Abstract
The patterning of vertebrate somitic muscle is regulated by signals from neighboring tissues. We examined the generation of slow and fast muscle in zebrafish embryos and show that Sonic hedgehog (Shh) secreted from the notochord can induce slow muscle from medial cells of the somite. Slow muscle derives from medial adaxial myoblasts that differentiate early, whereas fast muscle arises later from a separate myoblast pool. Mutant fish lacking shh expression fail to form slow muscle but do form fast muscle. Ectopic expression of shh, either in wild-type or mutant embryos, leads to ectopic slow muscle at the expense of fast. We suggest that Shh acts to induce myoblasts committed to slow muscle differentiation from uncommitted presomitic mesoderm.
Keywords: Zebrafish, muscle, fiber type, adaxial cells, sonic hedgehog, myoblast
All vertebrates have two classes of muscle fibers: slow and fast. Slow fibers have low-force, long-duration contractions because they express myosin isoforms that are specialized for slow contraction and an oxidative metabolism. Fast fibers have distinct fast myosins and glycolytic metabolism, ideal for high-force, short-duration contractions. Each muscle has a specific mix and spatial array of fast and slow fibers from its formation early in development. How such arrays are patterned is unknown, but evidence for two contrasting models exists. In one view, proliferative myoblasts are intrinsically committed to form either fast or slow fibers and accumulate in appropriate regions of the embryo. Clonal cell analysis in chicks shows that myoblasts are heterogeneous before their differentiation: Some are specialized to form slow muscle, whereas others form fast (Miller and Stockdale 1986; Schafer et al. 1987; DiMario et al. 1993). Alternatively, naive myoblasts could be instructed by their environment to express specific isoforms of muscle proteins at the time of differentiation, as occurs in postnatal rodent muscles (Hughes and Blau 1992). A resolution of this issue is suggested by studies in Drosophila where local extrinsic signals induce commitment of muscle founder myoblasts to the formation of a particular type of muscle in each location (Baylies et al. 1995). In this paper, we examine vertebrate muscle patterning in the zebrafish somite. We show that the secreted glycoprotein Sonic hedgehog (Shh) regulates the decision between fast and slow muscle formation and we suggest this decision involves induction of a specifically slow myoblast type.
Muscles are formed by the differentiation of mononucleate proliferative myoblasts into post-mitotic myocytes that subsequently fuse to form multinucleate muscle fibers. In amniotes, muscle fibers differentiate in two waves: The first-formed primary fibers are generally slow, whereas later secondary fibers, which form in close association with primary fibers, are fast (Kelly and Rubinstein 1980). These two fiber types are not separated spatially and, as they are formed over a considerable time period, the fate of individual cells as they mature has not been followed. In zebrafish, by contrast, somitic muscle fibers form in two temporally separated waves. The early differentiating cells are formed medially near the notochord and migrate laterally during late somitogenesis to become slow muscle (van Raamsdonk et al. 1978; Devoto et al. 1996). However, most somitic cells differentiate later and become fast muscle. How spatial information in the early somite generates this pattern is unclear.
The differentiation of somites is central to vertebrate mesoderm development. Somites are epithelial balls of mesoderm that arise from a mesenchymal mass of proliferative paraxial tissue in a rostro–caudal order. Once formed, somites differentiate rapidly into a ventral sclerotomal mesenchymal compartment and a dorsal epithelial structure, the dermomyotome. In lower vertebrates, such as fish, in which the sclerotome is small (Morin-Kensicki and Eisen 1997), the somite mainly gives rise to muscle. In amniotes, the dermomyotome contributes to trunk dermis and to several distinct populations of muscle cells. The dorsomedial lip of the dermomyotome, which is located next to the neural tube, forms differentiated muscle of the myotome that arises between the sclerotome and dermomyotome.
Signals from adjacent tissues regulate somitic muscle differentiation (for review, see Lassar and Munsterberg 1996). However, the precise source, nature, and roles of these signals is unclear. Axial structures (neural tube and notochord) are important as their removal leads to cell death and somite regression (Teillet and Le Douarin 1983; Rong et al. 1992), and they can enhance both myogenesis and chondrogenesis (Kenny-Mobbs and Thorogood 1987). Notochord can induce myogenesis in some assays of myogenic induction (Buffinger and Stockdale 1995; Gamel et al. 1995; Stern et al. 1995; Pownall et al. 1996), although ectopically positioned notochords in chick embryos can induce sclerotome at the expense of myogenic tissue (Pourquie et al. 1993; Bober et al. 1994; Fan and Tessier-Lavigne 1994; Goulding et al. 1994). Shh is a signaling molecule expressed in notochord at times when this tissue can influence muscle differentiation (Echelard et al. 1993; Krauss et al. 1993; Johnson et al. 1994; Roelink et al. 1994; Marti et al 1995). Shh can substitute for notochord in various assays of both sclerotome and muscle induction (Fan et al. 1995; Munsterberg et al. 1995), and induce ectopic muscle markers in vivo (Johnson et al. 1994; Concordet et al. 1996; Hammerschmidt et al. 1996; Weinberg et al. 1996). Moreover, mice homozygous for a targeted deletion of the shh gene have deficits in sclerotome and myotome precursor cell markers (Chiang et al. 1996). These data suggest that Shh may mediate notochord-dependent signals that induce myogenesis. However, two lines of evidence argue against this simple view. First, both the MyoD and Myf-5 muscle-specific transcription factors are still expressed in shh−/− mouse somites, although Myf-5 mRNA is reduced (Chiang et al. 1996). As Myf-5 and MyoD are myoblast markers in amniotes, this suggests that the myogenic program can be initiated in the absence of Shh. Second, ablation of all axial structures has little effect on limb and body wall muscle development although somitic myogenesis is reduced, partly attributable to regression of the somite (Rong et al. 1992). Therefore, although notochord-derived Shh is a strong candidate for a regulator of myotomal muscle formation, its precise role in myogenesis remains enigmatic.
A confounding factor in understanding myotomal muscle induction is the heterogeneity of myogenic cell populations within the somite (for review, see Cossu et al. 1996b). The phenotypes of mice with null mutations in members of the MyoD family of myogenic regulatory transcription factors (MRFs) suggest that several distinct populations of myogenic cells exist in different parts of the developing murine dermomyotome (Rudnicki et al. 1993; Tajbakhsh et al. 1997) and these populations appear to differ in their sensitivity to loss of Shh (Chiang et al. 1996). In addition to notochord, neural tube also contains inductive signals that can support somitic myogenesis (Teillet and Le Douarin 1983; Rong et al. 1992; Goulding et al. 1994; Buffinger and Stockdale 1995; Stern and Hauschka 1995), and dorsal neural tube can induce myogenesis, an effect that can be mimicked by some Wnt proteins (Gamel et al. 1995; Munsterberg et al. 1995; Stern et al. 1995). Moreover, inhibitory signals from lateral plate mesoderm and surface ectoderm have been suggested to influence myogenesis (Fan and Tessier-Lavigne 1994; Pourquie et al. 1996). Therefore, although several distinct signals and muscle cell populations exist, what signals induce each cell population in vivo is unclear.
In the zebrafish, the somite gives rise mainly to muscle, which is probably the primary fate of paraxial mesoderm during early chordate evolution (Holland et al. 1995). Even in this simple system, however, three muscle cell populations can be resolved. Adaxial cells form next to the notochord, eventually giving rise to slow muscle (van Raamsdonk et al. 1978; Devoto et al. 1996) and are the first cells in the embryo to express the muscle transcription factors myoD and mef2D, mef2A, and mef2C (Ticho et al. 1996; Weinberg et al. 1996). A specialized subpopulation of adaxial cells, the muscle pioneers, form at the dorsoventral midline of each somite (Felsenfeld et al. 1991), express engrailed proteins (Hatta et al. 1991), and appear to be induced by two sequential signals from outside the somite (Currie and Ingham 1996). The majority of the somite forms the third muscle cell population that both expresses myoD and differentiates later (Devoto et al. 1996; Weinberg et al. 1996).
Here, we describe the development of slow and fast muscle in the zebrafish embryo. We show that, as in amniotes, slow muscle differentiates first and that fast muscle is formed later in close association with differentiated slow fibers. We demonstrate that notochord-derived signals are required for formation of slow, but not fast, muscle. We find that Shh, a molecule expressed and secreted by early notochord, can induce slow muscle ectopically at the expense of fast muscle. Taken together, our results suggest that slow primary muscle in zebrafish somites is induced by Shh from the notochord, but that neither notochord nor primary slow muscle is required to control the timing of secondary fast fiber differentiation.
Results
Early zebrafish embryos have distinct fast and slow muscle cell populations
To examine the patterning of muscle in zebrafish somites, we screened a series of anti-MyHC monoclonal antibodies for reactivity with 1- to 2-day zebrafish muscle tissue. Two antibodies detected all differentiated skeletal and heart muscle, whereas three antibodies detected specific subpopulations of cells within the somites of 24-hr (prim-5) embryos (Fig. 1). BA-D5, an antibody that specifically detects slow MyHC in muscle fibers of all ages of mammals and chicks examined (Schiaffino et al. 1989; C.S. Blagden and S.M. Hughes, unpubl.), detects a single layer of cells in the superficial region of 24-hr zebrafish somites at all anteroposterior positions within the body axis (Fig. 1B,C,E,F). In contrast, EB165, an antibody that detects fast fibers in embryonic and adult chicken muscle (Gardahaut et al. 1992), detects an adjacent nonoverlapping population of medial somitic muscle fibers in the 1-day zebrafish embryo (Fig. 1D,F). A third monoclonal antibody, A4.1025, which reacts with a conserved epitope near the ATP-binding site of all striated muscle MyHC isoforms examined in a wide variety of species (Dan-Goor et al. 1990), detects both the BA-D5+ and EB165+ populations of cells (Fig. 1A,C). All three antibodies reacted with muscle fibers in a striated pattern typical of sarcomeric myosin and Western analysis of 24-hr zebrafish extracts separated by SDS-PAGE demonstrated that all three antibodies detect protein bands at or just under Mr 200,000, the size of MyHC isoforms (Fig. 1G). Therefore, these anti-MyHC antibodies distinguish slow and fast differentiated muscle cells in the zebrafish embryo.
Figure 1.

Slow and fast MyHC isoform-specific antibodies recognize discrete muscle fiber populations in developing embryonic 24-hr zebrafish. Slow BA-D5-reactive MyHC (green; B,C) is present in the outermost layer of A4.1025-reactive cells containing skeletal muscle MyHC (red; A,C) in a transverse cryosection through the trunk (A–C). A tail section (D–F) shows that fast EB165-reactive MyHC (red; D,F) was detected in medial fibers that do not express slow MyHC (green; E,F) so that muscle fibers at this stage can be divided into two subpopulations of slow lateral and fast medial cells (C,F). Western analysis (G) using protein extracted from 24-hr zebrafish embryos shows that A4.1025 and BA-D5 recognize major bands that migrate at the same rate as purified bovine cardiac muscle MyHC (∼220 kD). EB165 detects two bands ∼170 kD that could either reflect degradation of the fast MyHC or an unusual sized MyHC in zebrafish fast muscle. The lower ∼50-kD band is nonspecific because it is also detected with antibody F1.652 to mouse embryonic MyHC that does not react with fish sections. The ∼75-kD band in the A4.1025 lane reflects some degradation. (n) Notochord; (nt) neural tube.
Slow muscle differentiates before fast muscle in zebrafish embryos
We determined the timing and location of slow and fast muscle differentiation throughout zebrafish somite development. New somites separate from the presomitic paraxial mesoderm in an anterior to posterior order about every half hour between 10.5 and 26 hrs of development at 28°C (Westerfield 1995). We observed that MyHC+ adaxial cells appeared on each side of the notochord in an anterior-to-posterior order in each somite as it formed (Fig. 2A). Most, if not all, adaxial MyHC+ cells also express slow MyHC (Fig. 2B). Fast MyHC was undetectable in 15 somite embryos (Fig. 2C). Therefore, the first population of muscle cells to differentiate in the zebrafish somite are the adaxial cells, and these cells express slow, but not fast, characteristics from their inception.
Figure 2.
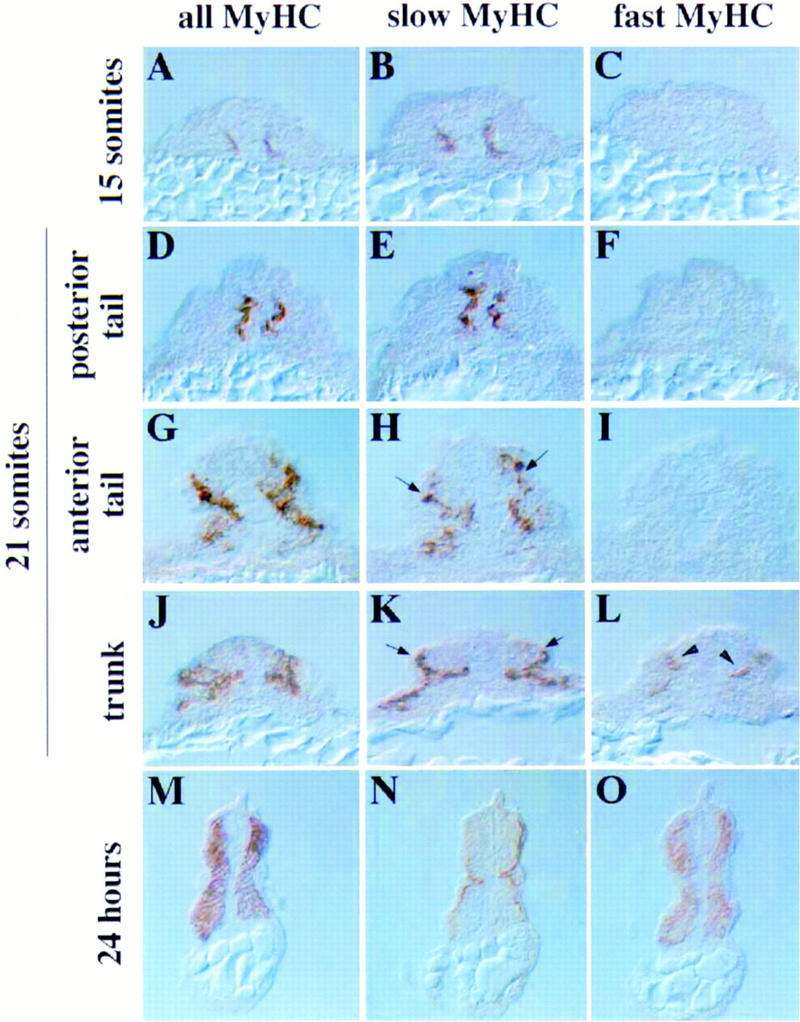
Slow muscle differentiates before fast in zebrafish embryos. (A–C) Serial transverse cryosections of a methanol-fixed 15-somite embryo show that muscle first differentiates next to the notochord (A), and expresses slow MyHC (B). Fast MyHC (C) is undetectable at any level of the embryo. (D–L) At the 21-somite stage, a developmental progression exists along the anteroposterior axis. Posteriorly, slow muscle cells are still medial (D,E) and fast MyHC is undetectable (F). However, more anterior sections of the same embryo show loss of slow MyHC+ cells medially and their appearance laterally (H,K; arrows). Somite cells medial to slow MyHC+ cells initiate differentiation (G,J) and fast MyHC expression (L; arrowheads). Therefore, medial fast muscle differentiates slightly before lateral in each individual somite. (M–O) By 24 hr, the slow muscle cells are aligned in a layer one cell thick at the lateral margin (N), and the remaining medial muscle expresses fast MyHC (O).
A recent study by Devoto et al. (1996) has elegantly shown that the differentiated adaxial cells of somites 16–20 in the gut extension region of zebrafish embryos migrate laterally through the somite 3–4 hr postsomitogenesis. Consistent with the results of Devoto et al. (1996), we find that before the 20-somite stage, adaxial slow MyHC+ cells in all somites remain medial, but that the adaxial slow MyHC+ cells of older somites appear to spread dorsally around the sides of the neural tube and ventrally past the hypochord to form a single layer of medial cells. At about the 20-somite stage the adaxial slow MyHC+ cells of the most anterior somites appear to migrate laterally, through the undifferentiated somitic mesoderm. Although it is possible that this apparent migration represents a wave of fiber type conversion, we think this unlikely from the earlier findings of Devoto et al. (1996) that early adaxial cells migrate and form slow muscle. The wave of migration sweeps rapidly along the embryo from anterior to posterior so that by the 21-somite stage, slow muscle cells of the anterior somites are located at the lateral edge of the somite under the epidermis (Fig. 2J,K), whereas slow muscle cells of mid-body somites are found in the center of the somite (Fig. 2G,H) and the most posterior slow muscle cells, are still in the adaxial position (Fig. 2D,E). During the lateral migration of slow muscle cells the differentiation of fast muscle cells commences (Fig. 2F,I,L). No differentiated fast muscle was observed lateral to migrating slow muscle cells. However, strikingly, fast muscle cells are detected medial to the slow muscle cells immediately after the migratory period in each somite (Fig. 2, cf. I and L). By the 26-somite stage, all slow muscle cells in somites 1–21 have migrated laterally (Fig. 2M,N; data not shown). At this stage fast muscle fills the medial bulk of the somite (Fig. 2O). Therefore, the differentiation of a distinct class of fast muscle cells rapidly succeeds the migration of the slow muscle cells past undifferentiated somitic cells.
Notochord defects correlate with lack of slow muscle differentiation
The formation of slow muscle next to notochord suggests that a notochord-derived signal induces slow muscle cells. To test this hypothesis we examined two mutant zebrafish strains that are defective in distinct stages of notochord development. Severely affected bozozok (bozi2) fish do not have visible notochord, lack the notochord and floor plate marker Shh, and completely lack differentiated slow muscle (Fig. 3A–C). At 24 hr of development, when in wild-type embryos adaxial cells have differentiated, migrated, and express slow MyHC in all somites, no slow MyHC is detected in trunk or tail regions of boz embryos that lack notochord (six of six embryos sectioned; Fig. 3B), although unaffected sibling embryos appear wild type (data not shown). Mutation of the boz gene does not prevent muscle differentiation per se, because a single fused somite of differentiated muscle is present beneath the neural tube, and this expresses fast MyHC (Fig. 3A,C). Normal boz function is required for formation of slow muscle, rather than maintenance, as both severely and more mildly affected embryos from a boz heterozygote cross failed to express detectable slow MyHC at the 15-somite stage, whereas morphologically normal siblings showed normal slow MyHC expression (data not shown). Therefore, the absence of notochord in the boz mutant is accompanied by the specific loss of slow muscle.
Figure 3.

Severe midline defects correlate with loss of slow muscle formation. (A–C) Serial transverse sections of severely affected 24-hr boz embryos, which have no axial mesoderm from gastrulation, have no detectable slow MyHC (B). A single fused somite (A) expressing fast MyHC (C) extends under the neural tube (cf. Fig. 2, M–O). (D–L) Successive transverse sections of a 24-hr ntlb160 embryo. Anterior tail shows normal distribution of muscle fiber types (D–F), except for the lack of muscle pioneers at the dorsoventral midline (E). More posteriorly (G–I), slow cells are missing or migrate abnormally (arrows in H,K). Fast MyHC appeared unaffected (F,I,L). (M–Q) Whole-mount in situ mRNA hybridization for shh at 15 somites (M–O,Q) or 24 hr (P). Notochord and floorplate shh expression is completely absent in severe boz embryos (N,Q), compared with wild type (M,O). In contrast, only the most posterior shh expression is lacking in ntl mutants (P). (M,N) Dorsal views of the tail; (O–Q) lateral views of the tail.
Although boz function is required for notochord formation, it is possible that the wild-type gene might also be required in paraxial mesoderm to permit differentiation of slow muscle cells. We therefore examined ntl mutant embryos in which midline mesodermal cells are present but fail to differentiate into mature notochord cells. Previous studies have shown that ntl embryos also lack muscle pioneer cells, a subpopulation of the adaxial cells (Halpern et al. 1993). ntl is the zebrafish homolog of the Brachyury transcription factor and is expressed in notochord but not in adaxial cells at the time of their differentiation and therefore muscle defects are unlikely to be attributable to a cell-autonomous action of ntl in paraxial mesoderm (Schulte-Merker et al. 1994). In ntlb160 embryos, notochord precursors are present in anterior regions but absent posteriorly in the region beyond the yolk tube, which is severely truncated (Halpern et al. 1993; Odenthal et al. 1996; Fig. 3P). We examined ntlb160 fish for slow MyHC expression anticipating that the loss of notochordal maturation might prevent slow muscle formation. Despite the absence of muscle pioneer cells at the dorsoventral midline, slow and fast muscle in anterior regions of ntl embryos appeared normal (Fig. 3D–F). Therefore, mature notochord is not required for slow muscle differentiation. However, more posterior regions of ntl embryos, in which axial mesoderm defects are more severe (Halpern et al. 1993), showed reduced slow muscle formation and aberrant positioning (Fig. 3G–I). In rare embryos (1/16 serially sectioned) a complete absence of slow muscle was observed in the most posterior somite at 24 hr of development, even though extensive differentiated fast muscle was present (Fig. 3J–L). The remaining ntl embryos (15/16) showed regional slow deficits. Therefore, ntl mutant fish demonstrate that although mature notochord is not necessary for slow muscle formation, severe defects in notochord establishment in the tail correlate with loss of slow muscle differentiation.
Shh induces ectopic slow muscle differentiation
Examination of the muscle phenotype of boz and ntl mutant embryos suggested that notochord-derived signals may determine the slow muscle fate, reminiscent of the induction of floorplate and motoneurons by notochord-derived Shh protein (Ericson et al. 1996) and of muscle pioneer cells by notochord-derived hedgehogs (Currie and Ingham 1996). Consistent with this, shh mRNA is absent from those regions of both boz and ntl embryos that show defects in slow muscle formation (Fig. 3M–Q). To test the possibility that Shh might be a notochord-derived inducer of the slow muscle fate, we injected shh mRNA into two- or four-cell zebrafish embryos to create animals chimeric for shh overexpressing cells. Such injections lead to an easily detectable reduced retina phenotype (Krauss et al. 1993). In animals affected for retinal development, we observed an induction of slow MyHC expression across the entire width of the somite in each of 12 serially-sectioned 24-hr embryos (Fig. 4A–C). Strikingly, this expansion occurs at the expense of differentiated fast muscle (Fig. 4C). In those animals in which mosaic segregation of shh mRNA causes partial slow muscle induction, residual fast muscle is observed in regions not expressing slow MyHC (data not shown). When the same experiment was repeated using an equivalent amount of echidna hedgehog (ehh) mRNA, no defect was detected in any part of 10 embryos serially sectioned (data not shown). Therefore, Shh is a notochord-derived signal capable of inducing slow muscle at the expense of fast.
Figure 4.
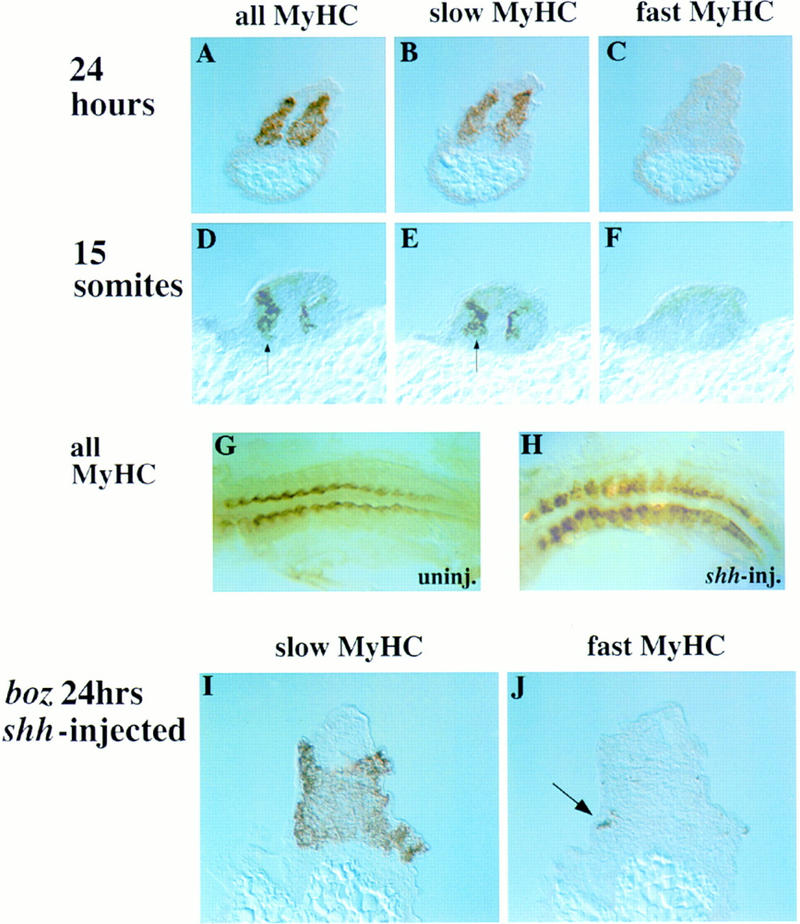
Ectopic overexpression of shh induces extra slow muscle cells in the myotome of wild-type and mutant embryos. (A–C) shh mRNA was microinjected into the zebrafish blastulae at the 2- or 4-cell stage and 24-hr embryos examined in transverse serial cryosections. All differentiated cells in both myotomes contained slow MyHC (A,B) and did not express fast MyHC (C). Some injected embryos showed ectopic slow MyHC and fast MyHC suppression on only one side, probably caused by mosaic expression of injected mRNA (data not shown). (D–H) Embryos injected with shh mRNA were analyzed at the 15-somite stage by whole-mount or cryosection immunohistochemistry. Compared with wild type (G and Fig. 2A), injected embryos showed bilateral (H) or unilateral (D, arrow) expansion of muscle differentiation. Ectopic muscle contained slow (E, arrow), but not fast, MyHC (F). Note that although ectopic lateral muscle is present in the anterior portion of shh-injected embryos (H, left), premature differentiation is not observed in presomitic mesoderm (H, right). (I,J) Embryos from boz mutant crosses were injected with shh mRNA and severely affected boz embryos (lacking eyes and notochord), were examined by serial cryosection immunohistochemistry. Widespread slow MyHC rescue (I) and supression of fast MyHC (J) was observed.
The wholesale conversion of large areas of somite to slow muscle by Shh has two possible explanations. Shh could induce somitic cells to differentiate as slow muscle prematurely. Alternatively, Shh might not affect the decision of when to differentiate, but simply determine what type of muscle is formed. To address this issue, we examined the effect of ectopic Shh on earlier stage zebrafish embryos. In 15-somite zebrafish embryos, Shh induces a wide region of ectopic lateral differentiated muscle within the somite (46/53 unselected injected embryos, Fig. 4G,H). Ectopic slow muscle differentiation occurred without premature induction of fast muscle tissue (Fig. 4D–F). The premature differentiation of lateral muscle tissue suggested that Shh might induce presomitic mesoderm to differentiate early. However, premature slow muscle differentiation before the normal time of adaxial cell differentiation was not observed in either presomitic mesoderm of any of 36 embryos examined at the 15-somite stage (Fig. 4H) or in any region of 22 embryos at tailbud stage (data not shown). Therefore, the earliest time somitic cells are competent to become slow muscle in response to Shh is when adaxial cells normally differentiate. However, at this stage, cells in all regions of the somite become competent.
The ability of Shh to induce slow muscle is consistent with the lack of slow muscle in regions of zebrafish mutants that lack midline shh expression. This correlation suggests strongly that the reason for the lack of slow muscle in the bozi2 and the tail of ntlb160 mutants is lack of notochord-derived Shh (Concordet et al. 1996). However, the boz gene has not been cloned, so its expression is unknown, and the ntl gene is expressed transiently in presomitic mesoderm, as well as in notochord (Odenthal et al. 1996). This raises the possibility that the lack of slow muscle in these mutants reflects a need for a cell autonomous action of the respective genes in paraxial mesoderm. To address this issue, we overexpressed shh in embryos from bozi2 mutant crosses and examined the resultant animals for slow MyHC expression. Five out of six severely affected boz mutants injected with shh mRNA showed induction of slow MyHC, and suppression of fast MyHC (Fig. 4I,J). Therefore, the bozi2 mutation does not affect the ability of somitic tissue to respond to Shh and form slow muscle. Moreover, even in the complete absence of notochord Shh is sufficient for the formation of slow muscle.
A limited source of Shh is sufficient to rescue slow muscle
One limitation of overexpression of Shh by mRNA injection is that ectopic Shh is expressed in regions of the animal never normally exposed to Shh and at above normal physiological levels. This might perturb signals necessary for muscle development from other embryonic tissues. To examine slow muscle differentiation in response to localized lower levels of shh expression we took advantage of the floating head (flh) mutation. Animals homozygous for flh are defective in notochord maturation because of mutation in a homeobox-containing gene expressed chiefly in the notochord (Halpern et al. 1995; Talbot et al. 1995) and, like ntl embryos, lack notochords and muscle pioneers. However, unlike ntl, flh exhibits transdifferentiation of notochord tissue into muscle (Halpern et al. 1995). We examined flh embryos for muscle differentiation and found that it occurs in an altered location. Cells in the embryonic midline, not those in the adaxial position, are the first to differentiate in flh embryos (Fig. 5A,B). This differentiation is immediately beneath the presumptive floorplate that expresses Shh sporadically (Fig. 5C). Despite the unusual location of these muscle cells, they express slow, but not fast, MyHC (Fig. 5D–F), spread dorsally around the neural tube and ventrally in the midline, and appear able to undergo lateral migration to take up a normal position beneath the ectoderm by 24 hr of development (Fig. 5G–I). Therefore, in flh embryos, apparently normal slow muscle cells differentiate beneath the residual floorplate—the sole remaining location where somitic mesoderm abuts shh-expressing tissue.
Figure 5.

Slow muscle forms beneath the floorplate in flh embryos. (A–C) Differentiated muscle visualized by MyHC-staining in whole-mount 15-somite wild-type (A) or flh (B,C) embryos observed after flat-mounting. Dual labeling for shh mRNA (red; C) and MyHC (green; C) confirmed that the most posterior differentiated muscle was at the midline (arrow, C). (D–I) Transverse cryosections show that differentiated muscle in flh midline mesoderm expresses slow but not fast MyHC at 15 somites (D–F). By 24 hr, flh embryos have a single fused myotome (G), with lateral slow and medial fast muscle (H,I). Despite the ectopic location of formation of slow muscle cells in the midline the slow muscle cells appear to migrate normally so that the only detectable residual slow muscle defect is lack of muscle pioneers. (J,K) Dual labeling for MyHC (green) and ptc1 mRNA (red) in the two most posterior MyHC-containing serial sections of a 15-somite flh embryo. Newly differentiating muscle cells close to the midline do not always express detectable ptc1 (arrows), even though ptc1 is expressed abundantly in the mesoderm underlying residual floor plate (K).
The discontinuous location of shh-expressing cells in the floorplate of flh mutants (Fig. 5C) allows an examination of the relationship between the location of Shh and slow muscle differentiation. At the posterior limit of MyHC-containing cells in flh we found no correlation between the location of remaining floorplate shh expression and medial slow myoblast differentiation. Muscle differentiates both immediately beneath and between islands of shh-expression (Fig. 5C). This data suggested that terminal differentiation of slow muscle is not induced directly by Shh. Further evidence that muscle differentiation, per se, is not induced by Shh came from examining the up-regulation of patched1 (ptc1) mRNA in flh mutant embryos. ptc1, a zebrafish homolog of Drosophila patched, is a Shh receptor (Stone et al. 1996), and is up-regulated adjacent to residual shh expression in flh embryos both at somitic and presomitic anteroposterior positions (Concordet et al. 1996). Therefore, mesodermal cells are first exposed to Shh long before muscle differentiation commences. Moreover, even the most recently differentiated muscle cells in flh embryos frequently do not express high levels of ptc1 mRNA, despite adjacent mesoderm expressing ptc1 abundantly (Fig. 5J,K). Therefore, although Shh induces ptc1 locally along the entire length of the flh embryo, there is a delay after Shh exposure before the appearance of differentiated slow muscle cells. In addition, by the time slow muscle differentiates, any spatial correlation between shh expression and myogenic cells has been lost. Taken together, these data suggest that Shh may initiate slow myoblast formation, but that continued exposure is not required to trigger the terminal differentiation of slow muscle fibers.
Discussion
Shh and slow muscle induction
Several lines of evidence show that adaxial slow muscle formation in zebrafish embryos is controlled by Shh. First, slow muscle differentiation occurs next to the notochord, which expresses shh. This observation confirms and extends the results of Devoto et al. (1996), who observed that adaxial cells give rise to slow muscle markers after they migrate laterally through the somite. Our data show that adaxial cells are already determined to form slow muscle as soon as they differentiate into skeletal muscle myosin-expressing myocytes. Second, a lack of slow muscle correlates with a lack of shh expression in mutant fish. Third, ectopic expression of shh can induce conversion of most, if not all, somitic cells to slow muscle, at the expense of fast muscle. Fourth, even in the absence of notochord, injection of shh mRNA can rescue formation of slow muscle. Fifth, in the absence of the normal Shh signal from notochord, a localized source of Shh correlates with induction of ectopic slow muscle cells, which then migrate in a similar fashion to slow muscle cells in wild-type embryos. These data are supported by the wild-type expression of a Shh receptor, ptc1, that is up-regulated in adaxial cells within presomitic mesoderm indicating that these cells are responding to hedgehog (hh) signaling (Concordet et al. 1996). Taken together, these findings make a strong case for notochord-derived Shh being the normal inducer of the differentiated slow adaxial muscle cell fate in the zebrafish.
Shh induces adaxial slow myoblasts
How might Shh induce the slow muscle fate in zebrafish? Muscle is formed in two steps—mesodermal commitment to the proliferative myoblast, followed by terminal differentiation into the postmitotic muscle fiber. Several lines of evidence suggest that Shh is responsible for induction of slow muscle precursor cells, rather than the terminal differentiation of slow muscle, per se. First, the muscle-specific transcription factor myoD is initially detectable in adaxial precursors located adjacent to shh-expressing cells within the embryonic shield several hours before their terminal differentiation at around the time of somitogenesis (Weinberg et al. 1996). This expression of myoD before terminal differentiation is also detected in adaxial cells at later stages when posterior somites arise from the tail bud. Second, zebrafish mutants like boz and ntl that lack slow muscle, also lack the early adaxial myoD expression, and this correlates with a lack of axial Shh (Fig. 3; Concordet et al. 1996; Odenthal et al. 1996; Weinberg et al. 1996; Schier et al. 1997). Third, Shh signaling can induce premature myoD in lateral presomitic cells (Concordet et al. 1996; Hammerschmidt et al. 1996; Weinberg et al. 1996). We show that these ectopic myoD-expressing cells in lateral somites have other features, such as the direction and timing of their differentiation and sensitivity to additional hh signals (Currie and Ingham 1996), suggesting that the terminal differentiation of these cells into slow muscle is prefigured at the myoblast level. Fourth, our examination of the flh mutant suggests that adaxial myoblasts differentiate into slow muscle fibers independent of their proximity to residual shh-expressing floor plate cells, and independent of their exposure to Shh during the period of terminal differentiation, as assayed by ptc1 expression (Concordet et al. 1996; Marigo and Tabin 1996). Therefore, at early stages MyoD may mark cells that, although not yet differentiated, have become committed to a slow myoblast lineage.
Previous data have suggested that the combined action of notochord-derived Shh and Ehh induces zebrafish muscle pioneer subset of the adaxial slow muscle cells (Currie and Ingham 1996). The data in the present paper demonstrate that Ehh is not required for production of the nonpioneer adaxial slow muscle cells. Ehh is not expressed in notochord of ntlb160(Currie and Ingham 1996), yet nonpioneer slow muscle cells form and migrate normally in the anterior of ntlb160 embryos where Shh alone is expressed (Fig. 3). Similarly, in flh mutants, which lack notochord and ehh expression (Currie and Ingham 1996), apparently normal nonpioneer adaxial cells are formed ectopically close to residual floorplate Shh. Moreover, Ehh does not appear able to substitute for Shh in the induction of nonpioneer adaxial cells as injection of ehh mRNA into wild-type embryos did not induce ectopic slow MyHC. These data support the hypothesis that in vivo Shh and Ehh serve distinct roles.
The finding that Shh induces slow myoblasts suggests a new view of the steps of muscle differentiation that contrasts with the traditional model in which somitic cells first become myoblasts and only subsequently specialize into one particular myoblast subclass. We suggest, as summarized in Figure 6, that the decision whether to form one type of muscle or another is made concurrently with myoblast commitment to the muscle lineage. This scheme concurs with conclusions from analysis of the you-type zebrafish mutants (van Eeden et al. 1996). Such a view also fits well with studies in Drosophila demonstrating that distinct extracellular signals serve to commit each founder myoblast to a particular muscle type (Baylies et al. 1995). However, it is possible that presomitic cells could be committed to myogenesis before myoD expression. Although MyoD is the first MRF to be expressed in birds, Myf-5 is the earliest MRF to appear at high levels in mammalian somites (Ott et al. 1991), and Pax-3 can induce myogenesis (Maroto et al. 1997; Tajbakhsh et al. 1997). Furthermore, whether all myoblasts are committed to form particular types of muscle from their inception is unclear. Whatever the case, our data show that Shh induces adaxial myoblasts that adopt a slow muscle fate.
Figure 6.

Three phases of zebrafish muscle development. A model in which presomitic mesoderm gains competence to respond to Shh early in development in wild-type zebrafish. The result of exposure to notochord-derived Shh at this stage is a slow myoblast phenotype involving myoD expression and up-regulation of ptc1 (orange). However, a second event, either intrinsically timed within the slow myoblast, or in the form of a signal from outside of the somite (indicated by ? on the diagram, but unlikely to be either Shh or notochord-derived), is required for the slow myoblast to differentiate into a fiber (red). Simultaneously, the MyoD-positive myoblast state in lateral somitic cells arises independent of notochord-derived signals (yellow). Later, adaxial slow fiber migration and concurrent differentiation of lateral myoblasts into fast muscle (green) is timed by a third event(s). Fast fiber differentiation is independent of slow fiber migration, although the converse may not be true.
Zebrafish fast muscle formation
Shh is not necessary for fast muscle formation. boz fish that lack Shh produce abundant fast muscle throughout the somite. Moreover, the normal myoD-expressing myoblasts stripes across the posterior border of the somite form at the normal time just before somitogenesis in embryos that lack shh expression (Odenthal et al. 1996). Therefore, in zebrafish, MyoD may mark commitment to a myoblast fate irrespective of the type of myoblast formed. We find that cells that are initially lateral within the somite differentiate into fast muscle. They may be committed to formation of fast muscle from the inception of myoD expression.
Timing of myoblast differentiation
We show that ectopic Shh can induce premature muscle differentiation in the lateral somite. However, premature differentiation was slow, rather than fast, and was only observed at the normal time of slow adaxial cell differentiation. Therefore, no somite cells are competent to differentiate in response to Shh until around the time of somitogenesis, even though myoD is expressed earlier. This may explain why slow muscle does not appear earlier in development even though shh is expressed in the developing notochord from gastrulation onwards (Krauss et al. 1993), and is presumably secreted because ptc1, a marker of Shh exposure, is expressed highly in adjacent presomitic cells (Concordet et al. 1996). Two alternative models could explain the delay between MyoD and slow myosin expression (Fig. 6). In one model, the delay is caused by an intrinsically timed maturation of the somitic cells. Although cell division is not extensive in zebrafish somites (Kimmel and Warga 1987), Shh might induce myoblasts committed to division followed by differentiation as it can be a somitic mitogen (Fan et al. 1995). Mammalian myoblasts show such behavior in vitro (Quinn et al. 1985), which is reminiscent of the induction of division followed by terminal differentiation in Drosophila lamina ganglia neurons in response to retinal neuron-derived hh (Huang and Kunes 1996). Alternatively, in the second model, extracellular signals may control terminal differentiation. In amniotes, other signals can cooperate with Shh to regulate myogenesis (Munsterberg et al. 1995; Stern et al. 1995; Pourquie et al. 1996), and a variety of growth factors repress myoblast differentiation in culture. Ventral axial structures are unlikely sources of such signals as Shh is sufficient to induce slow MyHC in boz embryos that fail to form notochord or floor plate. Regardless of the mechanism by which the timing of terminal adaxial slow muscle differentiation is controlled, our data show that similar mechanisms can operate in the lateral somite to control ectopic slow muscle differentiation in response to Shh.
Evolutionary conservation of muscle patterning
We found that in fish embryos the first skeletal muscle fibers to form are slow from the time of their inception. Later, a second wave of fibers, which ultimately constitute the majority of all fibers, differentiate as fast muscle. In amniote limbs muscle fibers also form in two waves, an early primary population that express slow (and embryonic) myosin and later secondary cells that, forming in close association with primary fibers, express fast (and embryonic) myosin from their inception (Kelly and Rubinstein 1980; Vivarelli et al. 1988; Cho et al. 1994). We suspect that zebrafish embryos may also express an embryonic myosin in both slow and fast fibers as the immunoreaction with our all-myosin antibody was stronger than with the specific slow and fast antibodies. Moreover, both adaxial and nonadaxial cells react from their inception with an antibody that detects embryonic myosin (Devoto et al. 1996). These analogies suggest that adaxial and nonadaxial somitic muscle cells in the zebrafish may be evolutionary homologs of amniote primary and secondary muscle fiber generations. Amniote secondary fibers form overlying the neuromuscular junctions of primary fibers and it has been suggested that signals from the forming neuromuscular junction region may be required to initiate secondary fiber formation (Duxson et al. 1989). This is not the case in the zebrafish as absence of differentiated slow primary fibers does not prevent differentiation of fast muscle despite the striking correlation between the lateral migration of slow fibers and the differentiation of fast fibers. The converse relationship, that fast fiber differentiation might cause slow fiber migration, remains a possibility. Nevertheless, the close similarities between fish and amniote fiber generation suggest that the common ancestor had two steps of muscle patterning: early fibers being slow and later fast.
There are further analogies between amniote and fish myogenesis. Amniote primary fibers are of several distinct fiber types that prefigure later muscle characteristics (Crow and Stockdale 1986), even though all express some form of slow MyHC (Kelly and Rubinstein 1980; Vivarelli et al. 1988; Page et al. 1992; Hughes et al. 1993). Slow adaxial cells in the zebrafish are also composed of two subpopulations, the muscle pioneer cells which express engrailed, and the nonpioneer adaxials. Engrailed proteins also mark a subpopulation of muscle cells in the jaw muscle of the zebrafish (Hatta et al. 1990). The data reported in the present paper, together with the previous findings that a second notochord-derived signal (Halpern et al. 1993), provided by Ehh (Currie and Ingham 1996), is responsible for regulating the formation of muscle pioneer cells, suggest that hh signaling molecules may regulate the diversity of muscle fiber types formed in the early fish embryo. Banded hedgehog is also expressed in particular regions of the Xenopus somite (Ekker et al. 1995). Whether similar signals control muscle patterning in amniotes remains to be determined.
Hedgehogs and vertebrate myogenesis
Secretion of Shh from notochord has been shown to induce floorplate markers in anterior (although not posterior) zebrafish central nervous system (CNS) and both floorplate and motoneurons in amniote neural tube (Echelard et al. 1993; Krauss et al. 1993; Roelink et al. 1994; Ericson et al. 1996). The role of Shh in somite patterning has been less clear. In amniotes, in which much of the somite becomes sclerotome, either ectopic notochord or shh-expressing cells can induce extra sclerotome at the expense of dermomyotome markers (Fan and Tessier-Lavigne 1994). Conversely, shh−/− mice have deficits in sclerotomal derivatives (Chiang et al. 1996). On the other hand, in both chick and zebrafish, overexpression of shh induces ectopic myoD expression, suggesting a myogenic action (Johnson et al. 1994; Concordet et al. 1996; Weinberg et al. 1996). Moreover, shh−/− mice show defects in medial muscle formation (Chiang et al. 1996) and notochord can induce avian myogenesis (Pownall et al. 1996). So Shh may regulate formation of both ventral and more dorsal somitic tissues. Action of Shh at distinct concentrations or times (Ericson et al. 1996), or in collaboration with other factors (Munsterberg et al. 1995; Stern et al. 1995; Pourquie et al. 1996), could determine the outcome of Shh signaling.
Induction of distinct myoblast types and the subsequent control of their terminal differentiation may account for the numerous signals capable of influencing somite myogenesis. If equivalents of Shh-dependent adaxial cells exist in amniotes, we would expect that particular muscle markers are not distributed uniformly between distinct muscle cell types in the developing dermomyotome. In amniotes, MRFs are the earliest known definitive myogenic markers. Expression of at least one MRF is obligatory for myogenesis in mice (Rudnicki et al. 1993). MRFs are expressed at low levels in presomitic mesoderm, which has the capacity to form muscle in dissociated cell culture (George-Weinstein et al. 1994; Lin-Jones and Hauschka 1996). However, two myoblast populations arise with distinct temporal and spatial patterns within the dermomyotome: The first initially expresses myf-5 in medial regions and the second myoD in lateral regions (Cossu et al. 1996a; Maroto et al. 1997; Tajbakhsh et al. 1997). That shh−/−mice have reduced expression of medial myf-5 but no detectable change in lateral myoD expression (Chiang et al. 1996) suggests a role for Shh in induction of the medial population. Inhibitory signals, such as BMP4 (Fan and Tessier-Lavigne 1994; Cossu et al. 1996b; Pourquie et al. 1996), may function in vivo to suppress overt myogenic phenotypes in the lateral compartment that generates limb and body wall muscle and may have no homologous process in most zebrafish somites. So generation of further diversity within the dorsomedial myogenic compartment could be a role of Shh in amniote myogenesis. Distinct populations of slow and fast fibers may be present in amniote myotome (Dhoot 1994). In this paper, we have shown that in zebrafish, Shh regulates formation of myotomal slow muscle. Much slow muscle in amniote limbs is located near developing bone that expresses indian hedgehog (Bitgood and McMahon 1995; Vortkamp et al. 1996). Moreover, motoneurons, which strongly influence muscle development, can express shh (Bitgood and McMahon 1995; Stone et al. 1996), raising the possibility that diverse hh proteins may regulate muscle fiber diversification.
Materials and methods
Zebrafish lines and maintenance
Wild-type and heterozygote mutant breeding fish were maintained at 28.5°C on a 14-hr/10-hr light cycle. We obtained flhn1 from the University of Newcastle-upon-Tyne, ntlb160 from the University of Oregon, and bozi2 was isolated in the Ingham laboratory (P.D. Currie., T. Schilling, G. Bergemann, and P.W. Ingham, unpubl.). bozi2 fish exhibit a variable phenotype with defects ranging from reduced notochords to a severe lack of axial mesoderm at all rostro–caudal levels. Of 142 progeny of a heterozygous cross examined at F2–F4, 20 (13%) showed complete absence of eyes and notochord. A further 25 (17%) showed a partial phenotype with variable eyes and the anterior half of the notochord missing. These, and a number of other aspects of the phenotype, are strongly reminiscent of boz mutant fish (Solnica-Krezel et al. 1996). Complementation analysis by a cross of heterozygous bozi2 with bozm168 has shown reduced eyes and notochord in 3 out of 30 progeny. We therefore tentatively conclude that these genes are allelic. However, because of the incomplete penetrance of boz, definitive demonstration of allelism awaits the mapping of the mutation. Embryos were collected by natural spawning and staged by anatomical markers according to Westerfield (1995). Prim-5 stage embryos are referred to as 24 hr.
RNA injection
RNA injections were performed as described (Currie and Ingham 1996).
Immunohistochemistry
The slow and fast MyHC antigens are destroyed by aldehyde fixatives, so embryos were fixed by incubating for 5 min each in graded methanols, rehydrated in 0.1% Tween 20, serially cryosectioned, and stained. However, preservation of younger embryos was better after staining in whole-mount, followed by post-fixation in 4% paraformaldehyde for 4 hr at 4°C before cryosectioning. Primary monoclonal antibody supernatants of A4.1025 (Dan-Goor et al. 1990) and BA-D5 (Schiaffino et al. 1989) were diluted 1:10. EB165 monoclonal ascites was used at 1:5000 (Gardahaut et al. 1992). First antibodies were detected with biotin-conjugated horse-derived anti-mouse IgG (Vector), Vectastain ABC Elite Peroxidase kit (Vector), and visualized using 0.5 mg/ml of diaminobenzidine with (black stains) or without (brown stains) 0.03% CoCl2 enhancement. Cryosections for dual immunofluorescence had IgG first antibodies detected with Cappell goat anti-mouse IgG (γ-specific) Texas red. After a mouse IgG block, biotinylated BA-D5, prepared using Pierce NHS-Biotin reagent, was detected with Dako streptavidin-FITC. Sections were mounted in 150 mg/ml of polyvinyl alcohol, 30% glycerol PBS with DABCO antifade, and photographed by confocal microscopy.
Western blots
Embryos were dechorionated, deyolked, and homogenized manually on ice for 10 min in 63 mm Tris-HCl (pH 6.8), 10% glycerol, 5% β-mercaptoethanol, 3.5% SDS, 0.2 mm PMSF, 0.5 μm aprotinin, and 0.5 μm leupeptin. Samples were microcentrifuged for 5 min at 4°C, 0.01% bromophenol blue added to the supernatant, the equivalent of 10 embryos run on each lane of a 7.5% acrylamide denaturing gel at 200 mV for 30 min, and electroblotted onto nitrocellulose (Amersham). Purified bovine cardiac myosin was a kind gift of Dr. John Sleep (The Randall Institute, London, UK). Nitrocellulose strips were blocked in 5% milk powder PBS/Az overnight, washed, and incubated with A4.1025 (1:10), BA-D5 (1:10), F1.652 [1:10; Webster et al. 1988)], or EB165 (1:250) for 2 hr at room temperature. After washing, primary antibody was detected with horseradish peroxidase-conjugated sheep anti-mouse IgG F(ab)2 and an ECL kit (Amersham).
Acknowledgments
We thank Steve Wilson and Nigel Holder for fish from their BBSRC-funded facility, Everett Bandman for EB165 ascites, John Sleep for bovine cardiac myosin, and Steve Devoto, Monte Westerfield, and Wolfgang Driever for showing us manuscripts before publication. We thank Steve Wilson, Patricia Salinas, and members of our laboratories for helpful comments on the manuscript, and T. Schilling and G. Bergemann for participation in the mutant screen. This work is supported by the Medical Research Council (S.M.H. and C.S.B.), the Imperial Cancer Research Fund, and a Human Frontier Science Program grant (P.W.I.).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL s.hughes@kcl.ac.uk; FAX 44-171 497 9078.
References
- Baylies MK, Martinez-Arias A, Bate M. wingless is required for the formation of a subset of muscle founder cells during Drosophila embryogenesis. Development. 1995;121:3829–3837. doi: 10.1242/dev.121.11.3829. [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol. 1995;172:126–138. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- Bober E, Brand-Saberi B, Ebensperger C, Wilting J, Balling R, Paterson BM, Arnold H-H, Christ B. Initial steps of myogenesis in somites are independant of influence from axial structures. Development. 1994;120:3073–3082. doi: 10.1242/dev.120.11.3073. [DOI] [PubMed] [Google Scholar]
- Buffinger N, Stockdale FE. Myogenic specification of somites is mediated by diffusible factors. Dev Biol. 1995;169:96–108. doi: 10.1006/dbio.1995.1130. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Cho M, Hughes SM, Karsch-Mizrachi I, Travis M, Leinwand LA, Blau HM. Fast myosin heavy chains expressed in secondary mammalian muscle fibres at the time of their inception. J Cell Sci. 1994;107:2361–2371. doi: 10.1242/jcs.107.9.2361. [DOI] [PubMed] [Google Scholar]
- Concordet J-P, Lewis KE, Moore JW, Goodrich LV, Johnson RL, Scott MP, Ingham PW. Spatial regulation of a zebrafish patched homologue reflects the roles of sonic hedgehog and protein kinase A in neural tube and somite patterning. Development. 1996;122:2835–2846. doi: 10.1242/dev.122.9.2835. [DOI] [PubMed] [Google Scholar]
- Cossu G, Kelly R, Tajbakhsh S, Di Donna S, Vivarelli E, Buckingham M. Activation of different myogenic pathways: myf-5 is induced by the neural tube and MyoD by the dorsal ectoderm in mouse paraxial mesoderm. Development. 1996a;122:429–437. doi: 10.1242/dev.122.2.429. [DOI] [PubMed] [Google Scholar]
- Cossu G, Tajbakhsh S, Buckingham M. How is myogenesis initiated in the embryo? Trends Genet. 1996b;12:218–222. doi: 10.1016/0168-9525(96)10025-1. [DOI] [PubMed] [Google Scholar]
- Crow MT, Stockdale FE. Myosin expression and specialization among the earliest muscle fibres of the developing avian limb. Dev Biol. 1986;113:238–254. doi: 10.1016/0012-1606(86)90126-0. [DOI] [PubMed] [Google Scholar]
- Currie PD, Ingham PW. Induction of a specific muscle cell type by a hedgehog-like protein in zebrafish. Nature. 1996;382:452–455. doi: 10.1038/382452a0. [DOI] [PubMed] [Google Scholar]
- Dan-Goor M, Silberstein L, Kessel M, Muhlrad A. Localization of epitopes and functional effects of two novel monoclonal antibodies against skeletal muscle myosin. J Muscle Res Cell Motil. 1990;11:216–226. doi: 10.1007/BF01843575. [DOI] [PubMed] [Google Scholar]
- Devoto SH, Melancon E, Eisen JS, Westerfield M. Identification of separate slow and fast muscle precursor cells in vivo, prior to somite formation. Development. 1996;122:3371–3380. doi: 10.1242/dev.122.11.3371. [DOI] [PubMed] [Google Scholar]
- Dhoot GK. Mammalian myoblasts become fast or slow myocytes within the somitic myotome. J Muscle Res Cell Motil. 1994;15:617–622. doi: 10.1007/BF00121069. [DOI] [PubMed] [Google Scholar]
- DiMario JX, Fernyak SE, Stockdale FE. Myoblasts transferred to the limbs of embryos are committed to specific fibre fates. Nature. 1993;362:165–167. doi: 10.1038/362165a0. [DOI] [PubMed] [Google Scholar]
- Duxson MJ, Usson Y, Harris AJ. The origin of secondary myotubes in mammalian skeletal muscles: Ultrastructural studies. Development. 1989;107:743–750. doi: 10.1242/dev.107.4.743. [DOI] [PubMed] [Google Scholar]
- Echelard Y, Epstein DJ, St.-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signalling molecules implicated in regulation of CNS and limb polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- Ekker SC, McGrew LL, Lai CJ, Lee JJ, Von Kessler DP, Moon RT, Beachy PA. Distinct expression and shared activities of members of the hedgehog gene family of Xenopus laevis. Development. 1995;121:2337–2347. doi: 10.1242/dev.121.8.2337. [DOI] [PubMed] [Google Scholar]
- Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87:661–673. doi: 10.1016/s0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- Fan C-M, Tessier-Lavigne M. Patterning of mammalian somites by surface ectoderm and notochord: Evidence for sclerotome induction by a hedgehog homolog. Cell. 1994;79:1175–1186. doi: 10.1016/0092-8674(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Fan C-M, Porter JA, Chiang C, Chang DT, Beachy PA, Tessier-Lavigne M. Long-range sclerotome induction by sonic hedgehog: Direct role of the amino-terminal cleavage product and modulation by the cAMP signalling pathway. Cell. 1995;81:457–465. doi: 10.1016/0092-8674(95)90398-4. [DOI] [PubMed] [Google Scholar]
- Felsenfeld AL, Curry M, Kimmel CB. The fub-1 mutation blocks initial myofibril formation in zebrafish muscle pioneer cells. Dev Biol. 1991;148:23–30. doi: 10.1016/0012-1606(91)90314-s. [DOI] [PubMed] [Google Scholar]
- Gamel AJ, Brand-Saberi B, Christ B. Halves of epithelial somites and segmental plate show distinct muscle differentiation behavior in vitro compared to entire somites and segmental plate. Dev Biol. 1995;172:625–639. doi: 10.1006/dbio.1995.8028. [DOI] [PubMed] [Google Scholar]
- Gardahaut MF, Fontaine-Perus J, Rouaud T, Bandman E, Ferrand R. Developmental modulation of myosin expression by thyroid hormone in avian skeletal muscle. Development. 1992;115:1121–1131. doi: 10.1242/dev.115.4.1121. [DOI] [PubMed] [Google Scholar]
- George-Weinstein M, Gerhart JV, Foti GJ, Lash JW. Maturation of myogenic and chondrogenic cells in the presomitic mesoderm of the chick embryo. Exp Cell Res. 1994;211:263–274. doi: 10.1006/excr.1994.1086. [DOI] [PubMed] [Google Scholar]
- Goulding M, Lumsden A, Paquette AJ. Regulation of Pax-3 expression in the dermamyotome and its role in muscle development. Development. 1994;120:957–971. doi: 10.1242/dev.120.4.957. [DOI] [PubMed] [Google Scholar]
- Halpern ME, Ho RK, Walker C, Kimmel CB. Induction of muscle pioneers and floor plate is distinguished by the zebrafish no tail mutation. Cell. 1993;75:99–111. [PubMed] [Google Scholar]
- Halpern ME, Thisse C, Ho RK, Thisse B, Riggleman B, Trevarrow B, Weinberg ES, Postlethwait JH, Kimmel CB. Cell-autonomous shift from axial to paraxial mesodermal development in zebrafish floating head mutants. Development. 1995;121:4257–4264. doi: 10.1242/dev.121.12.4257. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Bitgood MJ, McMahon AP. Protein kinase A is a common negative regulator of hedgehog signalling in the vertebrate embryo. Genes & Dev. 1996;10:647–658. doi: 10.1101/gad.10.6.647. [DOI] [PubMed] [Google Scholar]
- Hatta K, Schilling TF, BreMiller RA, Kimmel CB. Specification of jaw muscle identity in zebrafish: Correlation with engrailed-homeoprotein expression. Science. 1990;250:802–805. doi: 10.1126/science.1978412. [DOI] [PubMed] [Google Scholar]
- Hatta K, Bremiller R, Westerfield M, Kimmel CB. Diversity of expression of engrailed-like antigens in zebrafish. Development. 1991;112:821–832. doi: 10.1242/dev.112.3.821. [DOI] [PubMed] [Google Scholar]
- Holland LZ, Pace DA, Blink ML, Kene M, Holland ND. Sequence and expression of amphioxus alkali myosin light chain (AmphiMLC-alk) throughout development: Implications for vertebrate myogenesis. Dev Biol. 1995;171:665–676. doi: 10.1006/dbio.1995.1313. [DOI] [PubMed] [Google Scholar]
- Huang Z, Kunes S. Hedgehog, transmitted along retinal axons, triggers neurogenesis in the developing visual centers of the Drosophila brain. Cell. 1996;86:411–422. doi: 10.1016/s0092-8674(00)80114-2. [DOI] [PubMed] [Google Scholar]
- Hughes SM, Blau HM. Muscle fiber pattern is independent of cell lineage in postnatal rodent development. Cell. 1992;68:659–671. doi: 10.1016/0092-8674(92)90142-y. [DOI] [PubMed] [Google Scholar]
- Hughes SM, Cho M, Karsch-Mizrachi I, Travis M, Silberstein L, Leinwand LA, Blau HM. Three slow myosin heavy chains sequentially expressed in developing mammalian skeletal muscle. Dev Biol. 1993;158:183–199. doi: 10.1006/dbio.1993.1178. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Laufer E, Riddle RD, Tabin C. Ectopic expression of Sonic hedgehog alters dorsal-ventral patterning of somites. Cell. 1994;79:1165–1173. doi: 10.1016/0092-8674(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Rubinstein NA. Why are fetal muscles slow? Nature. 1980;288:266–269. doi: 10.1038/288266a0. [DOI] [PubMed] [Google Scholar]
- Kenny-Mobbs T, Thorogood P. Autonomy of differentiation in avian brachial somites and the influence of adjacent tissues. Development. 1987;100:449–462. doi: 10.1242/dev.100.3.449. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Warga RM. Cell lineages generating axial muscle in the zebrafish embryo. Nature. 1987;327:234–237. doi: 10.1038/327234a0. [DOI] [PubMed] [Google Scholar]
- Krauss S, Concordet J-P, Ingham PW. A functionally conserved homologue of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993;75:1431–1444. doi: 10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- Lassar AB, Munsterberg AE. The role of positive and negative signals in somite patterning. Curr Opin Neurobiol. 1996;6:57–63. doi: 10.1016/s0959-4388(96)80009-2. [DOI] [PubMed] [Google Scholar]
- Lin-Jones J, Hauschka SD. Myogenic determination factor expression in the developing avian limb bud: An RT-PCR analysis. Dev Biol. 1996;174:407–422. doi: 10.1006/dbio.1996.0084. [DOI] [PubMed] [Google Scholar]
- Marigo V, Tabin CJ. Regulation of patched by sonic hedgehog in the developing neural tube. Proc Natl Acad Sci. 1996;93:9346–9351. doi: 10.1073/pnas.93.18.9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroto M, Reshef R, Münsterberg AE, Koester S, Goulding M, Lassar AB. Ectopic Pax-3 activates MyoD and Myf-5 expression in embryonic mesoderm and neural tissue. Cell. 1997;89:139–148. doi: 10.1016/s0092-8674(00)80190-7. [DOI] [PubMed] [Google Scholar]
- Marti E, Takada R, Bumcrot DA, Sasaki H, McMahon AP. Distribution of Sonic hedgehog peptides in the developing chick and mouse embryo. Development. 1995;121:2537–2547. doi: 10.1242/dev.121.8.2537. [DOI] [PubMed] [Google Scholar]
- Miller JB, Stockdale FE. Developmental origins of skeletal muscle fibres: Clonal analysis of myogenic cell lineages based on expression of fast and slow myosin heavy chains. Proc Natl Acad Sci. 1986;83:3860–3864. doi: 10.1073/pnas.83.11.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin-Kensicki EM, Eisen JS. Sclerotome development and peripheral nervous system segmentation in embryonic zebrafish. Development. 1997;124:159–167. doi: 10.1242/dev.124.1.159. [DOI] [PubMed] [Google Scholar]
- Munsterberg AE, Kitajewski J, Bumcrot DA, McMahon AP, Lassar AB. Combinatorial signaling by sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes & Dev. 1995;9:2911–2922. doi: 10.1101/gad.9.23.2911. [DOI] [PubMed] [Google Scholar]
- Odenthal J, Haffter P, Vogelsang E, Brand M, Van Eeden FJM, Furutani-Seiki M, Granato M, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Warga RM, Allende ML, Weinberg ES, Nüsslein-Volhard C. Mutations affecting the formation of the notochord in the zebrafish, Danio rerio. Development. 1996;123:103–115. doi: 10.1242/dev.123.1.103. [DOI] [PubMed] [Google Scholar]
- Ott M-O, Bober E, Lyons G, Arnold H, Buckingham M. Early expression of the myogenic regulatory gene, myf-5, in precursor cells of skeletal muscle in the mouse embryo. Development. 1991;111:1097–1107. doi: 10.1242/dev.111.4.1097. [DOI] [PubMed] [Google Scholar]
- Page S, Miller JB, DiMario JX, Hager EJ, Moser A, Stockdale FE. Developmentally regulated expression of three slow isoforms of myosin heavy chain: Diversity among the first fibers to form in avian muscle. Dev Biol. 1992;154:118–128. doi: 10.1016/0012-1606(92)90053-j. [DOI] [PubMed] [Google Scholar]
- Pownall ME, Strunk KE, Emerson CP., Jr Notochord signals control the transcriptional cascade of myogenic bHLH genes in somites of quail embryos. Development. 1996;122:1475–1488. doi: 10.1242/dev.122.5.1475. [DOI] [PubMed] [Google Scholar]
- Pourquie O, Coltey M, Teillet M-A, Ordahl C, Le Douarin NM. Control of dorsoventral patterning of somitic derivatives by notochord and floor plate. Proc Natl Acad Sci. 1993;90:5242–5246. doi: 10.1073/pnas.90.11.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourquie O, Fan C-M, Coltey M, Hirsinger E, Watanabe Y, Breant C, Francis-West P, Brickell P, Tessier-Lavigne M, Le Douarin NM. Lateral and axial signals involved in avian somite patterning: A role for BMP4. Cell. 1996;84:461–471. doi: 10.1016/s0092-8674(00)81291-x. [DOI] [PubMed] [Google Scholar]
- Quinn LS, Holtzer H, Nameroff M. Generation of chick skeletal muscle cells in groups of 16 from stem cells. Nature. 1985;313:692–694. doi: 10.1038/313692a0. [DOI] [PubMed] [Google Scholar]
- Roelink H, Augsburger A, Heemskerk J, Korzh V, Norlin S, Ruiz i Altaba A, Tanabe Y, Placzek M, Edlund T, Jessell TM, Dodd J. Floor plate and motor neuron induction by vhh-1, a vertebrate homologue of hedgehog expressed by the notochord. Cell. 1994;76:761–775. doi: 10.1016/0092-8674(94)90514-2. [DOI] [PubMed] [Google Scholar]
- Rong PM, Teillet A-M, Ziller C, Le Douarin NM. The neural tube/notochord complex is necessary for vertebral but not limb and body wall striated muscle differentiation. Development. 1992;115:657–672. doi: 10.1242/dev.115.3.657. [DOI] [PubMed] [Google Scholar]
- Rudnicki MA, Schnegelsberg PNJ, Stead RH, Braun T, Arnold H-H, Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–1359. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- Schafer DA, Miller JB, Stockdale FE. Cell diversification within the myogenic lineage: In vitro generation of two types of myoblasts from a single myogenic progenitor cell. Cell. 1987;48:659–670. doi: 10.1016/0092-8674(87)90244-3. [DOI] [PubMed] [Google Scholar]
- Schier AF, Neuhauss SCF, Helde KA, Talbot WS, Driever W. The one-eyed pinhead gene functions in mesoderm and endoderm formation in zebrafish and interacts with no tail. Development. 1997;124:327–342. doi: 10.1242/dev.124.2.327. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Gundersen K, Lomo T. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989;10:197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, van Eeden FJM, Halpern ME, Kimmel CB, Nusslein-Volhard C. no tail (ntl) is the zebrafish homologue of the mouse T (Brachyury) gene. Development. 1994;120:1009–1015. doi: 10.1242/dev.120.4.1009. [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L, Stemple DL, Mountcastle-Shah E, Rangini Z, Neuhauss SCF, Malicki J, Schier AF, Stainier DYR, Zwartkruis F, Abdelilah S, Driever W. Mutations affecting cell fates and cellular rearrangements during gastrulation in zebrafish. Development. 1996;123:67–80. doi: 10.1242/dev.123.1.67. [DOI] [PubMed] [Google Scholar]
- Stern HM, Hauschka SD. Neural tube and notochord promote in vitro myogenesis in single somite explants. Dev Biol. 1995;167:87–103. doi: 10.1006/dbio.1995.1009. [DOI] [PubMed] [Google Scholar]
- Stern HM, Brown AMC, Hauschka SD. Myogenesis in paraxial mesoderm: Preferential induction by dorsal neural tube and by cells expressing Wnt-1. Development. 1995;121:3675–3686. doi: 10.1242/dev.121.11.3675. [DOI] [PubMed] [Google Scholar]
- Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL, Scott MP, Pennica D, Goddard A, Phillips H, Noll M, Hooper JE, De Sauvage F, Rosenthal A. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature. 1996;384:129–134. doi: 10.1038/384129a0. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, Rocancourt D, Cossu G, Buckingham M. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell. 1997;89:127–138. doi: 10.1016/s0092-8674(00)80189-0. [DOI] [PubMed] [Google Scholar]
- Talbot WB, Trevarrow B, Halpern ME, Melby AE, Farr G, Postlethwait TJ, Kimmel CB, Kimmelman D. A homeobox gene essential for zebrafish notochord development. Nature. 1995;378:150–157. doi: 10.1038/378150a0. [DOI] [PubMed] [Google Scholar]
- Teillet M-A, Le Douarin NM. Consequences of neural tube and notochord excision on the development of the peripheral nervous system in chick embryos. Dev Biol. 1983;98:192–211. doi: 10.1016/0012-1606(83)90349-4. [DOI] [PubMed] [Google Scholar]
- Ticho BS, Stainier DYR, Fishman MC. Three zebrafish MEF2 genes delineate somitic and cardiac muscle development in wild-type and mutant embryos. Mech Dev. 1996;59:205–218. doi: 10.1016/0925-4773(96)00601-6. [DOI] [PubMed] [Google Scholar]
- van Eeden FJM, Granato M, Schach U, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang Y-J, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Warga RM, Allende ML, Weinberg ES, Nüsslein-Volhard C. Mutations affecting somite formation and patterning in the zebrafish, Danio rerio. Development. 1996;123:153–164. doi: 10.1242/dev.123.1.153. [DOI] [PubMed] [Google Scholar]
- van Raamsdonk W, Pool CW, te Kronnie G. Differentiation of muscle fibre types in the teleost Brachydanio rerio. Anat Embryol. 1978;153:137–155. doi: 10.1007/BF00343370. [DOI] [PubMed] [Google Scholar]
- Vivarelli E, Brown WE, Whalen RG, Cossu G. The expression of slow myosin during mammalian somitogenesis and limb bud differentiation. J Cell Biol. 1988;107:2191–2197. doi: 10.1083/jcb.107.6.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian Hedgehog and PTH-related protein. Science. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- Webster C, Silberstein L, Hays AP, Blau HM. Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell. 1988;52:503–513. doi: 10.1016/0092-8674(88)90463-1. [DOI] [PubMed] [Google Scholar]
- Weinberg ES, Allende ML, Kelly CS, Abdelhamid A, Murakami T, Andermann P, Doerre OG, Grunwald DJ, Riggleman B. Developmental regulation of zebrafish MyoD in wild-type, no tail, and spadetail embryos. Development. 1996;122:271–280. doi: 10.1242/dev.122.1.271. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book. Eugene, OR: University of Oregon Press; 1995. [Google Scholar]


