Abstract
Background
Resistance of tumors to cell death signals poses a complex clinical problem. We explored the therapeutic potential and in vivo toxicity of a combination of bortezomib, a proteasome inhibitor, and MD5-1, a tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) receptor (DR5) agonist monoclonal antibody, in mouse carcinomas.
Methods
Mice bearing Renca-FLAG (renal) or 4T1 (mammary) tumors were treated with bortezomib and/or MD5-1 and examined for lung metastases (Renca-FLAG: n = 93; 4T1: n = 40) or monitored for survival (Renca-FLAG: n = 143). Toxicity was assessed by histopathology and hematology. Viability and apoptotic signaling in Renca-FLAG and 4T1 cells treated with bortezomib alone or in combination with TRAIL were analyzed using 3-[4,5-dimethyiazol-2-yl-5]-[3-carboxymethyloxyphenyl]-2-[4-sulfophenyl]-2H tetrazolium assay and by measuring mitochondrial membrane depolarization and caspase-8 and caspase-3 activation. All statistical tests were two-sided.
Results
Bortezomib (20 nM) sensitized Renca-FLAG and 4T1 cells to TRAIL-mediated apoptosis (mean percent decrease in numbers of viable cells, bortezomib + TRAIL vs TRAIL: Renca-FLAG, 95% vs 34%, difference = 61%, 95% confidence interval [CI] = 52% to 69%, P < .001; 4T1, 85% vs 20%, difference = 65%, 95% CI = 62% to 69%, P < .001). Sensitization involved activation of caspase-8 and caspase-3 but not mitochondrial membrane depolarization, suggesting an amplified signaling of the extrinsic cell death pathway. Treatment with bortezomib and MD5-1 reduced lung metastases in mice carrying Renca and 4T1 tumors (mean number of metastases, bortezomib + MD5-1 vs MD5-1: Renca-FLAG, 1 vs 8, difference = 7, 95% CI = 5 to 9, P < .001; 4T1, 1 vs 12, difference = 11, 95% CI = 9 to 12, P < .001) and increased median survival of mice bearing Renca-FLAG tumors (bortezomib + MD5-1 vs bortezomib + control isotype antibody: 22 of 30 [73%] were still alive at day 180 vs median survival of 42 days [95% CI = 41 to 44 days, P < .001]) in the absence of obvious toxicity.
Conclusion
Bortezomib combined with DR5 agonist monoclonal antibody may be a useful treatment for metastatic solid tumors.
Resistance of tumor cells to signals that trigger cell death is a major impediment in the treatment of cancer. Tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL/Apo2L) (1), which is implicated in host immunosurveillance against tumor development and metastasis (2–6), has tumoricidal activity in various models of human xenogeneic tumors in immunodeficient mice (7–11) without toxicity to most nontransformed cells (7,12,13). Agonist antibodies to the TRAIL death receptors have also been tested as potential therapeutic agents. These antibodies may offer distinct therapeutic advantages over TRAIL itself due to their long half-life and their lack of binding to decoy receptors on target cells (14). However, treatment with an agonist monoclonal antibody to the mouse TRAIL death receptor (DR5, TRAIL-R2, or CD262) only delays tumor development and does not improve the survival of mice bearing R331 renal carcinomas or 4T1 breast carcinomas (15,16), although the R331 clone is highly sensitive to TRAIL-mediated apoptosis in contrast to the parental Renca tumor (5,17).
Bortezomib, a specific and reversible inhibitor of the proteasome function that is crucial for protein degradation (18), has been approved by the Food and Drug Administration for the therapy of multiple myeloma (19) and has been shown by us and others (20–22) to sensitize tumors to TRAIL. However, to date no experimental evidence exists as to whether bortezomib can be combined with TRAIL or TRAIL receptor agonists in vivo for therapy of preexisting tumors within acceptable limits of toxicity.
Here we examined the therapeutic potential and the toxicity of the combination of bortezomib and the TRAIL receptor DR5 agonist monoclonal antibody MD5-1 in mice bearing Renca and 4T1 carcinomas. The molecular basis of bortezomib sensitization of tumor cells to TRAIL-mediated apoptosis was also investigated.
CONTEXT AND CAVEATS
Prior knowledge
Tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) is involved in host immunosurveillance against tumor development and metastasis and been shown to have antitumor activity in various mouse models of human cancers. Bortezomib is an inhibitor of protein degradation and has been approved by the Food and Drug Administration for the treatment of multiple myeloma.
Study design
Cell viability and signaling of apoptotic death of mouse renal adenocarcinoma and mammary carcinoma cells were assayed after treatment with TRAIL and/or bortezomib. Development of lung metastases and survival of mice carrying tumors derived from these cells were measured after treatment with bortezomib and/or MD5-1, a TRAIL receptor agonist antibody.
Contributions
More cells underwent apoptotic death after treatment with bortezomib and TRAIL than with TRAIL alone. Mice treated with bortezomib and MD5-1 lived longer and developed fewer lung metastases than mice treated with MD5-1 alone.
Implications
The combination of bortezomib and a TRAIL agonist antibody may be useful in the treatment of solid metastatic tumors.
Limitations
It is unknown whether results will be similar in humans or what nonspecific toxic effects a similar drug combination might have in humans.
Methods
Tumor Cell Lines
The renal cell adenocarcinoma (Renca) cell line of BALB/c origin was provided by Dr Robert H. Wiltrout (National Cancer Institute [NCI]–Frederick). To obtain Renca cells that were sensitive or relatively resistant to a TRAIL-mediated death signal, stable cell lines of TRAIL-sensitive Renca-FLAG and TRAIL-resistant Renca-FLIP were generated by transfecting complementary DNA (cDNA) encoding control vector FLAG or FLAG-tagged murine cellular Fas–associated death domain–like interleukin (IL)-1–converting enzyme–inhibitory protein (cFLIPL) under the control of a cytomegalovirus promoter (provided by Dr J. P. Medema, University of Leiden, Leiden, The Netherlands) with selection in G418 (400 µ g/mL) as described previously (5). The mouse mammary carcinoma cell line 4T1 was provided by Dr Suzanne Ostrand-Rosenberg (University of Maryland Baltimore County, Baltimore, MD). The murine neuroblastoma tumor cell line TBJ, which is a metastatic subclone of Neuro-2a cells, was provided by Dr Rosalba Salcedo (NCI–Frederick). Human renal cell carcinoma lines ACHN and A498, and breast carcinoma lines MDA-MB-231 and BT-549 were purchased from the DTP Molecular Targets Program, NCI–Frederick. All cell lines were maintained in RPMI-1640 supplemented with 10% fetal calf serum, 2 mM l-glutamine, 1× nonessential amino acids, 1 mM sodium pyruvate, 100 U/mL penicillin, 100 µg/mL streptomycin, 10 mM HEPES, and 5 × 10−5 M 2-mercaptoethanol, pH 7.4.
Retroviral Transduction of Bcl-2
High levels of the Bcl-2 protein can inhibit the intrinsic apoptotic cell death pathway (1). To assess the role of the intrinsic cell death pathway in bortezomib sensitization of tumor cells to TRAIL, a Bcl-2 retroviral expression vector was made by inserting human Bcl-2 cDNA (GenBank accession code NM_000633) into the EcoRI site of the pMIG-GFP plasmid (23) and transfecting the plasmid into the phoenix-Eco 293 packaging cell line (Orbigen, Inc, San Diego, CA) using FuGene 6 (Roche, Indianapolis, IN). The retrovirus-containing supernatant was collected 48 hours after transfection. 4T1 cells (1.0 × 106) were mixed with retroviral supernatant (4 mL) in the presence of polybrene (8 µg/mL) and centrifuged (900g, 1 hour, room temperature). Transduced cells (4T1-Bcl-2) were washed and cultured overnight before they were sorted for the expression of green fluorescence protein on a FACSAria (Becton Dickinson Immunocytometry Systems, San Jose, CA) using a 100-µm nozzle and a low-pressure set up. The laser used was a Coherent Sapphire solid-state laser at 488 nm and 13 mW of power. The two band-pass filters were a 530/30 on detector E for green fluorescence and on 575/26 detector D for phycoerythrin on the 488 emmision collection octagon. Only cells that stained positive were used in experiments evaluating the effect of Bcl-2 transduction in 4T1 cells on bortezomib sensitization to TRAIL.
Monoclonal Anti-DR5 Antibody Production
A hamster monoclonal IgG2 anti–mouse DR5 (MD5-1) was purified from concentrated tissue culture supernatants of MD5-1 hybridoma cells by a nonchromatographic procedure (24). Pooled fractions were dialyzed against phosphate-buffered saline (PBS; 2.68 mM KCl, 1.47 mM KH2PO4, 136.89 mM NaCl, and 8.1 mM Na2HPO4), sterile filtered (0.2-µm polyethersulfone membrane), and maintained at −20°C for long-term storage or at 4°C for use within 1 week.
Estimation of Viable Cell Numbers
To determine the cytotoxic activity of TRAIL (four independent experiments in triplicate), we plated Renca-FLAG, Renca-FLIP, TBJ, 4T1, and 4T1-Bcl-2 cells (5 × 103 cells per well, 37°C, 5% CO2) in flat-bottomed microtiter plates, and the next day cells were left untreated or were treated with bortezomib (provided by Millennium Pharmaceuticals, Cambridge, MA). A stock solution of 2.6 mM bortezomib in 0.9% NaCl was diluted to 10 or 20 nM in tissue culture media. After 3–12 hours, cells were treated overnight with soluble recombinant mouse or human TRAIL (1000 ng/mL) (BioMol, Plymouth Meeting, PA) that had been cross-linked by prior treatment with mouse monoclonal anti-polyhistidine (R&D Systems, Minneapolis, MN; 2 µg antibody per µg TRAIL). In experiments that included sequential treatment (seven independent experiments in triplicate), cells were washed with PBS to remove bortezomib before treatment with TRAIL for 12 or 24 hours. To assess the role of caspase activation in the reduction of tumor cell number (two independent experiments in triplicate), the pan-caspase inhibitor zVAD-FMK (R&D Systems) or the control zFA-FMK (ICN Pharmaceuticals Inc, Aurora, OH) was added at a final concentration of 40 µM, 2 hours before treatment with TRAIL.
To determine the cytotoxic activity of immobilized MD5-1 against Renca-FLAG, Renca-FLIP, and 4T1 cells (three independent experiments in triplicate), the cells were treated overnight with medium or with bortezomib (20 nM) and then were isolated and washed with tissue culture media. Cells were then plated (5 × 103 cells per well) on Reacti-Bind protein A–coated 96-well plates (Pierce, Rockford, IL) that had been precoated overnight at 4°C with hamster monoclonal anti–mouse MD5-1 antibody at 5 µg/mL and washed free of unbound antibody. The plates were centrifuged briefly (500g at room temperature) and incubated overnight (37°C, 5% CO2). In some experiments, staurosporine (0.2 µg/mL) was added to 4T1 and 4T1-Bcl-2 cells for 18 hours as a positive control, that is, to demonstrate that cells transduced with Bcl-2 were protected from an apoptosis-inducing agent (ie, staurosporine) whose action is known to be dependent on mitochondrial perturbation (three independent experiments in triplicate).
Viable cells were quantified by 3-[4,5-dimethyiazol-2-yl-5]-[3-carboxymethyloxyphenyl]-2-[4-sulfophenyl]-2H tetrazolium (Promega, Madison, WI) assay as described previously (20). Results are presented as viable cell number (absorbance at 490 nM) or as the percent decrease in cell number. Percentage decrease in cell number was calculated as 1 – [absorbance of treated cells – absorbance of media]/[absorbance of untreated cells – absorbance of media] × 100%.
Flow Cytometry to Measure DR5 Cell Surface Expression and Apoptosis
Renca-FLAG, Renca-FLIP, and 4T1 cells were treated overnight with bortezomib (20 nM) or medium. Nonspecific antibody binding was blocked by treatment with rat monoclonal anti–mouse CD16/32 (FcγR) (1:50; BD Biosciences Pharmingen, San Jose, CA). Cells were stained for surface DR5 immunofluorescence using purified hamster monoclonal anti–mouse MD5-1 (1 µg per 1 × 106 cells) and secondary biotinylated mouse anti–Armenian and Syrian hamster IgG (0.5 µg per 1 × 106 cells) or its isotype IgG2b (0.5 µg per 1 × 106 cells) and phycoerythrin-labeled streptavidin (1:2000; BD Pharmingen) according to standard procedures. Cells were stained with Annexin-V–fluorescein isothiocyanate and propidium iodide using a TACS apoptosis detection kit (R&D Systems) according to the manufacturer’s protocol. Stained cells were subjected to flow cytometry using a FACSCalibur flow cytometer (BD Biosciences), and data were analyzed using CellQuest (BD Biosciences) or FlowJo (Treestar Inc, San Carlos, CA) software. Four independent experiments were performed with single samples.
Evaluation of Mitochondrial Membrane Depolarization
Renca-FLAG, Renca-FLIP, 4T1, and TBJ cells were treated overnight with medium or with 10 nM of bortezomib. Cells were then stained with a bivariate mitochondrial membrane potential cationic dye 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolycarbocyanine iodide (JC-1) following the manufacturer’s protocol (Cell Technology Inc, Mountain View, CA). JC-1 is a potentiometric dye that exhibits a membrane potential–dependent loss as J-aggregates (polarized mitochondria) transition to JC-1 monomers (depolarized mitochondria); the loss of membrane potential is indicated by the fluorescence emission shift from red to green. Therefore, mitochondrial depolarization is indicated by an increase in the ratio of the green fluorescence to red fluorescence intensity. The data were acquired and analyzed by flow cytometry as described above. Five independent experiments were performed with single samples.
Assessment of Caspase-8 and Caspase-3 Enzyme Activities
Caspase-8 and -3 activities in Renca-FLAG, Renca-FLIP, and 4T1 cells were measured using the Caspase-Glo assay kit (Promega) as directed by the manufacturer but with some modifications. Briefly, the cells (5 × 103 cells per well) were plated in a white-walled 96-well luminometer plate and treated overnight with or without bortezomib (10 nM). The cells were then treated with mouse TRAIL (1 µg/mL) for 2 hours, followed by incubation with Caspase-Glo 8 or 3/7 buffer with luciferase and proluminescent substrates (provided by the manufacturer), containing either the amino acid sequence LETD or DEVD, which are cleaved by caspase-8 and caspase-3, respectively. A high concentration of bortezomib (20 µM) was included in the buffer–substrate mixture to block the endogenous caspase-like activity of the proteasome in the samples. An equal volume (100 µL) of the buffer–substrate mixture was added to each test well of the plate, which was incubated at room temperature for 10 minutes with shaking. The luminescence of each well in the plate after applying the white adhesive backing to the bottom of the plate was measured in a plate-reading luminometer (Wallac Victor 1420 Multilabel Counter, Perkin Elmer, Shelton, CT). The control for background luminescence was tissue culture media alone. Untreated cells were used as the control for levels of endogenous caspase activity. Luminescence (lumen) readings were taken after cells had been incubated with the buffer–substrate mixture for 30 minutes at room temperature. Four independent experiments were performed in triplicate.
Immunoblotting of Proteins Involved in Apoptotic Pathways
Immunoblotting was performed using NuPage 4%–12% Bis-Tris gels (Invitrogen, Carlsbad, CA) as described previously (5). Briefly, proteins (20 µg) from total cell lysates of Renca-FLAG, Renca-FLIP, and 4T1 cells that had been treated overnight with 20 nM bortezomib were separated by gel electrophoresis under reducing conditions, transferred to polyvinylidene difluoride membranes (Invitrogen), and probed with the mouse monoclonal antibodies FLIP Dave-2 (1:1000; Kamiya Biomedical, Seattle, WA), cIAP-1 (1:1000; R&D Systems), XIAP (1:250; BD Biosciences, San Diego, CA), or anti –β-actin AC74 (1:5000; Sigma, St Louis, MO) overnight at 4°C, followed by appropriate horseradish peroxidase–conjugated secondary antibodies (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA) (four independent experiments with individual samples). To assess caspase-8 activation, cells that had been incubated overnight in the presence or absence of 10 nM bortezomib were treated for 5 hours with recombinant mouse TRAIL (1000 ng/mL) before cell lysates were prepared. Monoclonal mouse anti–caspase-8 1G12 (1:2000; Alexis Biochemicals, San Diego, CA) was used to detect the cleaved (ie, active) form of caspase-8 (three independent experiments with individual samples). Antibody–protein complexes were detected using SuperSignal West Dura extended duration or Femto maximum sensitivity substrate (Pierce, Woburn, MA) according to the manufacturer’s instructions.
Mice
Female 7- to 12-week-old BALB/c (H2d) wild-type (WT) mice (n = 412) and severe combined immunodeficiency (SCID) mice (n = 60) were obtained from the Animal Production Area of the NCI–Frederick. SCID mice were used to gauge the dependence of the therapeutic benefit of the tested regimen on the host’s immune system. All mice were maintained under pathogen-free conditions. NCI–Frederick is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International and follows the Public Health Service Policy for the Care and Use of Laboratory Animals. Mice were cared for in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals (25).
Experimental Lung Metastasis and Combination Therapy With Bortezomib and Anti-DR5
For experimental lung metastases, BALB/c WT mice were injected intravenously on day 0 with Renca-FLAG cells (5 × 104, n = 63; 2 × 104, n = 30) or with 4T1 cells (2 × 104, n = 40). For long-term survival studies, BALB/c WT (n = 238) were injected intravenously with Renca-FLAG cells (1 × 104, n = 188) or with Renca-FLIP cells (1 × 104, n = 50), and BALB/c SCID (n = 60) mice were injected intravenously with Renca-FLAG cells (1 × 104).
A total of four injections of bortezomib and/or MD5-1 or its isotype control hamster IgG2 UC8-1B9 (HY, Tokyo, Japan) were given on days 4, 7, 11, and 15 after injection of tumor cells. Due to their heightened sensitivity to bortezomib for reasons not yet explored, SCID mice only received two injections, on days 4 and 11. Bortezomib was injected 5 hours before MD5-1. Different doses of bortezomib and MD5-1 were attempted. In some experiments, depletion antibodies anti–asialo-GM1 (200 µL of a 1:20 dilution; Wako Chemicals USA, Inc, Richmond, VA), anti-CD8α.2 (19/178; 200 µL of a 1:10 dilution), or anti-CD4 (GK1.5; 200 µL of a 1:4 dilution) (provided by Dr Robert H. Wiltrout, NCI–Frederick) were administered to mice intraperitoneally on days –1, 1, 8, and 15 with respect to the day of tumor injection. All mice were monitored for the relevant outcome (ie, tumor metastasis or survival) and were killed by CO2 inhalation when moribund (survival studies; 10 independent experiments) or on day 18 for analysis of lung metastases (tumor metastasis studies; four independent experiments), at which time lungs were surgically removed and fixed using Bouin’s fluid, and the number of lung tumor nodules as a measure of tumor burden were counted using a dissection microscope.
Assessment of Toxicity Following Treatments With Bortezomib and Anti-DR5
In experiment 1, 7- to 8-week-old BALB/c WT mice were injected intraperitoneally with saline (two mice) or with bortezomib (1.5 mg/kg) on days 1, 4, 8, 16, 22, and 29 and MD5-1 (50 µg per mouse) on days 2, 5, 9, 17, 23, and 30 (three mice). Mice were analyzed on day 31, 2 days after the final bortezomib injection. In experiment 2, mice were injected on day 0 with Renca-FLAG cells (1 × 104) and injected intravenously with saline (13 mice) or with bortezomib (1 mg/kg body weight) plus MD5-1 (50 µg per mouse) (13 mice) on days 4, 7, 12, and 15. Bortezomib was injected 5 hours before MD5-1.
Blood was collected by cardiac puncture on day 18 from three mice that were randomly selected and killed from each group in experiment 2. Blood was subjected to automated hematology analysis (KX-21, Sysmex Corporation, Kobe, Japan), and numbers of red and white blood cells, lymphocytes, and platelets were calculated. Serum levels of liver enzymes serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured using a CDCHemavet (Oxford, CT), which was calibrated for mouse blood.
Mice in experiments 1 (five mice) and 2 (six mice) were weighed, killed by CO2 inhalation, and subjected to necropsy examination. Livers and thymus glands were weighed. A comprehensive set of organs and tissues were collected and fixed in 10% neutral buffered formalin. Tissues were processed by standard methods, paraffin embedded, sectioned at 5-µm thickness, and stained with hematoxylin and eosin. Brains, kidneys, thymus, livers, spleens, lungs, small and large intestines, vertebrae, and spinal cords from all five mice from experiment 1 (114 days of age) as well as thymus and livers from all six mice from experiment 2 (68 days of age) were evaluated by a board-certified veterinary pathologist (Dr Diana C. Haines, NCI–Frederick).
Statistical Analysis
Comparisons of mean values between the groups were analyzed using GraphPad Instat software (GraphPad Prism, GraphPad Software Inc, San Diego, CA). Statistical significance of the differences was analyzed by using unpaired Student t test for comparisons of two groups or by one-way analysis of variance for comparisons of more than two groups. Comparisons of survival curves estimated by Kaplan–Meier plots using GraphPad Prism were performed by the log-rank (Mantel–Cox) test. All statistical tests were two-sided; P values less than .05 were considered statistically significant.
Results
Bortezomib-Induced Sensitization of Renal and Mammary Mouse Tumors to TRAIL
We evaluated the effect of bortezomib-induced proteasome inhibition on TRAIL-mediated death of Renca-FLAG, Renca-FLIP (Supplementary Figure 1, available online), and 4T1 cells. No substantial decrease in cell number was observed following TRAIL or bortezomib treatment alone. However, combination treatment with bortezomib (20 nM) and TRAIL (1 µg/mL) led to a decrease in the number of viable tumor cells (mean percent decrease in numbers of viable cells among bortezomib + TRAIL vs TRAIL-treated cells, Renca-FLAG, 95% vs 34%, difference = 61%, 95% confidence interval [CI] = 52% to 69%, P < .001; 4T1, 85% vs 20%, difference = 65%, 95% CI = 62% to 69%, P < .001; Figure 1, A). Similar effects were seen when bortezomib at 10 nM was combined with TRAIL, whereas the combination of bortezomib at 2.5 nM and TRAIL was without effect (data not shown). Experiments in which different schedules of these agents were tested showed that the pretreatment of these tumors with bortezomib sensitized them to TRAIL-mediated death and not vice versa (data not shown).
Figure 1.
Bortezomib (Bzb)-induced sensitization of renal and mammary mouse tumors to tumor necrosis factor–related apoptosis-inducing ligand (TRAIL). A) Renca-FLAG, Renca-FLIP, and 4T1 tumor cells were untreated or pretreated with 20 nM Bzb for 3 hours. Cells were then treated with soluble recombinant mouse TRAIL (1 µg/mL) or medium. B) The cells were treated the same way except that instead of TRAIL they were treated with protein A–bound immobilized TRAIL receptor agonist monoclonal antibody MD5-1 (5 µg/mL). Cell viability was determined 18 hours later using 3-[4,5-dimethyiazol-2-yl-5]-[3-carboxymethyloxyphenyl]-2-[4-sulfophenyl]-2H tetrazolium assay. Percent decrease in cell number of treated cells compared with untreated cells is shown as means and 95% confidence intervals (n = 3) from one representative experiment of four (A) and three (B) independent experiments. *P < .001 (analysis of variance, two-sided) with respect to single agents.
We next investigated whether the mouse TRAIL receptor DR5 monoclonal antibody MD5-1 could be cytotoxic in a manner similar to recombinant TRAIL. Soluble MD5-1 had little cytotoxic effect, even when combined with bortezomib (data not shown). However, exposure of cells that were pretreated with bortezomib (20 nM) to immobilized protein A–bound MD5-1 (5 µg/mL) led to a statistically significant reduction in cell numbers (mean percent decrease in cell number, bortezomib + MD5-1 vs MD5-1: Renca-FLAG, 79% vs 27%, difference = 52%, 95% CI = 46% to 55%, P < .001; Renca-FLIP, 39% vs 4.9%, difference = 34.1%, 95% CI = 29% to 38%, P < .001; 4T1, 67% vs 30%, difference = 37%, 95% CI = 26% to 45%, P < .001) (Figure 1, B), confirming previous findings that appropriate cross-linking of MD5-1 is crucial for its optimal biologic effects (17).
Tumor cells exhibited apoptotic morphology following bortezomib and TRAIL treatments (data not shown). Addition of the pan-caspase inhibitor zVAD-FMK to Renca-FLAG and 4T1 cells before incubation with TRAIL blocked TRAIL-mediated apoptosis as assessed by flow cytometry of annexin-V and propidium iodide–stained cells (Figure 2), suggesting that the combination of bortezomib and TRAIL induced caspase-dependent apoptosis in these tumor cells. Furthermore, the enhanced cytotoxicity of the combination treatment of these cells was also confirmed by the 111In-oxine release (Supplementary Figure 2, available online). Thus, pharmacologically relevant bortezomib concentrations [10–20 nM (26)] reduced the threshold for apoptosis in response to a death receptor–mediated signal in both renal and mammary carcinoma cells.
Figure 2.
Bortezomib (Bzb)-induced sensitization to tumor necrosis factor–related apoptosis-inducing ligand (TRAIL)–mediated apoptosis. Renca-FLAG and 4T1 tumor cells were untreated (Med) or treated with 20 nM Bzb for 3 hours before the addition of soluble recombinant mouse TRAIL (1 µg/mL) for a further 14–18 hours. The pan-caspase inhibitor zVAD-FMK (zVAD) or control analog zFA-FMK (zFA) (both at 40 µM final concentration) was added 2 hours before TRAIL treatment. Cell death was measured by annexin-V and propidium iodide staining using flow cytometry. Representative dot plots from one experiment of two independent experiments are shown. Numbers represent percentage of propidium iodide–stained and annexin-V–positive cells in the respective quadrants.
Molecular Basis of Bortezomib-Induced Sensitization of umor Cells to Apoptotic Signals
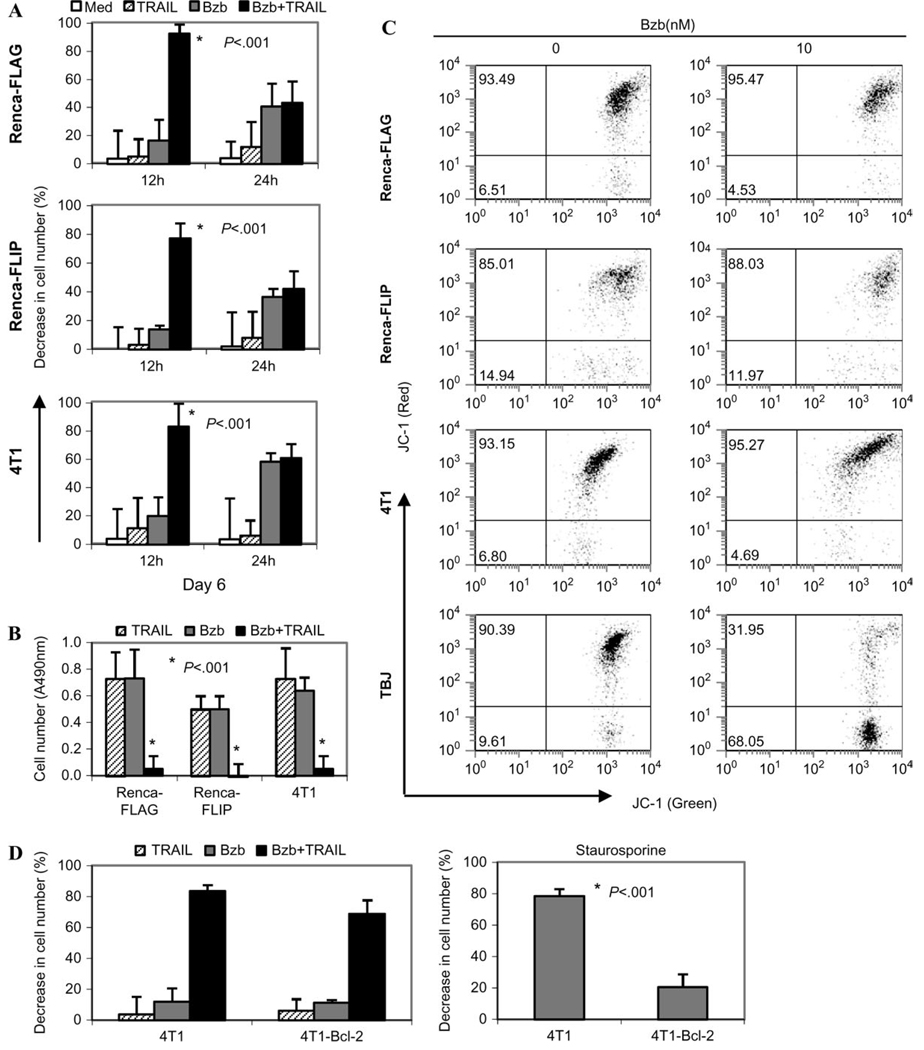
The bortezomib-mediated sensitization of Renca-FLAG and 4T1 tumor cells to TRAIL-mediated apoptotic signals was a transient phenomenon. The dramatic decrease in cell number in response to TRAIL was still evident 12 hours after removal of bortezomib but was lost by 24 hours (Figure 3, A). The bortezomib concentration (20 nM) that was required to sensitize these tumor cells to apoptosis was itself cytostatic. Long-term (6-day) culture of the treated tumor cells showed that the cytostatic effect of bortezomib was transient—at day 6 after bortezomib removal, we observed substantial cell proliferation. By contrast, the combination of bortezomib and TRAIL was cytotoxic, resulting in a dramatic reduction of tumor cell numbers on day 6 (Figure 3, B).
Figure 3.
Cytotoxicity of combination treatment with bortezomib (Bzb) and tumor necrosis factor–related apoptosis-inducing ligand (TRAIL). Renca-FLAG, Renca-FLIP, and 4T1 cells were untreated (Med) or treated with 20 nM Bzb for 12 hours and washed with phosphate-buffered saline. Recombinant mouse TRAIL (1 µg/mL) was added to cells 12 or 24 hours later. Cell number was determined using the 3-[4,5-dimethyiazol-2-yl-5]-[3-carboxymethyloxyphenyl]-2-[4-sulfophenyl]-2H tetrazolium assay. A) Percent decrease in cell number following 18 hours of TRAIL treatment. B) Numbers of viable cells in the respective cell lines shown as absorbance (A) at 490 nm 6 days after Bzb treatment in the presence or absence of TRAIL, added at 12 hours after Bzb. Means and 95% confidence intervals from one representative experiment of four (A) and seven (B) independent experiments are shown. *P < .001 (analysis of variance, two-sided) with respect to single agents or medium. C) Renca-FLAG, Renca-FLIP, 4T1, and TBJ cells treated overnight with 0 or 10 nM of Bzb were analyzed by flow cytometry following 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolycarbocyanine iodide (JC-1) bivariate staining. A loss of J-aggregates (red fluorescence) was observed only in TBJ cells. Numbers represent percentage of JC-1-red–positive cells (upper quadrant, polarized mitochondria) and percentage of JC-1-red–negative cells (lower quadrant, depolarized). Representative dot plots from one experiment of five independent experiments are shown. D) Bcl-2–transduced (4T1-Bcl-2) or parental 4T1 cells were untreated or treated with 10 nM Bzb for 3 hours and then treated with mouse TRAIL (500 ng/mL). Percent decrease in cell number after 18 hours of treatment with TRAIL is shown (left). Percent decrease in cell number in 4T1-Bcl-2 or parental 4T1 cells following 18-hour exposure to staurosporine (0.2 µg/mL) is shown (right); *P < .001 (two-sided Student t test). One representative experiment out of three independent experiments is shown. For all graphs, means and 95% confidence intervals (error bars) are shown.
To investigate the molecular mechanism(s) underlying this transient sensitization of tumors to apoptotic signals, we assessed the activity of various components of apoptotic signaling pathways. Apoptotic signaling can follow a mitochondria-dependent (intrinsic) or -independent (extrinsic) pathway after engagement of the TRAIL receptor with its cognate ligands (12, 27). Renca-FLAG, Renca-FLIP, or 4T1 cells treated with bortezomib alone (10 nM, overnight) did not show any mitochondrial membrane depolarization (Figure 3, C), whereas the neuroblastoma TBJ tumor cell line did (28). Also, no major changes in the protein levels of Bcl-2 or Bax, which normally regulate mitochondria-dependent apoptosis, were detected in these tumor cells following bortezomib treatment (data not shown). Furthermore, both Bcl-2–transduced 4T1 cells (Supplementary Figure 3, available online) and parental 4T1 cells showed a similar decrease in cell number when treated with the combination of bortezomib and TRAIL (Figure 3, D). By contrast, fewer 4T1-Bcl-2 cells than parental 4T1 cells died after treatment with staurosporine, a trigger of the intrinsic apoptotic pathway (Figure 3, D, right). The lack of an effect of Bcl-2 expression on the bortezomib-induced sensitization of 4T1 cells to TRAIL-mediated cytotoxicity implies that 4T1 cells did not require a mitochondria-dependent amplification of the apoptotic signal in response to this combination of agents. Moreover, bortezomib (20 nM) had no effect on the cellular levels of the antiapoptotic proteins cFLIP, cIAP, and XIAP in Renca-FLAG, Renca-FLIP, and 4T1 cells (Figure 4, D). Therefore, there were no obvious changes in the cellular levels of a variety of proteins involved in apoptosis following bortezomib treatment.
Figure 4.
Bortezomib (Bzb)-induced sensitization to the extrinsic tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) receptor–mediated pathway of apoptosis in renal and mammary carcinomas. Renca-FLAG, Renca-FLIP, and 4T1 cells were treated overnight with Bzb (10 or 20 nM) or medium. A) Surface expression of death receptor DR5 by MD5-1 staining on tumor cells sensitized with 20 nM Bzb. Representative histograms from four independent experiments with similar results are shown (thin line, isotype control; filled histogram, untreated; heavy line, Bzb treated). B) Caspase-8 and caspase-3 activation in cells that were treated with a combination of Bzb (10 nM, overnight) and TRAIL (TR, 1000 ng/mL, 2 hours), with either agent alone, or left untreated (Med). Caspase activation was assayed by the luciferase-based enzymatic reaction. Mean luminescence and 95% confidence intervals (error bars) from one representative experiment of four independent experiments are shown. *P < .001 (analysis of variance, twosided) compared with single agents and untreated cells. C) Immunoblots of caspase-8 expression in whole-cell lysates of Renca-FLAG, Renca-FLIP, and 4T1 cells that were treated with media (Med) or treated overnight with 20 nM Bzb and then treated with or without recombinant mouse TRAIL (TR, 1000 ng/mL, 5 hours). Representative blots from three independent experiments are shown. D) Immunoblots of cFLIP, cIAP-1, and XIAP protein expression in whole-cell lysates of Renca-FLAG, Renca-FLIP, and 4T1 cells treated overnight with 0 or 20 nM Bzb. Representative blots from three independent experiments are shown.
Furthermore, these tumor cell lines exhibit constitutive cell surface expression of death receptor DR5, and expression was only slightly enhanced following overnight sensitization with bortezomib (mean fluorescence intensity, untreated cells vs bortezomib-treated cells: Renca-FLAG, 127 vs 157, difference = 30, 95% CI = 54 to 113, P = .4; Renca-FLIP, 139 vs 189, difference = 50, 95% CI = 77 to 177, P = .3; and 4T1, 190 vs 272, difference = 82, 95% CI = 1.4 to 163, P = .04) (Figure 4, A). However, a critical step in TRAIL signaling after receptor aggregation is thought to be the dimerization of pro-caspase-8 in the death-inducing signaling complex (29), which confers a conformational change that is required for its enzymatic activity (30). Increased enzymatic activation of caspase-8 (Figure 4, B) and accumulation of the cleaved form (43 kDa) of caspase-8 (Figure 4, C) was seen in all three tumor cell lines treated with the combination of bortezomib and TRAIL. These treated tumor cells also had elevated caspase-3 enzyme activity (Figure 4, B). The major molecular mechanism for bortezomib-sensitization to TRAIL-mediated apoptosis in these renal and breast tumor cells therefore seems to be a direct increase in caspase-8 enzyme activity, thus amplifying upstream signaling of the extrinsic apoptotic pathway.
Therapeutic Benefit of Bortezomib Plus Anti-DR5 dministration
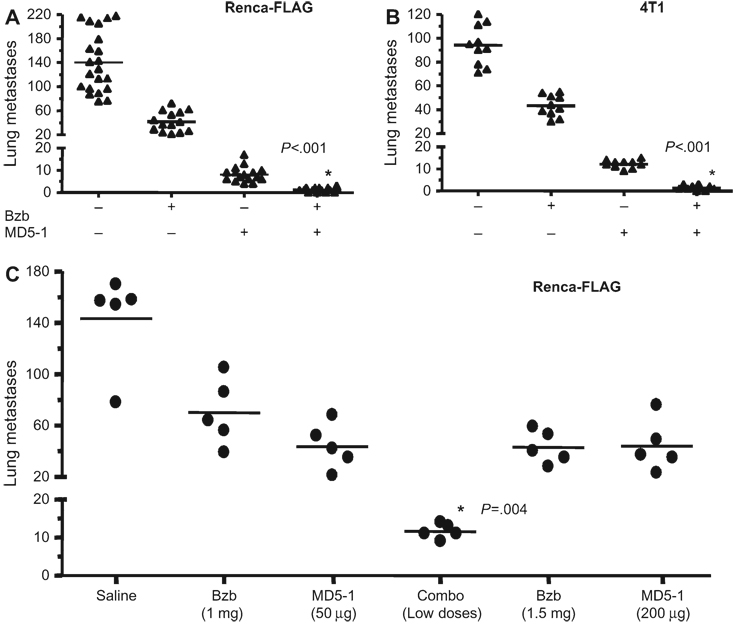
Mice with Renca-FLAG tumors were treated with bortezomib, followed 5 hours later by agonistic TRAIL-receptor monoclonal anti-body MD5-1. Mice treated with the combination of bortezomib (0.5 mg/kg) and MD5-1 (5 µg per mouse) had fewer lung metastases than mice that were treated with either single agent (mean no. of lung metastases, combination treatment vs MD5-1 alone: 1 vs 8, difference = 7, 95% CI = 5 to 9, P < .001) (Figure 5, A). Administration of even higher doses of bortezomib (1.5 mg/kg) or MD5-1 (200 µg per mouse) alone failed to reduce metastases as much as the combination treatment (Figure 5, C). Furthermore, the combination treatment also caused a substantial reduction in lung metastases of 4T1, a highly metastatic mammary tumor line (Figure 5, B), suggesting that this combination regimen may have therapeutic benefits in different tumor types.
Figure 5.
Effect on lung metastases in tumor-bearing mice of combination treatment with bortezomib (Bzb) and death receptor (DR5) agonist antibody vs MD5-1 treatment with single agent. BALB/c wild-type (WT) mice bearing tumors derived from Renca-FLAG and 4T1 tumor cells were injected intravenously with Bzb (0.5 mg/kg) and/or MD5-1 (5 µg per mouse) on days 4, 7, 11, and 15 after tumor cells were injected (ie, day 0). Lung metastases counts are shown on day 18 following therapy. A) Renca-FLAG (5 × 104) (n = 14 per treatment group; n = 21 in the untreated group). B) 4T1 (2 × 104) (n = 10). *P < .001 (analysis of variance [ANOVA], two-sided) compared with other treatment groups. Data from three independent experiments are shown. C) Lung metastases counts are shown on day 18 after therapy in Renca- FLAG (5 × 104) tumor–bearing BALB/c WT mice treated with the combination regimen (Combo) of Bzb (1 mg/kg) and MD5-1 (50 µg per mouse) or individual agents at the same or higher doses of Bzb (1.5 mg/kg) or MD5-1 (200 µg per mouse); n = 5 mice per group. *P = .004 (ANOVA, two-sided) when compared with all other treatment groups.
We next examined the long-term effects of the combination treatment. No long-term survival benefit was observed in mice treated with the combination or bortezomib at 0.5 mg/kg and MD5-1 at 5 µg (data not shown). To optimize the antitumor therapeutic potential of the combination treatment, we titrated the doses of bortezomib and MD5-1 and the frequency of their administration. Four cycles of combination treatment with bortezomib (1 mg/kg) and MD5-1 (50 µg per mouse) administered on days 4, 7, 11, and 15 after tumor injection was optimal for long-term therapy in WT mice. At day 181, 22 of 30 (73%) mice bearing experimental lung metastases of Renca-FLAG were alive and tumor free following combination therapy, whereas median survival of mice treated with bortezomib and control isotype antibody was 42 days (95% CI = 41 to 44 days, P < .001) and that of mice treated with MD5-1 alone was 57 days (95% CI = 51 to 64 days, P < .001) (Fig. 6, A). A statistically significant extension in the median survival time, albeit with few long-term survivors, was also observed among Renca-FLIP tumor–bearing mice with the combination treatment (combination vs MD5-1 alone, 60 days vs 41 days, difference = 19 days, 95% CI = 17 to 24 days; P = .001) (Figure 6, B). The increased long-term survival by the combination of bortezomib with MD5-1 in vivo suggests that the therapeutic mechanism is dependent on enhanced apoptotic signaling and can still act even on tumors that are relatively refractory to death receptor–mediated killing.
Figure 6.
Effect on long-term survival of tumor-bearing mice treated with the combination of bortezomib (Bzb) and death receptor (DR5) agonist antibody. Tumor-bearing BALB/c wild-type (WT) mice were treated with the combination of Bzb (1 mg/kg) and MD5-1 (50 µg per mouse) on days 4, 7, 11, and 15 after tumor cell injection. A) Survival of Renca-FLAG (1 × 104) tumor–bearing mice (n = 30) following Bzb (1 mg/kg) and MD5-1 (50 µg per mouse) therapy compared with the groups treated with saline (n = 36), Bzb (n = 17), or MD5-1 (n = 20) or a combination of Bzb and control isotype antibody UC8-1B9 (n = 10). *P < .001 (two-sided log-rank test). B) Survival of Renca-FLIP (1 × 104) tumor–bearing mice (n = 15) following the combination therapy compared with the groups treated with saline (n = 15), Bzb (n = 10), or MD5-1 (n = 10) treatments; *P = .001 (two-sided log-rank test). C) Survival of Renca-FLAG (1 × 104) tumor–bearing BALB/c WT mice following combination treatments (Combo) compared with the groups treated with the combination with coinjections of anti-CD4 (aCD4), anti-CD8α (aCD8), or anti–asialo-GM1 (aGM1) antibodies (see “Methods”) (n = 15 in each group). *P = .5, **P = .2 (two-sided log-rank test). D) Survival of Renca-FLAG (1 × 104) tumor–bearing BALB/c severe combined immunodeficiency mice following combination treatments (days 4 and 11 only after tumor cell injection) compared with the groups treated with saline (n = 30 each). *P < .001 (two-sided log-rank test). Data from three (A), two (B and C), and three (D) independent experiments are shown. Numbers of mice at risk on various days are given below each graph.
We further evaluated whether the therapeutic benefit of the combination regimen depended on the host’s immune system. About 60% of the Renca-FLAG tumor–bearing SCID mice treated with bortezomib and MD5-1 showed long-term tumor-free survival (median survival: combination vs saline, 181 days vs 49 days, difference = 132 days, 95% CI = 128 to 139 days; P < .001) (Figure 6, D). We also evaluated the effect of the combination regimen in the Renca-FLAG tumor–bearing WT mice following depletion treatments with anti-CD8α, anti-CD4, and anti–asialo-GM1 antibodies. No reduction of the therapeutic benefit of the combination regimen was observed following the lymphocyte depletions (Figure 6, C). In addition, mice surviving after combination therapy did not reject the tumor on rechallenge (data not shown). Therefore, the therapeutic effect of the combination regimen did not require any obvious contribution of the host’s immune response but seemingly involved enhanced direct cytotoxic effects on the tumor cells themselves.
Lack of Toxicity of Bortezomib and Anti-DR5 Therapy
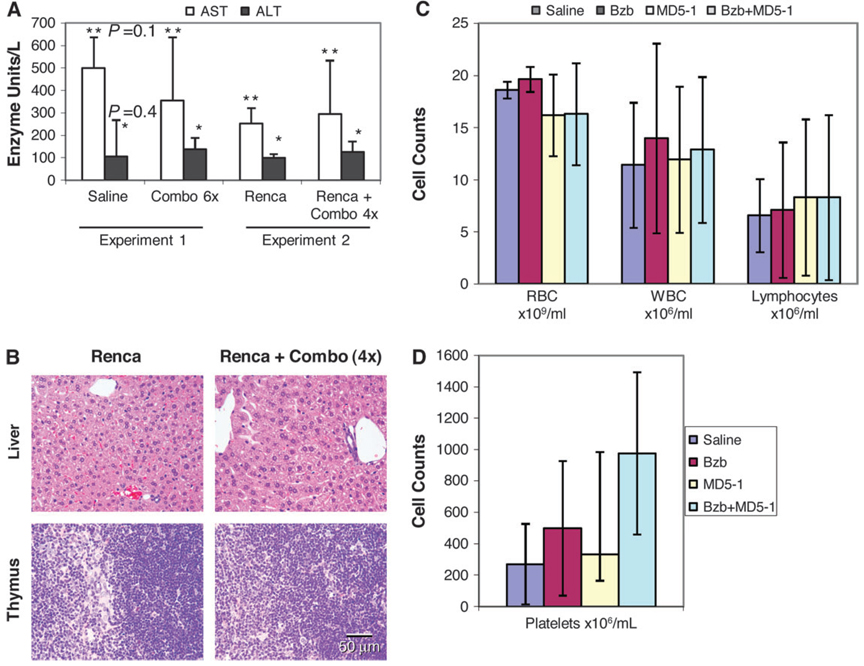
To assess hepatotoxicity, levels of serum AST and ALT in mice following six combination treatments with bortezomib (1.5 mg/kg) plus MD5-1 (50 µg per mouse intraperitoneally) in non–tumor-bearing mice (experiment 1) or four combination treatments with bortezomib (1 mg/kg) plus MD5-1 (50 µg per mouse intravenously) in Renca-FLAG tumor–bearing mice (experiment 2) were measured 2 days (experiment 1) or 3 days (experiment 2) after the last injection of bortezomib. No major change in these enzyme levels was observed (Figure 7, A). Treated mice from experiments 1 and 2 showed no clinically abnormal behavior or any difference in body or organ weight compared with control mice. No gross abnormalities were noted, nor was there any histologic evidence of tissue pathology in the liver, thymus, kidney, brain, lungs, intestine, or skin (Figure 7, B and data not shown). Also, no substantial changes in the numbers of red or white blood cells or lymphocytes in the peripheral blood evaluated 3 days after the fourth treatment of the combination regimen (bortezomib 1 mg/kg plus MD5-1 200 µg per mouse intravenously) were observed in experiment 2 (Figure 7, C). A slight but non–statistically significant increase in platelet counts was seen in mice treated with bortezomib or with bortezomib and MD5-1, compared with control mice at this time point (Figure 7, D). Necropsies of mice in experiment 2 showed abundant tumor metastases only in the untreated mice (data not shown).
Figure 7.
Potential toxicity of the combination of bortezomib (Bzb) and death receptor (DR5) agonist antibody regimen in mice. Toxicity in non–tumor-bearing or Renca-FLAG tumor–bearing mice injected intraperitoneally with Bzb (1.5 mg/kg) on days 1, 4, 8, 16, 22, and 29 plus MD5-1 (50 µg per mouse) on days 2, 5, 9, 17, 23, and 30 (Combo 6×) (experiment 1) or intravenously with Bzb (1 mg/kg) plus MD5-1 (50 µg per mouse) on days 4, 7, 12, and 15 (Combo 4×) (experiment 2). A) Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels in untreated and treated mice. Data represent means and 95% confidence intervals (n = 3); *P = .4 (ALT), **P = .1 (AST), (treated vs saline; analysis of variance, two-sided). B) Representative sections of liver and thymus stained with hematoxylin and eosin from mice in experiment 2 are shown (bar = 50 µm corresponds to all four images). C and D) Red blood cells (RBCs), white blood cells (WBCs), and lymphocytes (C) and platelets (D) were counted in blood collected from Renca-FLAG tumor–bearing mice from experiment 2 on day 18 after tumor cell injection that were untreated or treated with Bzb (1 mg/kg body weight), MD5-1 (50 µg per mouse), or the combination on days 4, 7, 11, and 15. Values are means and 95% confidence intervals from three mice in each group. Representative data from two independent experiments are shown.
Sensitization of Human Renal and Mammary Tumors to RAIL
We evaluated also the effect of bortezomib on TRAIL-mediated death of human renal cell carcinoma cells ACHN and A498 or mammary carcinomas MDA-MB-231 and BT-549. A decrease in tumor cell number in response to the bortezomib and TRAIL combination occurred in all these human tumor lines (Figure 8). This decrease was due to enhanced tumor cell apoptosis as assessed by annexin-V and propidium iodide staining of cells (data not shown), thereby suggesting that bortezomib augmented TRAIL-mediated apoptotic signaling in these human tumor cells.
Figure 8.
Bortezomib (Bzb)-induced sensitization of human tumors to tumor necrosis factor–related apoptosis-inducing ligand (TRAIL). Cell viability of human cancer cell lines treated with or without 20 nM of Bzb for 3 hours was determined 18 hours after treatment with soluble recombinant human TRAIL in the continued presence of Bzb. A) Renal cell carcinomas ACHN and A498. B) Breast carcinomas MDA-MB-231 and BT-549. Percent decrease in cell number is shown as means and 95% confidence intervals (n = 3) from one representative experiment of five independent experiments. *P < .001 (analysis of variance, two-sided) with respect to single agents.
Discussion
We examined the ability of a combination of bortezomib with a death ligand TRAIL receptor agonist monoclonal antibody MD5-1 to promote cancer cell death in vivo. This combination regimen increased survival among the mice with lung metastases of Renca-FLAG, whereas bortezomib or MD5-1 alone provided no survival benefit compared with saline. Also, this regimen showed no evidence of host toxicity. These results thus provide a rationale for the administration of bortezomib in vivo to sensitize tumor cells to the apoptotic effects of TRAIL receptor agonist antibodies or TRAIL ligand to promote solid tumor regression.
Because proteasome inhibition can have many biologic effects, multiple molecular changes could underlie sensitization of tumor cells to TRAIL-mediated apoptosis. For example, the blocking of NF-κB activation following proteasome inhibition may sensitize cells to TRAIL-mediated apoptosis (31, 32). However, blocking NF-κB activation is not always required for sensitization of tumor cells to TRAIL (20, 33, 34). Bortezomib-induced sensitization of tumor cells to TRAIL has been reported to involve increases in TRAIL receptors (22, 35, 36), decreases in antiapoptotic proteins c-FLIP (20, 37) and XIAP (34), effects on members of the Bcl-2 family (22, 38, 39), the release of proapoptotic factors from the mitochondria (34, 40), and increased p21 expression (41). However, few studies have assessed the effects of proteasome inhibition alone or in combination with TRAIL on long-term survival of tumor cells. Moreover, most of these studies used high concentrations (100–1000 nM) of bortezomib in vitro that cannot be extrapolated to the therapeutic situation in vivo. Consequently, high concentrations of proteasome inhibitors alone can damage cells through increasing calcium levels (42), endoplasmic reticulum stress (43), and aggresome formation (44) and changing mitochondrial function (42). Higher concentrations (>100 nM) of bortezomib show cytotoxic effects on most tumor cells after 48–96 hours. Therefore, in studies using high concentrations of bortezomib, subsequent exposure to TRAIL may merely be increasing the kinetics of an apoptotic process that has already been initiated by bortezomib. The maximum concentration of bortezomib (20 nM) used in our studies was transiently cytostatic to tumors and became cytotoxic only in the presence of TRAIL. Therefore, the molecular basis of the sensitization of tumor cells to TRAIL following bortezomib treatment could be clearly separated from the direct cytotoxic effects of bortezomib alone. Furthermore, 10–20 nM concentrations of bortezomib are attainable in vivo (26), and the level of proteasome inhibition [about 80% in Renca (20) and 4T1 cells (data not shown)] that occurs at these concentrations can be tolerated in both mice and humans without appreciable toxicity (45, 46).
Because no changes in the mitochondrial membrane potential were observed after treatment with low concentrations of bortezomib and because Bcl-2 overexpression did not abrogate bortezomib sensitization, it appears that bortezomib-induced amplification of the caspase-8 activation in response to TRAIL is probably important for enhanced apoptosis of Renca-FLAG and 4T1 cells treated with bortezomib and TRAIL. This crucial role for caspase-8 activation is in agreement with previous studies on bortezomib sensitization of human hepatoma cells to TRAIL (36). The molecular basis of this increased caspase-8 activity is currently under investigation. In contrast to previous studies (22, 38, 39), we observed no role for Bcl-2 family members in the bortezomib-induced sensitization to TRAIL. However, individual cells may differ in their requirements for a Bid-mediated mitochondrial contribution to the apoptotic cascade (12, 27). From this study and other reports (22, 33), it is encouraging that some tumors can be sensitized to TRAIL-mediated apoptosis by bortezomib even when they express high levels of antiapoptotic proteins, such as c-FLIP or Bcl-2. Additional studies of a possible molecular link between cell cycle inhibition and apoptosis sensitization are merited. Identification of the molecular changes that are necessary for bortezomib-induced sensitization to TRAIL could allow for a molecular profiling of tumors to select for those most responsive to bortezomib and TRAIL therapies (47).
The combination treatment of bortezomib and MD5-1 worked well in SCID mice. This finding suggests that the major therapeutic effect of this combination involves direct tumor cytotoxicity, without dependence on an adaptive immune response.
This study has some limitations. We evaluated therapeutic efficacy of bortezomib and MD5-1 antibody in 4-day mouse tumors. The therapeutic efficacy of this combination regimen may be limited in advanced tumors. Moreover, little is known about the effects of bortezomib on immune responses. Bortezomib may have immunosuppressive effects, which may hamper the capacity of the host to mount an antitumor immune response in advanced tumors. Inhibitory effects of bortezomib on dendritic cells in vitro (48, 49) and on the generation of graft-versus-host disease in vivo (50) have been reported. These effects may be indications of a suppression of adaptive immune responses by bortezomib, limiting T cell–mediated eradication of tumor cells.
Despite these limitations, we recently reported that bortezomib could enhance the immunotherapeutic efficacy of the cytokines IL-2 and IL-12 (28). Therefore, with appropriate scheduling, it may be possible to combine molecular targeted tumor cytotoxicity using bortezomib and TRAIL receptor agonists with immunotherapy regimens for increased therapeutic benefit. Because bortezomib can also sensitize tumor cells to apoptosis in response to TNF-α or Fas ligand (data not shown), another possible treatment strategy could be to combine bortezomib with the adoptive transfer of T cells or natural killer cells as sources of multiple death ligands (51, 52).
In summary, this preclinical study has provided both a molecular basis and a translationally relevant proof of principle for the therapeutic combination of the proteasome inhibitor bortezomib with death receptor agonist monoclonal antibodies or recombinant TRAIL/Apo2L in vivo to promote tumor cell apoptosis.
Acknowledgments
Funding
National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
National Health and Medical Research Council of Australia (Research Fellowship to M.J.S.); Cancer Council of Victoria (Project Grant to M.J.S.).
We thank Dr Diana Haines for the histopathology, Dr W. Gregory Alvord and members of the Computer and Statistical Services (NCI–Frederick) for statistical consultations, Dr Wenqing Li for transduction of 4T1-Bcl-2 cells, and Teresa Ramirez, Kristen Jacobsen, and Evan Lowery (NCI–Frederick) for technical assistance. We are grateful to Kathleen Noer and Roberta Matthai of the Flow Cytometry Core Laboratory (NCI–Frederick) for assistance with cell sorting. We also thank Dr William Gillette and Ralph Hopkins of the Protein Expression Laboratory (NCI–Frederick) for purification of the MD5-1 antibody.
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the decision to submit the manuscript for publication; or the writing of the manuscript.
Footnotes
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
References
- 1.Jin Z, el Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4(2):139–163. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- 2.Hayakawa Y, Smyth MJ, Kayagaki N, et al. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001;7(1):94–100. doi: 10.1038/83416. [DOI] [PubMed] [Google Scholar]
- 3.Takeda K, Smyth MJ, Cretney E, et al. Critical role for tumor necrosis factor-related apoptosis-inducing ligand in immune surveillance against tumor development. J Exp Med. 2002;195(2):161–169. doi: 10.1084/jem.20011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol. 2002;168(3):1356–1361. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- 5.Seki N, Hayakawa Y, Brooks AD, et al. Tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis is an important endogenous mechanism for resistance to liver metastases in murine renal cancer. Cancer Res. 2003;63(1):207–213. [PubMed] [Google Scholar]
- 6.Smyth MJ, Takeda K, Hayakawa Y, Peschon JJ, van den Brink MRM, Yagita H. Nature’s TRAIL—on a path to cancer Iimmunotherapy. Immunity. 2003;18(1):1–6. doi: 10.1016/s1074-7613(02)00502-2. [DOI] [PubMed] [Google Scholar]
- 7.Almasan A, Ashkenazi A. Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 2003;14(3–4):337–348. doi: 10.1016/s1359-6101(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 8.Walczak H, Miller RE, Ariail K, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5(2):157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 9.Mitsiades CS, Treon SP, Mitsiades N, et al. TRAIL/Apo2L ligand selectively induces apoptosis and overcomes drug resistance in multiple myeloma: therapeutic applications. Blood. 2001;98(3):795–804. doi: 10.1182/blood.v98.3.795. [DOI] [PubMed] [Google Scholar]
- 10.Naka T, Sugamura K, Hylander BL, Widmer MB, Rustum YM, Repasky EA. Effects of tumor necrosis factor-related apoptosis-inducing ligand alone and in combination with chemotherapeutic agents on patients’ colon tumors grown in SCID mice. Cancer Res. 2002;62(20):5800–5806. [PubMed] [Google Scholar]
- 11.Hylander BL, Pitoniak R, Penetrante RB, et al. The anti-tumor effect of Apo2L/TRAIL on patient pancreatic adenocarcinomas grown as xenografts in SCID mice. J Transl Med. 2005;3(1):22. doi: 10.1186/1479-5876-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cretney E, Shanker A, Yagita H, Smyth MJ, Sayers TJ. TNF-related apoptosis-inducing ligand as a therapeutic agent in autoimmunity and cancer. Immunol Cell Biol. 2006;84(1):87–98. doi: 10.1111/j.1440-1711.2005.01413.x. [DOI] [PubMed] [Google Scholar]
- 13.Ashkenazi A, Pai RC, Fong S, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104(2):155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motoki K, Mori E, Matsumoto A, et al. Enhanced apoptosis and tumor regression induced by a direct agonist antibody to tumor necrosis factor-related apoptosis-inducing ligand receptor 2. Clin Cancer Res. 2005;11(8):3126–3135. doi: 10.1158/1078-0432.CCR-04-1867. [DOI] [PubMed] [Google Scholar]
- 15.Smyth MJ, Hayakawa Y, Cretney E, et al. IL-21 enhances tumor-specific CTL induction by anti-DR5 antibody therapy. J Immunol. 2006;176(10):6347–6355. doi: 10.4049/jimmunol.176.10.6347. [DOI] [PubMed] [Google Scholar]
- 16.Uno T, Takeda K, Kojima Y, et al. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med. 2006;12(6):693–698. doi: 10.1038/nm1405. [DOI] [PubMed] [Google Scholar]
- 17.Takeda K, Yamaguchi N, Akiba H, et al. Induction of tumor-specific T cell immunity by anti-DR5 antibody therapy. J Exp Med. 2004;199(4):437–448. doi: 10.1084/jem.20031457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nandi D, Tahiliani P, Kumar A, Chandu D. The ubiquitin-proteasome system. J Biosci. 2006;31(1):137–155. doi: 10.1007/BF02705243. [DOI] [PubMed] [Google Scholar]
- 19.Joazeiro CA, Anderson KC, Hunter T. Proteasome inhibitor drugs on the rise. Cancer Res. 2006;66(16):7840–7842. doi: 10.1158/0008-5472.CAN-06-2033. [DOI] [PubMed] [Google Scholar]
- 20.Sayers TJ, Brooks AD, Koh CY, et al. The proteasome inhibitor PS-341 sensitizes neoplastic cells to TRAIL-mediated apoptosis by reducing levels of c-FLIP. Blood. 2003;102(1):303–310. doi: 10.1182/blood-2002-09-2975. [DOI] [PubMed] [Google Scholar]
- 21.An J, Sun YP, Adams J, Fisher M, Belldegrun A, Rettig MB. Drug interactions between the proteasome inhibitor bortezomib and cytotoxic chemotherapy, tumor necrosis factor (TNF) alpha, and TNF-related apoptosis-inducing ligand in prostate cancer. Clin Cancer Res. 2003;9(12):4537–4545. [PubMed] [Google Scholar]
- 22.Johnson TR, Stone K, Nikrad M, et al. The proteasome inhibitor PS-341 overcomes TRAIL resistance in Bax and caspase 9-negative or Bcl-xL overexpressing cells. Oncogene. 2003;22(32):4953–4963. doi: 10.1038/sj.onc.1206656. [DOI] [PubMed] [Google Scholar]
- 23.Li WQ, Jiang Q, Khaled AR, Keller JR, Durum SK. Interleukin-7 inactivates the pro-apoptotic protein bad promoting T cell survival. J Biol Chem. 2004;279(28):29160–29166. doi: 10.1074/jbc.M401656200. [DOI] [PubMed] [Google Scholar]
- 24.Reik LM, Maines SL, Ryan DE, Levin W, Bandiera S, Thomas PE. A simple, non-chromatographic purification procedure for monoclonal antibodies. Isolation of monoclonal antibodies against cytochrome P450 isozymes. J Immunol Methods. 1987;100(1–2):123–130. doi: 10.1016/0022-1759(87)90180-3. [DOI] [PubMed] [Google Scholar]
- 25.Clark JD, Baldwin RL, Bayne KA, et al. Guide for the Care and Use of Laboratory Animals: National Research Council. Washington, DC: National Academy Press; 1996. [Google Scholar]
- 26.Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61(7):3071–3076. [PubMed] [Google Scholar]
- 27.Scaffidi C, Fulda S, Srinivasan A, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17(6):1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan T, Stauffer JK, Williams R, et al. Proteasome inhibition to maximize the apoptotic potential of cytokine therapy for murine neuroblastoma tumors. J Immunol. 2006;176(10):6302–6312. doi: 10.4049/jimmunol.176.10.6302. [DOI] [PubMed] [Google Scholar]
- 29.Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Diff. 2003;10(1):26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- 30.Boatright KM, Renatus M, Scott FL, et al. A unified model for apical caspase activation. Mol Cell. 2003;11(2):529–541. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 31.Khanbolooki S, Nawrocki ST, Arumugam T, et al. Nuclear factor-kappaB maintains TRAIL resistance in human pancreatic cancer cells. Mol Cancer Ther. 2006;5(9):2251–2260. doi: 10.1158/1535-7163.MCT-06-0075. [DOI] [PubMed] [Google Scholar]
- 32.Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation-induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6(3):297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Nencioni A, Wille L, Dal Bello G, et al. Cooperative cytotoxicity of proteasome inhibitors and tumor necrosis factor-related apoptosis-inducing ligand in chemoresistant Bcl-2-overexpressing cells. Clin Cancer Res. 2005;11(11):4259–4265. doi: 10.1158/1078-0432.CCR-04-2496. [DOI] [PubMed] [Google Scholar]
- 34.Leverkus M, Sprick MR, Wachter T, et al. Proteasome inhibition results in TRAIL sensitization of primary keratinocytes by removing the resistance-mediating block of effector caspase maturation. Mol Cell Biol. 2003;23(3):777–790. doi: 10.1128/MCB.23.3.777-790.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He Q, Huang Y, Sheikh MS. Proteasome inhibitor MG132 upregulates death receptor 5 and cooperates with Apo2L/TRAIL to induce apoptosis in Bax-proficient and -deficient cells. Oncogene. 2004;23(14):2554–2558. doi: 10.1038/sj.onc.1207351. [DOI] [PubMed] [Google Scholar]
- 36.Ganten TM, Koschny R, Haas TL, et al. Proteasome inhibition sensitizes hepatocellular carcinoma cells, but not human hepatocytes, to TRAIL. Hepatology. 2005;42(3):588–597. doi: 10.1002/hep.20807. [DOI] [PubMed] [Google Scholar]
- 37.Kabore AF, Sun J, Hu X, McCrea K, Johnston JB, Gibson SB. The TRAIL apoptotic pathway mediates proteasome inhibitor induced apoptosis in primary chronic lymphocytic leukemia cells. Apoptosis. 2006;11(7):1175–1193. doi: 10.1007/s10495-006-8048-9. [DOI] [PubMed] [Google Scholar]
- 38.Zhu H, Guo W, Zhang L, et al. Proteasome inhibitors-mediated TRAIL resensitization and Bik accumulation. Cancer Biol Ther. 2005;4(7):781–786. doi: 10.4161/cbt.4.7.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nikrad M, Johnson T, Puthalalath H, Coultas L, Adams J, Kraft AS. The proteasome inhibitor bortezomib sensitizes cells to killing by death receptor ligand TRAIL via BH3-only proteins Bik and Bim. Mol Cancer Ther. 2005;4(3):443–449. doi: 10.1158/1535-7163.MCT-04-0260. [DOI] [PubMed] [Google Scholar]
- 40.Nencioni A, Hua F, Dillon CP, et al. Evidence for a protective role of Mcl-1 in proteasome inhibitor-induced apoptosis. Blood. 2005;105(8):3255–3262. doi: 10.1182/blood-2004-10-3984. [DOI] [PubMed] [Google Scholar]
- 41.Lashinger LM, Zhu K, Williams SA, Shrader M, Dinney CP, McConkey DJ. Bortezomib abolishes tumor necrosis factor-related apoptosis-inducing ligand resistance via a p21-dependent mechanism in human bladder and prostate cancer cells. Cancer Res. 2005;65(11):4902–4908. doi: 10.1158/0008-5472.CAN-04-3701. [DOI] [PubMed] [Google Scholar]
- 42.Landowski TH, Megli CJ, Nullmeyer KD, Lynch RM, Dorr RT. Mitochondrial-mediated disregulation of Ca2+ is a critical determinant of Velcade (PS-341/bortezomib) cytotoxicity in myeloma cell lines. Cancer Res. 2005;65(9):3828–3836. doi: 10.1158/0008-5472.CAN-04-3684. [DOI] [PubMed] [Google Scholar]
- 43.Nawrocki ST, Carew JS, Pino MS, et al. Bortezomib sensitizes pancreatic cancer cells to endoplasmic reticulum stress-mediated apoptosis. Cancer Res. 2005;65(24):11658–11666. doi: 10.1158/0008-5472.CAN-05-2370. [DOI] [PubMed] [Google Scholar]
- 44.Nawrocki ST, Carew JS, Pino MS, et al. Aggresome disruption: a novel strategy to enhance bortezomib-induced apoptosis in pancreatic cancer cells. Cancer Res. 2006;66(7):3773–3781. doi: 10.1158/0008-5472.CAN-05-2961. [DOI] [PubMed] [Google Scholar]
- 45.Lightcap ES, McCormack TA, Pien CS, Chau V, Adams J, Elliott PJ. Proteasome inhibition measurements: clinical application. Clin Chem. 2000;46(5):673–683. [PubMed] [Google Scholar]
- 46.Adams J, Palombella VJ, Sausville EA, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59(11):2615–2622. [PubMed] [Google Scholar]
- 47.Sayers TJ, Murphy WJ. Combining proteasome inhibition with TNF-related apoptosis-inducing ligand (Apo2L/TRAIL) for cancer therapy. Cancer Immunol Immunother. 2006;55(1):76–84. doi: 10.1007/s00262-005-0676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nencioni A, Schwarzenberg K, Brauer KM, et al. Proteasome inhibitor bortezomib modulates TLR4-induced dendritic cell activation. Blood. 2006;108(2):551–558. doi: 10.1182/blood-2005-08-3494. [DOI] [PubMed] [Google Scholar]
- 49.Nencioni A, Garuti A, Schwarzenberg K, et al. Proteasome inhibitor-induced apoptosis in human monocyte-derived dendritic cells. Eur J Immunol. 2006;36(3):681–689. doi: 10.1002/eji.200535298. [DOI] [PubMed] [Google Scholar]
- 50.Sun K, Welniak LA, Panoskaltsis-Mortari A, et al. Inhibition of acute graft-versus-host disease with retention of graft-versus-tumor effects by the proteasome inhibitor bortezomib. Proc Natl Acad Sci U S A. 2004;101(21):8120–8125. doi: 10.1073/pnas.0401563101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seki N, Brooks AD, Carter CR, et al. Tumor-specific CTL kill murine renal cancer cells using both perforin and Fas ligand-mediated lysis in vitro, but cause tumor regression in vivo in the absence of perforin. J Immunol. 2002;168(7):3484–3492. doi: 10.4049/jimmunol.168.7.3484. [DOI] [PubMed] [Google Scholar]
- 52.Hallett WH, Ames E, Motarjemi M, et al. Sensitization of tumor cells to NK cell-mediated killing by proteasome inhibition. J Immunol. 2008;180(1):163–170. doi: 10.4049/jimmunol.180.1.163. [DOI] [PubMed] [Google Scholar]