Abstract
Acute lung injury (ALI) is a prevalent disease associated with high mortality. 12/15 lipoxygenase (12/15-LO) is an enzyme producing 12-hydroxy-eicosatetraenoic acid (HETE) and 15-HETE from arachidonic acid. To test whether 12/15-LO is involved in increasing vascular permeability in the lung, we investigated the role of 12/15-LO in murine models of lipopolysaccharide (LPS)-induced pulmonary inflammation and clinically relevant acid-induced ALI. The vascular permeability increase upon LPS inhalation was abolished in Alox15−/− mice lacking 12/15-LO and in WT mice after pharmacological blockade of 12/15-LO. Alox15−/− mice also showed improved gas exchange, reduced permeability increase, and prolonged survival in the acid-induced ALI model. Bone marrow chimeras and reconstitution experiments revealed that 12-HETE produced by hematopoietic cells regulates vascular permeability through a CXCR2-dependent mechanism. Our findings suggest that 12/15-LO-derived 12-HETE is a key mediator of vascular permeability in acute lung injury.
Keywords: lipid mediators, vascular permeability, acute lung injury
Introduction
Acute lung injury (ALI) is a common disease with an incidence of 79 per 100,000 person-years in the United States (1). Despite improved treatment, this disease is associated with a high mortality of up to 38%. The 3.6 million hospital days per year associated with ALI have a large impact on the health system (1). ALI has intrapulmonary causes like pneumonia and aspiration of gastric content, and extrapulmonary causes like sepsis and massive transfusion. In ALI, the alveolar capillary and epithelial membranes are damaged by infiltration of polymorphonuclear neutrophils and monocytes, leading to leakage of protein-rich edema fluid into the alveolar space, formation of hyaline membranes and impaired gas exchange (2). Currently available treatments are only marginally effective (3).
Tissue injury of many etiologies changes vascular barrier function and consequently leads to fluid loss, edema, and organ dysfunction (4-7). The vascular endothelium presents the predominant barrier to prevent movement of molecules across the blood vessel wall (5, 8). Under physiological conditions, vascular permeability is tightly regulated. In response to different inflammatory stimuli, the increase of endothelial permeability is regulated by several receptors including the chemokine receptor CXCR2, the adenosine receptor A2B, and the thromboxane A2 receptor (7, 9, 10). Sphingosine 1-phosphate (S1P), a biologically active lipid, also regulates endothelial cell activation and vascular permeability by binding to several G-protein coupled receptors (11, 12).
In addition to chemokines, lipid mediators play a role in pulmonary inflammation (13-15). Lipoxygenases incorporate oxygen into unsaturated fatty acids and are named according to the position of the carbon double bonds they oxidize (16). Humans and rabbits express 15-lipoxygenase (15-LO) (17, 18), whereas pigs, rats, and mice express “leukocyte-type” 12-lipoxygenase (12-LO) (19) with some 15-LO activity. Mouse leukocyte 12/15-LO is highly related to 15-LO in humans and probably represents the mouse orthologue of human 15-LO (20, 21). 12/15-LO catalysis of arachidonic acid yields short-lived peroxidized products, which are reduced or enzymatically converted to 12-hydroxyeicosatetraenoic acid (12-HETE), lipoxins, hepoxilins, and others (22). 12/15-lipoxygenase mRNA expression is highest in monocytes and macrophages (23), and some is found in endothelial cells (24) and other cells. 12-HETE is a pro-inflammatory chemoattractant for neutrophils (25), regulates endothelial cell cytoskeleton rearrangement (26), induces cytokine production (27), expression of adhesion molecules on endothelial cells (28), and is involved in chronic inflammatory processes (23). Alox15−/− mice were found to be protected from the development of allergic sensitization and airway inflammation (29). However, leukotrienes and other lipoxygenases, such as the 5-lipoxygenase, are also involved in modulating inflammation. Elimination of 5-LO preserves gas exchange and increases survival in a ventilator-induced lung injury (30).
The present study was designed to investigate the role of 12/15-lipoxygenase and 12-HETE on vascular permeability in murine models of LPS-induced pulmonary inflammation and acid-induced lung injury. In order to investigate the main source of 12/15-LO relevant to vascular permeability, chimeric mice were generated by bone marrow transplantation that expressed 12/15-LO in hematopoietic cells, nonhematopoietic cells, both, or neither. To address the mechanism by which 12/15-LO products influence vascular permeability in ALI, we tested the interaction between 12-HETE and the CXCL1-CXCR2 axis of inflammatory chemokine signaling.
Materials and Methods
Animals
We used 8-12 weeks old C57BL/6 mice (Jackson Laboratory, Bar Harbor, USA) and Alox15 deficient mice (31) backcrossed to C57BL/6 for at least 10 generations (UVA colony) (32). Furthermore, Cxcr2−/− mice (Jackson Laboratory, Bar Harbor, USA) on the BALB/c background and BALB/c controls were used. Mice were housed in a barrier facility under specific pathogen-free conditions. The Animal Care and Use Committee of the University of Virginia (Charlottesville) approved all animal experiments.
Murine Model of LPS-induced pulmonary inflammation and acid-induced ALI
LPS from Salmonella enteritidis (500 μg/ml, Sigma-Aldrich) was nebulized to induce pulmonary inflammation as previously described (33). Briefly, mice were exposed to 30 minutes of aerosolized LPS or saline aerosol as a control.
In some experiments 12/15-LO activity was pharmacologically inhibited by CDC (Cinnamyl-3,4-Dihydroxy-α-Cyanocinnamate, Biomol International, Philadelphia, PA). Mice were injected one hour (8mg/kg, i.p.) before induction of the pulmonary inflammation.
Acid-induced ALI was induced by injection of 2 μl/g of HCl (pH = 1.5) intratracheally, followed by a bolus of air (30 μl/g) as previously described (7). Following a tracheotomy, mice were ventilated with a respirator (MiniVent, Type 845; Hugo Sachs Elektronik, March-Hugstetten, Germany) for 2 hours (tidal volume, 10 μl/g; respiration rate, 140/min; fraction ofinspiratory oxygen [FiO2], 0.21). Control animals received saline instead of HCl in the same manner.
Pulmonary microvascular permeability
We determined pulmonary microvascular permeability in WT, Alox15−/−, and chimeric mice using the Evans blue dye extravasation technique as described previously (10). Briefly, Evans blue (20 mg/kg; Sigma-Aldrich) was injected intravenously 30 minutes prior to euthanasia. Lungs were perfused, removed, and Evans blue was extracted. The absorption of Evans blue was measured and calculated. The results were corrected for hemoglobin. Extravasated Evans blue was determined in the different animal groups 6 hours after LPS or saline inhalation and expressed as μg/g lung. Vascular permeability in the acid-induced lung injury model was determined by measuring protein concentration in the supernatant of the bronchoalveolar lavage (BAL) (Lowry's method). In some experiments CXCL1 was neutralized by a polyclonal antibody (R&D). Mice were injected either with the antibody or a preimmune control six hours (25mg/kg, i.p.) before induction of the pulmonary inflammation.
In order to measure pulmonary blood volume, we injected Evans blue five minutes before the end of the experiment. After this time, mice were killed, the pulmonary vessels ligated, the vessels transected proximal to the ligation, and the lungs removed. The weight of the blood-filled lungs, the Evans blue concentration in lungs and blood were measured. The time period between the injection of the Evans blue and the end of the experiment was too short for the Evans blue to leave the intravascular compartment, and we calculated blood volume based on indicator dilution:
where Vpul. is the blood volume in the lung, MEB is the concentration of EB in whole lung tissue including the trapped blood, CEB is the concentration of EB in the blood and VB is the blood volume measured.
In some experiments, the lungs were removed, wet weight was measured, and the lungs were dried in an incubator set at 70°C. The dry weight was obtained after 72 hours of incubation and the ratio of wet/dry weight was calculated.
Pulmonary function — oxygenation
Blood was obtained from an arterial catheter, and standard arterial blood gas analyses were accomplished 2h after the induction of the acid-induced ALI (Rapidlab 800 System; Bayer HealthCare). Partial pressure of arterial oxygen (PaO2) was normalized to the fraction of inspiratory oxygen (FiO2) to yield the oxygenation index (PaO2/FiO2).
In vitro endothelial and epithelial_cell assay
To assess the distribution of F-actin upon 12-HETE stimulation (Sigma-Aldrich), HPMECs (ATCC, Manassas, VA) or human alveolar epithelial cells (HAEC, ATCC, Manassas, VA) were cultured on glass coverslips. After stimulation, cells were fixed, permeabilized, and stained with FITC-phalloidin (Invitrogen) as previously described (7). Coverslips were mounted on glass slides, and microscopy was accomplished on a Nikon Diaphot inverted fluorescence microscope.
Generation of 12/15-Lipoxygenase chimeric mice
To distinguish the role of non- and hematopoietic 12/15-Lipoxygenase in pulmonary inflammation, chimeric mice were generated following a previously described protocol (7). C57BL/6 (on CD45.2 and CD45.1 background) and Alox15 deficient mice (CD45.2) were used as donors and/or recipients. Recipient mice were lethally irradiated in two doses of 600 rad each (separated by 4 hours). Bone marrow was isolated from donor mice under sterile conditions, and approximately 5 × 106 were injected intravenously into recipient mice. Experiments were performed 6 weeks after BMT.
Quantitative real-time RT-PCR
Total RNA from whole lung tissue was extracted using Trizol (Invitrogen, Carlsbad, CA). Reverse transcription and PCR steps were performed using QuantiTect SYBR Green RT-PCR Kit (Qiagen) on an iCycler iQ Real-Time Detection System (Qiagen) and sequence specific primers designed on Beacon Designer 2.06 software. Samples used to construct the standard curve consisted of wild type LPS stimulated mesenteric peritoneal macrophages using 20, 6.3 and 2 nanograms of RNA. One and a half micrograms of total RNA were used for all lung samples. Values were determined using iCycler iQ Real-Time Detection System Software v3.0 (Qiagen). The corresponding values were normalized to 18s mRNA.
The primers for CXCR-2 (forward 5‘ATGCCCTCTATTCTGCCAGAT’3, reverse 5‘GTGCTCCGGTTGTATAAGATGAC’3) were selected form NCBI Primer Bank.
Chemokine Measurments
CXCL1 in the BAL fluid were measured in triplicates using enzyme-linked immunosorbent assay kits, following the procedures supplied by the manufacturer (R&D Systems, Minneapolis, MN). Chemokines were determined in control mice (saline) and LPS treated mice.
Histology
In order to visualize morphological changes during LPS-induced lung injury, paraffin-embedded lung sections (5 μm) were stained for 12/15-LO (polyclonal rabbit anti-porcine Ab) (34) using the avidin-biotin technique (Vector Laboratories, Burlingame, CA) as described previously (35).
For immunofluorescence labeling, biotin-labeled Mac-2 (Clone M3/38, Accurate Chemicals, Westbury, NY) was visualized with streptavidin Alexa Fluor 555 (Molecular Probes), 12/15-LO was visualized by using anti-rabbit-Alexa Fluor 488 (Molecular Probes), and nuclei were stained with diamidino-2-phenylindole (DAPI, Vector Laboratories).
Statistics
Statistical analysis was performed with SPSS (version 9.0, Chicago, IL) and included one-way analysis of variance, Student-Newman-Keuls test, and t-test where appropriate. Kaplan-Meier method was used for analyzing survival rate. All data are presented as mean ± SEM. P < 0.05 was considered significant.
Results
12/15-lipoxygenase is involved in LPS-induced regulation of vascular permeability
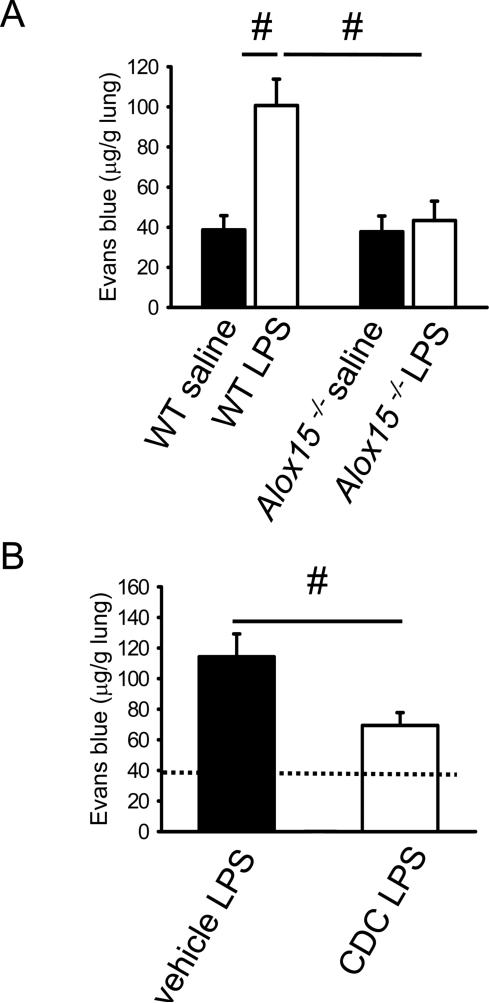
In order to investigate the role of 12/15-lipoxygenase in pulmonary inflammation, mice were exposed to aerosolized LPS for 30 minutes and vascular permeability was determined. Vascular permeability as measured by the Evans blue method (10) significantly increased in WT mice 6h after LPS stimulation compared to saline control mice (Figure 1A). Baseline vascular permeability of Alox15−/− mice (31) was similar to WT mice, but Alox15−/− mice were completely protected from permeability increase upon LPS inhalation (Figure 1A).
Figure 1. 12/15-lipoxygenase is involved in LPS-induced regulation of vascular permeability.
(A) Vascular permeability of WT mice and Alox15−/− (Alox15−/−) mice was measured 6h after LPS inhalation by the Evans blue method (n=6-7). (B) After pharmacological blockade of 12/15-LO by the inhibitor CDC (Cinnamyl-3,4-Dihydroxy-a-Cyanocinnamate, open bars) or in vehicle control mice (black bars), vascular permeability (6h after LPS) was measured by the Evans blue method. Vascular permeability in WT mice pre-treated with the 12/15-LO inhibitor or vehicle (n=4). Dotted line indicates Evans blue in saline-treated WT mice for comparison. #P < 0.05.
In order to investigate whether the lack of 12/15-lipoxygenase had an effect on the lung vascular surface area, we measured pulmonary blood volume in WT mice and Alox 15−/− mice. Pulmonary blood volume was similar in WT mice and Alox 15−/− mice (WT: 141±13 μl, Alox 15−/−: 153±21 μl, n = 4, n.s.). There was no difference in lung wet-to-dry weight ratio (data not shown). These data suggest that the lack of 12/15-lipoxygenase does not influence lung vascular surface area.
Alox15−/− mice lack the Alox15 gene and all its products from conception. In order to investigate whether acute blockade of 12/15-LO by a pharmacological inhibitor also reduces vascular permeability, mice were injected with the 12/15-LO inhibitor Cinnamyl-3,4-Dihydroxy-α-Cyanocinnamate (CDC) one hour before LPS exposure. A single injection of 8 mg/kg CDC was previously shown to significantly reduce 12/15-LO activity, as measured by urinary 12-HETE concentration (36). Similar to the observation in Alox15−/− mice, acute pharmacological inhibition of 12/15-LO also significantly reduced vascular permeability (Figure 1B).
LPS inhalation induces 12/15-LO expression in Mac-2+ cells in the lung
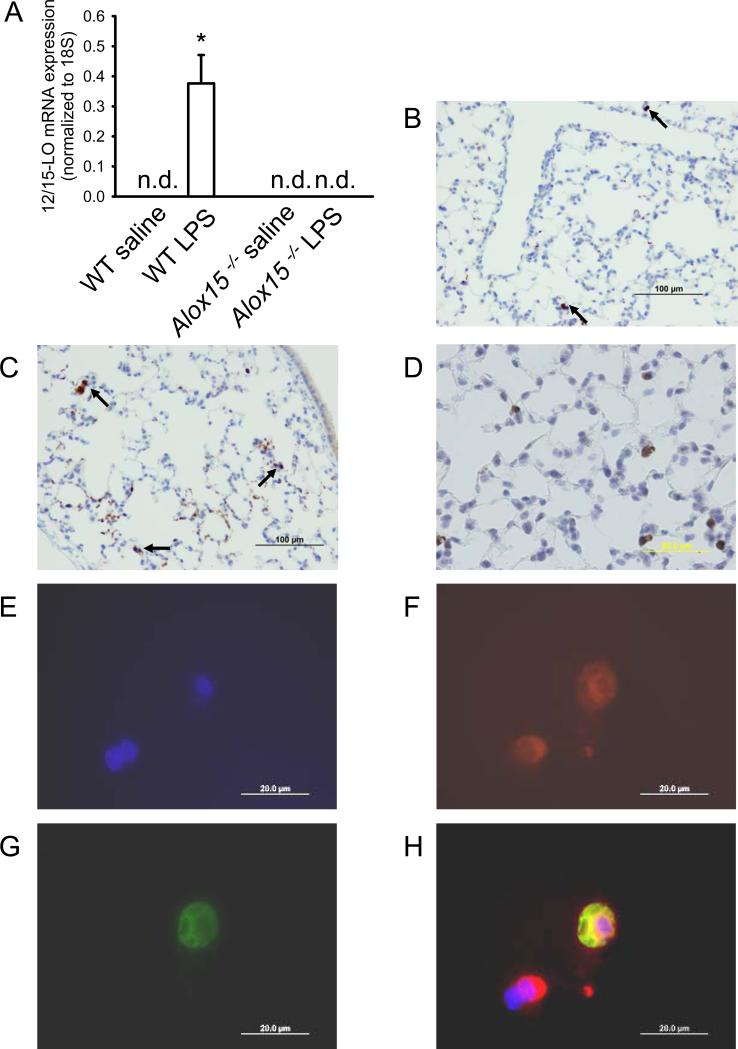
In order to determine 12/15-LO expression in lungs, we measured 12/15-LO mRNA levels by quantitative RT-PCR. Without stimulation, 12/15-LO mRNA expression was not detectable in lungs from WT mice, but significantly increased 3h after LPS exposure (Figure 2A). As expected, 12/15-LO mRNA was undetectable in Alox15−/− mice. 12/15-LO protein expression in the lung was visualized by immunohistochemistry with and without LPS stimulation. Rare 12/15-LO-expressing cells were detectable in lungs of untreated mice (Figure 2B). After LPS inhalation, more 12/15-LO expressing cells were found (Figure 2C + D). In order to investigate whether lung macrophages are the main source of 12/15-LO, we used immunofluorescence microscopy of the lung. LPS-treated lungs were stained for both 12/15-LO and Mac-2, a macrophage marker. The co-localization (Figure 2H) of the nucleus (Figure 2E), Mac-2 (Figure 2F), and 12/15-LO (Figure 2G) showed that some but not all Mac-2+ cells express 12/15-LO in the lung after LPS exposure. In untreated lungs from WT mice, only 0.75% of macrophages were positive for 12/15-lipoxygenase. Three hours following LPS exposure the percentage increased to 4% (n = 3, p < 0.05).
Figure 2. 12/15-LO produced in lung macrophages is up-regulated following LPS inhalation.
(A) 12/15-LO mRNA expression in lungs of unstimulated and stimulated WT mice and Alox15 deficient mice was investigated by quantitative RT-PCR (n.d., not detectable). 12/15-LO protein expression shown by immunohistochemistry in lungs from untreated WT (B) mice and WT mice 3h after LPS inhalation (C+D). (E-H) Fluorescence microscopy images of monocytes/macrophages in the lung upon LPS stimulation. Nuclei (blue, DAPI, E), Mac-2 (red, clone M3/38, F), and 12/15-LO (green, polyclonal rabbit anti-porcine, G). (H) The merged image demonstrates co-localization (yellow) of Mac-2 and 12/15-LO in one of the two monocytes/macrophages. Scale bar equals 20 μm. (E-H)
Hematopoietic 12/15-LO is responsible for regulation of vascular permeability following LPS-inhalation
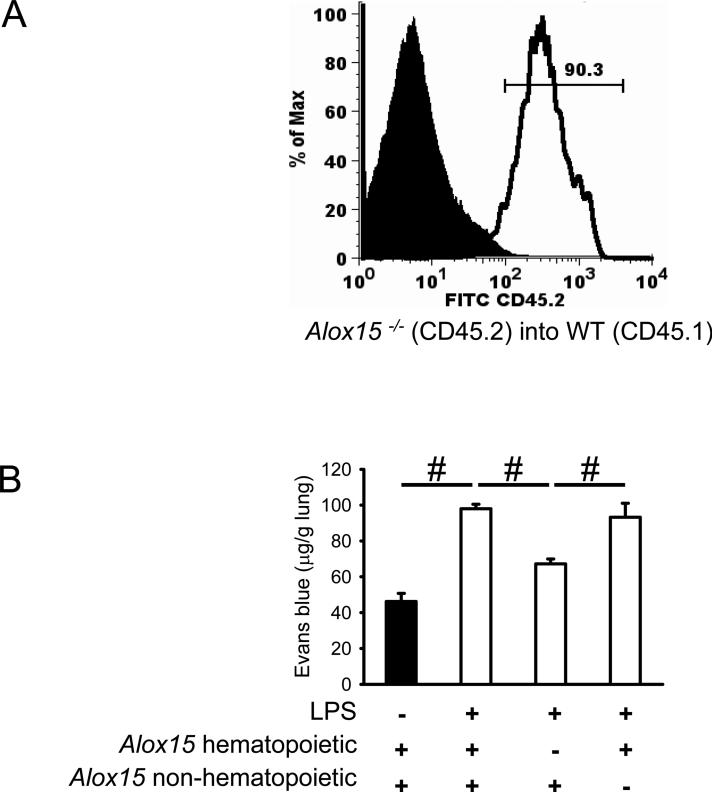
To determine whether 12/15-LO expression in bone marrow-derived cells is important in lung inflammation, we generated chimeric mice using Alox15−/− mice (CD45.2) and CD45.1 WT mice. CD45.1 and CD45.2 are functionally equivalent alleles of the CD45 antigen expressed by all leukocytes. Six weeks after the bone marrow transplantation, more then 90% of all macrophages/monocytes in the lung were reconstituted with cells from donor mice (Figure 3A).
Figure 3. Hematopoietic 12/15-LO is responsible for regulation of vascular permeability following LPS-inhalation.
Alox15−/− mice on a CD45.2 background and CD45.1 WT mice were used in bone marrow transplant experiments. (A) Monocytes and macrophages in single cell lung preparations were identified by gating on CD45+F4/80+ cells and the percentage of reconstituted cells were shown in the histogram by CD45.2 expression (donor-derived). (B) Chimeric mice lacking hematopoietic Alox15 are protected from increased vascular permeability as measured by Evans Blue extravasation upon LPS inhalation (n ≥ 4). #P < 0.05.
Mice lacking hematopoietic Alox15 were almost completely protected from increased vascular permeability after LPS inhalation (Figure 3B). Chimeric mice lacking non-hematopoietic Alox15 showed a similar increase of vascular permeability after LPS exposure as control mice (Figure 3B). No 12/15-lipoxygenase mRNA was detectable in blood leukocytes (data not shown), suggesting that monocyte-derived resident lung macrophages are the main source of 12/15-lipoxygenase.
12-HETE regulates vascular permeability
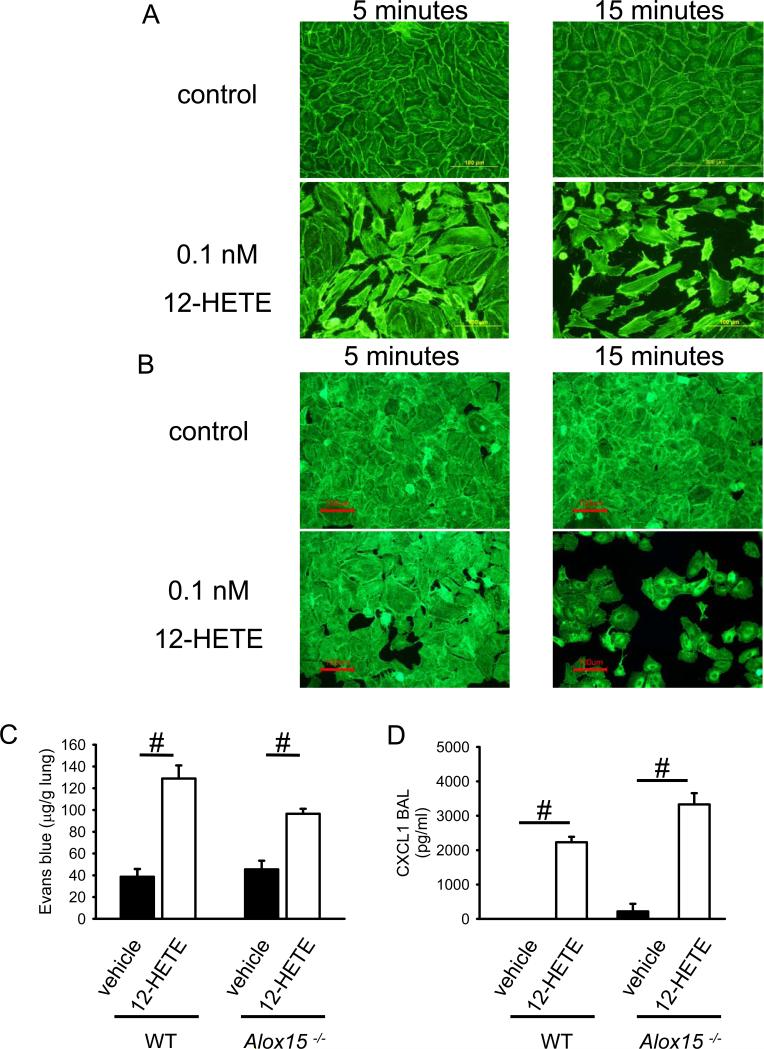
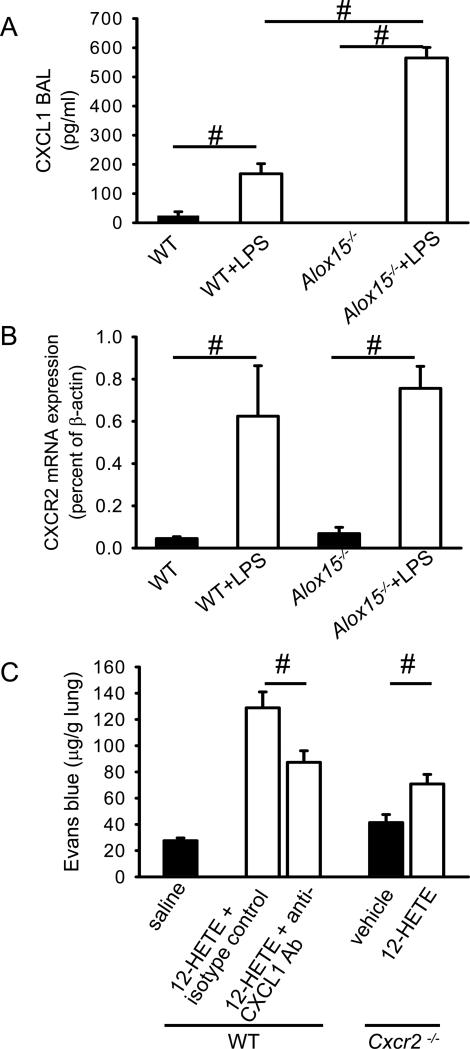
12/15-LO generates a number of oxidized lipids from various substrates (22). The main arachidonic acid product is 12-HETE. Therefore, we investigated 12-HETE concentration in BAL of WT mice and Alox15−/− mice three hours after LPS inhalation. In WT mice, the concentration of 12-HETE was 42±4 pg/ml, but 12-HETE was undetectable in Alox15−/− mice. Cultured human pulmonary endothelial cells and human alveolar epithelial cells stimulated with 0.1nM (32 pg/ml) 12-HETE, a concentration similar to that found in the BAL of WT mice after LPS inhalation, induced a striking increase in F-actin. F-actin rings appeared at the edge of the cells and caused retraction. These changes reached a peak at 15min (Figure 4A + B, and data not shown), suggesting that 12-HETE is a major regulator of vascular permeability following LPS inhalation. Consistent with these in vitro data, intratracheal 12-HETE application of 5 pg (100μl of 50 pg/ml solution) induced increased vascular permeability in WT and Alox15−/− mice (Figure 4C). CXCL1 is an important ligand for CXCR2, a receptor known to be involved in regulating lung endothelial permeability (10). CXCL-1 levels measured by ELISA increased in response to intratracheal injection of 12HETE in WT mice and Alox15−/− mice (p < 0.05; Figure 4D). The levels of CXCL1 in Alox15−/− mice and WT mice were similar.
Figure 4. 12-HETE regulates endothelial and epithelial integrity and vascular permeability.
Endothelial (A) and alveolar (B) cell response to 12-HETE activation as reflected by F-actin localization. Human pulmonary endothelial (A) and alveolar (B) cells were treated with 0.1 nM of 12-HETE, and F-actin was localized by phalloidin staining. Images are representative of 3 experiments with similar results. (C) Vascular permeability was measured by the Evans blue method 6h after intratracheal injection of 12-HETE or vehicle into WT or Alox15−/− mice (n = 4). #P < 0.05. (D) CXCL1 levels in the lung of WT and Alox15−/− mice after 12-HETE application.
Role of CXCR2 and CXCL1 in 12-HETE-induced permeability
In order to address the mechanism through which 12-HETE induces vascular permeability of the lung, we investigated the role of the chemokine receptor CXCR2 and one of its ligands, CXCL1. CXCR2 expressed by endothelial and epithelial cells plays an important role in regulating vascular permeability following LPS-exposure of the lung (10). In order to investigate the role of the CXCL1/CXCR2 axis in WT mice and Alox15−/− mice, we determined the CXCL1 levels in the BAL and mRNA expression of CXCR2 in the lung following LPS inhalation. CXCL1 levels in the BAL of WT mice and Alox15−/− were similar under baseline conditions (Figure 5A). The levels of CXCL1 in the BAL significantly increased in WT and Alox15−/− after LPS exposure with a more pronounced increase in Alox15−/− mice compared to WT mice (Figure 5A). CXCR2 mRNA expression in WT mice and Alox15−/− mice significantly increased following LPS stimulation (Figure 5B). It appears that CXCR2 has a special role among chemoattractant receptors in that it seems to control neutrophil access to the lung (37). Since vascular permeability in the lung is almost completely dependent on CXCR2 after LPS inhalation (10), but also on 12/15-LO (present data), we reasoned that CXCR2 may also be involved in 12-HETE-dependent permeability. In order to test this hypothesis, 12-HETE (100μl of 50 pg/ml) was injected intratracheally into CXCR2-deficient mice and vascular permeability was measured by Evans blue after 6h. Following 12-HETE instillation, vascular permeability slightly increased in CXCR2-deficient mice compared to vehicle treated mice (Figure 5 C), but the increase was not as pronounced as in WT mice reconstituted with 12-HETE (Figure 5 C). Antibody blockade of CXCL1, a CXCR2 ligand, for 6h before induction of ALI, reduced vascular permeability in WT mice (Figure 5). These data suggest that 12-HETE regulates vascular permeability through a largely CXCR2- and CXCL1-dependent mechanism.
Figure 5. 12-HETE-induced vascular leakage requires CXCL1 and CXCR2.
CXCL1 levels in the BAL (A) and CXCR2 mRNA expression in the lung of WT mice and Alox15−/− mice (B). Vascular permeability was measured by the Evans blue method 6h after intratracheal injection of 12-HETE (open bars) or vehicle (black bars) into WT or Cxcr2−/− mice, mice (n = 4). Neutralization of CXCL1 by antibody (n = 3-4) reduced the vascular permeability increase in response to 12-HETE, similar to the level seen in Cxcr2−/− mice.
12/15-LO determines outcome of acid-induced acute lung injury
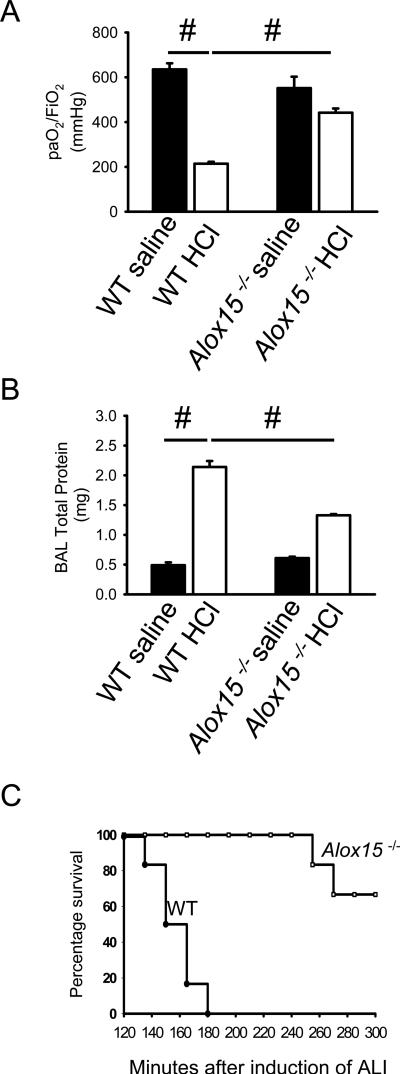
Based on our finding that the absence of 12/15-LO reduces vascular permeability in response to aerosolized LPS, we hypothesized that this enzyme and its products may also determine disease outcome in acid-induced ALI (7), which mimics aspiration of gastric contents that can occur in anesthetized or unconscious patients. This is a much more severe model than LPS-induced lung injury, leading to 100% lethality within 3 hours (7). Two hours after induction of ALI, WT mice showed significantly reduced gas exchange (Figure 6A) and increased accumulation of proteins in the alveolar compartment (Figure 6B). Protein accumulation, as a marker of vascular permeability, was blunted in Alox15−/− mice. This was associated with a dramatically improved lung function as reflected by paO2/FiO2. These protective effects resulted in a significant survival benefit to Alox15−/− mice after acid-induced ALI (Figure 6C).
Figure 6. Absence of 12/15-LO improves acid-induced acute lung injury.
Two hours after initiation of acid-induced ALI, gas exchange was measured in Alox15−/− and wild-type mice before and after HCl instillation (A). Permeability measured by BAL protein contents (B) compared to control mice (n ≥ 3 mice). #P < 0.05. (C) Alox15−/− mice showed significantly prolonged survival after induction of acid-induced ALI compared to HCl-treated WT mice (n = 6 per group). P < 0.001 by log rank test.
Discussion
The results of our study suggest that the enzyme 12/15-LO plays a crucial role in the regulation of vascular permeability in LPS-induced pulmonary inflammation and acid-induced acute lung injury (ALI). Absence of 12/15-LO greatly improves survival in an acid-induced model of ALI that mimics aspiration of gastric contents. Elimination of hematopoeitic Alox15 by bone marrow reconstitution experiments demonstrate a dominant role of myeloid-derived 12-HETE in the regulation of vascular permeability in a largely CXCR2-dependent manner.
12/15-LO has been implicated in a variety of chronic inflammatory diseases including atherosclerosis (23, 38, 39) and β cell destruction in diabetes (40). Furthermore, the two lipoxygenase products, 5- and 12-HETE, have been proposed to be involved in modulating the vascular permeability during pulmonary inflammation caused by gram-negative endotoxemia (41). In most studies, 12/15-LO had deleterious effects, and eliminating 12/15-LO by gene targeting improved the disease outcome. Inhibiting 12/15-LO has been proposed as a viable target for anti-inflammatory therapy (42, 43). In analyzing different studies investigating the role of Alox15, it is important to recognize that the products of this enzyme are different in mice (mostly 12-HETE) and humans (mostly 15-HETE). Here, we show that 12/15-LO also plays a deleterious role in acute lung inflammation in mice.
Previous studies have demonstrated that macrophage-derived 12/15-LO plays a key role in atherosclerosis development in the Apoe−/− model (23). Here, we show that 12/15-LO expression in hematopoietic-derived cells plays a dominant role in regulating vascular permeability in acute lung injury. This is consistent with a role for the 12/15-LO product 12-HETE in regulating endothelial and epithelial cell contraction and permeability. Although the receptor for 12-HETE is unknown, its signaling is pertussis toxin-sensitive, indicating it is coupled through members of the Gαi family of G-proteins (44). Our studies suggest that 12-HETE induces increased lung vascular permeability through a CXCR2-dependent mechanism, but absence of Alox15 does not reduce CXCL1 secretion into the BAL. Further research will be needed to determine the exact relationship between the 12-HETE and CXCR2 pathways. It has been shown that 12-HETE causes pulmonary vasoconstriction, an effect that may contribute to protein leakage independent of lung vascular permeability (45). In this in vivo study, we did not directly measure the pulmonary surface area and therefore, we cannot fully rule out that the vascular permeability may also be modulated by the vasoconstrictor properties of 12-HETE.
Since CXCR2 on endothelial cells is known to regulate vascular permeability following LPS exposure (10), we reasoned that CXCR2 may be involved in 12-HETE-induced permeability. We show that eliminating CXCR2 (in Cxcr2−/− mice) or blocking one of its major ligands, CXCL1, indeed curbs 12-HETE-induced permeability. However, the response to 12-HETE in Cxcr2−/− mice is not completely abolished, suggesting that direct effects of 12-HETE or other chemokines and chemokine receptors are involved in regulating vascular permeability. Our data suggest that LPS activates 12/15-lipoxygenase in lung macrophages and leads to the release of 12-HETE with the subsequent production and release of CXCL1. CXCL1 then binds to endothelial CXCR2 in a paracrine manner and regulates vascular permeability. Translating this finding to humans may be complicated by the expression of an additional chemokine, CXCL8 (46).
Stimulation of cells with 12-HETE activates a cascade of downstream targets, such as p38 mitogen-activated protein kinase (47), c-Jun amino terminal kinase (48), phosphoinositide 3-kinase, and p21-activated kinase (PAK) (49). Although the details of the signaling cascade downstream of 12-HETE are not known, the observation that PAK is involved in regulating vascular permeability (51) provides a possible mechanism for the endothelial effects of 12-HETE.
The biology of 12/15 lipoxygenase is complicated, because products of this enzyme have pro- and anti-inflammatory effects (50). In addition to producing 12-HETE and 13-HODE, this enzyme is involved in the biosynthesis of lipoxins (51). Although it is well established that the mouse enzyme encoded by Alox15 has more 12-lipoxygenase activity than human ALOX15 (52), both enzymes produce pro- and anti-inflammatory mediators. 15-lipoxygenase products like 15-HETE and lipoxins have anti-inflammatory activities (53, 54). Incorporation of 15-HETE into phosphatidylinositol can modulate intracellular signaling that subsequently leads to reduced activation and recruitment of inflammatory cells and altered adhesion molecule expression (55, 56). The published data suggest that certain stimuli may induce mainly the 12-lipoxgenase activity and subsequently the production of inflammatory mediators, whereas other stimuli predominantly activate the anti-inflammatory pathway (15-HETE and lipoxins). However, further studies will be necessary to address this hypothesis. Furthermore, specific inhibitors for the (largely unknown) receptors of its products will be needed to fully dissect the functional role of 12/15 lipoxygenase and to determine whether this enzyme is a valid target for pharmacological intervention in human disease.
Our study suggests that blocking or eliminating 12-HETE production improved arterial oxygen partial pressure and resulted in a survival benefit in acute lung injury as shown by Alox15 deficient mice. CDC, the 12/15-LO inhibitor used in our study, was effective when given as a pre-treatment. However, high does of CDC are required to inhibit 12/15-LO (57-59). More specific and effective pharmacological inhibitors of 12/15-LO may be valuable in the treatment of ALI.
Acknowledgements
The authors wish to thank Tracy Burcin for technical support.
Footnotes
This study was supported by Else Kröner-Fresenius-Stiftung (grant A69/07 to A.Z.), by German Research Foundation (ZA428/2-1 to A.Z., VI508/1-1 to S.v.V), by NIH grant PO1 HL 73361 to Joel Linden (project 2 to K.L.) and by NIH grant PO1 HL 55798 to J.N. (project 4 to K.L.).
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 3.Levitt JE, Matthay MA. Treatment of acute lung injury: historical perspective and potential future therapies. Semin Respir Crit Care Med. 2006;27:426–437. doi: 10.1055/s-2006-948296. [DOI] [PubMed] [Google Scholar]
- 4.Webb AR. Capillary leak. Pathogenesis and treatment. Minerva Anestesiol. 2000;66:255–263. [PubMed] [Google Scholar]
- 5.Stevens T, Garcia JG, Shasby DM, Bhattacharya J, Malik AB. Mechanisms regulating endothelial cell barrier function. Am J Physiol Lung Cell Mol Physiol. 2000;279:L419–422. doi: 10.1152/ajplung.2000.279.3.L419. [DOI] [PubMed] [Google Scholar]
- 6.Michel CC, Curry FE. Microvascular permeability. Physiol Rev. 1999;79:703–761. doi: 10.1152/physrev.1999.79.3.703. [DOI] [PubMed] [Google Scholar]
- 7.Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 2006;116:3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens T, Creighton J, Thompson WJ. Control of cAMP in lung endothelial cell phenotypes. Implications for control of barrier function. Am J Physiol. 1999;277:L119–126. doi: 10.1152/ajplung.1999.277.1.L119. [DOI] [PubMed] [Google Scholar]
- 9.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reutershan J, Morris MA, Burcin TL, Smith DF, Chang D, Saprito MS, Ley K. Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J Clin Invest. 2006;116:695–702. doi: 10.1172/JCI27009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson JR, Garcia JG. Novel therapies for microvascular permeability in sepsis. Curr Drug Targets. 2007;8:509–514. doi: 10.2174/138945007780362719. [DOI] [PubMed] [Google Scholar]
- 12.McVerry BJ, Garcia JG. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell Signal. 2005;17:131–139. doi: 10.1016/j.cellsig.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Zarbock A, Schmolke M, Spieker T, Jurk K, Van Aken H, Singbartl K. Acute uremia but not renal inflammation attenuates aseptic acute lung injury: a critical role for uremic neutrophils. J Am Soc Nephrol. 2006;17:3124–3131. doi: 10.1681/ASN.2006040358. [DOI] [PubMed] [Google Scholar]
- 14.Frank JA, Matthay MA. Leukotrienes in acute lung injury: a potential therapeutic target? Am J Respir Crit Care Med. 2005;172:261–262. doi: 10.1164/rccm.2505008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raby KE, Barry J, Creager MA, Cook EF, Weisberg MC, Goldman L. Detection and significance of intraoperative and postoperative myocardial ischemia in peripheral vascular surgery. JAMA. 1992;268:222–227. [PubMed] [Google Scholar]
- 16.Conrad DJ. The arachidonate 12/15 lipoxygenases. A review of tissue expression and biologic function. Clin Rev Allergy Immunol. 1999;17:71–89. doi: 10.1007/BF02737598. [DOI] [PubMed] [Google Scholar]
- 17.Belkner J, Stender H, Kuhn H. The rabbit 15-lipoxygenase preferentially oxygenates LDL cholesterol esters, and this reaction does not require vitamin E. J Biol Chem. 1998;273:23225–23232. doi: 10.1074/jbc.273.36.23225. [DOI] [PubMed] [Google Scholar]
- 18.Kuhn H, Belkner J, Suzuki H, Yamamoto S. Oxidative modification of human lipoproteins by lipoxygenases of different positional specificities. J Lipid Res. 1994;35:1749–1759. [PubMed] [Google Scholar]
- 19.Takahashi Y, Glasgow WC, Suzuki H, Taketani Y, Yamamoto S, Anton M, Kuhn H, Brash AR. Investigation of the oxygenation of phospholipids by the porcine leukocyte and human platelet arachidonate 12-lipoxygenases. Eur J Biochem. 1993;218:165–171. doi: 10.1111/j.1432-1033.1993.tb18362.x. [DOI] [PubMed] [Google Scholar]
- 20.Funk CD. The molecular biology of mammalian lipoxygenases and the quest for eicosanoid functions using lipoxygenase-deficient mice. Biochim Biophys Acta. 1996;1304:65–84. doi: 10.1016/s0005-2760(96)00107-5. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimoto T, Takahashi Y. Arachidonate 12-lipoxygenases. Prostaglandins Other Lipid Mediat. 2002;68-69:245–262. doi: 10.1016/s0090-6980(02)00034-5. [DOI] [PubMed] [Google Scholar]
- 22.Chiang N, Serhan CN, Dahlen SE, Drazen JM, Hay DW, Rovati GE, Shimizu T, Yokomizo T, Brink C. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 23.Huo Y, Zhao L, Hyman MC, Shashkin P, Harry BL, Burcin T, Forlow SB, Stark MA, Smith DF, Clarke S, Srinivasan S, Hedrick CC, Pratico D, Witztum JL, Nadler JL, Funk CD, Ley K. Critical role of macrophage 12/15-lipoxygenase for atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;110:2024–2031. doi: 10.1161/01.CIR.0000143628.37680.F6. [DOI] [PubMed] [Google Scholar]
- 24.Hatley ME, Srinivasan S, Reilly KB, Bolick DT, Hedrick CC. Increased production of 12/15 lipoxygenase eicosanoids accelerates monocyte/endothelial interactions in diabetic db/db mice. J Biol Chem. 2003;278:25369–25375. doi: 10.1074/jbc.M301175200. [DOI] [PubMed] [Google Scholar]
- 25.Cunningham FM, Woollard PM. 12(R)-hydroxy-5,8,10,14-eicosatetraenoic acid is a chemoattractant for human polymorphonuclear leucocytes in vitro. Prostaglandins. 1987;34:71–78. doi: 10.1016/0090-6980(87)90264-4. [DOI] [PubMed] [Google Scholar]
- 26.Tang DG, Timar J, Grossi IM, Renaud C, Kimler VA, Diglio CA, Taylor JD, Honn KV. The lipoxygenase metabolite, 12(S)-HETE, induces a protein kinase C-dependent cytoskeletal rearrangement and retraction of microvascular endothelial cells. Exp Cell Res. 1993;207:361–375. doi: 10.1006/excr.1993.1203. [DOI] [PubMed] [Google Scholar]
- 27.Wen Y, Gu J, Chakrabarti SK, Aylor K, Marshall J, Takahashi Y, Yoshimoto T, Nadler JL. The Role of 12/15-Lipoxygenase in the Expression of IL-6 and TNF-{alpha} in Macrophages. Endocrinology. 2006 doi: 10.1210/en.2006-0665. [DOI] [PubMed] [Google Scholar]
- 28.Bolick DT, Orr AW, Whetzel A, Srinivasan S, Hatley ME, Schwartz MA, Hedrick CC. 12/15-lipoxygenase regulates intercellular adhesion molecule-1 expression and monocyte adhesion to endothelium through activation of RhoA and nuclear factor-kappaB. Arterioscler Thromb Vasc Biol. 2005;25:2301–2307. doi: 10.1161/01.ATV.0000186181.19909.a6. [DOI] [PubMed] [Google Scholar]
- 29.Hajek AR, Lindley AR, Favoreto S, Jr., Carter R, Schleimer RP, Kuperman DA. 12/15-Lipoxygenase deficiency protects mice from allergic airways inflammation and increases secretory IgA levels. J Allergy Clin Immunol. 2008;122:633–639. e633. doi: 10.1016/j.jaci.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caironi P, Ichinose F, Liu R, Jones RC, Bloch KD, Zapol WM. 5-Lipoxygenase deficiency prevents respiratory failure during ventilator-induced lung injury. Am J Respir Crit Care Med. 2005;172:334–343. doi: 10.1164/rccm.200501-034OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Funk CD, Chen XS, Johnson EN, Zhao L. Lipoxygenase genes and their targeted disruption. Prostaglandins Other Lipid Mediat. 2002;68-69:303–312. doi: 10.1016/s0090-6980(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 32.Reilly KB, Srinivasan S, Hatley ME, Patricia MK, Lannigan J, Bolick DT, Vandenhoff G, Pei H, Natarajan R, Nadler JL, Hedrick CC. 12/15-Lipoxygenase activity mediates inflammatory monocyte/endothelial interactions and atherosclerosis in vivo. J Biol Chem. 2004;279:9440–9450. doi: 10.1074/jbc.M303857200. [DOI] [PubMed] [Google Scholar]
- 33.Reutershan J, Stockton R, Zarbock A, Sullivan GW, Chang D, Scott D, Schwartz MA, Ley K. Blocking p21-activated Kinase Reduces Lipopolysaccharide-induced Acute Lung Injury by Preventing Polymorphonuclear Leukocyte Infiltration. Am J Respir Crit Care Med. 2007;175:1027–1035. doi: 10.1164/rccm.200612-1822OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pei H, Gu J, Thimmalapura PR, Mison A, Nadler JL. Activation of the 12-lipoxygenase and signal transducer and activator of transcription pathway during neointima formation in a model of the metabolic syndrome. Am J Physiol Endocrinol Metab. 2006;290:E92–E102. doi: 10.1152/ajpendo.00133.2005. [DOI] [PubMed] [Google Scholar]
- 35.Singbartl K, Bockhorn SG, Zarbock A, Schmolke M, Van Aken H. T Cells Modulate Neutrophil-Dependent Acute Renal Failure during Endotoxemia: Critical Role for CD28. J Am Soc Nephrol. 2005;16:720–728. doi: 10.1681/ASN.2004050381. [DOI] [PubMed] [Google Scholar]
- 36.Ma J, Natarajan R, LaPage J, Lanting L, Kim N, Becerra D, Clemmons B, Nast CC, Surya Prakash GK, Mandal M, Adler SG. 12/15-lipoxygenase inhibitors in diabetic nephropathy in the rat. Prostaglandins Leukot Essent Fatty Acids. 2005;72:13–20. doi: 10.1016/j.plefa.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Strieter RM, Keane MP, Burdick MD, Sakkour A, Murray LA, Belperio JA. The role of CXCR2/CXCR2 ligands in acute lung injury. Curr Drug Targets Inflamm Allergy. 2005;4:299–303. doi: 10.2174/1568010054022178. [DOI] [PubMed] [Google Scholar]
- 38.George J, Afek A, Shaish A, Levkovitz H, Bloom N, Cyrus T, Zhao L, Funk CD, Sigal E, Harats D. 12/15-Lipoxygenase gene disruption attenuates atherogenesis in LDL receptor-deficient mice. Circulation. 2001;104:1646–1650. doi: 10.1161/hc3901.095772. [DOI] [PubMed] [Google Scholar]
- 39.Cyrus T, Witztum JL, Rader DJ, Tangirala R, Fazio S, Linton MF, Funk CD. Disruption of the 12/15-lipoxygenase gene diminishes atherosclerosis in apo E-deficient mice. J Clin Invest. 1999;103:1597–1604. doi: 10.1172/JCI5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bleich D, Chen S, Zipser B, Sun D, Funk CD, Nadler JL. Resistance to type 1 diabetes induction in 12-lipoxygenase knockout mice. J Clin Invest. 1999;103:1431–1436. doi: 10.1172/JCI5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brigham KL. Pulmonary dysfunction caused by diffuse lung inflammation. Roles of metabolites of arachidonic acid. Prog Biochem Pharmacol. 1985;20:26–37. [PubMed] [Google Scholar]
- 42.Cornicelli JA, Trivedi BK. 15-Lipoxygenase and its inhibition: a novel therapeutic target for vascular disease. Curr Pharm Des. 1999;5:11–20. [PubMed] [Google Scholar]
- 43.Dailey LA, Imming P. 12-Lipoxygenase: classification, possible therapeutic benefits from inhibition, and inhibitors. Curr Med Chem. 1999;6:389–398. [PubMed] [Google Scholar]
- 44.Hampson AJ, Grimaldi M. 12-hydroxyeicosatetrenoate (12-HETE) attenuates AMPA receptor-mediated neurotoxicity: evidence for a G-protein-coupled HETE receptor. J Neurosci. 2002;22:257–264. doi: 10.1523/JNEUROSCI.22-01-00257.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu D, Medhora M, Campbell WB, Spitzbarth N, Baker JE, Jacobs ER. Chronic hypoxia activates lung 15-lipoxygenase, which catalyzes production of 15-HETE and enhances constriction in neonatal rabbit pulmonary arteries. Circ Res. 2003;92:992–1000. doi: 10.1161/01.RES.0000070881.65194.8F. [DOI] [PubMed] [Google Scholar]
- 46.Pease JE, Sabroe I. The role of interleukin-8 and its receptors in inflammatory lung disease: implications for therapy. Am J Respir Med. 2002;1:19–25. doi: 10.1007/BF03257159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Natarajan R, Yang DC, Lanting L, Nadler JL. Key role of P38 mitogen-activated protein kinase and the lipoxygenase pathway in angiotensin II actions in H295R adrenocortical cells. Endocrine. 2002;18:295–301. doi: 10.1385/ENDO:18:3:295. [DOI] [PubMed] [Google Scholar]
- 48.Bleich D, Chen S, Wen Y, Nadler JL. The stress-activated c-Jun protein kinase (JNK) is stimulated by lipoxygenase pathway product 12-HETE in RIN m5F cells. Biochem Biophys Res Commun. 1997;230:448–451. doi: 10.1006/bbrc.1996.5981. [DOI] [PubMed] [Google Scholar]
- 49.Wen Y, Gu J, Knaus UG, Thomas L, Gonzales N, Nadler JL. Evidence that 12-lipoxygenase product 12-hydroxyeicosatetraenoic acid activates p21-activated kinase. Biochem J. 2000;349:481–487. doi: 10.1042/0264-6021:3490481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuhn H, Chan L. The role of 15-lipoxygenase in atherogenesis: pro- and antiatherogenic actions. Curr Opin Lipidol. 1997;8:111–117. doi: 10.1097/00041433-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 51.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. Faseb J. 2008 doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Funk CD, Cyrus T. 12/15-lipoxygenase, oxidative modification of LDL and atherogenesis. Trends Cardiovasc Med. 2001;11:116–124. doi: 10.1016/s1050-1738(01)00096-2. [DOI] [PubMed] [Google Scholar]
- 53.Serhan CN. Lipoxins and novel aspirin-triggered 15-epi-lipoxins (ATL): a jungle of cell-cell interactions or a therapeutic opportunity? Prostaglandins. 1997;53:107–137. doi: 10.1016/s0090-6980(97)00001-4. [DOI] [PubMed] [Google Scholar]
- 54.Takata S, Matsubara M, Allen PG, Janmey PA, Serhan CN, Brady HR. Remodeling of neutrophil phospholipids with 15(S)-hydroxyeicosatetraenoic acid inhibits leukotriene B4-induced neutrophil migration across endothelium. J Clin Invest. 1994;93:499–508. doi: 10.1172/JCI116999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brezinski ME, Serhan CN. Selective incorporation of (15S)-hydroxyeicosatetraenoic acid in phosphatidylinositol of human neutrophils: agonist-induced deacylation and transformation of stored hydroxyeicosanoids. Proc Natl Acad Sci U S A. 1990;87:6248–6252. doi: 10.1073/pnas.87.16.6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Legrand AB, Lawson JA, Meyrick BO, Blair IA, Oates JA. Substitution of 15-hydroxyeicosatetraenoic acid in the phosphoinositide signaling pathway. J Biol Chem. 1991;266:7570–7577. [PubMed] [Google Scholar]
- 57.Zhou W, Wang XL, Kaduce TL, Spector AA, Lee HC. Impaired arachidonic acid-mediated dilation of small mesenteric arteries in Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol. 2005;288:H2210–2218. doi: 10.1152/ajpheart.00704.2004. [DOI] [PubMed] [Google Scholar]
- 58.Ye H, Bi HR, Lu CL, Tang XB, Zhu DL. 15-hydroxyeicosatetraenoic acid depressed endothelial nitric oxide synthase activity in pulmonary artery. Sheng Li Xue Bao. 2005;57:612–618. [PubMed] [Google Scholar]
- 59.Lu C, Liu Y, Tang X, Ye H, Zhu D. Role of 15-hydroxyeicosatetraenoic acid in phosphorylation of ERK1/2 and caldesmon in pulmonary arterial smooth muscle cells. Can J Physiol Pharmacol. 2006;84:1061–1069. doi: 10.1139/y06-057. [DOI] [PubMed] [Google Scholar]