10.1128/iai.61.7.2763-2773.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenton MJ, Vermeulen MW. Immunopathology of tuberculosis: roles of macrophages and monocytes. Infect Immun. 1996;64:683–90. doi: 10.1128/iai.64.3.683-690.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rojas M, Olivier M, Gros P, Barrera LF, García LF. TNF-α and IL-10 modulate the induction of apoptosis by virulent Mycobacterium tuberculosis in murine macrophages. J Immunol. 1999;162:6122–31. [PubMed] [Google Scholar]
- 10.Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette–Guérin. J Exp Med. 1994;180:1499–509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keane J, Balcewicz-Sablinska MK, Remold HG, Chupp GL, Meek BB, Fenton MJ, Kornfeld H. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect Immun. 1997;65:298–304. doi: 10.1128/iai.65.1.298-304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fratazzi C, Arbeit RD, Carini C, Balcewicz-Sablinska MK, Keane J, Kornfeld H, Remold HG. Macrophage apoptosis in mycobacterial infections. J Leukoc Biol. 1999;66:763–4. doi: 10.1002/jlb.66.5.763. [DOI] [PubMed] [Google Scholar]
- 13.Keane J, Remold HG, Kornfeld H. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J Immunol. 2000;164:2016–20. doi: 10.4049/jimmunol.164.4.2016. [DOI] [PubMed] [Google Scholar]
- 14.Porcelli SA, Jacobs WR., Jr Tuberculosis: unsealing the apoptotic envelope. Nat Immunol. 2008;9:1101–2. doi: 10.1038/ni1008-1101. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Jiang R, Takayama H, Tanaka Y. Survival of virulent Mycobacterium tuberculosis involves preventing apoptosis induced by Bcl-2 upregulation and release resulting from necrosis in J774 macrophages. Microbiol Immunol. 2005;49:845–52. doi: 10.1111/j.1348-0421.2005.tb03673.x. [DOI] [PubMed] [Google Scholar]
- 16.Ríos-Barrera VA, Campos-Penã V, Aguilar-León D, Lascurain LR, Meraz-Ríos MA, Moreno J, Figueroa-Granados V, Hernández-Pando R. Macrophage and T lymphocyte apoptosis during experimental pulmonary tuberculosis: their relationship to mycobacterial virulence. Eur J Immunol. 2006;36:345–53. doi: 10.1002/eji.200535202. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Remold HG, Ieong MH, Kornfeld H. Macrophage apoptosis in response to high intracellular burden of Mycobacterium tuberculosis is mediated by a novel caspase-independent pathway. J Immunol. 2006;176:4267–74. doi: 10.4049/jimmunol.176.7.4267. [DOI] [PubMed] [Google Scholar]
- 18.Klingler K, Tchou-Wong KM, Brändli O, Aston C, Kim R, Chi C, Rom WN. Effects of mycobacteria on regulation of apoptosis in mononuclear phagocytes. Infect Immun. 1997;65:5272–8. doi: 10.1128/iai.65.12.5272-5278.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mogga SJ, Mustafa T, Sviland L, Nilsen R. Increased Bcl-2 and reduced Bax expression in infected macrophages in slowly progressive primary murine Mycobacterium tuberculosis infection. Scand J Immunol. 2002;56:383–91. doi: 10.1046/j.1365-3083.2002.01140.x. [DOI] [PubMed] [Google Scholar]
- 20.Sly LM, Hingley-Wilson SM, Reiner NE, McMaster WR. Survival of Mycobacterium tuberculosis in host macrophages involves resistance to apoptosis dependent upon induction of antiapoptotic Bcl-2 family member Mcl-1. J Immunol. 2003;170:430–7. doi: 10.4049/jimmunol.170.1.430. [DOI] [PubMed] [Google Scholar]
- 21.Keane J, Shurtleff B, Kornfeld H. TNF-dependent BALB/c murine macrophage apoptosis following Mycobacterium tuberculosis infection inhibits bacillary growth in an IFN-gamma independent manner. Tuberculosis. 2002;82:55–61. doi: 10.1054/tube.2002.0322. [DOI] [PubMed] [Google Scholar]
- 22.Spira A, Carroll JD, Liu G, Aziz Z, Shah V, Kornfeld H, Keane J. Apoptosis genes in human alveolar macrophages infected with virulent or attenuated Mycobacterium tuberculosis. a pivotal role for tumor necrosis factor. Am J Respir Cell Mol Biol. 2003;29:545–51. doi: 10.1165/rcmb.2002-0310OC. [DOI] [PubMed] [Google Scholar]
- 23.Balcewicz-Sablinska MK, Gan H, Remold HG. Interleukin 10 produced by macrophages inoculated with Mycobacterium avium attenuates mycobacteria-induced apoptosis by reduction of TNF-α activity. J Infect Dis. 1999;180:1230–7. doi: 10.1086/315011. [DOI] [PubMed] [Google Scholar]
- 24.Nigou J, Gilleron M, Rojas M, García LF, Thurnher M, Puzo G. Mycobacterial lipoarabinomannans: modulators of dendritic cell function and the apoptotic response. Microbes Infect. 2002;4:945–53. doi: 10.1016/s1286-4579(02)01621-0. [DOI] [PubMed] [Google Scholar]
- 25.Schaible UE, Winau F, Sieling PA, et al. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat Med. 2003;9:1039–46. doi: 10.1038/nm906. [DOI] [PubMed] [Google Scholar]
- 26.Winau F, Kaufmann SH, Schaible UE. Apoptosis paves the detour path for CD8 T cell activation against intracellular bacteria. Cell Microbiol. 2004;7:599–607. doi: 10.1111/j.1462-5822.2004.00408.x. [DOI] [PubMed] [Google Scholar]
- 27.Fulton SA, Martin TD, Redline RW, Henry BoomW. Pulmonary immune responses during primary Mycobacterium bovis-Calmette–Guérin bacillus infection in C57BL/6 mice. Am J Respir Cell Mol Biol. 2000;22:333–43. doi: 10.1165/ajrcmb.22.3.3776. [DOI] [PubMed] [Google Scholar]
- 28.Bekker LG, Moreira AL, Bergtold A, Freeman S, Ryffel B, Kaplan G. Immunophathologic effects of tumor necrosis factor alpha in murine mycobacterial infection are dose dependent. Infect Immun. 2000;68:6954–61. doi: 10.1128/iai.68.12.6954-6961.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–56. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Rijt LS, Kuipers H, Vos N, Hijdra D, Hoogsteden HC, Lambrecht BN. A rapid flow cytometric method for determining the cellular composition of bronchoalveolar lavage fluid cells in mouse models of asthma. J Immnunol Methods. 2004;288:111–21. doi: 10.1016/j.jim.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Winau F, Weber S, Sad S, et al. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity. 2006;24:105–17. doi: 10.1016/j.immuni.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Pan H, Yan BS, Rojas M, et al. Ipr1 gene mediates innate immunity to tuberculosis. Nature. 2005;434:767–72. doi: 10.1038/nature03419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–75. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 34.O’Sullivan MP, O’Leary S, Kelly DM, Keane J. A caspase-independent pathway mediates macrophage cell death in response to Mycobacterium tuberculosis infection. Infect Immun. 2007;75:1984–93. doi: 10.1128/IAI.01107-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arcila ML, Sánchez MD, Ortiz B, Barrera LF, García LF, Rojas M. Activation of apoptosis, but not necrosis, during Mycobacterium tuberculosis infection correlated with decreased bacterial growth: role of TNF-α, IL-10, caspases and phospholipase A2. Cell Immunol. 2007;249:80–93. doi: 10.1016/j.cellimm.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Balcewicz-Sablinska MK, Keane J, Kornefeld H, Remold HG. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-α. J Immunol. 1998;161:2636–41. [PubMed] [Google Scholar]
- 37.Cohen SB, Crawlwy JB, Kahan MC, Feldmann M, Foxwell BM. Interleukin-10 rescues T cells from apoptotic cell death: association with an upregulation of Bcl-2. Immunology. 1997;92:1–5. doi: 10.1046/j.1365-2567.1997.00348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levy Y, Brouet JC. Interleukin-10 prevents spontaneous death of germinal center B cells by induction of the Bcl-2 protein. J Clin Invest. 1994;93:424–8. doi: 10.1172/JCI116977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hickman SP, Chan J, Salgame P. Mycobacterium tuberculosis induces differential cytokine production from dendritic cells and macrophages with divergent effects on naive T cell polarization. J Immunol. 2002;168:4636–42. doi: 10.4049/jimmunol.168.9.4636. [DOI] [PubMed] [Google Scholar]
- 40.Pompei L, Jang S, Zamlynny B, Ravikumar S, McBride A, Hickman SP, Salgame P. Disparity in IL-12 release in dendritic cells and macrophages in response to Mycobacterium tuberculosis is due to use of distinct TLRs. J Immunol. 2007;178:5192–9. doi: 10.4049/jimmunol.178.8.5192. [DOI] [PubMed] [Google Scholar]