Abstract
DNA vaccination is a novel immunization strategy that has great potential for the development of vaccines and immune therapeutics. This strategy has been highly effective in mice, but is less immunogenic in non-human primates and in humans. Enhancing DNA vaccine potency remains a challenge. It is likely that antigen-presenting cells (APCs), and especially dendritic cells (DCs), play a significant role in the presentation of the vaccine antigen to the immune system. A new study reports the synergistic recruitment, expansion and activation of DCs in vivo by high-mobility group box 1 (HMGB1) protein. Such combinational strategies for delivering vaccine in a single, simple platform will hypothetically bolster the cellular immunity in vivo. Here, we combined plasmid encoding human immunodeficiency virus-1 (HIV-1) Gag and Env with an HMGB1 plasmid as a DNA adjuvant in BALB/c mice (by intramuscular immunization via electroporation), and humoral and cellular responses were measured. Co-administration of this potent immunostimulatory adjuvant strongly enhanced the cellular interferon-γ (IFN-γ) and humoral immune response compared with that obtained in mice immunized with vaccine only. Our results show that co-immunization with HMGB1 can have a strong adjuvant activity, driving strong cellular and humoral immunity that may be an effective immunological adjuvant in DNA vaccination against HIV-1.
Keywords: cellular and humoral immune response, dendritic cell maturation, DNA adjuvant, electroporation, high-mobility group box 1 protein, human immunodeficiency virus-1 vaccines
Introduction
DNA vaccination is a novel immunization strategy that has great potential for the development of vaccines and immune therapeutics.1,2 The principle behind a DNA vaccine is expression of the target antigen gene (or genes) followed by intracytoplasmic processing, producing peptides that bind to major histocompatibility complex (MHC) class I molecules.1 The presentation of these MHC-bound peptides on the cell surface stimulates CD8 T-lymphocyte responses.3,4 Theoretically, DNA vaccines combine the most desirable attributes of standard vaccine approaches in order to achieve functional enhancement of antigen-specific immune cells.5,6 The efficiency of DNA vaccination depends on the interaction amongst genetic material, lymphocytes and antigen-presenting cells (APCs).5,7 Regardless of the underlying mechanism, it has become clear that in the context of DNA vaccination, APCs are key inducers of immunity, as they are the pivotal mediators of immune responses between resident somatic cells and T cells in the lymph nodes. By directing antigen from the site of injection to the secondary lymphoid organs, APCs serve to present antigen efficiently to naive T cells.1,3,5,7–10
Studies conducted with single-gene DNA vaccines alone showed that the number of dendritic cells (DCs) present at the site of inoculation during antigen expression is a possible rate-limiting factor for DNA vaccine efficacy.1,11 Combinational vaccine strategies have aimed to heighten the potency of DNA alone by combining DNA with adjuvant, boosting with a recombinant viral vector or protein, or by both adjuvant effect and boosting.6,9,11–14 Adjuvants can enhance antigen delivery to the APCs followed by efficient antigen processing by the MHC class molecules.3–5,7,9,14,15 They can also induce the production of immunomodulatory cytokines targeting lymphocytes and favor the development of antigen-specific T-helper 1 (Th1) responses.6,9,12,14 Novel antigens that have been used to induce CD8+ cytotoxic T-lymphocyte (CTL) responses may do so by release of antigen directly into the cytosol for presentation to MHC class I molecules, which may mimic the way that antigen is presented during viral infection.16 DNA vaccines mimic infection with intracellular pathogens in that they are produced within host cells and thereby gain direct access to MHC class I molecules. It has been shown that the antigen–adjuvant complex, once injected, allows the antigen to cross the endosomal membranes and enter the cytosol for presentation to MHC class I.3–5,10,16
High mobility group box 1 (HMGB1) protein is a small 25-kDa protein of 215 amino acids that is highly conserved.17 The HMGB1 molecule is organized into three domains: two DNA-binding domains; HMG box A and box B; and an acidic COOH terminus composed of 30 glutamic and aspartic acid residues.17–19 A ubiquitous and abundant chromatin component, HMGB1 has been shown to act as a mediator of inflammation.20 Previous work shows that HMGB1 is necessary for the up-regulation of costimulatory molecules CD80, CD83, and CD86 on human DCs and for the production of interleukin (IL)-18.21 Additionally, HMGB1 promotes inflammation and induces DC maturation.20,21 Therefore, we hypothesized that HMGB1 plasmid DNA may act as an adjuvant when combined with antigen-encoding DNA, increasing inflammation and stimulating the recruitment and activation of DCs to mediate a cytotoxic immune response. In order to understand the adjuvant effect of HMGB1, we tested the HMGB1 plasmid DNA in mice.
Materials and methods
DNA constructs and protein expression
Mouse HMGB1 DNA was optimized for expression, including codon and RNA optimization (GeneArt, Regensburg, Germany). Optimized constructs were then synthesized and inserted into the pVax1 expression vector (Invitrogen, Carlsbad, CA).22,23
HMGB1 expression was confirmed by utilizing a T7 promoter in the pVax1 backbone and a T7-based coupled transcription/translation system (Promega, Madison, WI) containing 35S-methionine-labelled HMGB1. The synthesized protein was immunoprecipitated using rabbit polyclonal antibody to HMGB1 (Abcam, Cambridge, MA). The immunoprecipitated protein was electrophoresed on a 10% sodium dodecyl sulfate (SDS) polycrylamide gel (NuPAGE; Invitrogen) and subsequently fixed and dried. Autoradiography was performed to detect incorporation of a 35S-labelled gene product.24In vivo expression was confirmed by transfecting the HMGB1 DNA into 293T cells (106) using the Fugene transfection method (Roche, Nutley, NJ). Seventy-two hours after transfection, proteins (50 μg) were fractioned on 10% SDS polyacrylamide gels and transferred to a poly(vinylidene difluoride) (PVDF) membrane (Bio-Rad, Hercules, CA). Immunoblot analyses were performed with anti-HMGB1 antiserum, as described previously,24 and visualized using horseradish peroxidase (HRP)-coupled goat anti-rabbit IgG using an enhanced chemiluminescence (ECL) detection system (Amersham Pharmacia Biotech, Piscataway, NJ) for visualization.24
Generation of bone marrow-derived DC and induction of dendritic cell maturation
Bone marrow (BM) was prepared from tibia and femur bones of BALB/c mice. The bones were placed for 2 min in a 100-mm dish containing 70% alcohol, after removing the muscle tissue using gauze, then the bones were washed twice in phosphate-buffered saline (PBS) and transferred into a fresh dish containing RPMI. Both ends of the bones were cut using scissors and the BM was flushed into a new dish using a syringe fitted with a 19-gauge needle and 5–10 ml of RPMI. Red blood cells (RBC) were lysed with ACK lysing buffer (Invitrogen). The remaining BM cells were washed twice and passed through a cell strainer (70 μm; Becton Dickinson, Bedford, MA). BM cells were resuspended in culture medium (RPMI) supplemented with 2 mm l-glutamine, 50 μm 2-mercaptoethanol (2-ME), 10 mm HEPES, penicillin (100 U/ml)–streptomycin (100 μg/ml) and 5% human AB serum (Gemini Bio-Products, Woodland, CA), supplemented with mouse granulocyte–macrophage colony-stimulating factor (GM-CSF) (5 ng/ml) and mouse IL-4 (5 ng/ml) (BD Biosciences, San Jose, CA), and plated on six-well plates in 3 ml of culture medium per well at a density of 5 × 105 cells/ml.25 Immature DC (iDC) were plated in 96-well round-bottomed microtiter plates at 5 × 104cells/well and were given 100 μl (100 μg/ml) of cell lysates from either pVax vector-transfected cells or HMGB1 plasmid-transfected cells. After 2 days, DCs were harvested and assessed for expression of the maturation markers CD83, CD86 and CCR7. As a control, optimal DC maturation was induced by the addition of tumour necrosis factor-α (TNF-α) (50 ng/ml) (R&D Systems, Minneapolis, MN). The culture supernatants were then collected for chemokine analysis.26
Staining for surface antigens and flow cytometry analysis
The staining of surface molecules on bone marrow-derived dendritic cells (BMDC) with fluorochrome-conjugated monoclonal antibodies (mAbs) was performed on ice. Single-cell suspensions (2–4 × 105) were washed in PBS (pH 7·2) containing 0·2% bovine serum albumin (BSA) and 0·1% NaN3. After pre-incubation for 5 min with FcBlock (BD Biosciences) to avoid non-specific binding, BMDC were stained for 30 min at 4° with saturating amounts of the following Abs: phycoerythrin (PE)-conjugated anti-CD83 (Michel-19; Rat IgG1, κ) and anti-CD86 (GL1; Rat IgG2a, κ) (purchased from BD Biosciences) and PE-conjugated anti-CCR7 (cat. no. 12-1971) (eBioscience, San Diego, CA). After washing twice with fluorescence-activated cell sorter (FACS) buffer, the cells were analyzed using flow cytometry. Forward scatter area (FSC-A) versus forward scatter height (FSC-H) was used to gate out the cell aggregates. In addition, ViViD dye staining was used to exclude the dead and dying cells and analyzed directly on a modified LSRII flow cytometry (BD Immunocytometry Systems, San Jose, CA) or a Coulter EPICS Flow Cytometer (Coulter, Hialeah, FL) using flowjo software (TreeStar, San Carlos, CA). All samples were compared with their isotype-matched controls. In the case of dual flow cytometry, individual samples treated with each isotype alone were used to determine the background levels of autofluorescence.27 Immunofluorescence assay (IFA) was analyzed to detect HMGB1 protein in the transfected cells, utilizing rabbit polyclonal antibody against HMGB1 (Abcam) for 1 hr at room temperature and detected with Alexa-Fluor-488-conjugated secondary antibody (Molecular Probes, Carlsbad, CA) as described previously.24
Enzyme-linked immunosorbent assay for detection of HMGB1 and macrophage inflammatory protein-2 and for quantification of antibodies to Gag and Env
The antibody levels following each DNA-priming injection, and the humoral immune response to vaccination, were determined for each DNA construct. Briefly, 96-well high-binding polystyrene plates (Costar, Corning Incorporated, Corning, NY) were coated overnight at 4° with Gag or Env protein (2 μg/ml), which was diluted in PBS. The next day, plates were washed with PBS containing 0·05% Tween-20 (PBST), blocked for 1 hr with 3% BSA in PBST and then incubated with 1:100 dilutions of serum from immunized and naïve mice for 1 hr at 37°. Bound IgG was detected using goat anti-mouse IgG–HRP (GE Healthcare, formerly Amersham Biosciences, Piscataway, NJ) at a dilution of 1:5000. Bound enzyme was detected by the addition of the chromogen substrate solution tetramethyl benzidine (TMB) (R&D Systems), and read at 450 nm on a Biotek EL312e Bio-Kinetics reader (Bio-Tek Instruments, Winooski, VT). All serum samples were tested in triplicate.26 The levels of mouse CXCl2/macrophage inflammatory protein-2 (MIP-2) (cat. no. MM200; R&D Systems) and HMGB1 (cat. no. ST51011; IBL International, Hamburg, Germany) in the culture medium were determined using a commercially available enzyme-linked immunosorbent assay (ELISA) kit following the manufacturer’s instructions. All samples were analyzed in triplicate.
Mice studies: immunization/electroporation/splenocyte isolation
The quadriceps muscles of 6–8-week-old female BALB/c mice (Jackson Laboratory, Bar Harbor, ME) were injected three times, 2 weeks apart, with 25 μg of plasmid-encoded antigen (pHIV-1 Gag or pHIV-1 Env; pHIV-1 Gag + pHMGB1 or pHIV-1 Env + pHMGB1; pHMGB1 and expression vector pVax1) and electroporated as previously described.22,23,28 All plasmid DNA was made using endotoxin-free Qiagen columns (Qiagen, Valencia, CA). Briefly, square-wave pulses were delivered through a triangular three-electrode array consisting of 26-gauge solid stainless-steel electrodes. Two constant-current pulses of 0·1 Amps were delivered for 52 ms/pulse separated by a 1 second delay using the CELLECTRA® adaptive constant-current device (VGX Pharmaceuticals, The Woodlands, TX).28 Mice were housed and treated in a temperature-controlled, light-cycled facility at the University of Pennsylvania, and cared for under the guidelines of the National Institute of Health (NIH) and the University of Pennsylvania (PA, USA). The mice were killed 1 week following the third immunization and the spleens were pooled according to group (n= 4). The spleens were crushed using a Stomacher machine (Seward stomacher 80; Seward Laboratory Systems Inc., Bohemia, NY) and the resulting product was put through a 40 μm cell strainer to isolate the splenocytes. The cells were treated for 5 min with ACK lysing buffer (Invitrogen) to clear the RBCs. Following lysis the splenocytes were resuspended in RPMI supplemented with 10% fetal bovine serum (FBS). The cell number was determined using a haemocytometer.23,28
Interferon-γ enzyme-linked immunosorbent spot-forming cell assay
An enzyme-linked immunosorbent spot-forming cell assay (ELISPOT) was conducted as previously described.23,28,29 Briefly, 96-well ELISPOT plates (Millipore, Billerica, MA) were coated with anti-mouse IFN-γ capture antibody and incubated for 24 hr at 4° (R&D Systems). The following day, plates were washed and blocked for 2 hr with 1% BSA. Splenocytes (2 × 105) from immunized mice were added to each well and stimulated overnight at 37° in 5% CO2 in the presence of RPMI (negative control), concanavalin A (Con A) (positive control), or specific peptide antigens (10 μg/ml) [NIH-AIDS Research & Reference Reagent Program (NIHARRP)]. Peptide pools consist of 15-mer peptides overlapping by 11 amino acids. After 24 hr of stimulation, the cells were washed and incubated for 24 hr at 4° with detection antibody (biotinylated anti-mouse IFN-γ) immunoglobulin (R&D Systems). The plates were washed, and streptavidin–alkaline phosphatase was added to each well and incubated for 2 hr at room temperature. The plates were washed, and 5-bromo-4-chloro-3′-indolylphosphate p-toluidine salt and nitroblue tetrazolium chloride (chromogen color reagent) were added to each well (R&D Systems). The plates were then rinsed with distilled water and dried at room temperature. Spots were counted using an automated ELISPOT reader (Cellular Technology Ltd., Shaker Heights, OH).6
Statistical analysis
Statistical analyses were performed using one-way and two-way anovas and the Student’s two-tailed unpaired t-test from graph pad prism 3·0 software (GraphPad Software, Inc., La Jolla, CA). The differences were considered statistically significant at a P-value of<0·05.
Results
Construction and expression of HMGB1
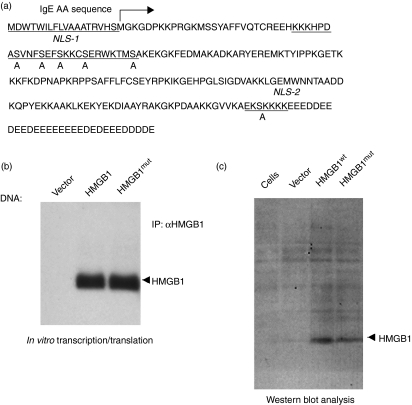
HMGB1 contains two nuclear localization signals (NLS) and two putative nuclear export signals for controlled nuclear transport, and the acetylation of both NLS are involved in nuclear export and secretion.30 NLS in many proteins influence their binding to nuclear import proteins and consequently increase or decrease their nuclear accumulation. Mutating serine residues to glutamic acid inside either or both NLS of HMGB1 has been shown to stimulate a phosphorylated state.30 Here we show that the mutation of serine residues to alanine in both NLS decreases the binding to nuclear importin protein and permits relocation to the cytoplasm and subsequent secretion.30Figure 1(a) shows an amino acid alignment of wild-type mouse HMGB1 with the NLS mutant. Because our vaccination strategy entailed harnessing the potent adjuvant activity of HMGB1, and because secretion was required for this activity, we generated an HMGB1wt construct and an HMGB1 construct lacking the NLS (HMGB1mut). The HMGB1 construct was also optimized by codon and RNA optimization, the addition of an immunoglobulin E (IgE) leader sequence and the replacement of the existing Kozak sequence with a stronger sequence (GCCGCCACC), and was inserted into the clinical expression vector, pVax1, under the control of the cytomegalovirus (CMV) promoter and between HindIII and Not1.
Figure 1.
Cloning and expression of high-mobility group box 1 (HMGB1) protein. (a) Schematic representation of the strategy for cloning the immunoglobulin G (IgE)-leader HMGB1 gene into the pVax1 vector and complete amino acid (AA) sequence of the HMGB1 gene. NLS, nuclear localization signal. (b) The in vitro translated HMGB1 reaction mixture was immunoprecipitated (IP) using the anti-HMGB1 product and subjected to electrophoresis on a 10% sodium dodecyl sulfate (SDS) polyacrylamide gel, demonstrating a band of 29 000 molecular weight. (c) Western blot analysis of HMGB1. Wild-type and mutant HMGB1 DNA (HMGB1wt and HMGB1mut, respectively) was constructed as described in the Materials and methods. Human 293T cells were transiently transfected with 10 μg of control vector, or with HMGB1wt or HMGB1mut expression constructs, as indicated. Cell lysates were extracted 48 hr after transfection, and immunoblotting was performed using rabbit polyclonal antibody to HMGB1.
Analysis of HMGB1 expression
The expression of pHMGB1 was confirmed using different methods. A 35S-labeled in vitro transcription and translation assay was performed. The product was immunoprecipated with anti-HMGB1 polyclonal antibody. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and radiographic analysis of the 35S-labeled translated product showed that the HMGB1 construct runs at its theoretically predicted molecular weight (29 000) upon autoradiography (Fig. 1b). Next, following transfection into cells, the proteins were extracted 2 days post-transfection and HMGB1 expression was detected using Western blotting with rabbit polyclonal HMGB1 antibody as the probe (Fig. 1c).
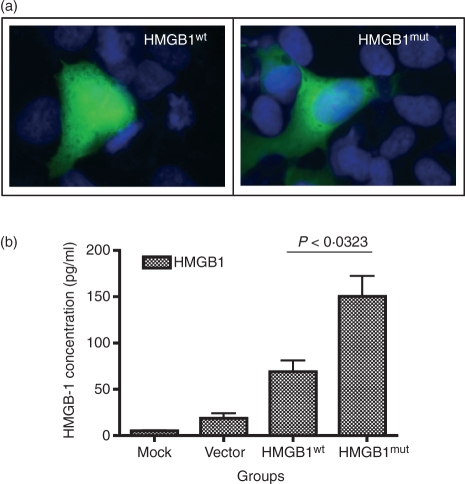
After verification of expression, the HMGB1 NLS was mutated in order to avoid interference with nuclear cytoplasmic shuttling.30 To analyze the expression and localization of HMGB1mut we performed immunohistochemistry on HeLa cells transfected with construct HMGB1wt or construct HMGB1mut (Fig. 2). HMGB1wt was mainly present in the nucleus. To confirm if the wild-type HMGB1wt is not secreted, but rather stored intracellularly, appearing in nuclear components, whereas the HMGB1mut is associated with secreted HMGB1, we conducted immunofluorescent staining of transfected cells using antibodies specific for HMGB1, as described in the Materials and methods. Cells transfected with the NLS mutant (HMGB1mut) expressed HMGB1 and this was clearly visible throughout the cytoplasm (Fig. 2a). Such staining was absent in cells transfected with the wild-type NLS construct (HMGB1wt). Furthermore, we observed (by ELISA analyses) a substantial increase in HMGB1 protein secreted from the HMGB1mut construct; HMGB1mut produced 150 pg/ml of HMGB1 protein, whereas HMGB1wt secreted lower levels (69 pg/ml). These data illustrate that the NLS mutant construct increases (by about twofold) the secretion of HMGB1 protein compared with the native HMGB1 construct (Fig. 2b).
Figure 2.
Comparison of wild-type and mutated high-mobility group box 1 (HMGB1) expression and functional activity. (a) Immunofluorescence analysis of the transfected HeLa cells expressing wild-type or mutant HMGB1 (HMGB1wt and HMGB1mut, respectively), showing the expressed protein in nucleus or cytoplasm. Cells were fixed and stained using rabbit polyclonal antibody to HMGB1, followed by fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit secondary antibody, as described in the Materials and methods. (b) Expression levels of plasmid constructs were tested. Forty-eight hours post-transfection, cell supernatants were harvested and analyzed, using enzyme-linked immunosorbent assay (ELISA), for the presence of secreted HMGB1 protein. The data shown are representative of three independent experiments.
HMGB1 protein induces phenotypic maturation of dendritic cells
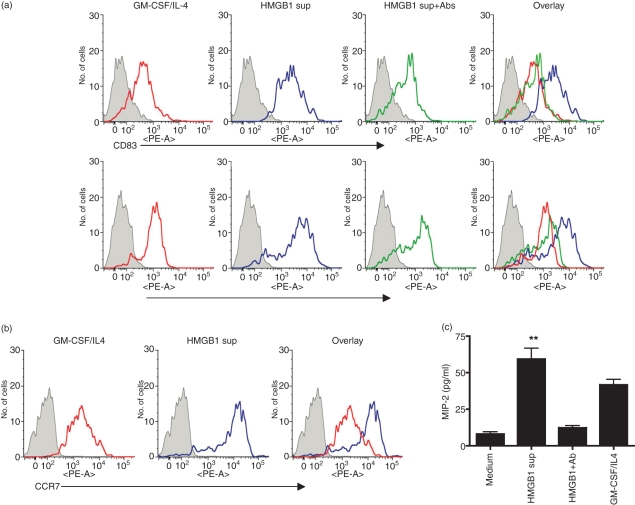
HMGB1, a nuclear and cytosolic protein, was originally identified as an intranuclear factor with an important structural function in chromatin organization.30,31 Recently, HMGB1 was identified as a pro-inflammatory cytokine that mediates inflammation, macrophage activation and DC maturation.20,21 DCs are the most potent APCs, and the manipulation of DC maturation provides a strategy for enhancing the immune response.32 In this study, we examined the activation/maturation of DC by HMGB1 protein.21,32 To determine whether HMGB1 can induce DC maturation, supernatant from the HMGB1mut transfected cells was added to mouse BM-derived iDCs, and the up-regulation of CD83 and CD86 was observed (Fig. 3a). In this study, we also treated DC with supernatant from HMGB1mut-transfected cells in the presence of rabbit polyclonal HMGB1 antibody. In this assay, polyclonal HMGB1 antibody completely inhibited the ability of HMGB1 protein to activate DC, suggesting that this effect was specific to HMGB1 protein. The protein-induced changes were quantitatively similar to those produced by TNF-α (data not shown). Furthermore, iDCs express CCR1, CCR2, CCR5 and CCR6 in response to inflammatory chemokines, whereas mature DCs express CCR7, which is bound by the chemokines CCL19 (ELC/MIP-3) and CCL21 (SLC/6Ckine).27,32 The expression patterns of chemokine receptors in DCs are tightly regulated as a function of maturation and account for the differences observed in the roles of immature and mature DCs. Therefore, we tested whether HMGB1 can induce the expression of CCR7 on mature DCs. As shown in Fig. 3b, the fact that HMGB1 was sufficient to up-regulate CCR7 in mature DC suggests that HMGB1 dictates the ability of DC to mature. As we observed an increase in DC maturation activity by HMGB1, we investigated whether HMGB1 also induced the increase in chemokine secretion in BMDC, in addition to its effect on maturation. Again, HMGB1 showed consistently higher MIP-2 production in BMDC cultured with HMGB1 protein (Fig. 3c). Together, these data indicate that HMGB1 is able to maturate the iDC in this in vitro culture model. These results also suggest that the activation/maturation of DC is useful for attracting more APCs towards the site of DNA injection and also increases antigen-presentation activity in vivo.
Figure 3.
High-mobility group box 1 (HMGB1) induces the phenotypic maturation of dendritic cells (DCs). Fluorescence-activated cell sorter (FACS) analysis of immature DC cultured in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF)/interleukin-4 (IL-4) medium or HMGB1 plasmid-transfected cell supernatant. (a) Bone marrow-derived dendritic cells (BMDC) of BALB/c mice were resuspended in culture medium supplemented with GM-CSF/IL-4 (red line) or in the presence of HMGB1mut plasmid-transfected cell supernatant (HMGB1 sup) (blue line) or cell supernatant depleted with rabbit polyclonal antibody to HMGB1 (HMGB1 sup + Ab) (green line) and cultured for 7 days. On day 7, the cells were harvested, washed and analyzed for surface expression, by flow cytometry, using the indicated markers. (b) Treatment of HMGB1 in up-regulation of chemokine receptor expression. Cells were stained for CCR7 and analyzed using FACS. Histograms show the staining of specific surface markers, and filled histograms represent the isotype-matched control antibody staining. The FACS data are representative of three separate experiments, with similar results obtained on each occasion. (c) Chemokine secretions in response to HMGB1 treatment. The concentration of macrophage inflammatory protein-2 (MIP-2) was determined in the supernatant using enzyme-linked immunosorbent assay (ELISA) analysis. All error bars represent standard deviation (SD) and are representative of three independent experiments. **Significant differences by comparison with the medium control (P < 0.001; Student’s unpaired t-test). PE, phycoerythrin.
Antibody responses to immunization with HMGB1 DNA vaccine constructs
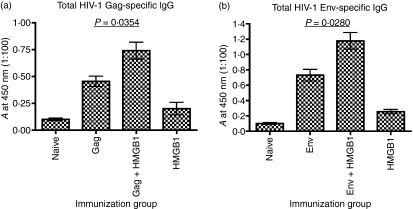
We hypothesized that stronger immune responses could be elicited by a combination of electroporation and conventional adjuvant, especially when the adjuvant utilized the stimulatory mechanisms of APCs for amplifying the response to the vaccine.1,7 To test this hypothesis, we examined the role of the adjuvant HMGB1 in enhancing the antibody responses in BALB/c mice vaccinated with human immunodeficiency virus-1 (HIV-1) Gag or HIV-1 Env. Sera obtained after immunization with DNA were tested for antibody response using ELISA. Gag or Env antibodies titers in the sera of mice immunized with HIV-1 Gag + HMGB1mut or HIV-1 Env + HMGB1mut were significantly higher than in the sera of mice immunized with HMGB1wt, HIV-1 Gag (P= 0·0354) or HIV-1 Env (P =0·0280) only (Fig. 4). These results were further supported by the increase observed in the specific IgG response to the DNA vaccine.
Figure 4.
Humoral responses enhanced by combining DNA vaccination with high-mobility group box 1 (HMGB1). BALB/c mice were immunized with human immunodeficiency virus-1 (HIV-1) Gag or HIV-1 Env DNA, alone or co-immunized with HMGB1, as indicated. The serum pool of each group (n= 4) was diluted to 1:100 for reaction with Gag or Env. The absorbance (A) was measured at 405 nm. The assay was performed in triplicate. All error bars represent the standard deviation (SD) and are representative of three independent experiments. Student's t-test indicates P-values are statistically significant (P < 0·05).
HMGB1 significantly induces cellular immunogenicity of the HIV-Gag vaccine
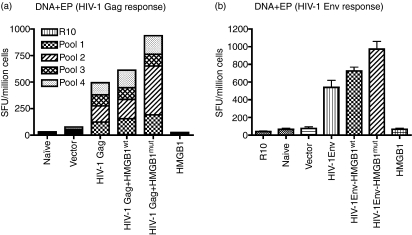
Next, we examined the effects of HMGB1 on enhancing the HIV-1-specific immune response, as determined using IFN-γ ELISPOTs. The mice were killed 1 week following the third immunization and cellular immune responses to the construct were determined using IFN-γ ELISPOTs.6 Splenocytes from vaccinated mice were stimulated with four HIV-1 Gag pools of 15-mer peptides overlapping by eight amino acids and spanning the sequence of HIV-1 Gag.6,26 As shown in Figure 5a, HIV-1 Gag + HMGB1mut is able to induce potent cellular immune responses. The magnitude of the response, as measured by the number of spot-forming units per million splenocytes (SFU), was 491 SFU/million cells for the Gag antigen. However, inclusion of HMGB1 as part of the HIV-1 DNA vaccine strategy results in an increased Gag response (933 SFU/million cells), which indicated a high level of Gag immunogenicity.
Figure 5.
High-mobility group box 1 (HMGB1) induces strong human immunodeficiency virus (HIV)-specific T-cell responses in mice. Interferon-γ enzyme-linked immunosorbent spot-forming cell assay (ELISPOT). BALB/c mice were immunized three times, each 2 weeks apart, with 25 μg of pVax vector or with pHMGB1 and were killed 1 week later. Splenocytes were harvested and cultured overnight in the presence of R10 (negative control), 10 μg/ml of HIV-1 Gag peptide pools or 10 μg/ml of HIV-1 Env peptide pools against a library of peptides spanning HIV-1 subtype B. (a) Responses to HIV-1 Gag are shown as a stacked group. (b) The total additive response to HIV-1 Env. Spot-forming units (SFU) were quantified using an automated ELISPOT reader, and the raw values were normalized to the number of SFU per million splenocytes. Error bars represent the standard deviation (SD) of ELISPOT results in triplicate wells and the data are representative of three independent experiments.
Similarly for HIV-1 envelope, the ELISPOT was performed against a library of peptides spanning HIV-1 subtype B Env. Two-hundred and eleven 15-mer peptides with 11 amino acid overlaps between them, which span residues 1–700 of HIV-1 Env B protein, were used.6 The results showed that the total additive average response was 540 SFU/million cells for the positively reacting Env peptides; however, inclusion of HMGB1 with the Env vaccine resulted in an increase in the number of antigen-specific T cells, to 973 SFU/million cells, which indicated a high level of Env immunogenicity (Fig. 5b).
Discussion
The current estimate of people suffering worldwide with human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS) is around 38 million. With approximately 14 000 new infections occurring every day and 2·8 million lives being lost already, AIDS is one of the most important infectious diseases that needs immediate attention (The Joint United Nations Programme on HIV-AIDS/World Health Organization statistics).33,34 Vaccines can be highly effective in preventing diseases caused by infectious agents; however, inducing an adequate immune response without significant adverse events represents a considerable challenge to vaccine development.35,36 In the coming years there will be an ongoing search for a vaccine that elicits an antibody response capable of neutralizing diverse HIV strains, along with continuous effort being directed at generating effective regimens for inducing HIV-1-directed CTL responses. In animal models, DNA vaccines have been shown to induce both humoral and cellular immune responses to the antigen of interest and then to confer protective immunity against certain viral pathogens.6,22,23,37 In several studies, the co-inoculation of plasmids encoding cytokine genes as adjuvants enhanced significantly the immune response to DNA vaccines.5–7,12,14,38,39 In this report we demonstrate that co-immunization of HMGB1 enhances the antibody response as well as the T-cell cellular immune response to DNA compared with the immune response induced by pHIV-1 Gag or pHIV-1 Env DNA immunogen alone.
HMGB1, a nuclear and cytosolic protein, is also defined as a pro-inflammatory cytokine that mediates macrophage attraction and activation.40 The intracellular abundance of HMGB1 and its pro-inflammatory activities suggest that its release at sites of cell injury or damage plays a role in the initiation and/or perpetuation of an immune response. We report here that an NLS mutated HMGB1 protein leaves the nucleus from transfected cells and is secreted in the extracellular environment, facilitating attraction of APCs. The receptor involved in HMGB1-mediated DC activation/attraction is not yet fully defined, although HMGB1 is a specific ligand for receptor for advanced glycation end-products (RAGE) and several studies raise the possibility that HMGB1 signals DC maturation through RAGE.40 Another possible receptor for HMGB1 is the Toll-like receptor 2 (TLR2), which is sufficient for DC maturation.40 A number of studies have reported enhancement of cellular or humoral response from cytokine codelivery.5,6,8,10,12,14,28,39 DCs play a critical role in the stimulation of the immune response by DNA vaccines,3 and their potential is not fully utilized in the DNA vaccine setting because they take up only a minor fraction of the injected DNA in the muscle. Therefore DC-intensifying approaches have been utilized, including growth-facilitative, function-stimulating, or life-prolonging strategies.3,9,21,39 We employed a natural DC-chemoattractant molecule, HMGB1, to recruit more DCs to the antigen-expression site to facilitate improved antigen processing and presentation. Interestingly, based on the results presented in this report, we propose that administration of HMGB1 DNA could stimulate increased recruitment of iDCs to the injection site and that subsequently these DCs acquired an elevated maturation phenotype, leading to an enhanced immune response to an HIV-1 plasmid vaccine, as shown by both humoral and cellular immune assays. These results support the fact that further studies on the use of HMGB1 to enhance DNA vaccine immune responses are warranted.
Acknowledgments
Support from the National Institutes of Health AIDS Research and Reference Reagents program and University of Pennsylvania Centers for AIDS Research (CFAR), is also acknowledged. GM thanks Dr Eileen F. Lynch, EdD, Guidance Counselor, Cherry Hill High School East, Cherry Hill, NJ, for assistance with the Research in Science (RIS) academic program.
Disclosures
The authors declare having no financial or commercial conflicts of interest.
References
- 1.Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet. 2008;9:776–88. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laddy DJ, Weiner DB. From plasmids to protection: a review of DNA vaccines against infectious diseases. Int Rev Immunol. 2006;4:99–123. doi: 10.1080/08830180600785827. [DOI] [PubMed] [Google Scholar]
- 3.Hung CF, Yang M, Wu TC. Modifying professional antigen-presenting cells to enhance DNA vaccine potency. Methods Mol Med. 2006;127:199–220. doi: 10.1385/1-59745-168-1:199. [DOI] [PubMed] [Google Scholar]
- 4.Hovav AH, Panas MW, Rahman S, Sircar P, Gillard G, Cayabyab MJ, Letvin NL. Duration of antigen expression in vivo following DNA immunization modifies the magnitude, contraction, and secondary responses of CD8+ T lymphocytes. J Immunol. 2007;179:6725–33. doi: 10.4049/jimmunol.179.10.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chattergoon MA, Muthumani K, Tamura Y, et al. DR5 activation of caspase-8 induces DC maturation and immune enhancement in vivo. Mol Ther. 2008;16:419–26. doi: 10.1038/sj.mt.6300373. [DOI] [PubMed] [Google Scholar]
- 6.Boyer JD, Robinson TM, Kutzler MA, et al. Protection against simian/human immunodeficiency virus (SHIV) 89.6P in macaques after coimmunization with SHIV antigen and IL-15 plasmid. Proc Natl Acad Sci USA. 2007;104:18648–53. doi: 10.1073/pnas.0709198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurunathan S, Wu CY, Freidag BL, Seder RA. DNA vaccines: a key for inducing long-term cellular immunity. Curr Opin Immunol. 2000;12:442–7. doi: 10.1016/s0952-7915(00)00118-7. [DOI] [PubMed] [Google Scholar]
- 8.Audibert FM, Lise LD. Adjuvants: current status, clinical perspectives and future prospects. Immunol Today. 1993;14:281–4. doi: 10.1016/0167-5699(93)90046-N. [DOI] [PubMed] [Google Scholar]
- 9.Morrow MP, Weiner DB. Cytokines as adjuvants for improving anti-HIV responses. AIDS. 2008;22:333–8. doi: 10.1097/QAD.0b013e3282f42461. [DOI] [PubMed] [Google Scholar]
- 10.Nchinda G, Kuroiwa J, Oks M, et al. The efficacy of DNA vaccination is enhanced in mice by targeting the encoded protein to dendritic cells. J Clin Invest. 2008;118:1427–36. doi: 10.1172/JCI34224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kutzler MA, Weiner DB. Developing DNA vaccines that call to dendritic cells. J Clin Invest. 2004;114:1241–4. doi: 10.1172/JCI23467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JJ, Nottingham LK, Sin JI, et al. CD8 positive T cells influence antigen-specific immune responses through the expression of chemokines. J Clin Invest. 1998;102:1112–24. doi: 10.1172/JCI3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacGregor RR, Boyer JD, Ugen KE, et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J Infect Dis. 1998;178:2–100. doi: 10.1086/515613. [DOI] [PubMed] [Google Scholar]
- 14.Hirao LA, Wu L, Khan AS, et al. Combined effects of IL-12 and electroporation enhances the potency of DNA vaccination in macaques. Vaccine. 2008;26:3112–20. doi: 10.1016/j.vaccine.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovacsovics-Bankowski M, Rock KL. Presentation of exogenous antigens by macrophages: analysis of major histocompatibility complex class I and II presentation and regulation by cytokines. Eur J Immunol. 1994;24:2421–8. doi: 10.1002/eji.1830241024. [DOI] [PubMed] [Google Scholar]
- 16.Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–6. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 17.Degryse B, Bonaldi T, Scaffidi P, Muller S, Resnati M, Sanvito F, Arrigoni G, Bianchi ME. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol. 2001;152:1197–206. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardman CH, Broadhurst RW, Raine AR, Grasser KD, Thomas JO, Laue ED. Structure of the A-domain of HMG1 and its interaction with DNA as studied by heteronuclear three- and four-dimensional NMR spectroscopy. Biochemistry. 1995;34:16596–607. doi: 10.1021/bi00051a007. [DOI] [PubMed] [Google Scholar]
- 19.Weir HM, Kraulis PJ, Hill CS, Raine AR, Laue ED, Thomas JO. Structure of the HMG box motif in the B-domain of HMG1. EMBO J. 1993;12:1311–9. doi: 10.1002/j.1460-2075.1993.tb05776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dumitriu IE, Baruah P, Valentinis B, et al. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol. 2005;174:7506–15. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- 21.Semino C, Angelini G, Poggi A, Rubartelli A. NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood. 2005;106:609–16. doi: 10.1182/blood-2004-10-3906. [DOI] [PubMed] [Google Scholar]
- 22.Muthumani K, Lankaraman KM, Laddy DJ, et al. Immunogenicity of novel consensus-based DNA vaccines against Chikungunya virus. Vaccine. 2008;26:5128–34. doi: 10.1016/j.vaccine.2008.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laddy DJ, Yan J, Corbitt N, Kobasa D, Kobinger GP, Weiner DB. Immunogenicity of novel consensus-based DNA vaccines against avian influenza. Vaccine. 2007;25:2984–9. doi: 10.1016/j.vaccine.2007.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muthumani K, Choo AY, Zong WX, et al. The HIV-1 Vpr and glucocorticoid receptor complex is a gain-of-function interaction that prevents the nuclear localization of PARP-1. Nat Cell Biol. 2006;8:170–9. doi: 10.1038/ncb1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talmor M, Mirza A, Turley S, Mellman I, Hoffman LA, Steinman RM. Generation or large numbers of immature and mature dendritic cells from rat bone marrow cultures. Eur J Immunol. 1998;28:811–7. doi: 10.1002/(SICI)1521-4141(199803)28:03<811::AID-IMMU811>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 26.Muthumani K, Hwang DS, Choo AY, Mayilvahanan S, Dayes NS, Thieu KP, Weiner DB. HIV-1 Vpr inhibits the maturation and activation of macrophages and dendritic cells in vitro. Int Immunol. 2005;17:103–16. doi: 10.1093/intimm/dxh190. [DOI] [PubMed] [Google Scholar]
- 27.Muthumani K, Choo AY, Hwang DS, et al. HIV-1 Nef-induced FasL induction and bystander killing requires p38 MAPK activation. Blood. 2005;106:2059–68. doi: 10.1182/blood-2005-03-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirao LA, Wu L, Khan AS, Satishchandran A, Draghia-Akli R, Weiner DB. Intradermal/subcutaneous immunization by electroporation improves plasmid vaccine delivery and potency in pigs and rhesus macaques. Vaccine. 2008;26:440–8. doi: 10.1016/j.vaccine.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 29.Kutzler MA, Robinson TM, Chattergoon MA, et al. Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially ndependent of CD4 T cell help. J Immunol. 2005;175:112–23. doi: 10.4049/jimmunol.175.1.112. [DOI] [PubMed] [Google Scholar]
- 30.Youn JH, Shin JS. Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion. J Immunol. 2006;177:7889–97. doi: 10.4049/jimmunol.177.11.7889. [DOI] [PubMed] [Google Scholar]
- 31.Zong WX, Ditsworth D, Bauer DE, Wang ZQ, Thompson CB. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 2004;18:1272–82. doi: 10.1101/gad.1199904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rovere-Querini P, Capobianco A, Scaffidi P. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5:825–30. doi: 10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen J. HIV/AIDS. Treatment and prevention exchange vows at international conference. Science. 2008;321:902–3. doi: 10.1126/science.321.5891.902. [DOI] [PubMed] [Google Scholar]
- 34.Cohen J. HIV gets by with a lot of help from human host. Science. 2008;319:143–4. doi: 10.1126/science.319.5860.143. [DOI] [PubMed] [Google Scholar]
- 35.Sekaly RP. The failed HIV Merck vaccine study: a step back or a launching point for future accine development? J Exp Med. 2008;205:7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen J. HIV/AIDS. The high cost of stolen funds. Science. 2008;321:523. doi: 10.1126/science.321.5888.523. [DOI] [PubMed] [Google Scholar]
- 37.Lang KA, Yan J, Draghia-Akli R, Khan A, Weiner DB. Strong HCV NS3- and NS4A-specific cellular immune responses induced in mice and Rhesus macaques by a novel HCV genotype 1a/1b consensus DNA vaccine. Vaccine. 2008;26:6225–31. doi: 10.1016/j.vaccine.2008.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eo SK, Lee S, Kumaraguru U, Rouse BT. Immunopotentiation of DNA vaccine against herpes simplex virus via co-delivery of plasmid DNA expressing CCR7 ligands. Vaccine. 2001;19:4685–93. doi: 10.1016/s0264-410x(01)00241-9. [DOI] [PubMed] [Google Scholar]
- 39.Pinto AR, Reyes-Sandoval A, Ertl HC. Chemokines and TRANCE as genetic adjuvants for a DNA vaccine to rabies virus. Cell Immunol. 2003;224:106–13. doi: 10.1016/j.cellimm.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Park JS, Svetkauskaite D, He Q, Kim JY. Strassheim, D., Ishizaka A. and Abraham E., Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]