Abstract
Objective
To examine sleep patterns and influencing factors (age, gender, Tanner Stage, weekday vs. weekend, and pre-sleep activity) among rural Chinese adolescents.
Methods
This is a prospective study among 621 adolescents aged 11–20 years (341 males) using both a questionnaire and sleep diary to obtain bedtime, wake-up time, sleep latency, and total sleep time (TST).
Results
The median TST was 8.6 hours on weekdays and 9.4 hours on weekends. Despite absence of late night social pressure and computers, a U-shaped TST pattern was observed across age and Tanner stage, with a nadir around age 15–16 years or Tanner IV. Bedtimes became progressively later with age and Tanner Stage, while wake-up time was considerably earlier for school students or up to Tanner IV. Later wake-up times and longer TST on weekends were seen in school students, but not in non-school adolescents (>17 years). Pre-sleep activity, like reading or studying, was related to later bedtime, earlier wake-up time, and shorter TST in both genders.
Conclusions
Age, Tanner stage, and pre-sleep activity affected sleep patterns in this sample of rural Chinese adolescents. Later bedtime coupled with earlier wake-up time associated with academic demand appear to be important contributors to sleep loss among school students.
Keywords: Age, gender, puberty, Tanner stages, sleep patterns, wake-up time, bedtime, total sleep time
INTRODUCTION
There is increasing evidence that optimal sleep is important for both physical and mental health [1, 2]. Recent studies have demonstrated a link between short sleep duration and increased risk of metabolic and cardiovascular disorders [2, 3]. Yet sleep loss is prevalent in our society, and adolescents are at particular risk for sleep deprivation and its negative consequences on health, productivity and safety, due to the interactions between biological, psychological, social and environmental factors. Sleep patterns change considerably from childhood to early adulthood, with dramatic shifts occurring during adolescence [4], with a tendency towards delayed sleep times and wake-up times [5–7]. Self-reported sleep measures showed that older adolescents had later bedtime, and they woke up later when daily schedule permitted, primarily on a non-school day [7, 8]. A combination of biological factors, such as a delay in the timing of circadian rhythms [6, 9, 10] and psychosocial factors, interact to re-shape adolescent sleep patterns [11–13]. Two-process model data (sleep/wake homeostatic process, assessed by electroencephalographic (EEG) and slow wave sleep, and the circadian timing system estimated by melatonin, light sensitivity and morningness/eveningness) suggested that biological maturation may be, either as “compelling” or “permissive” factors, related to the delayed sleep timing in more mature adolescents [5, 8]. Also, sleep patterns have been found to be sensitive to academic pressure (school schedules and demands) [13], reduced parental influence [14], social opportunity at night [5], and evening activities (television, computer and video games) [15], and were different between weekdays and weekends [8].
Adolescence is also a period when gender differences become more apparent in growth and developmental parameters such as height, body composition, and pulmonary function [16]. Studies on gender difference in sleep patterns in adolescents (based on chronological age) have produced somewhat inconsistent results [17–19]. For example, a study of US urban adolescents found that weekday wake-up time in boys was significantly later than in girls [17], whereas LaBerge and colleagues did not find gender difference for weekday sleep time, but reported that girls woke up later and slept longer than boys on weekends [18]. A recent study of Japanese adolescents showed that for a majority of sleep measures girls had better sleep than boys [19]. These apparent discrepancies may be explained partly by ethnic, cultural and environmental differences between the study populations. Another explanation may be that chronological age and sexual maturation are not comparable between boys and girls during adolescence and that perhaps differences in sexual maturity could in part account for the inconsistent findings between studies [18, 20, 21].
Improved understanding of biological and psychosocial influences on sleep requires studies in different populations, cultures and environments. To date, most epidemiologic studies of sleep patterns have been conducted in Western adolescents [8, 15]. The few studies in urban Chinese adolescents showed that when compared to their Western counterparts, they had decreased sleep time [13, 22], which might in part be explained by the high level of academic competition [13, 22]. No sleep data is available on rural Chinese adolescents.
In this study we describe sleep patterns and several potentially influential factors – age, gender, Tanner Stage, weekday vs. weekend, and pre-sleep activity – in a community-based cohort of rural Chinese adolescents. This rural population provides a unique opportunity to study sleep patterns which are less influenced by psychosocial and environmental factors linked to delayed sleep time in western or urban adolescents, [5, 15] thus, more likely reflecting a “natural” sleep pattern.
METHODS
Study population and procedures
This study is part of an ongoing study of metabolic syndrome (MS) in a large population-based twin cohort. This cohort was originally designed to study environmental and genetic determinants of complex human diseases such as MS. The study protocol was approved by the Institutional Review Boards of Children’s Memorial Hospital and the Institute of Biomedicine, Anhui Medical University in Hefei, China.
The baseline study was carried out in eight counties of the Anqing region, Anhui Province, China in 1998–2000, and the follow-up study has been conducted since 2005. This report used the data obtained at the follow-up survey. For the baseline study, as described in detail by Wang et al.[23], twins recruited were (1) 6 years or older and (2) both twins were available. For the follow-up study, eligible twins met the following criteria: (1) both twins participated in the baseline survey, and (2) both twins agreed and consented to participate. Participants were invited to a central office to complete a questionnaire interview and physical examination and were asked to record sleep diaries. Epidemiological information, including occupation, education, smoking and alcohol consumption, and menarche status was obtained via a standard questionnaire interview. Physical examinations were conducted by well-trained study physicians. Tanner Stages (I to V) were assessed based on visual inspection of pubic hair, genitals (boys) and breasts (girls) by three physicians [24, 25]. The concordances of assessments between the raters were higher than 90%. Less than 1% of adolescents rejected these assessments. This report includes 621 twin individuals (males 341) aged 11–20 years. Mean (SD) age was 15.9 (2.0) years; 86.4% of females had experienced menarche and 65.8% (n=358) were monozygotic twins.
In this rural community electricity was available for all households. All families had TV, but they rarely had computers. Internet was not available in this community. The common activities at night or after dinner were watching TV, reading, studying/doing homework, playing cards, and in-person social conversation. The participants rarely had evening social activities outside the home on either weekdays or weekends. Few participants reported smoking (2.8%) or alcohol use (2.9%). Most of the twins’ parents were farmers.
Average family size was 5–6 persons/household, i.e., grandparents (mostly father side), parents and 1–2 children. In general, twins shared a bedroom among themselves, but not with their parents or other family members.
Most (84.1%) participants were students, especially those 11–17 years old (94.2%). Among the students, the majority (80.0 %) was in junior high or high school, and 16.4% were in elementary school. Only 3.6% of them were college students. Among the older adolescents (18–20 years), one-third were students (18 in high school and 17 in college).
Typically, children started elementary school at age 7 (or 6, if parents decided); elementary school consists of 1st – 6th grade (or 1st – 5th grade before 2004). The school hour was from 8:00 a.m. to 4:30 p.m. The bedtimes, around 9:00 p.m., were usually determined by parents. Then, for junior high (7th – 9th grade) and high school (10th – 12th), most students lived in school from Monday to Thursday and at home from Friday to Sunday. The school hour was from 8:00 a.m. to 5:00 p.m. plus a morning exercise time (6:15–7:30 a.m.) and a self-study time (7:00–9:00 p.m.). The bedtime schedules, around 10:30 p.m., were set by schools. After completing high school, some students attended college. Elementary schools were usually located within 15 minutes walking distance from students’ homes. There was no school during weekends (Saturday and Sunday). This sample from a population-based twin cohort was representative of local children with regard to demographic characteristic and school settings.
Sleep Measures
Sleep parameters were obtained by sleep diaries and questionnaires. Questionnaires were designed to capture the average sleep characteristics over the past month, whereas sleep diaries were kept for 4–7 consecutive days prospectively.
Questionnaires included the following items: (1) “During last month, when did you usually go to bed for sleep?” (2) “During last month, how long were you able to fall asleep (after you go to bed for sleep) each night?” (3) “During last month, when did you usually wake up in the morning?” (4) “During last month, how many hours did you actually sleep during the night?” Children were asked to answer questions first and then parents were asked to confirm and verify their children’s answers during a joint interview, especially for children under 18 years old.
In the diary, participants were asked the following questions: (1) “When did you wake up today?” (2) “When did you go to bed for sleep today?” (3) “How long were you able to fall asleep (after you go to bed for sleep) today?” and (4) “How many hours did you sleep tonight?” In addition, the participants were also asked to record pre-sleep activities which are defined as the activities one hour before bedtime. Specifically, the participants were asked to respond to a multiple choice question: "What activities did you do one hour before bedtime today? 1=doing nothing; 2=playing card game or Majiang; 3=doing Chaos; 4=watching TV; 5=working; 6=reading; 7=driving/sitting on an automobile; 8=in person social conversation; 9=study/doing homework; 10=running; 11=knitting; 12=taking a bath; 13=listening to radio; 14=walking; 15=fighting; 16=drinking; 17=playing; 18=taking a rest; 19=at hospital; 20=boating; 21=having a haircut; 22=dancing; 23=babysitting; 24=eating; 25=attending a wedding; 26=using Internet; 27=sending phone message; 28=religious worship; 29=singing; 30=chess; 31=visiting friends; 32=others.” Research staffs kept contact with participants’ parents or classroom teachers by phone, asking them to remind participants to record in their diaries on time. Research staffs collected diary sheets when completed. Of the 621 subjects, 241 completed 7-day sleep diaries and 380 completed 4-day sleep diaries; 66.3% (n= 252) of the 4-day diaries had weekend days. We analyzed sleep data by including all the subjects and by excluding subjects with only 4-day sleep diary data. We found that sleep patterns from the 241 subjects who completed 7-day diaries were similar to those from all 621 subjects. Thus, data from all the subjects were presented in this report.
Definition of major sleep parameters
Sleep parameters included bedtime, wake-up time, sleep latency, and total sleep time (TST).
We calculated TST as sleep duration from bedtime to wake-up time minus sleep latency (or from today’s bedtime to tomorrow’s wake-up time minus today’s sleep latency in sleep diary), but did not take into account wake time after sleep onset (WASO) and naps. We defined weekdays as Monday through Friday and weekends as Saturday and Sunday for wake-up time, while we defined weekdays as Sunday through Thursday and weekends as Friday and Saturday for bedtime, sleep latency and TST. For example, weekday TST is the mean TST of Sunday night through Thursday night. The TST directly reported by participants was highly correlated with the TST calculated from the above formula (Spearman correlation coefficient (r), 0.71 –0.79, for each day of week (Monday – Sunday) and over past one month; p<0.001 for all).
Weekday and weekend sleep parameters based on diary data were both correlated with those based on questionnaire data (p<0.001 for all). The degree of correlations was higher for weekdays than for weekends (r for weekdays vs. r for weekends: bedtime 0.74 vs. 0.69; sleep latency 0.68 vs. 0.65; wake-up time 0.73 vs. 0.49; TST 0.60 vs. 0.50). Sleep patterns based on questionnaire were similar to those of diary (data not shown). Therefore, in the subsequent report we only presented sleep diary data.
Statistical analyses
The frequency distribution was plotted for weekday vs. weekend TST integer values (<=6, 7, 8, 9,>=10 hours), stratified by gender.
Gender-specific median of sleep parameters on weekdays vs. weekends were plotted against age and Tanner stage, respectively. To examine weekday-weekend pair difference in sleep parameters, paired t-tests were used for normally distributed variables and paired signed rank tests for non-normally distributed data. Linear regressions were used to test the trend of sleep parameters across gender, age and Tanner stages.
Gender-specific multivariate linear regressions were conducted to examine the independent effect of Tanner stage and pre-sleep activity on sleep parameters. Fourteen 18–20 year-olds were excluded due to their history of shift-work which might have potential long-term impact on sleep regulation. Nevertheless, the results were quite similar by including or excluding these 14 individuals. Covariates in the models included pre-sleep activity (watching TV, reading, study/homework, and the others), Tanner stages (1–5), age (11–12, 13-, 14-, 15-, 16-, 17-, 18–20 years), and day of week (Monday–Sunday). We also repeated the multivariate regressions by replacing Tanner stage by menarche status (yes/no) in females.
Generalized estimating equations (GEE) were applied to all regression models to adjust for intra-twin pair correlation, with an independent working correlation structure using the SAS GENMOD procedure [26]. Intra-pair correlation coefficients [27] in weekdays (weekends) are as follows: bedtime 0.67 (0.59), sleep latency 0.43 (0.31), wake-up time 0.75 (0.66), and TST 0.57 (0.56). To examine potential co-twin influence on the results, we repeated the analyses by only including one twin per family (the first born twin). As the results were similar (data not shown), data for all the twins are presented in this report.
RESULTS
Sleep Patterns during Weekdays and Weekends
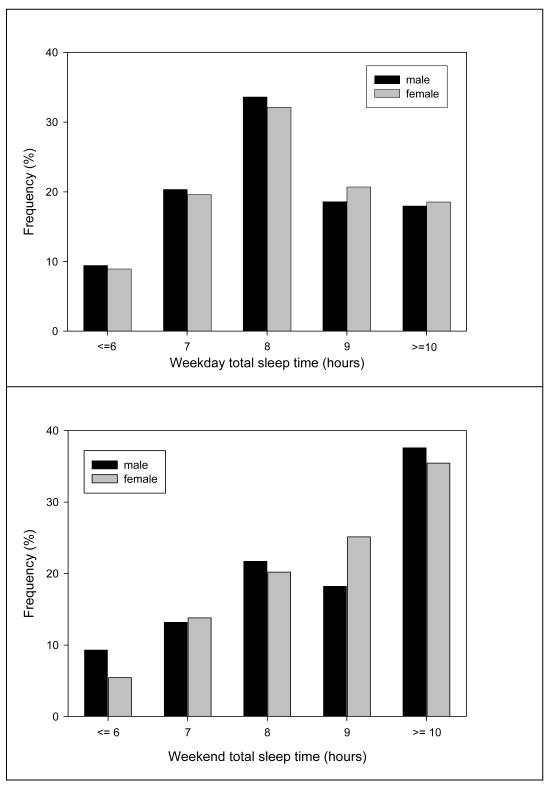
The median TST was 8.6 hours on weekdays and 9.4 hours on weekends. The most frequent weekday TST was 8.0–8.9 hours (33.6% in males and 32.1% in females) (Figure 1). The most frequent weekend TST was 2 hours longer: 37.60% males and 35.5% females had a weekend TST ≥ 10 hours.
Figure 1.
Distribution of weekday and weekend total sleep time (TST) stratified by gender in 621 Chinese adolescents aged 11–20 years.
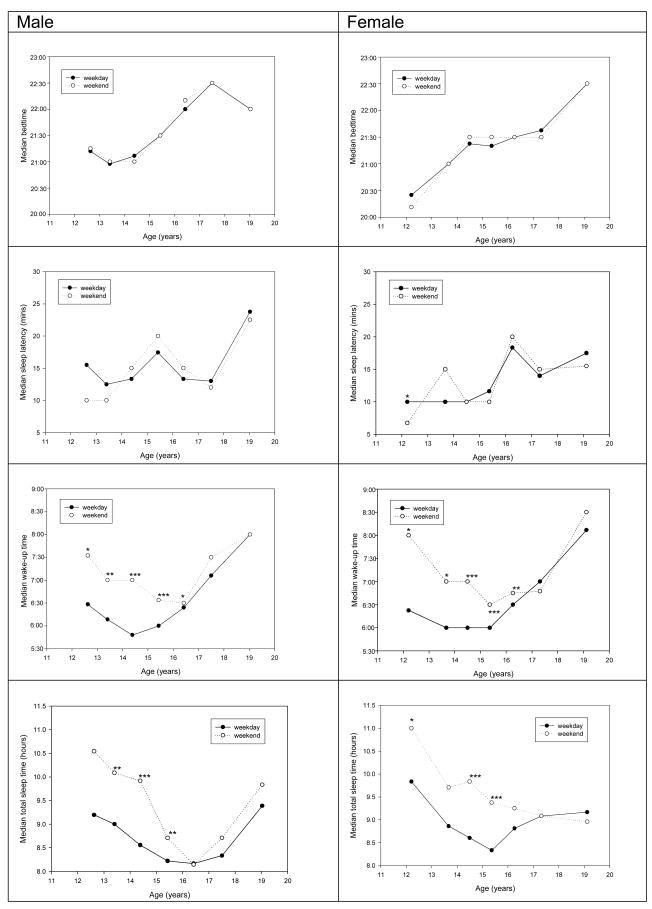
Median bedtimes were not significantly different between weekdays and weekends in both genders (all p> 0.05) (Figure 2). In contrast, there was a notable delay in wake-up time on weekends (p<0.05) among younger groups (age ≤ 16 years), accounting for significantly longer weekend TSTs than weekday TSTs among those groups (p<0.05 for most age groups < 16 years, except for males 11–12 years and females 13 years) in both genders (Figure 2). As students, they were expected to wake-up early on weekdays for school.
Figure 2.
Weekday and weekend sleep parameters by age stratified by gender.
* p<0.05; ** p < 0.01; *** p<0.001. Pair-matched tests for the differences between weekdays and weekends.
The weekday-weekend discrepancy in wake-up times and TST disappeared in the older groups (age ≥ 17 years or Tanner V, Figure 2; online figure a), of which most participants had left school and were no longer constrained by early school wake-up time.
Weekday Sleep Patterns by Age vs. Tanner Stage
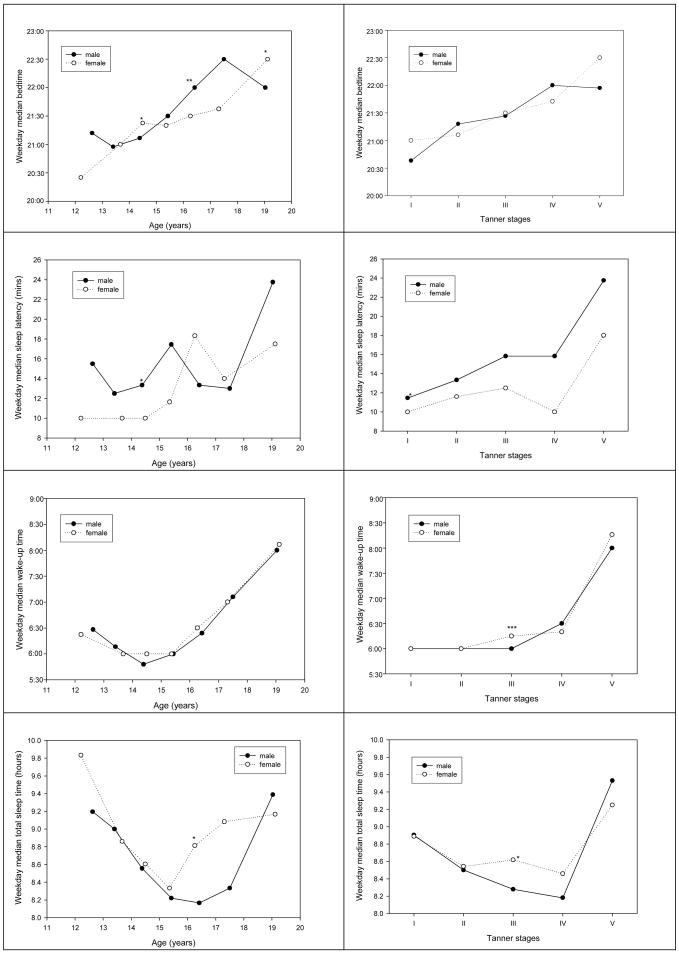
As shown in Figure 3 and Table 1, weekday bedtime delayed progressively with age (ptrend<0.001 for both genders), while wake-up time moved earlier from ages 11–14 and did not become considerably later until ages 15–16, accounting for a “U”-shaped pattern in weekday TST in both genders. The oldest group (age ≥ 18) had late bedtimes, very late wake-up times, and increased TST in both genders.
Figure 3.
Weekday sleep parameters by age vs. Tanner stages stratified by gender.
* p<0.05; ** p < 0.01; *** p<0.001. Linear regression models were used to test difference of each sleep parameter means between males and female.
Table 1.
Weekday sleep characteristics of 621 Chinese adolescents aged 11–20 years based on sleep diary by age and gender.
| Age (years)/ gender |
Median age (years) |
Bedtime |
Sleep latency (mins) |
Wake-up time |
TST * (hour) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Median (IQR†) |
10th | 90th | Median (IQR†) |
10th | 90th | Median (IQR†) |
10th | 90th | Median (IQR†) |
10th | 90th | ||
| Male | ||||||||||||||
| 11–12 | 12.6 | 18 | 21:12 (49) | 19:35 | 22:00 | 15.5 (13.0) | 2.6 | 58.0 | 6:28 (55) | 6:00 | 7:35 | 9.2 (1.5) | 8.3 | 11.0 |
| 13- | 13.4 | 32 | 20:58 (61) | 19:45 | 21:36 | 12.5 (9.0) | 7.5 | 24.0 | 6:08 (30) | 5:53 | 6:40 | 9.0 (1.1) | 8.3 | 11.0 |
| 14- | 14.4 | 81 | 21:07 (56) | 19:50 | 21:50 | 13.3 (10.0) | 5.0 | 34.8 | 5:48 (34) | 5:10 | 7:00 | 8.6 (1.0) | 7.7 | 10.2 |
| 15- | 15.4 | 76 | 21:30 (59) | 20:10 | 22:45 | 17.5 (17.5) | 7.5 | 32.0 | 6:00 (38) | 5:30 | 6:45 | 8.2 (1.4) | 7.0 | 9.8 |
| 16- | 16.4 | 45 | 22:00 (78) | 21:00 | 23:30 | 13.3 (10.0) | 5.0 | 30.0 | 6:24 (56) | 5:42 | 7:36 | 8.2 (2.0) | 6.1 | 9.8 |
| 17- | 17.5 | 25 | 22:30 (67) | 21:12 | 23:30 | 13.0 (10.0) | 5.0 | 30.0 | 7:06 (108) | 5:30 | 8:42 | 8.3 (2.7) | 5.9 | 11.3 |
| 18–20 | 19.0 | 64 | 22:00 (118) | 20:36 | 23:48 | 23.8 (20.5) | 8.0 | 56.0 | 8:00 (92) | 6:20 | 9:30 | 9.4 (2.9) | 7.1 | 11.8 |
| Female | ||||||||||||||
| 11–12 | 12.2 | 13 | 20:25 (88) | 19:43 | 21:30 | 10.0 (11.8) | 3.7 | 20.0 | 6:23 (53) | 5:30 | 7:00 | 9.8 (1.3) | 8.0 | 10.9 |
| 13- | 13.7 | 26 | 21:00 (62) | 19:30 | 22:30 | 10.0 (10.5) | 4.0 | 30.0 | 6:00 (60) | 5:00 | 7:00 | 8.9 (2.0) | 6.9 | 10.7 |
| 14- | 14.5 | 48 | 21:23 (75) | 20:12 | 22:30 | 10.0 (10.7) | 5.0 | 28.0 | 6:00 (36) | 5:30 | 7:06 | 8.6 (1.3) | 7.5 | 9.7 |
| 15- | 15.4 | 72 | 21:20 (60) | 20:30 | 22:48 | 11.6 (7.9) | 5.0 | 30.0 | 6:00 (49) | 5:30 | 7:18 | 8.3 (1.2) | 6.7 | 10.3 |
| 16- | 16.3 | 53 | 21:30 (60) | 20:30 | 22:38 | 18.3 (16.7) | 5.0 | 30.0 | 6:30 (120) | 5:30 | 8:15 | 8.8 (1.4) | 7.5 | 10.6 |
| 17- | 17.3 | 27 | 21:38 (94) | 20:36 | 23:18 | 14.0 (25.0) | 5.0 | 64.0 | 7:00 (110) | 5:48 | 8:22 | 9.1 (3.3) | 6.4 | 11.4 |
| 18–20 | 19.1 | 41 | 22:30 (64) | 21:43 | 24:00 | 17.5 (21.0) | 5.0 | 49.0 | 8:07 (90) | 6:36 | 10:00 | 9.2 (1.8) | 7.8 | 10.4 |
TST (Total Sleep Time) = wake-up time − bedtime − sleep latency.
IQR: Intraquartile range in minutes for bedtime and wake-up time and in hours for TST.
Sleep patterns by Tanner stage, in comparison to chronological age, were less erratic and more consistent for all four sleep parameters in either males or females, although the overall results were similar (Figure 3 and Table 2).
Table 2.
Weekday sleep characteristics of 583 Chinese adolescents* aged 11–20 years based on sleep diary by gender and Tanner stages.
| Gender/Tanner stages |
Age (years) mean (SD) |
Bedtime |
Sleep latency |
Wake-up time |
TST§ (hour) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | median (IQR†) |
10th | 90th | median (IQR†) |
10th | 90th | median (IQR†) |
10th | 90th | median (IQR†) |
10th | 90th | ||
| Male | ||||||||||||||
| I | 13.9 (1.1) | 58 | 20:38 (80) | 19:30 | 22:00 | 11.5 (10.0) | 5.0 | 30.0 | 6:00 (46) | 5:20 | 7:00 | 8.9 (1.8) | 7.8 | 11.3 |
| II | 15.2 (1.3) | 63 | 21:18 (60) | 20:18 | 23:00 | 13.3 (13.5) | 5.0 | 30.0 | 6:00 (60) | 5:19 | 7:35 | 8.5 (1.8) | 6.5 | 9.8 |
| III | 15.2 (1.1) | 68 | 21:27 (61) | 20:00 | 23:00 | 15.8 (15.8) | 6.7 | 32.5 | 6:00 (40) | 5:20 | 6:50 | 8.3 (1.3) | 6.9 | 9.8 |
| IV | 16.6 (1.6) | 62 | 22:00 (75) | 21:00 | 23:08 | 15.8 (14.8) | 8.0 | 36.0 | 6:30 (84) | 5:35 | 8:12 | 8.2 (1.8) | 6.6 | 11.0 |
| V | 19.0 (1.1) | 56 | 21:57 (115) | 20:24 | 23:54 | 23.8 (18.6) | 5.0 | 56.0 | 8:00 (90) | 6:34 | 9:30 | 9.5 (2.7) | 7.3 | 11.5 |
| Female | ||||||||||||||
| I | 12.9 (0.7) | 9 | 21:00 (43) | 19:45 | 21:17 | 10.0 (4.6) | 5.0 | 23.3 | 6:00 (3) | 5:30 | 7:00 | 8.9 (1.0) | 8.0 | 9.9 |
| II | 14.6 (1.2) | 71 | 21:06 (90) | 19:50 | 22:30 | 11.6 (15.0) | 5.0 | 30.0 | 6:00 (40) | 5:30 | 7:00 | 8.5 (1.8) | 7.1 | 10.2 |
| III | 15.9 (1.3) | 110 | 21:30 (68) | 20:30 | 23:00 | 12.5 (16.0) | 5.0 | 30.0 | 6:15 (119) | 5:30 | 8:12 | 8.6 (1.8) | 6.9 | 10.7 |
| IV | 16.6 (1.7) | 65 | 21:43 (90) | 20:30 | 23:30 | 10.0 (12.7) | 5.0 | 46.0 | 6:20 (116) | 5:30 | 8:22 | 8.5 (1.3) | 7.0 | 10.5 |
| V | 19.2 (1.4) | 21 | 22:30 (42) | 21:08 | 23:12 | 18.0 (13.0) | 5.0 | 32.0 | 8:16 (102) | 6:08 | 9:18 | 9.3 (1.9) | 7.6 | 10.6 |
Sample size varied from 621 to 583 due to missing Tanner stage data.
TST (Total Sleep Time) = wake-up time − bedtime − sleep latency.
IQR: Intraquartile range in minutes for bedtime and wake-up time and in hours for TST.
Gender Difference in Weekday Sleep Patterns by Age vs. Tanner Stage
As shown in Figure 3, while some gender differences in sleep patterns were noted when plotted by chronological age, very similar patterns were observed when the data were plotted by Tanner stage. For example, the nadir of TST occurred about 1–2 years later in males than in females. However, both males and females showed a TST nadir at Tanner IV (Figure 3 and Table 2).
Influence of Tanner Stage or Menarche Status (female only) on Sleep Patterns
After adjusting for age, day of the week, and pre-sleep activities, higher Tanner stage was associated with later bedtime and later wake-up time in males but not in females (Table 3). The potential explanation for this was limited sample size of Tanner stage I in females (only 9 children). Females who had experienced menarche were associated with 30.6 minutes later bedtime than those who had not experienced menarche (95% CI: 4.3–57.0 minutes, p=0.023) after adjusting for the covariates. Sleep latency and TST did not differ across Tanner stages in both genders (Table 3). Wake-up time, sleep latency and TST were not associated with menarche status in females after adjusting for the covariates (data not shown).
Table 3.
Multivariate linear regression models of sleep parameters (minutes) as a function of pre-sleep activity, Tanner stage, age and day of week (Monday-Sunday).
| Sample size (days) | Bedtime |
wake-up time |
sleep latency |
TST |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | se | p value | β | se | p value | β | se | p value | β | se | p value | ||
| male | |||||||||||||
| Presleep activity | |||||||||||||
| Wacthing TV | 692 | Ref. | Ref. | Ref. | Ref. | ||||||||
| Reading | 482 | 27.3 | 6.8 | <0.001 | −51.6 | 6.0 | <0.001 | −1.2 | 1.3 | 0.347 | −76.6 | 10.2 | <0.001 |
| Study/Homework | 130 | 29.9 | 7.3 | <0.001 | −39.1 | 6.0 | <0.001 | −5.4 | 1.6 | <0.001 | −74.7 | 9.9 | <0.001 |
| Others† | 92 | 54.9 | 21.6 | 0.011 | −8.7 | 17.4 | 0.616 | 2.7 | 3.5 | 0.440 | −70.2 | 16.6 | <0.001 |
| Tanner stage | |||||||||||||
| I | 238 | Ref. | Ref. | Ref. | Ref. | ||||||||
| II | 280 | 20.6 | 9.3 | 0.028 | 4.2 | 6.8 | 0.533 | −0.6 | 2.3 | 0.779 | −10.9 | 11.1 | 0.326 |
| III | 297 | 26.0 | 9.7 | 0.007 | 12.9 | 6.5 | 0.046 | 2.7 | 2.6 | 0.284 | −17.7 | 10.9 | 0.105 |
| IV | 286 | 31.7 | 9.9 | 0.001 | 32.8 | 7.6 | <0.001 | 1.8 | 2.8 | 0.516 | 3.3 | 13.6 | 0.810 |
| V | 295 | 39.9 | 20.9 | 0.056 | 54.5 | 14.2 | <0.001 | 6.8 | 4.1 | 0.093 | 20.1 | 25.6 | 0.432 |
| Age | |||||||||||||
| 11–12 | 89 | Ref. | Ref. | Ref. | Ref. | ||||||||
| 13- | 141 | −19.3 | 15.1 | 0.201 | −11.1 | 9.9 | 0.263 | −1.3 | 2.6 | 0.630 | 8.9 | 19.4 | 0.645 |
| 14- | 312 | −8.6 | 14.0 | 0.538 | −32.4 | 9.5 | <0.001 | 1.0 | 3.1 | 0.746 | −20.4 | 18.0 | 0.255 |
| 15- | 258 | 10.6 | 14.6 | 0.466 | −30.0 | 9.2 | 0.001 | 3.1 | 2.9 | 0.284 | −41.2 | 18.4 | 0.025 |
| 16- | 160 | 46.8 | 15.7 | 0.003 | −22.1 | 11.3 | 0.05 | 0.5 | 3.7 | 0.886 | −69.0 | 21.0 | 0.001 |
| 17- | 106 | 40.4 | 18.3 | 0.027 | 1.3 | 15.5 | 0.934 | −2.8 | 3.5 | 0.427 | −44.8 | 26.7 | 0.093 |
| 18–20 | 330 | 35.9 | 22.1 | 0.104 | 21.6 | 14.4 | 0.133 | 4.5 | 4.2 | 0.282 | −27.7 | 28.6 | 0.333 |
| Day of week | |||||||||||||
| Monday | 166 | Ref. | Ref. | Ref. | Ref. | ||||||||
| Tuesday | 193 | −3.5 | 4.7 | 0.450 | −19.8 | 4.4 | <0.001 | −2.7 | 1.7 | 0.114 | 0.1 | 6.5 | 0.984 |
| Wednesday | 222 | −0.4 | 5.6 | 0.939 | −20.9 | 4.8 | <0.001 | −3.2 | 1.7 | 0.061 | −5.2 | 6.6 | 0.430 |
| Thurday | 213 | −0.7 | 5.4 | 0.899 | −20.1 | 5.1 | <0.001 | −1.4 | 2.0 | 0.507 | −12.4 | 6.7 | 0.064 |
| Friday | 226 | 2.5 | 5.4 | 0.647 | −24.4 | 4.7 | <0.001 | −1.8 | 1.9 | 0.323 | 21.3 | 8.2 | 0.01 |
| Saturday | 204 | 0.9 | 5.8 | 0.877 | 1.6 | 5.5 | 0.779 | −2.9 | 1.9 | 0.138 | 31.2 | 8.2 | <0.001 |
| Sunday | 172 | 1.3 | 4.7 | 0.777 | 20.3 | 6.2 | 0.001 | −2.3 | 1.7 | 0.187 | 9.1 | 6.9 | 0.191 |
| female | |||||||||||||
| Presleep activity | |||||||||||||
| Wacthing TV | 550 | Ref. | Ref. | Ref. | Ref. | ||||||||
| Reading | 469 | 14.5 | 6.4 | 0.025 | −56.0 | 6.6 | <0.001 | −4.5 | 1.6 | 0.005 | −69.4 | 10.6 | <0.001 |
| Study/Homework | 125 | 33.6 | 9.8 | <0.001 | −52.8 | 7.1 | <0.001 | −1.0 | 2.7 | 0.714 | −89.3 | 13.0 | <0.001 |
| Others† | 49 | 23.3 | 21.7 | 0.283 | −21.6 | 16.6 | 0.193 | 0.4 | 2.9 | 0.895 | −52.6 | 27.2 | 0.053 |
| Tanner stage | |||||||||||||
| I | 33 | Ref. | Ref. | Ref. | Ref. | ||||||||
| II | 345 | 0.3 | 15.1 | 0.984 | 0.8 | 13.6 | 0.953 | 0.6 | 3.1 | 0.847 | −7.7 | 22.8 | 0.735 |
| III | 490 | 6.4 | 16.7 | 0.702 | 11.6 | 14.5 | 0.423 | 0.2 | 3.2 | 0.947 | 1.8 | 25.5 | 0.944 |
| IV | 248 | 6.0 | 17.8 | 0.736 | −9.1 | 15.0 | 0.546 | 0.6 | 3.5 | 0.876 | −22.4 | 26.0 | 0.388 |
| V | 77 | 16.7 | 21.9 | 0.444 | 25.3 | 22.8 | 0.267 | 7.0 | 5.6 | 0.208 | −4.1 | 30.0 | 0.891 |
| Age | |||||||||||||
| 11–12 | 48 | Ref. | Ref. | Ref. | Ref. | ||||||||
| 13- | 118 | 34.7 | 16.0 | 0.030 | −4.2 | 11.2 | 0.71 | 4.4 | 2.8 | 0.108 | −33.0 | 22.1 | 0.136 |
| 14- | 224 | 47.2 | 15.1 | 0.002 | 12.6 | 11.0 | 0.253 | 4.0 | 2.6 | 0.132 | −31.6 | 19.9 | 0.111 |
| 15- | 302 | 59.0 | 14.9 | <0.001 | 6.0 | 10.8 | 0.583 | 7.2 | 2.7 | 0.008 | −61.3 | 20.7 | 0.003 |
| 16- | 221 | 56.8 | 15.4 | <0.001 | 14.0 | 11.4 | 0.218 | 11.0 | 2.7 | <0.001 | −51.4 | 21.5 | 0.017 |
| 17- | 114 | 71.7 | 17.2 | <0.001 | 37.8 | 14.8 | 0.011 | 9.5 | 4.5 | 0.036 | −35.3 | 25.6 | 0.169 |
| 18–20 | 166 | 124.6 | 17.3 | <0.001 | 84.2 | 15.1 | <0.001 | 4.2 | 3.5 | 0.223 | −44.8 | 22.6 | 0.047 |
| Day of week | |||||||||||||
| Monday | 137 | Ref. | Ref. | Ref. | Ref. | ||||||||
| Tuesday | 152 | 1.4 | 4.6 | 0.770 | −8.0 | 4.8 | 0.094 | −0.4 | 1.1 | 0.718 | −3.7 | 7.5 | 0.618 |
| Wednesday | 191 | 9.2 | 5.5 | 0.095 | −16.9 | 5.4 | 0.002 | 2.9 | 1.9 | 0.118 | −20.0 | 8.4 | 0.017 |
| Thurday | 191 | 6.6 | 5.5 | 0.235 | −20.3 | 5.9 | <0.001 | 1.9 | 2.0 | 0.349 | −12.1 | 8.7 | 0.164 |
| Friday | 214 | 2.3 | 5.4 | 0.676 | −22.8 | 5.2 | <0.001 | 0.6 | 1.3 | 0.670 | 20.5 | 10.4 | 0.050 |
| Saturday | 173 | −0.1 | 6.0 | 0.987 | 1.5 | 6.9 | 0.832 | 0.1 | 1.7 | 0.947 | 30.3 | 11.0 | 0.006 |
| Sunday | 135 | −6.8 | 5.1 | 0.184 | 19.0 | 6.6 | 0.004 | 1.6 | 1.5 | 0.308 | 8.7 | 8.1 | 0.281 |
other pre-sleep activities included: doing nothing, taking a rest, in person social conversation, working, playing, listening to radio, playing card game or Majiang, doing Chaos, taking a bath, walking, et al.
Pre-sleep activity: activities one hour before bedtime.
Fourteen 18–20 year-old were excluded due to shift-work history.
Influence of Pre-Sleep Activities on Sleep Patterns
There were three common pre-sleep activities: watching TV, reading, and studying/doing homework. For the 11–17 year-olds, 37.3% watched TV, 46.1% read, and 13.6% reported studying/doing homework on weekdays. On weekends, more of them watched TV (57.8%) and fewer read (31.2%) or reported studying/doing homework (6.7%). For older adolescents (≥ 18 years), the majority of them watched TV (67.2% weekday, 71.4% weekend), less than one-sixth (14.4% weekday and 13.7% weekend) read, and almost none of them reported study/doing homework (weekday 0.5%; weekend 0%).
Pre-sleep activities independently influenced sleep patterns in both genders, even after 14 individuals with a history of shift-work were excluded (Table 3). Compared to those who watched TV, children who read or studied/did homework went to bed later, woke up earlier and got fewer TSTs after adjusting for covariates, regardless of gender. For example, in males, those who studied or did homework, on average, went to sleep 29.9 minutes later, woke up 39.1 minutes earlier, fell asleep 5.4 minutes faster, and slept 74.7 minutes shorter than those who watched TV after adjusting for Tanner stage, age, and day of week. These associations were also observed when data was limited to the 11–17 year-olds (data not shown).
Individual Variability of Sleep Parameters
The variability of each sleep parameter among individuals was substantial for a given age and gender group (Table 1 and online Figure b). For example, the differences between 10th and 90th percentiles of weekday TST for each age group were 2.5–5.3 hours in males and 2.2–5.0 hours in females. Considerable variations for the sleep parameters also existed for each gender-specific Tanner stage (Table 2). For example, 10th percentile of TST in Tanner I males was 7.8 hours, while its 90th percentile was 11.3 hours (the difference was 3.5 hours).
DISCUSSION
To our knowledge, this is the first prospective, sleep diary-based study of sleep patterns among rural Chinese adolescents. Several interesting findings were noted. As compared to urban or western populations, these rural adolescents were less exposed to psychosocial and environmental factors often linked to delayed sleep phase (no computer or video games, no internet, absent evening social activities), thus, they had earlier weekday bedtimes and relatively longer weekday TST. For example, age-specific weekday TSTs were ~1 hour longer than those reported in urban Shandong China [22], more than one hour longer than those for Japanese children [19], Korean teenagers [7], or Americans aged 14–19 years [8], and similar to Swiss urban adolescents [28] and Americans aged 11–13 years [8]. Also, similar bedtimes were found between weekdays and weekends in this study while bedtimes were found later on weekends in developed countries [15, 26], which might be attributed to rural environment in this study.
Our data confirmed the age-related delay in bedtimes across adolescence as reported in previous studies. Normative data from a 16-year longitudinal study of 493 participants showed that bedtimes were delayed and TST decreased with age during adolescence [28]. Also, a study of 1457 urban Korean students aged 9–19 years (grades 5–12) reported that students in higher grades had later weekday bedtime, earlier wake-up time and much shorter TST than students in lower grades [7].
After adjusting for age and other covariates, we found independent effect of pubertal development (Tanner stage in males and menarche status in females) on delayed bedtime. Similarly, in a study of 1146 Canadian adolescents (aged 13 years, Tanner I–IV; 588 males), higher pubertal stage was associated with later bedtime, later wake-up time, and longer sleep time on weekends [18]. This suggests that biological changes associated with sexual maturation may have a robust influence on sleep timing during adolescence. Intrinsic circadian rhythm and sleep-wake process profoundly change during adolescent development [5, 11], which may account for later bedtimes in older adolescents [5, 29]. However, in a study of 1547 children aged 9–16 years conducted in urban Taiwan, where sleep patterns were highly shaped by extremely high academic demands, no association was found between self-assessed pubertal status and sleep-wake patterns [13]. Our findings differed from those in Taiwan, which might be due to the lower degree of academic pressure in the sample.
Interestingly, in this study TST went up dramatically in the oldest group, while it decreased with age in early and mid-adolescence (Figure 3). This differs from those of previous studies [7, 30]. In a meta-analysis of sleep data derived from 65 studies, school-day TST decreased during adolescence [30]. The higher TST in the oldest teens (18–20 year-olds) is likely an indication of release from sleep deprivation imposed by school schedule or academic pressure in this study. The majority (66.0%) of these teens had left school (most of them (71.7%) and had worked outside their hometown; they were at home, away from work, when this study was conducted). Fourteen subjects reported shift-work in their jobs outside the home, but the TST pattern by age remained even after excluding the 14 subjects (data not shown). Our findings indicate that when given the opportunity to sleep later, teenagers will do so and get the sleep that they need.
No independent associations were found between Tanner stage and TST after adjustment for the covariates, despite their “U”-shaped pattern in crude analysis (Fig 3). Consistently, a study of 19 children (8 females) conducted in a summer camp, where environmental constraints were removed, found that nocturnal sleep time remained constant across Tanner stages [20].
Another interesting finding is that similar to their counterparts in other countries and in urban settings, school students in this rural Chinese sample may have chronic sleep deprivation. We found a weekday-weekend discrepancy in younger adolescents (but not older teens), which may reflect compensation for sleep loss and a return to an instinctual circadian rhythm lost during the weekdays [31]. Later wake-up time and longer TST on weekends were also found in urban Koreans, especially for the high graders (12th-graders, about 17 years old) who were under severe sleep deprivation (sleep time less than 5 hours on school-nights) [7]. These rural Chinese adolescents were similar in that they endured fixed, early rise times imposed by school even during an age in development when teenagers would rather sleep later. The teens did not compensate by going to bed earlier. Instead, they became sleep deprived. These data seem to support arguments for later school start times during adolescence.
Previous studies reported watching TV had a negative influence on sleep. [15] A survey study of 2546 students in Belgium (means (SD) age 13.2 (0.4) and 16.4 (0.7) years) found that television watching, computer game playing and internet use were related to later bedtimes for the entire week, less sleep time on weekdays and later wakeup time on weekends [15]. Compared to those watching TV, we found that hard-working students might spend more time studying and getting less sleep, which further suggested the influence of academic demand on sleep schedules.
Potential limitations need to be considered when interpreting our results. First, sleep data were based on sleep diaries and questionnaires, which may be subject to reporting bias. Secondly, the napping time and WASO was not taken into account when calculating TST. However, a very small fraction of adolescents in this study took naps, especially during the non-summer season when this study was conducted. None of them reported napping in the diaries and 82% reported no naps during the past month in a sub-dataset analysis of adolescents from the same area. Thus, not accounting for nap time should not affect TST significantly in this study population. While the lack of accurate measure of WASO in this study may result in overestimation of TST, this should be a relatively minor factor given the study participants are generally healthy adolescents. On the other hand, TST patterns across age, gender and Tanner stage should not be affected.
In summary, the findings that higher Tanner stage associated with later bedtime and wake-up time in males and having experienced menarche associated with delayed bedtime in females suggest a robust effect of developmental maturation on delayed sleep times. More importantly, the results of this prospective study indicated that rural Chinese adolescents –despite less exposure to late night social pressures and computers – were sleep deprived, as evidenced by the need to “compensate” (later wake up times and higher TST) on weekends, and the longer TST (return to a “natural schedule”) seen in the oldest participants, who were no longer in school. Pre-sleep activity (reading/studying) was related to less sleep. Taken together, our data suggest that early school start time coupled with later bedtime associated with academic demand appear to be important contributors to chronic partial sleep deprivation in this rural Chinese adolescent population.
Supplementary Material
Acknowledgments
This study is supported in part by grant R01 HD049059 from the National Institute of Child Health and Human Development; R01 HL0864619 and 5T32HL007909-08 from the National Heart, Lung, and Blood Institute; and R01 AG032227 from the National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mindell JA, Owens JA, Carskadon MA. Developmental features of sleep. Child Adolesc Psychiatr Clin N Am. 1999;8:695–725. [PubMed] [Google Scholar]
- 2.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 3.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–19. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 4.Timing is everything. Nature. 2003;425:885. doi: 10.1038/425885a. [DOI] [PubMed] [Google Scholar]
- 5.Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: implications for behavior. Ann N Y Acad Sci. 2004;1021:276–91. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- 6.Carskadon MA. Patterns of sleep and sleepiness in adolescents. Pediatrician. 1990;17:5–12. [PubMed] [Google Scholar]
- 7.Yang CK, Kim JK, Patel SR, Lee JH. Age-related changes in sleep/wake patterns among Korean teenagers. Pediatrics. 2005;115:250–6. doi: 10.1542/peds.2004-0815G. [DOI] [PubMed] [Google Scholar]
- 8.Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007 doi: 10.1016/j.sleep.2006.12.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Carskadon MA. Adolescent sleep patterns: biological, social and psychological influences. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- 10.Wolfson AR, Carskadon MA. Understanding adolescents’ sleep patterns and school performance: A critical approach. Sleep Medicine Review. 2003;7:491–506. doi: 10.1016/s1087-0792(03)90003-7. [DOI] [PubMed] [Google Scholar]
- 11.Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998;21:871–81. doi: 10.1093/sleep/21.8.871. [DOI] [PubMed] [Google Scholar]
- 12.Jenni OG, O’Connor BB. Children’s sleep: an interplay between culture and biology. Pediatrics. 2005;115:204–16. doi: 10.1542/peds.2004-0815B. [DOI] [PubMed] [Google Scholar]
- 13.Gau SF, Soong WT. The transition of sleep-wake patterns in early adolescence. Sleep. 2003;26:449–54. doi: 10.1093/sleep/26.4.449. [DOI] [PubMed] [Google Scholar]
- 14.Ferber R. Sleep schedule-dependent causes of insomnia and sleepiness in middle childhood and adolescence. Pediatrician. 1990;17:13–20. [PubMed] [Google Scholar]
- 15.Van den Bulck J. Television viewing, computer game playing, and Internet use and self-reported time to bed and time out of bed in secondary-school children. Sleep. 2004;27:101–4. doi: 10.1093/sleep/27.1.101. [DOI] [PubMed] [Google Scholar]
- 16.Tanner J. Growth at adolescence. 2. Springfield, I11: Charles C. Thomas; 1962. [Google Scholar]
- 17.Lee KA, McEnany G, Weekes D. Gender differences in sleep patterns for early adolescents. J Adolesc Health. 1999;24:16–20. doi: 10.1016/s1054-139x(98)00074-3. [DOI] [PubMed] [Google Scholar]
- 18.Laberge L, Petit D, Simard C, Vitaro F, Tremblay RE, Montplaisir J. Development of sleep patterns in early adolescence. J Sleep Res. 2001;10:59–67. doi: 10.1046/j.1365-2869.2001.00242.x. [DOI] [PubMed] [Google Scholar]
- 19.Gaina A, Sekine M, Hamanishi S, Chen X, Kagamimori S. Gender and temporal differences in sleep-wake patterns in Japanese schoolchildren. Sleep. 2005;28:337–42. [PubMed] [Google Scholar]
- 20.Carskadon MA, Harvey K, Duke P, Anders TF, Litt IF, Dement WC. Pubertal changes in daytime sleepiness. Sleep. 1980;2:453–60. doi: 10.1093/sleep/2.4.453. [DOI] [PubMed] [Google Scholar]
- 21.Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–62. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Zhou H. Sleep duration, insomnia and behavioral problems among Chinese adolescents. Psychiatry Res. 2002;111:75–85. doi: 10.1016/s0165-1781(02)00131-2. [DOI] [PubMed] [Google Scholar]
- 23.Wang B, Necheles J, Ouyang F, Ma W, Li Z, Liu X, Yang J, Xing H, Xu X, Wang X. Monozygotic co-twin analyses of body composition measurements and serum lipids. Prev Med. 2007;45:358–365. doi: 10.1016/j.ypmed.2007.07.014. Epub 2007 Jul 18. [DOI] [PubMed] [Google Scholar]
- 24.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine B, Moyles T, Roehrs T, Fortier J, Roth T. Actigraphic and polygraphic recordings in determination of sleep and wake. In: Chase MH, McGinty DJ, Crane G, editors. Sleep research. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1986. p. 247. [Google Scholar]
- 27.Srivastava MS. Estimation of the intraclass correlation coefficient. Ann Hum Genet. 1993;57:159–65. doi: 10.1111/j.1469-1809.1993.tb00897.x. [DOI] [PubMed] [Google Scholar]
- 28.Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111:302–7. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- 29.Taylor DJ, Jenni OG, Acebo C, Carskadon MA. Sleep tendency during extended wakefulness: insights into adolescent sleep regulation and behavior. J Sleep Res. 2005;14:239–44. doi: 10.1111/j.1365-2869.2005.00467.x. [DOI] [PubMed] [Google Scholar]
- 30.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 31.Hansen M, Janssen I, Schiff A, Zee PC, Dubocovich ML. The impact of school daily schedule on adolescent sleep. Pediatrics. 2005;115:1555–61. doi: 10.1542/peds.2004-1649. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.