Abstract
Mitosis involves a generalized repression of gene expression. In the case of RNA polymerase III transcription, this is due to phosphorylation-mediated inactivation of TFIIIB, an essential complex comprising the TATA-binding protein TBP and the TAF subunits Brf1 and Bdp1. In HeLa cells, this repression is mediated by a mitotic kinase other than cdc2–cyclin B and is antagonized by protein phosphatase 2A. Brf1 is hyperphosphorylated in metaphase-arrested cells, but remains associated with promoters in condensed chromosomes, along with TBP. In contrast, Bdp1 is selectively released. Repression can be reversed by raising the concentration of Brf1 or Bdp1. The data support a model in which hyperphosphorylation disrupts TFIIIB during mitosis, compromising its ability to support transcription.
Keywords: cell cycle/mitosis/pol III/TFIIIB/transcription
Introduction
Nuclear transcription is repressed during mitosis (reviewed by Gottesfeld and Forbes, 1997). An important step towards characterizing this regulation came with the discovery that mitotic repression can be reproduced in vitro using extracts prepared from synchronized cells. Hartl et al. (1993) demonstrated that RNA polymerase (pol) III transcription is inhibited substantially when Xenopus egg extracts are shifted into mitosis by addition of cyclin B. Although it had been postulated that chromatin condensation might be responsible for mitotic repression, pre-incubation with non-specific DNA to titrate out histones made no difference to the extent of inhibition (Hartl et al., 1993). The topoisomerase II inhibitor VM26, which blocks mitotic chromosome condensation, also failed to prevent repression (Hartl et al., 1993). Therefore, transcriptional inhibition during mitosis may not require condensed chromatin. In support of this, immunofluorescent and electron spectroscopic imaging has shown that pol II transcription of viral genes can be repressed at mitosis in the absence of chromatin condensation (Spencer et al., 2000).
Cyclin-dependent kinases (cdks) from mitotic frog extracts inhibit expression of pol III templates, an effect that is blocked by the kinase inhibitor 6-dimethylaminopurine (DMAP) (Hartl et al., 1993; Gottesfeld et al., 1994; Leresche et al., 1996). In contrast, cdks purified from interphase extracts do not inhibit transcription (Hartl et al., 1993; Gottesfeld et al., 1994). Gottesfeld et al. (1994) showed that cdc2–cyclin B is sufficient to repress expression of a Xenopus 5S rRNA gene. Although this implicated cdc2–cyclin B in pol III repression, removal of cdc2 from metaphase-arrested Xenopus egg extracts does not prevent the DMAP-sensitive inhibition of a tRNA gene (Hartl et al., 1993). Furthermore, cdc2-depleted mitotic extract can inhibit transcription when mixed with interphase extract (Hartl et al., 1993). The authors concluded that mitotic extracts contain at least one other kinase besides cdc2 that is capable of repressing pol III transcription.
TFIIIB is responsible for recruiting pol III to the initiation site of its templates (Kassavetis et al., 1990). It is a complex containing the TATA-binding protein (TBP) and two TBP-associated factors (TAFs) called Bdp1 and Brf1; the latter bears homology to the pol II-specific factor TFIIB (reviewed by Schramm and Hernandez, 2002). At most pol III promoters, TFIIIB is recruited by protein–protein interactions with the assembly factor TFIIIC, that binds downstream of the initiation site (reviewed by Geiduschek and Kassavetis, 2001; Schramm and Hernandez, 2002; White, 2002). Gottesfeld et al. (1994) showed that repression of Xenopus pol III transcription by cdc2 can be reversed by addition of highly purified TFIIIB, whereas TFIIIC has no effect. Although TFIIIB isolated from interphase extracts is highly efficient in this respect, TFIIIB from mitotic extracts is inactive, unless it is pre-treated with alkaline phosphatase (Gottesfeld et al., 1994). Furthermore, affinity-purified interphase TFIIIB can be inactivated using cdc2 (Gottesfeld et al., 1994). The data indicate that repression of pol III transcription in metaphase frog eggs involves inactivation of TFIIIB through the redundant action of cdc2 and one or more additional mitotic kinase. Kinases isolated from mitotic extracts using p13suc1–beads phosphorylate polypeptides of 33–34, 42 and 90–92 kDa that are present in affinity-purified TFIIIB fractions (Leresche et al., 1996). The 33 kDa species co-migrates with Xenopus TBP (Leresche et al., 1996). Furthermore, recombinant TBP can be phosphorylated in vitro using mitotic kinases, but not with purified cdc2 (Gottesfeld et al., 1994). The 92 kDa species is the same size as a TAF present in Xenopus TFIIIB (McBryant et al., 1996).
Whereas the cell cycle in early Xenopus embryos consists of a simple fluctuation between S and M phases, somatic cells undergo a more complex cycle that involves sequential passage through G1, S, G2 and M. Nevertheless, mitotic HeLa cells repress pol III transcription by a mechanism that resembles the situation in frog eggs (White et al., 1995b). Thus, TFIIIB is inactivated specifically in extracts prepared from HeLa cells synchronized in M phase (White et al., 1995b). TBP becomes hyperphosphorylated during mitosis, but this does not account for the reduction in pol III transcription, since expression cannot be restored by the addition of recombinant TBP to mitotic extracts (White et al., 1995b). In contrast, affinity-purified TFIIIB TAFs reverse repression efficiently, and can restore transcription in M phase extracts (White et al., 1995b). Direct assays confirmed that the TAF component of TFIIIB is inactivated at mitosis (White et al., 1995b), but vertebrate pol III TAF sequences had not been cloned when that study was conducted and so reagents were not available to allow more precise analysis.
Extensive purification of human TFIIIB has since allowed cloning of cDNAs that encode Brf1 (Wang and Roeder, 1995; Mital et al., 1996). Human Bdp1 cDNAs were isolated subsequently on the basis of homology with the yeast sequence (Schramm et al., 2000). Although Brf1 makes direct contacts with both TFIIIC and pol III (Geiduschek and Kassavetis, 2001; Schramm and Hernandez, 2002; White, 2002), Bdp1 is also necessary for productive polymerase recruitment and initiation at supercoiled class III genes (Kassavetis et al., 2001). Now that these human TAFs are available for molecular analysis, we have investigated in more detail how TFIIIB behaves at mitosis. We find that Brf1 is hyperphosphorylated when HeLa cells are arrested in metaphase, but remains associated with template DNA. In contrast, Bdp1 is released from promoters, which precludes the productive recruitment of pol III. We propose a model in which hyperphosphorylation of Brf1 at mitosis is associated with disruption of TFIIIB and release of one of its essential subunits.
Results
Pol III transcriptional activity in extracts of mitotic HeLa cells is determined by a balance between kinases and phosphatases
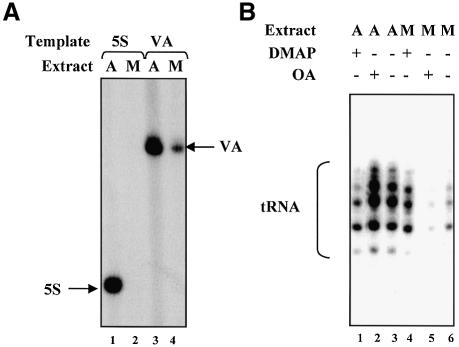
Cycling HeLa cells were arrested in metaphase by treatment with nocodozole. Extracts of these mitotic cells were compared with extracts of asynchronous cells, which are predominantly (96–98%) in interphase (Terasima and Tolmach, 1963). As expected, extracts of mitotic HeLa cells are severely compromised in their ability to support transcription by pol III (Figure 1A).
Fig. 1. Mitotic repression of pol III transcription is mediated by phosphorylation. (A) Class III genes are transcribed more efficiently by asynchronous extracts than by mitotic extracts. pHu5S3.1 (lanes 1 and 2) or pVA1 (lanes 3 and 4) templates (500 ng) were transcribed using 14 µg of extract prepared from either asynchronous (lanes 1 and 3) or mitotic (lanes 2 and 4) cells. (B) Mitotic repression of pol III transcription can be relieved using a kinase inhibitor and accentuated with a phosphatase inhibitor. pGlu6 (500 ng) was transcribed using 30 µg of either asynchronous (lanes 1–3) or mitotic extract (lanes 4–6). Reactions in lanes 1 and 4 contained 2.4 mM DMAP; those in lanes 2 and 5 contained 2 µM okadaic acid.
To assess the importance of protein kinases in maintaining repression in the mitotic extract, we tested the effect of adding DMAP, a nucleotide analogue that inhibits a broad range of protein kinases (Vesely et al., 1994). When added to extracts of asynchronous cells, DMAP inhibited transcription of a tRNA gene (Figure 1B, lanes 1 and 3). This is consistent with the fact that CK2 and ERK kinases stimulate pol III transcription in HeLa cells (Johnston et al., 2002; Felton-Edkins et al., 2003). However, pre-incubating DMAP with mitotic extract resulted in a substantial increase in tRNA gene expression (compare lanes 4 and 6). Indeed, in the presence of DMAP, the transcriptional activity of the mitotic extract was comparable with that of asynchronous extract (lanes 3 and 4). These results suggest that one or more kinases repress the pol III transcription apparatus at mitosis, but do not operate during interphase.
The ability of DMAP to activate transcription implies the presence in mitotic extracts of protein phosphatase(s) that can dephosphorylate transcription factors once kinase activity is suppressed. To examine this possibility, we tested the effect of adding okadaic acid, a selective inhibitor of certain protein phosphatases (Cohen et al., 1990). The low level of tRNA transcription in mitotic extracts was reduced even further by okadaic acid (Figure 1B, lanes 5 and 6). This confirms that one or more phosphatases antagonizes the repressive effect of the mitotic kinase(s). In contrast, okadaic acid did not depress transcription when added to asynchronous extracts (lanes 2 and 3). We conclude that the activity of the pol III transcription apparatus is controlled in mitotic HeLa cells by a balance between kinase and phosphatase activities.
Cdc2 activity is not required to maintain repression of pol III transcription in extracts of mitotic HeLa cells
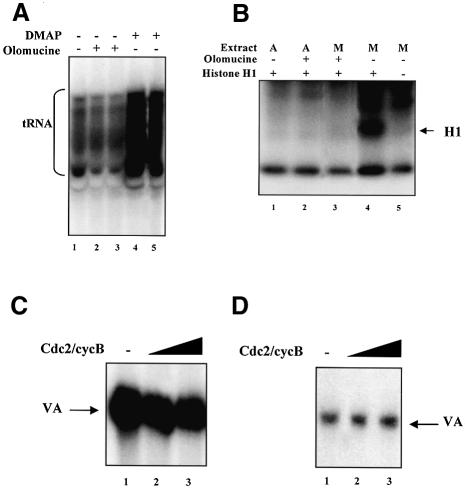
The ability of DMAP to restore pol III activity in a mitotic HeLa cell extract (Figure 1B) demonstrates that one or more protein kinases are required to maintain repression. Since the mitotic kinase cdc2–cyclin B can inactivate TFIIIB in Xenopus (Gottesfeld et al., 1994), we investigated whether it performs a similar function in a human cell system. For this purpose, we employed the purine analogue olomoucine, a potent inhibitor of cdc2–cyclin B (IC50 ∼7 µM) and cdc2–cyclin A (IC50 ∼50 µM) that is ineffective against most kinases that are not cdks (Vesely et al., 1994). Olomoucine caused no activation of tRNA synthesis when pre-incubated with a mitotic extract, even at a final concentration of 500 µM (Figure 2A). In contrast, the same concentration of DMAP produced a strong increase in tRNA expression, even though DMAP is much less effective than olomoucine against cdc2–cyclin B (IC50 ∼120 µM) (Vesely et al., 1994). To confirm the efficacy of olomoucine in our system, we tested its ability to inhibit phosphorylation of histone H1, a well-documented substrate of cdc2–cyclin B (Figure 2B). As expected, mitotic extracts contain a strong H1 kinase activity that is not seen in asynchronous extracts (compare lanes 1 and 4); this activity is inhibited efficiently by olomoucine concentrations that cause no increase in tRNA expression (lanes 3 and 4). We conclude that the mitotic repression of pol III transcription is maintained even if cdc2 is inhibited.
Fig. 2. Cyclin B-dependent kinases are not required to maintain mitotic repression of human pol III transcription. (A) Mitotic repression of pol III transcription can be relieved using DMAP but not olomoucine. pGlu6 (500 ng) was transcribed using 25 µg of mitotic extract that had been pre-incubated for 10 min at 30°C in the presence of buffer alone (lane 1), 0.5 mM olomoucine (lanes 2 and 3), 0.5 mM DMAP (lane 4) or 3 mM DMAP (lane 5). (B) Mitotic H1 kinase activity is inhibited efficiently by 0.5 mM olomoucine. Asynchronous (lanes 1 and 2) or mitotic (lanes 3–5) extracts (10 µg) were incubated for 10 min at 30°C in the presence of [γ-32P]ATP. Histone H1 (200 ng) was included in lanes 1–4. Olomoucine (0.5 mM) was included in lanes 2 and 3. (C) Recombinant cdc2–cyclin B has little effect on VA1 transcription in HeLa extract. Extract of asynchronous HeLa cells (10 µg) was incubated for 15 min at 30°C in the presence of 0.5 mM ATP and either buffer (lane 1), or 1 and 2 µl of baculovirus-expressed cdc2–cyclin B (lanes 2 and 3, respectively). Transcription was then started by addition of pVA1 (250 ng) and nucleotides. (D) Cdc2–cyclin B purified from mitotic HeLa cells has little effect on VA1 transcription using partially purified factors. CHep-1.0 (2.5 µl) and DE-1.0 (2.5 µl) fractions were incubated for 15 min at 30°C in the presence of 0.6 mM ATP, 4 mM vanadate and either buffer (lane 1), or 1 and 3 µl of immunopurified cyclin B-dependent kinase (lanes 2 and 3, respectively). Transcription was then started by addition of pVA1 (250 ng) and nucleotides.
The apparent lack of a requirement for cdc2–cyclin B to maintain inhibition in a mitotic extract may reflect redundancy in the control mechanisms. We therefore tested directly whether cdc2–cyclin B will repress pol III transcription in HeLa extracts. Baculovirus-expressed recombinant cdc2–cyclin B was pre-incubated with asynchronous extract in the presence of ATP; after 15 min, we added nucleotides and template and then assayed for transcription (Figure 2C). Inclusion of cdc2–cyclin B made little difference to the level of VA1 expression. Since asynchronous extract might contain activities which antagonize the action of cdc2–cyclin B, we repeated these experiments using extracts of mitotic cells, but again the kinase had little effect (data not shown). We also tested cdc2–cyclin B that had been immunopurified from mitotic HeLa cells. As with the recombinant protein, this kinase had little effect on pol III output when added to crude extract. It was also used in a reconstituted system composed of partially purified transcription factors, but again failed to inhibit expression (Figure 2D). Several preparations of recombinant and natural cdc2–cyclin B were tested and the kinase activity of these was confirmed using histone H1 as substrate. Some kinase preparations produced weak inhibitory effects that did not require ATP and could not be blocked by DMAP; this was presumed to be caused by contaminants. However, we have been unable to detect any consistent response to added cdc2–cyclin B that is dependent on kinase activity.
The inhibitory effect of mitotic kinase(s) on pol III transcription is antagonized specifically by PP2A
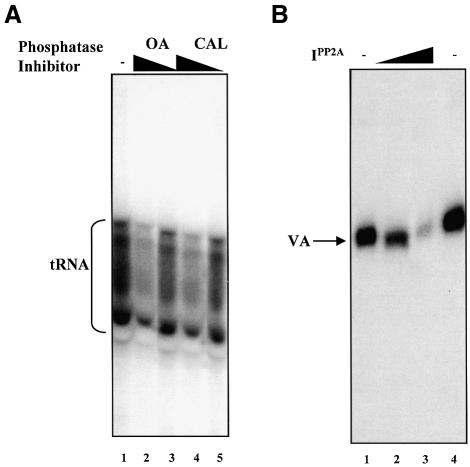
As the phosphatase inhibitor okadaic acid depresses the level of tRNA synthesis in mitotic extracts (Figure 1B), one or more endogenous phosphatases must antagonize the repressive effect of the kinase(s) present at metaphase. We adopted a pharmacological approach to identify the phosphatase(s) responsible for this effect. In Figure 1B, we used a concentration of okadaic acid (2.5 µM) that will inhibit a range of protein phosphatases (Cohen et al., 1990). This decreased tRNA production by ∼2-fold when added to mitotic extract. When used in the nanomolar range, okadaic acid displays greater specificity for the PP1 and PP2A families of serine/threonine phosphatases (Cohen et al., 1990). We found that 100 nM okadaic acid was just as effective at depressing tRNA synthesis, again producing an ∼2-fold decrease (Figure 3A, lanes 1 and 2). Indeed, a slight reduction (1.3-fold) was seen with only 0.2 nM okadaic acid (lane 3). We also tested the unrelated toxin calyculin A, another potent inhibitor of PP1 and PP2A (Cohen et al., 1990). Calyculin A produced a 2-fold decrease in transcription of the tRNA gene at 100 nM (lane 4) and a 1.5-fold decrease at 0.2 nM (lane 5). These results suggest that PP1 and/or PP2A may counteract repression of TFIIIB in mitotic HeLa cells.
Fig. 3. PP2A inhibitors depress pol III transcription in extracts of mitotic HeLa cells. (A) Mitotic repression of pol III transcription can be accentuated using okadaic acid or calyculin A. pGlu6 (500 ng) was transcribed using 25 µg of mitotic extract that had been pre-incubated for 10 min at 30°C in the presence of buffer alone (lane 1), 100 or 0.2 nM okadaic acid (lanes 2 and 3, respectively), and 100 or 0.2 nM calyculin A (lanes 4 and 5, respectively). (B) PP2A inhibitor polypeptide depresses pol III transcription in extracts of mitotic HeLa cells. pVAI (250 ng) was transcribed with 25 µg of mitotic extract pre- incubated for 10 min at 30°C in the presence of buffer alone (lanes 1 and 4), and 25 or 100 ng of I1PP2A (lanes 2 and 3, respectively).
Whereas PP2A displays a similar sensitivity to okadaic acid and calyculin A, PP1 is 10- to 100-fold more sensitive to the latter (Cohen et al., 1990). The fact that these toxins were equally potent in reducing tRNA synthesis by a mitotic extract (Figure 3A) suggests that the phosphatase responsible is PP2A rather than PP1. To confirm this, we tested I1PP2A, a polypeptide inhibitor with high potency and apparently absolute specificity for PP2A (Li et al., 1995). This decreased pol III transcription efficiently when added to mitotic extract (Figure 3B). These results implicate endogenous PP2A as being responsible for antagonizing the inhibitory effect on TFIIIB of kinase(s) active in metaphase-arrested HeLa cells.
The TFIIIB-specific TAF Brf1 is hyperphosphorylated during mitosis
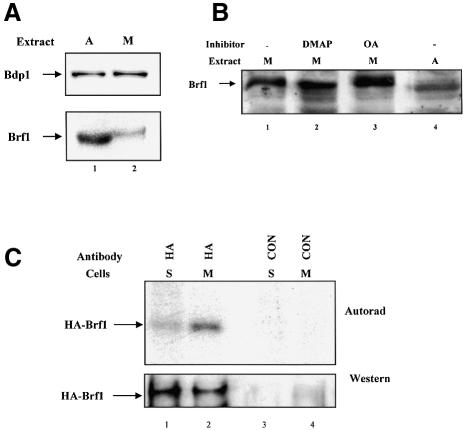
It was found previously that repression of pol III transcription in mitotic extracts can be reversed by titration of affinity-purified fractions containing TFIIIB TAFs, whereas TFIIIC, pol III or recombinant TBP do not restore expression (White et al., 1995b). This led to the model that mitotic repression is mediated through specific inactivation of one or more of the TAF subunits of TFIIIB (White et al., 1995b). Further characterization was not possible at that time, as the TAF components of human TFIIIB had not been identified. However, it is now established that the TAF component of human TFIIIB comprises the essential subunits Brf1 and Bdp1 (Schramm and Hernandez, 2002). As the results above suggest that repression is mediated through phosphorylation, we used immunoblotting to look for changes in the electrophoretic mobility of the TFIIIB TAFs, that might result from addition of phosphate groups. Although the mobility of Bdp1 remains constant after entry into mitosis, Brf1 in mitotic extracts migrates more slowly than Brf1 in asynchronous cell extracts (Figure 4A). That this shift in mobility is due to phosphorylation was suggested by using kinase and phosphatase inhibitors (Figure 4B). Pre- incubation with DMAP increased the mobility of mitotic Brf1 (lane 2), whereas inclusion of okadaic acid had the opposite effect, accentuating the slow migrating forms of mitotic Brf1 (lane 3). Thus, kinase and phosphatase inhibitors that alter the activity of the pol III transcription apparatus have a parallel effect on the electrophoretic mobility of Brf1.
Fig. 4. The Brf1 subunit of TFIIIB is hyperphosphorylated during mitosis. (A) The electrophoretic mobility of Brf1 decreases at mitosis. Asynchronous (lane 1) and mitotic (lane 2) extracts (50 µg) were resolved on a 7.8% SDS–polyacrylamide gel and analysed by western immunoblotting with anti-Bdp1 antibody 2663 (upper panel) and anti-Brf1 antibody 330 (lower panel). (B) The altered mobility of Brf1 at mitosis is due to phosphorylation. Extracts (30 µg) of mitotic (lanes 1–3) or asynchronous (lane 4) cells were incubated for 35 min at 30°C in the presence of 0.5 mM ATP. Reactions shown in lanes 2 and 3 also contained 2.4 mM DMAP or 2 µM okadaic acid, respectively. Samples were resolved on a 10% SDS–polyacrylamide gel and immunoblotted with anti-Brf1 antibody CSH409. (C) Brf1 is hyperphosphorylated in metaphase-arrested cells. HeLa cells were transiently transfected with pcDNA3HA.BRF (10 µg) and then synchronized in S or M phases and labelled with [32P]orthophosphate for 3 h prior to harvesting. Proteins immunoprecipitated with anti-HA antibody F-7 (lanes 1 and 2) or non-immune serum (lanes 3 and 4) were resolved on a 7.8% SDS–polyacrylamide gel, transferred to nitrocellulose and then visualized by autoradiography (top) and western blotting with F-7 (bottom).
In vivo labelling was used to test if Brf1 becomes hyperphosphorylated at mitosis in living cells. HeLa cells were transfected with haemagglutinin (HA)-tagged Brf1, synchronized in S or M phases and then labelled with [32P]orthophosphate; S phase synchronization was used to remove the ∼2% of mitotic cells that are present in an asynchronous population. Protein immunoprecipitated using either anti-HA antibody or a pre-immune control was resolved on a denaturing gel and subjected to autoradiography and western blotting. Although comparable amounts of Brf1 were immunoprecipitated from the two cell populations, phosphate labelling was ∼6-fold higher in the mitotic cells (Figure 4C). Specificity was confirmed by the absence of Brf1 in the control immunoprecipitates. We conclude that Brf1 is hyperphosphorylated by one or more kinases that become active at metaphase.
Promoter occupancy by TFIIIB is compromised during mitosis
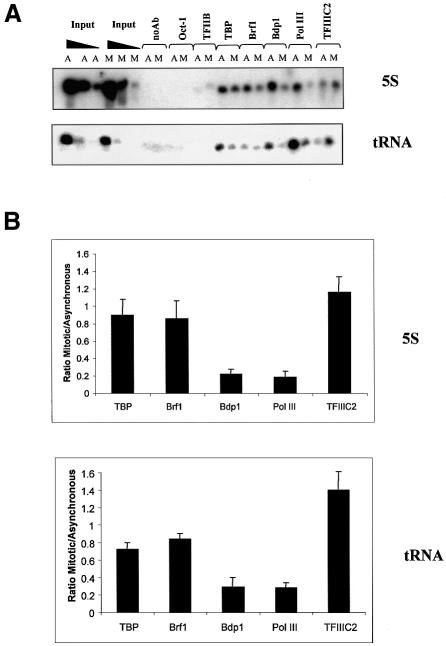
Mitotic phosphorylation has been shown to dissociate several transcription factors from condensing chromosomes, such as Oct-1, Sp1 and Ikaros (Segil et al., 1991; Martinez-Balbas et al., 1995; Dovat et al., 2002). We therefore investigated whether TFIIIB might also be released in this way. To this end, chromatin immunoprecipitation (ChIP) assays were used to compare promoter occupancy between asynchronous and mitotic cells in vivo (Figure 5). As expected, TFIIIC2, TFIIIB and pol III were all detected at 5S rRNA and tRNA genes, whereas little or no Oct-1 or TFIIB was found at these sites. Antibodies against the individual subunits of TFIIIB revealed a slight reduction (∼20%) in the amounts of TBP and Brf1 associated with promoters in mitotic cells compared with asynchronous populations. However, occupancy of the third TFIIIB subunit, Bdp1, was more substantially diminished. Levels of bound pol III paralleled those of Bdp1, consistent with the fact that Bdp1 is necessary for efficient recruitment of pol III onto templates (Kassavetis et al., 2001). In contrast to these results, TFIIIC2 occupancy was undiminished at mitosis. Indeed, we reproducibly observed an increase in TFIIIC2 signals in mitotic cells. Although this might reflect increased binding, it might also result from an increase in antibody accessibility due to diminished association of pol III and TFIIIB. Whatever the cause, it confirms that the observed reductions in occupancy by TFIIIB and pol III are specific. The data suggest that Bdp1 and pol III are preferentially released from mitotic chromatin.
Fig. 5. Promoter occupancy by TFIIIB is compromised during mitosis. (A) ChIP assay showing levels of Oct-1, TFIIB, TBP, Brf1, Bdp1, pol III (BN51 subunit) and TFIIIC2 (TFIIIC110 subunit) associated with 5S rRNA and tRNAArg genes in asynchronous (A) or mitotic (M) HeLa cells, as indicated. Serial dilutions of input DNA confirm that PCRs are within the linear range and that A and M samples utilize equivalent amounts of input. (B) Quantitation of ChIP assays. PCR products from three independent ChIP experiments were quantified for 5S rRNA (top) and tRNAArg genes (bottom); after normalization to input, the mean and standard deviations are shown for the ratio of mitotic/asynchronous signals.
As an independent test of this, purified metaphase chromosome preparations were examined by immunoblotting. As reported (Segil et al., 1991), Oct-1 was barely detectable in the metaphase chromosomes, showing that they are relatively free of contamination (Figure 6). In contrast, TBP was readily detected, consistent with previous evidence that some TFIID remains tightly associated with condensed mitotic chromosomes (Segil et al., 1996; Christova and Oelgeschläger, 2001; Chen et al., 2002). Similarly, immunoblotting revealed the presence of Brf1, as shown previously (Chen et al., 2002). A substantial proportion of this chromosomally associated Brf1 was found to migrate more slowly than the Brf1 present in asynchronous cells, consistent with its reduced mobility in mitotic extracts (Figure 4A). Some faster migrating Brf1 was also detected in the metaphase chromosomes; it is unclear whether this reflects the existence of distinct forms in vivo, or partial dephosphorylation during harvesting. Nevertheless, these observations suggest that hyperphosphorylation does not release Brf1 from chromosomes during M phase. In contrast to TBP and Brf1, the third TFIIIB subunit, Bdp1, was barely detectable in the same chromosome preparations (Figure 6). This was also true of RPC155, the largest subunit of pol III. However, TFIIIC220, the DNA-binding subunit of TFIIIC2, was found associated with the purified metaphase chromosomes (Figure 6). These observations therefore support the ChIP data and point to a differential displacement from mitotic chromatin of components of the pol III machinery, with Bdp1 and pol III being released preferentially.

Fig. 6. TBP and Brf1 remain associated with purified metaphase chromosomes, whereas Bdp1 is not detectable. Metaphase chromosomes (50 µl; lane 1) and asynchronous cell extracts (50 µg; lane 2) were resolved on a 7.8% SDS–polyacrylamide gel and then analysed by western immunoblotting with anti-TBP antibody 58C9, anti-Brf1 antibody 128, anti-Bdp1 antibody 2663, anti-pol III antibody 1900, anti-TFIIIC220 antibody Ab2 and anti-Oct-1 antibody C-21, as indicated.
The affinity of Brf1 for Bdp1 may be diminished during mitosis
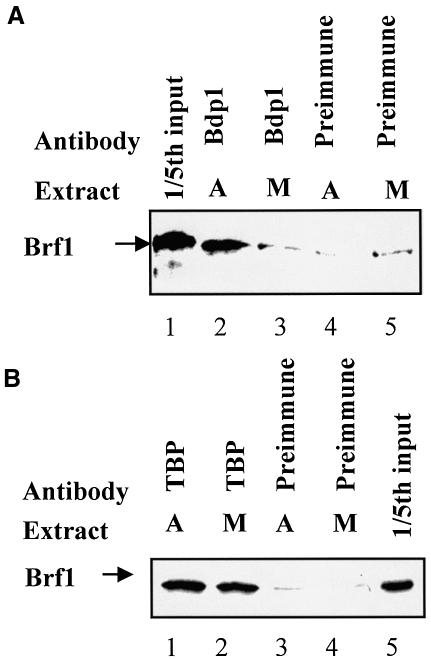
On TATA-less promoters such as 5S rRNA and tRNAArg, TFIIIB is recruited through binding of Brf1 to DNA-bound TFIIIC2; the presence of Bdp1 in turn depends on its interaction with Brf1 (Geiduschek and Kassavetis, 2001; Schramm and Hernandez, 2002). The mitotic release of Bdp1 from these genes suggests that its affinity for Brf1 may be diminished during this phase of the cell cycle. To test this, we carried out co-immunoprecipitations to monitor the Brf1–Bdp1 interaction. Brf1 was labelled with 35S by translation in vitro and then mixed with extract of asynchronous or metaphase-arrested HeLa cells. Although not apparent under the running conditions used for Figure 7, a differential shift in electrophoretic mobility indicates that the exogenous Brf1 becomes hyperphosphorylated in the mitotic extract (data not shown). With asynchronous extracts, antiserum against Bdp1 was able to co-immunoprecipitate Brf1, but with mitotic extracts this was reduced to the background levels observed with pre-immune serum (Figure 7A). In contrast, similar amounts of Brf1 were co-immunoprecipitated with TBP from mitotic and asynchronous extracts (Figure 7B). These observations suggest that the interaction between Bdp1 and Brf1 may be selectively destabilized at M phase. This might explain the observed release of Bdp1 from metaphase chromosomes.
Fig. 7. The Brf1–Bdp1 interaction may be compromised specifically at mitosis. (A) Reticulocyte lysate (10 µl) containing in vitro-translated Brf1 was mixed with extract (150 µg) of asynchronous (lanes 2 and 4) or metaphase-arrested HeLa cells (lanes 3 and 5) prior to immunoprecipitation with anti-Bdp1 antiserum (lanes 2 and 3) or pre-immune serum (lanes 4 and 5). Proteins retained after extensive washing were resolved by SDS–PAGE and visualized by autoradiography. Lane 1 shows 20% of the input reticulocyte lysate. (B) As above, except that 20% input is shown in lane 5 and the immunoprecipitations used anti-TBP antibody (lanes 1 and 2) or pre-immune serum (lanes 3 and 4).
Mitotic repression of pol III transcription can be overcome by raising the concentration of Brf1 or Bdp1
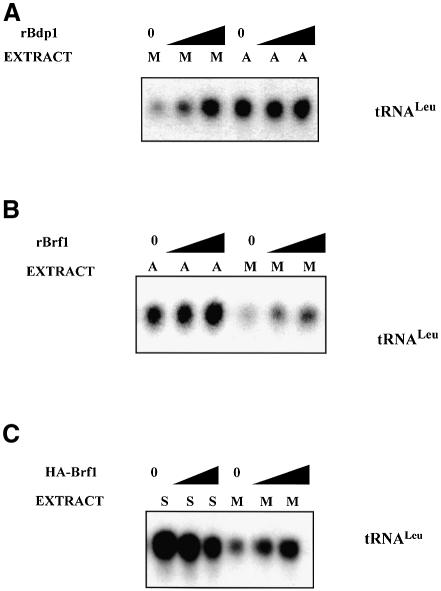
If mitotic repression of class III genes reflects dissociation of TFIIIB due to a compromised interaction between its subunits, it might be possible to recover active transcription by raising the concentration of one or more of these subunits, thereby favouring assembly of TFIIIB complexes. Although this was unsuccessful with recombinant TBP (White et al., 1995b), the rate of pol III transcription was increased significantly when recombinant Bdp1 or Brf1 was titrated into mitotic cell extracts (Figure 8A and B). These effects are specific to mitosis, since neither TFIIIB subunit produced significant stimulation when titrated into asynchronous cell extracts at the same concentrations. This is consistent with previous evidence that TFIIIC is limiting for pol III transcription in HeLa cells during most of interphase (White et al., 1995a). Although we have not yet succeeded in expressing Bdp1 in transfected cells, it was possible to confirm in vivo that raising Brf1 levels can overcome mitotic repression of pol III templates. Thus, HeLa cells were transfected with a vector encoding Brf1 and then synchronized and harvested in either S or M phase. Overexpressing Brf1 in S phase cells caused a slight reduction in tRNA gene transcription, perhaps because it created an imbalance or squelching effect (Figure 8C). In contrast, the tRNA gene was clearly induced by raising the Brf1 concentration in mitotic cells. A similar response was obtained using the VA1 gene as template (data not shown). We conclude that repression of pol III transcription can be relieved by raising the concentration of Brf1 in metaphase-arrested HeLa cells.
Fig. 8. Overexpression of Bdp1 or Brf1 can rescue pol III transcription in mitotic cell extracts. (A) Transcription of pLeu using 10 µg of mitotic (lanes 1–3) or asynchronous (lanes 4–6) cell extract in the presence of 1.5 (lanes 2 and 5) or 3 µl (lanes 3 and 6) of bacterially expressed recombinant Bdp1. (B) Transcription of pLeu using 10 µg of asynchronous (lanes 1–3) or mitotic (lanes 4–6) cell extract in the presence of 1.5 (lanes 2 and 5) or 3 µl (lanes 3 and 6) of bacterially expressed recombinant Brf1. (C) Transcription of pLeu using 10 µg of extract made from HeLa cells transfected with empty vector (lanes 1 and 4), 5 µg of pcDNA3HA.BRF (lanes 2 and 5) or 10 µg of pcDNA3HA.BRF (lanes 3 and 6). After transfection, the cells were synchronized in S (lanes 1–3) or M (lanes 4–6) phases prior to harvesting.
Discussion
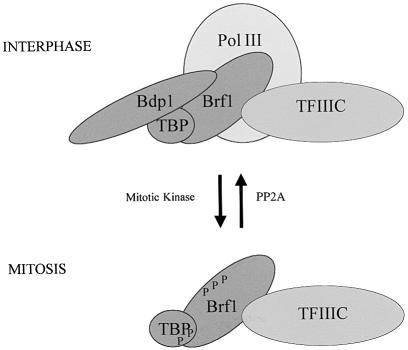
Use of kinase and phosphatase inhibitors strongly implicates protein phosphorylation in suppressing pol III transcription in mitotic HeLa cells. Unlike the situation in Xenopus eggs, we find no evidence that this is a direct effect of cdc2–cyclin B. However, PP2A is implicated in counteracting the inhibitory effect. Previous work demonstrated a specific inactivation of the TAF component of TFIIIB at mitosis (White et al., 1995b). In support of this, we find that Brf1 is hyperphosphorylated during metaphase. Furthermore, overexpression of Brf1 can overcome mitotic repression both in vitro and in vivo. This suggests that metaphase-arrested cells have insufficient Brf1 activity to support efficient pol III transcription. However, recombinant Bdp1 can also restore expression in mitotic extracts. Indeed, Bdp1 is displaced from condensed metaphase chromosomes, whereas Brf1 remains associated with tRNA and 5S rRNA genes in vivo. Co-immunoprecipitation experiments suggest that the Brf1–Bdp1 interaction is weakened at mitosis. On the basis of these observations, we propose a model in which hyperphosphorylation of Brf1 during M phase may reduce its affinity for Bdp1, allowing release of the latter (Figure 9). This can be expected to compromise pol III recruitment and thereby inhibit transcription. Raising the concentration of either Brf1 or Bdp1 can push the equilibrium towards complex formation and thereby recover transcription despite kinase activity. Brf1 makes direct contact with pol III, and it is possible that this interaction is also affected by mitotic phosphorylation, independently of the effect on Bdp1. Although we do not exclude this, it is the changes to TFIIIB that appear to be crucial, since mitotic repression cannot be relieved by titrating pol III (White et al., 1995b).
Fig. 9. Model to explain the repression of pol III transcription in mitotic HeLa cells. Hyperphosphorylation of Brf1 by one or more kinases at mitosis may lead to a diminished interaction with Bdp1 and release of the latter from chromosomal templates; this will compromise pol III recruitment and gene expression. TBP is also hyperphosphorylated during M phase, but there is no evidence that this has functional significance. Other components of the pol III machinery are also likely to undergo mitotic phosphorylation and this may contribute to transcriptional repression.
The apparent loss of Brf1 function correlates with hyperphosphorylation of this TAF in vivo. The effects of DMAP and okadaic acid on the phosphorylation state of Brf1, as determined by its electrophoretic mobility, parallel the effects of these inhibitors on pol III transcription. Thus, okadaic acid depresses tRNA expression in metaphase extracts, whereas DMAP raises it to levels obtained with asynchronous cell extracts (Figure 1B). Neither of these responses are seen when DMAP or okadaic acid are added to extracts of asynchronous cells. We conclude that metaphase cells contain one or more kinases that phosphorylate and inactivate Brf1 and repress pol III transcription. Neither electrophoretic mobility analysis nor in vivo labelling have provided any evidence that Bdp1 is subject to differential phosphorylation during mitosis, but we cannot exclude the possibility of subtle changes.
Our results with HeLa cells suggest clear similarities with the situation in metaphase-arrested frog eggs, despite the substantial differences between the early embryonic cell cycle of amphibians and the more complex cycle of somatic mammalian cells. In both situations, mitotic repression of pol III transcription is achieved through specific phosphorylation of TFIIIB. TBP is hyperphosphorylated during metaphase in both frogs and humans, but in neither case is this thought to be sufficient to account for the inactivation of TFIIIB (White et al., 1995b; Leresche et al., 1996). Instead, mitotic repression involves the phosphorylation-dependent inactivation of TAF subunits of TFIIIB (White et al., 1995b; Leresche et al., 1996). Leresche et al. (1996) found that mitotic kinases phosphorylate a 90–92 kDa polypeptide that is present in affinity-purified preparations of Xenopus TFIIIB. Since human Brf1 is ∼90 kDa, they speculated that this polypeptide may correspond to the Xenopus homologue of this TAF (Leresche et al., 1996). However, this could not be confirmed, since a gene encoding Brf1 has yet to be isolated from amphibians. Our data clearly support this hypothesis, by demonstrating that Brf1 is subject to mitotic phosphorylation in HeLa cells. It therefore seems likely that phosphorylation of Brf1 is a conserved mechanism for the cell cycle control of pol III transcription.
Metaphase-arrested Xenopus eggs contain at least two protein kinase activities that are capable of repressing pol III transcription (Hartl et al., 1993; Gottesfeld et al., 1994; Leresche et al., 1996). One of these is cdc2–cyclin B, which can inactivate purified TFIIIB in vitro (Gottesfeld et al., 1994; Leresche et al., 1996). However, there is redundancy in the Xenopus system, since repression is maintained if mitotic egg extracts are depleted of cdc2–cyclin B (Hartl et al., 1993). Despite repeated attempts, we find no consistent evidence that phosphorylation by cdc2 has a direct effect on the human pol III machinery, although cdc2 might have an effect under different assay conditions. Nevertheless, use of the specific inhibitor olomoucine provides evidence that cdc2 is not required to maintain repression in a mitotic cell extract. Thus, concentrations of olomoucine that block cdc2 activity have no stimulatory effect on pol III transcription. In contrast, the general kinase inhibitor DMAP, when tested in parallel and at the same concentration, restores transcription in extracts of metaphase-arrested HeLa cells. We conclude that cdc2 is not necessary to maintain mitotic repression of class III gene expression in this human system.
Extracts of metaphase HeLa cells contain phosphatase activity that stimulates pol III transcription and appears to antagonize the effect of the mitotic kinase(s). Thus, three unrelated inhibitors of serine/threonine phosphatases can depress expression when added to extracts of M phase cells. Use of a polypeptide inhibitor with apparently absolute specificity for PP2A provided clear evidence that this group of phosphatases has a positive effect on pol III transcription during mitosis. This conclusion was reinforced by the observation that the PP2A inhibitors okadaic acid and calyculin A also decrease tRNA synthesis in mitotic cell extracts. This is consistent with genetic and biochemical analyses in yeast, which found that mutation of a regulatory subunit of PP2A causes Saccharomyces cerevisiae to stop synthesizing tRNA at the non-permissive temperature (Van Zyl et al., 1992). In extracts prepared from the mutant strain, the defect could be reversed by addition of partially purified TFIIIB (Van Zyl et al., 1992). These results implicate PP2A as a regulator of TFIIIB in vivo, a feature which may have been conserved through evolution.
Sp1 and Ikaros are both displaced from chromatin at mitosis due to phosphorylation of the linkers between their C2H2 zinc fingers (Dovat et al., 2002). It has been suggested that linker phosphorylation provides a global mechanism for inactivation of the C2H2 family of transcription factors (Dovat et al., 2002). The founding member of this family was TFIIIA, which has nine C2H2 zinc fingers (Miller et al., 1985). This factor nucleates initiation complex assembly on 5S rRNA genes and is essential for subsequent recruitment of TFIIIC and TFIIIB (reviewed by Geiduschek and Kassavetis, 2001; Schramm and Hernandez, 2002; White, 2002). Although we have not tested TFIIIA directly, its binding to 5S rRNA genes in mitotic chromosomes can be inferred from the presence of TFIIIC2 and Brf1. Our ChIP results therefore suggest that TFIIIA escapes the global mitotic displacement of C2H2 DNA-binding proteins that has been postulated. Perhaps its role as a platform for TFIIIC shields TFIIIA from mitotic kinases.
As well as changes to regulatory factors such as Sp1 and Ikaros, two components of the basal pol II transcription machinery have been shown to be inactivated at mitosis. One of these is the cdk7 subunit of TFIIH, which loses its ability to trigger initiation by phosphorylating pol II (Akoulitchev and Reinberg, 1998; Long et al., 1998). The second is TFIID, which becomes hyperphosphorylated on several TAFs and thereby loses responsiveness to upstream activators (Segil et al., 1996; Long et al., 1998). Similarly, mitotic repression of pol I transcription involves hyperphosphorylation of TAFI110 within the TBP-containing complex SL1, which compromises its interaction with the upstream activator UBF (Heix et al., 1998). Phosphorylation of TAFs at mitosis therefore appears to provide a mechanism for controlling transcription by all three nuclear RNA polymerases, since our data demonstrate that this is also the case for Brf1. Like the Brf1–TBP subcomplex of TFIIIB, SL1 and TFIID remain associated with promoters in metaphase chromatin (Roussel et al., 1996; Segil et al., 1996; Christova and Oelgeschläger, 2001; Chen et al., 2002). However, in contrast to TFIIIB, no evidence has been reported that SL1 or TFIID are disrupted at mitosis (Segil et al., 1996; Heix et al., 1998). This difference in behaviour between the three TBP-containing complexes probably reflects the fact that TFIIIB is much less stable than SL1 or TFIID, with Bdp1 dissociating readily during chromatography or immunoprecipitation (Kassavetis et al., 1991; Huet et al., 1994; Schramm et al., 2000). The affinity of Bdp1 for TFIIIB seems to be diminished further during mitosis, resulting in transcriptional repression. The pol III system also appears to differ from pols I and II in terms of the mitotic kinase that mediates control. Cdc2–cyclin B has been clearly implicated in suppressing transcription by pols I and II (Heix et al., 1998; Long et al., 1998). Although this kinase can also inhibit pol III transcription in Xenopus eggs, we have found no evidence that this is the case for a human system. Our data suggest that one or more alternative kinases regulate TFIIIB at mitosis. There is also evidence that an unknown kinase can suppress pol III transcription in Xenopus, where repression persisted after cdc2 was depleted from mitotic extracts (Hartl et al., 1993). It would be interesting to determine whether this alternative kinase in frogs is the same as the kinase that inactivates TFIIIB in HeLa cells; if so, our data suggest that it may have subsumed primary responsibility for suppressing pol III transcription in mitotic human cells.
Materials and methods
Cell culture and transfection
HeLa cells were cultured and synchronized as previously described (White et al., 1995b). Cell synchronization was confirmed by fluorescence-activated cell sorting (FACS) analysis of DNA content. Transient transfection of pcDNA3HA.BRF, an expression vector encoding full-length human Brf1 with an N-terminal HA tag, was achieved with Lipofectamine.
Transcription assays
Pol III transcription was carried out as previously described (White et al., 1989), except that transcriptions were for 60 min at 30°C. Templates pVA1, pHu5S3.1, pLeu and pGlu6 have all been described (White et al., 1989, 1995b>).
Western blotting and immunoprecipitation
Western immunoblotting was performed as described by White et al. (1995b). Anti-Brf1 antibody 330 was raised by immunizing rabbits with synthetic peptide SNDYGCDGDEDDGY (human Brf1 residues 664–677) coupled to keyhole limpet haemocyanin (KLH). Anti-Brf1 antibodies CSH409 and 128 have been described previously (Mital et al., 1996; Cairns and White, 1998). Antiserum 2663 was raised by immunizing rabbits with synthetic peptide CSDRYRIYKAQKLRE (human Bdp1 residues 139–153) coupled to KLH. Antiserum 1900 was raised by immunizing rabbits with synthetic peptide PKRPLTFDTNEFHIPLVT (residues 1374–1391 of the RPC155 subunit of human pol III) coupled to KLH. Ab2 against the TFIIIC220 subunit of TFIIIC2 has been described (Shen et al., 1996). Anti-HA antibody F-7, anti-Oct-1 antibody C-21 and anti-TBP antibody 58C9 were obtained from Santa Cruz Biotechnology. Immunoprecipitation was carried out as descibed previously (Johnston et al., 2002).
Cell extracts and protein fractions
Whole-cell extracts were prepared as previously by a freeze–thaw protocol (White et al., 1995b). Protein fractions were prepared from asynchronous HeLa cells to generate the CHep-1.0 fraction containing TFIIIC and pol III (White et al., 1995b) and the DE-1.0 fraction containing TFIIIB and pol III (White and Jackson, 1992), produced as previously.
Recombinant Bdp1 and Brf1 were expressed in bacteria and purified according to published protocols (Mital et al., 1996; Schramm et al., 2000). Cdc2–cyclin B kinase was prepared by immunoprecipitation from mitotic cells using antibody against cyclin B1. Baculovirus-expressed cdc2–cyclin B was a generous gift from Kathryn Ball and was also obtained commercially from New England Biolabs.
Kinase assays
For H1 kinase assays, extract (10 µg) was incubated for 10 min at 30°C with 200 ng of histone H1 (Boehringer Mannheim) and 5 µCi of [γ-32P]ATP in LDB buffer (White et al., 1995b). Samples were then resolved on a 10% SDS–polyacrylamide gel and visualized by autoradiography.
Phosphate labelling in vivo
HeLa cells were transiently transfected with pCDNA3HA.BRF1, synchronized in S phase using a double thymidine block or in mitosis with nocodozole (White et al., 1995b) and then harvested 72 h post-transfection. Three hours prior to harvesting, cells were labelled with 0.5 mCi/ml [32P]orthophosphate in phosphate-free medium. Harvested cells were washed twice in ice-cold phosphate-buffered saline (PBS) and then solubilized in 0.5 ml of IP buffer (Johnston et al., 2002). After 60 min on a rotating wheel, insoluble material was removed by centrifugation at 14 000 g for 15 min prior to immunoprecipitation.
Chromatin immunoprecipitation assays
ChIP assays were performed as previously (Gomez-Roman et al., 2003). Antibodies used were C-21 against Oct-1 and C-18 against TFIIB (Santa Cruz Biotechnologies), 128 against Brf1, 2663 against Bdp1, 114 against the BN51 subunit of pol III, and 4286 against the TFIIIC110 subunit of TFIIIC2. 114, 128 and 4286 have been described and characterized previously (Sutcliffe et al., 2000).
Metaphase chromosomes
Isolated human mitotic chromosomes were prepared as detailed by Bickmore and Oghene (1996). HeLa cells were treated with 0.1 mg/ml colcemid for 16 h prior to harvest. Cells were harvested, washed in PBS and resuspended at 2 × 106 cells/ml in 75 mM KCl for 10 min. Cells were then pelleted and resuspended in cold polyamine (PA) buffer (15 mM Tris–HCl, 0.2 mM spermine, 0.5 mM spermidine, 2 mM EDTA, 0.5 mM EGTA, 80 mM KCl, 20 mM NaCl, 0.1 mM CuSO4 pH 7.2) at 8 × 106 cells/ml. After centrifugation at 1200 r.p.m. for 5 min, the pellet was resuspended at 107 cells/ml in cold PA buffer containing 1 mg/ml digitonin and vortexed for 30 s to release the mitotic chromosomes. Nuclei were spun out at 1200 r.p.m. for 5 min and the supernatant containing isolated chromosomes was collected. Pellets were resuspended in fresh PA and digitonin and respun. The two supernatants were combined, and the isolated chromosomes were pelleted at 7000 r.p.m. for 5 min and then resuspended in PA containing 40% glycerol.
Acknowledgments
Acknowledgements
We are very grateful to Wendy Bickmore for purified metaphase chromosomes, Kathryn Ball for baculovirus-expressed cdc2–cyclin B, Yuhong Shen and Arnie Berk for antibody Ab2, Michael Ittmann for antibody 114, and Nouria Hernandez for the GST–Brf1 expression vector and antibodies CSH407 and CSH409. This work was funded by grant 17/C13725 to R.J.W. from the Biotechnology and Biological Sciences Research Council.
References
- Akoulitchev S. and Reinberg,D. (1998) The molecular mechanism of mitotic inhibition of TFIIH is mediated by phosphorylation of CDK7. Genes Dev., 12, 3541–3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickmore W.A. and Oghene,K. (1996) Spatial relationships between defined DNA sequences and the axial region of extracted metaphase chromosomes. Cell, 84, 95–104. [DOI] [PubMed] [Google Scholar]
- Cairns C.A. and White,R.J. (1998) p53 is a general repressor of RNA polymerase III transcription. EMBO J., 17, 3112–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Hinkley,C.S., Henry,R.W. and Huang,S. (2002) TBP dynamics in living human cells: constitutive association of TBP with mitotic chromosomes. Mol. Biol. Cell, 13, 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Holmes,C.F.B. and Tsukitani,Y. (1990) Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem. Sci., 15, 98–102. [DOI] [PubMed] [Google Scholar]
- Christova R. and Oelgeschläger,T. (2001) Association of human TFIID–promoter complexes with silenced mitotic chromatin in vivo. Nature Cell Biol., 4, 79–82. [DOI] [PubMed] [Google Scholar]
- Dovat S., Ronni,T., Russell,D., Ferrini,R., Cobb,B.S. and Smale,S.T. (2002) A common mechanism for mitotic inactivation of C2H2 zinc finger DNA-binding domains. Genes Dev., 16, 2985–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton-Edkins Z.A., Fairley,J.A., Graham,E.L., Johnston,I.J., White,R.J. and Scott,P.H. (2003) The mitogen-activated protein (MAP) kinase ERK induces tRNA synthesis by phosphorylating TFIIIB. EMBO J., 22, 2422–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiduschek E.P. and Kassavetis,G.A. (2001) The RNA polymerase III transcription apparatus. J. Mol. Biol., 310, 1–26. [DOI] [PubMed] [Google Scholar]
- Gomez-Roman N., Grandori,C., Eisenman,R.N. and White,R.J. (2003) Direct activation of RNA polymerase III transcription by c-Myc. Nature, 421, 290–294. [DOI] [PubMed] [Google Scholar]
- Gottesfeld J.M. and Forbes,D.J. (1997) Mitotic repression of the transcriptional machinery. Trends Biochem. Sci., 22, 197–202. [DOI] [PubMed] [Google Scholar]
- Gottesfeld J.M., Wolf,V.J., Dang,T., Forbes,D.J. and Hartl,P. (1994) Mitotic repression of RNA polymerase III transcription in vitro mediated by phosphorylation of a TFIIIB component. Science, 263, 81–84. [DOI] [PubMed] [Google Scholar]
- Hartl P., Gottesfeld,J. and Forbes,D.J. (1993) Mitotic repression of transcription in vitro. J. Cell Biol., 120, 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heix J., Vente,A., Voit,R., Budde,A., Michaelidis,T.M. and Grummt,I. (1998) Mitotic silencing of human rRNA synthesis: inactivation of the promoter selectivity factor SL1 by cdc2/cyclin B-mediated phosphorylation. EMBO J., 17, 7373–7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet J., Conesa,C., Manaud,N., Chaussivert,N. and Sentenac,A. (1994) Interactions between yeast TFIIIB components. Nucleic Acids Res., 22, 3433–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston I.M., Allison,S.J., Morton,J.P., Schramm,L., Scott,P.H. and White,R.J. (2002) CK2 forms a stable complex with TFIIIB and activates RNA polymerase III transcription in human cells. Mol. Cell. Biol., 22, 3757–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassavetis G.A., Braun,B.R., Nguyen,L.H. and Geiduschek,E.P. (1990) S.cerevisiae TFIIIB is the transcription initiation factor proper of RNA polymerase III, while TFIIIA and TFIIIC are assembly factors. Cell, 60, 235–245. [DOI] [PubMed] [Google Scholar]
- Kassavetis G.A., Bartholomew,B., Blanco,J.A., Johnson,T.E. and Geiduschek,P.E. (1991) Two essential components of the Saccharomyces cerevisiae transcription factor TFIIIB: transcription and DNA-binding properties. Proc. Natl Acad. Sci. USA, 88, 7308–7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassavetis G.A., Letts,G.A. and Geiduschek,E.P. (2001) The RNA polymerase III transcription initiation factor TFIIIB participates in two steps of promoter opening. EMBO J., 20, 2823–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leresche A., Wolf,V.J. and Gottesfeld,J.M. (1996) Repression of RNA polymerase II and III transcription during M phase of the cell cycle. Exp. Cell Res., 229, 282–288. [DOI] [PubMed] [Google Scholar]
- Li M., Guo,H. and Damuni,Z. (1995) Purification and characterization of two potent heat-stable protein inhibitors of protein phosphatase 2A from bovine kidney. Biochemistry, 34, 1988–1996. [DOI] [PubMed] [Google Scholar]
- Long J.J., Leresche,A., Kriwacki,R.W. and Gottesfeld,J.M. (1998) Repression of TFIIH transcriptional activity and TFIIH-associated cdk7 kinase activity at mitosis. Mol. Cell. Biol., 18, 1467–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Balbas M.A., Dey,A., Rabindran,S.K., Ozato,K. and Wu,C. (1995) Displacement of sequence-specific transcription factors from mitotic chromatin. Cell, 83, 29–38. [DOI] [PubMed] [Google Scholar]
- McBryant S.J., Meier,E., Leresche,A., Sharp,S.J., Wolf,V.J. and Gottesfeld,J.M. (1996) TATA-box DNA binding activity and subunit composition of RNA polymerase III transcription factor IIIB from Xenopus laevis. Mol. Cell. Biol., 16, 4639–4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., McLachlan,A.D. and Klug,A. (1985) Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J., 4, 1609–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mital R., Kobayashi,R. and Hernandez,N. (1996) RNA polymerase III transcription from the human U6 and adenovirus type 2 VAI promoters has different requirements for human BRF, a subunit of human TFIIIB. Mol. Cell. Biol., 16, 7031–7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel P., André,C., Comai,L. and Hernandez-Verdun,D. (1996) The rDNA transcription machinery is assembled during mitosis in active NORs and absent in inactive NORs. J. Cell Biol., 133, 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm L. and Hernandez,N. (2002) Recruitment of RNA polymerase III to its target promoters. Genes Dev., 16, 2593–2620. [DOI] [PubMed] [Google Scholar]
- Schramm L., Pendergrast,P.S., Sun,Y. and Hernandez,N. (2000) Different human TFIIIB activities direct RNA polymerase III transcription from TATA-containing and TATA-less promoters. Genes Dev., 14, 2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segil N., Boseman Roberts,S. and Heintz,N. (1991) Mitotic phosphorylation of the Oct-1 homeodomain and regulation of Oct-1 DNA binding activity. Science, 254, 1814–1816. [DOI] [PubMed] [Google Scholar]
- Segil N., Guermah,M., Hoffmann,A., Roeder,R.G. and Heintz,N. (1996) Mitotic regulation of TFIID: inhibition of activator-dependent transcription and changes in subcellular localization. Genes Dev., 10, 2389–2400. [DOI] [PubMed] [Google Scholar]
- Shen Y., Igo,M., Yalamanchili,P., Berk,A.J. and Dasgupta,A. (1996) DNA binding domain and subunit interactions of transcription factor IIIC revealed by dissection with poliovirus 3C protease. Mol. Cell. Biol., 16, 4163–4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer C.A., Kruhlak,M.J., Jenkins,H.L., Sun,X. and Bazett-Jones,D.P. (2000) Mitotic transcription repression in vivo in the absence of nucleosomal chromatin condensation. J. Cell Biol., 150, 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J.E., Brown,T.R.P., Allison,S.J., Scott,P.H. and White,R.J. (2000) Retinoblastoma protein disrupts interactions required for RNA polymerase III transcription. Mol. Cell. Biol., 20, 9192–9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasima T. and Tolmach,L.J. (1963) Growth and nucleic acid synthesis in synchronously dividing populations of HeLa cells. Exp. Cell Res., 30, 344–362. [DOI] [PubMed] [Google Scholar]
- Van Zyl W., Huang,W., Sneddon,A.A., Stark,M., Camier,S., Werner,M., Marck,C., Sentenac,A. and Broach,J.R. (1992) Inactivation of the protein phosphatase 2A regulatory subunit A results in morphological and transcriptional defects in Saccharomyces cerevisiae. Mol. Cell. Biol., 12, 4946–4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesely J. et al. (1994) Inhibition of cyclin-dependent kinases by purine analogues. Eur. J. Biochem., 224, 771–786. [DOI] [PubMed] [Google Scholar]
- Wang Z. and Roeder,R.G. (1995) Structure and function of a human transcription factor TFIIIB subunit that is evolutionarily conserved and contains both TFIIB- and high-mobility-group protein 2-related domains. Proc. Natl Acad. Sci. USA, 92, 7026–7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R.J. (2002) RNA Polymerase III Transcription, 3rd edn. Landes Bioscience, Austin, TX. http://www.eurekah.com. [Google Scholar]
- White R.J. and Jackson,S.P. (1992) Mechanism of TATA-binding protein recruitment to a TATA-less class III promoter. Cell, 71, 1041–1053. [DOI] [PubMed] [Google Scholar]
- White R.J., Stott,D. and Rigby,P.W.J. (1989) Regulation of RNA polymerase III transcription in response to F9 embryonal carcinoma stem cell differentiation. Cell, 59, 1081–1092. [DOI] [PubMed] [Google Scholar]
- White R.J., Gottlieb,T.M., Downes,C.S. and Jackson,S.P. (1995a) Cell cycle regulation of RNA polymerase III transcription. Mol. Cell. Biol., 15, 6653–6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R.J., Gottlieb,T.M., Downes,C.S. and Jackson,S.P. (1995b) Mitotic regulation of a TATA-binding-protein-containing complex. Mol. Cell. Biol., 15, 1983–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]