Abstract
It has been known for over a decade that inhibition of protein phosphatase 1 (PP1) activity prevents entry into M phase, but the relevant substrate has not been identified. We report here that PP1 is required for dephosphorylation of the Cdc2-directed phosphatase Cdc25 at Ser287 (of Xenopus Cdc25; Ser216 of human Cdc25C), a site that suppresses Cdc25 during interphase. Moreover, PP1 recognizes Cdc25 directly by interacting with a PP1-binding motif in the Cdc25 N-terminus. We have also found that 14-3-3 binding to phospho-Ser287 protects Cdc25 from premature dephosphorylation. Upon entry into M phase, 14-3-3 removal from Cdc25 precedes Ser287 dephosphorylation, suggesting the existence of a phosphatase- independent pathway for 14-3-3 removal from Cdc25. We show here that this dissociation of 14-3-3 from Cdc25 requires the activity of the cyclin-dependent kinase Cdk2, providing a molecular explanation for the previously reported requirement for Cdk2 in promoting mitotic entry. Collectively, our data clarify several steps important for Cdc25 activation and provide new insight into the role of PP1 in Cdc2 activation and mitotic entry.
Keywords: 14-3-3/Cdc25/Cdk2/PP1/Xenopus
Introduction
Both entry into mitosis in somatic cells and initiation of meiotic M phase in oocytes rely upon the properly timed activation of the Cdc2–cyclin B kinase (reviewed in Lew and Kornbluth, 1996). The activity of this complex is controlled by the antagonistic actions of Wee1 family kinases, which suppress Cdc2 activity, and the Cdc25 phosphatase, which promotes Cdc2 activation (reviewed in Coleman and Dunphy, 1994; Lew and Kornbluth, 1996).
Cdc25 is, itself, the target of multiple regulatory pathways. Most notably, Cdc25 activation is restrained by DNA-responsive checkpoints that prevent mitosis in the presence of incompletely replicated or damaged DNA (Sanchez et al., 1997; Kumagai et al., 1998a; Zeng et al., 1998). Checkpoint suppression of Cdc25 is mediated largely through phosphorylation of Cdc25 at Ser287 (S287 of Xenopus Cdc25/S216 of human Cdc25C) which is required for docking of the small acidic protein, 14-3-3 (Peng et al., 1997; Sanchez et al., 1997; Kumagai et al., 1998b). The extent to which S287 phosphorylation inhibits Cdc25 independently of 14-3-3 binding is not entirely clear. However, a mutant Cdc25 which blocks both S287 phosphorylation and 14-3-3 binding (S287 to alanine) can override the G2 arrest produced by either DNA damage or DNA replication inhibitors (Peng et al., 1997; Kumagai et al., 1998a). The S287A mutant Cdc25 exhibits ∼2-fold higher phosphatase activity than its wild-type counterpart and is imported more efficiently into nuclei, providing possible explanations for its increased ability to induce mitosis (Kumagai et al., 1998b; Kumagai and Dunphy, 1999; Yang et al., 1999; Graves et al., 2001). However, as the S287A mutation abrogates both phosphorylation and 14-3-3 binding, loss of either or both of these may contribute to checkpoint override.
In somatic cells, S287 can be phosphorylated by several kinases, including the checkpoint kinases Chk1 and Cds1/Chk2, and the c-TAK1 kinase (Furnari et al., 1997, 1999; Kumagai et al., 1998a; Peng et al., 1998; Zeng et al., 1998). It has also been shown in Xenopus oocytes that protein kinase A (PKA) can phosphorylate this site (Duckworth et al., 2002). This is of particular interest as PKA blocks oocyte maturation, and progesterone-induced M phase entry is preceded by a drop in cAMP concentration in these cells (Maller et al., 1979; Mulner et al., 1979; Finidori-Lepicard et al., 1981; Huchon et al., 1981b; Sadler and Maller, 1981). Consistent with these findings, S287A Cdc25 can override the ability of PKA to prevent oocyte maturation (Duckworth et al., 2002).
Cdc25 activation at M phase is accompanied by increased Cdc25 phosphorylation at multiple residues (Izumi et al., 1992; Izumi and Maller, 1993). Some of these phosphorylations occur through a Cdc2–cyclin B-mediated feed-forward loop, increasing Cdc25 activation (Hoffmann et al., 1993; Izumi and Maller, 1993; Karaiskou et al., 1998; Bulavin et al., 2003). In addition, Polo-like kinase (Plx) has been implicated in the direct phosphorylation and activation of Cdc25 at G2/M (Kumagai and Dunphy, 1996; Qian et al., 2001). As these mitotic phosphorylations are appearing, S287 phosphorylation is lost, as is Cdc25 binding to 14-3-3 (Peng et al., 1997). This suggests a possible link between S287 dephosphorylation and phosphorylation of Cdc25 at other sites. As shown by Duckworth et al. (2002), in the early embryonic cell cycles in Xenopus, S287 dephosphorylation precedes detectable Cdc25-mediated tyrosine dephosphorylation (and hence activation) of Cdc2. These data are consistent with an ordering of events in which S287 is dephosphorylated, Cdc2 is activated and then further Cdc2-mediated mitotic phosphorylations of Cdc25 occur. The precise timing of 14-3-3 dissociation from Cdc25, relative to these events, has not been carefully documented, nor have the factors involved in each Cdc25 activation step been fully elucidated.
Given the intensive study of Cdc2–cyclin B regulation, it is perhaps surprising that several molecules well documented to modulate Cdc2 activation have not been placed into any known Cdc2 regulatory pathway. It has, for example, been known for over a decade that injection of Xenopus oocytes with the protein phosphatase 1 (PP1) inhibitor-1 (I-1) impedes Cdc2 activation and oocyte maturation (Huchon et al., 1981a). However, it is not clear how PP1 regulates mitotic entry. Similarly, Guadagno and Newport (1996) reported that the cyclin-dependent kinase Cdk2, thought to function primarily at G1/S, is also required for mitotic entry and may act as a positive regulator of Cdc2–cyclin B. Why this is the case has not yet been reported.
From an analysis of the individual steps in Cdc25 activation, we report here that PP1 is responsible for dephosphorylating S287, thereby derepressing Cdc25 at the time of mitotic entry. In addition, our data demonstrate that removal of 14-3-3 from Cdc25 precedes S287 dephosphorylation and can occur even when S287 dephosphorylation is inhibited. Moreover, 14-3-3 binding inhibits S287 dephosphorylation, and a Cdc25 mutant that avidly binds 14-3-3 is not dephosphorylated at S287. Finally, we demonstrate that Cdk2 is required for removal of 14-3-3 from Cdc25, providing a molecular explanation for Cdk2 involvement in Cdc2 activation.
Results
PP1 dephosphorylates S287 of Cdc25
As described previously, entry into M phase in both Xenopus cycling extracts and progesterone-treated oocytes is preceded by the dephosphorylation of S287 on Cdc25 (Duckworth et al., 2002). As phosphorylation at this site suppresses the ability of Cdc25 to promote M phase, we speculated that the phosphatase acting on this site might represent a possible locus of G2/M control. Hence, we became interested in the identification and characterization of the S287-directed phosphatase. Recent studies using a short synthetic phosphopeptide encompassing the Cdc25 S287 phosphorylation site have implicated PP2A as a potential protein Ser/Thr phosphatase regulating Cdc25 S287 dephosphorylation and mitotic entry (Hutchins et al., 2002). However, previous studies indicate that such synthetic phosphopeptides may yield misleading results as they are poor substrates of many other phosphatases, such as PP1 (Pinna and Donella-Deana, 1994). Moreover, PP2A may dephosphorylate a model peptide in vitro without being truly responsible for dephosphorylation of the full-length parent protein. Thus, in this study, we wished to re-evaluate the regulation of Cdc25 S287 by protein phosphatases, focusing on the intact wild-type (WT) Cdc25 as substrate.
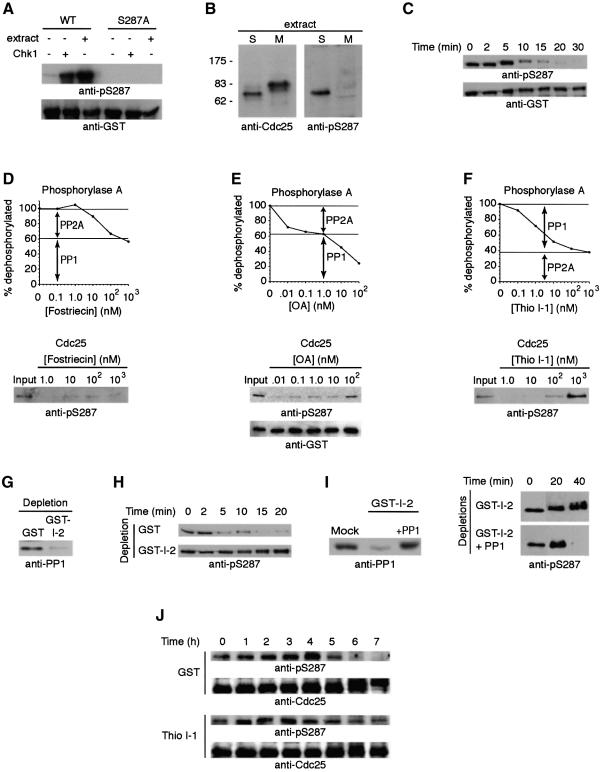
Initially, to monitor Cdc25 dephosphorylation, we constructed a GST–Cdc25 protein that could be produced in bacteria in a fully dephosphorylated form. Following incubation with either purified Chk1 or interphase Xenopus egg extracts, the GST–Cdc25, like endogenous Cdc25 from interphase egg extract, was phosphorylated on S287, as evidenced by immunoreactivity with an antibody directed against phosphorylated S287 (anti-pSer287) (Figure 1A and B). In contrast, GST–Cdc25 containing the S287A substitution or endogenous Cdc25 from mitotic egg extracts was not recognized by this antibody (Figure 1A and B). WT GST–Cdc25 phosphorylated at S287 was then incubated in interphase egg extract and retrieved on glutathione–Sepharose at various times after addition of non-degradable cyclin B1 (to convert the extract to a mitotic state). This resulted in a time-dependent dephosphorylation of S287 as evidenced by loss of anti-pSer287 reactivity (Figure 1C). Similar results were obtained using GST–Cdc25 (1–322) lacking the Cdc25 phosphatase domain or endogenous Cdc25 immunoprecipitated from an interphase extract as substrates (data not shown). As recently reported, Chk1/Cds1 cannot re-phosphorylate this site once it is dephosphorylated due to Cdc2-induced phosphorylation at S285 on Cdc25 (Bulavin et al., 2003).
Fig. 1. S287 is dephosphorylated by PP1. (A) Full-length GST–Cdc25 and GST–Cdc25 S287A proteins were incubated with Xenopus interphase egg extract at 4°C or Chk1 + ATP at 30°C for 1.5 h. Samples were collected, washed and resolved by SDS–PAGE for immunoblotting with either anti-pSer287 or anti-GST antibodies. (B) Total interphase or mitotic extracts were resolved by SDS–PAGE and immunoblotted with the indicated antisera. (C) Following GST–Cdc25 incubation in interphase egg extract, non-degradable cyclin was added to drive the extract into mitosis. GST–Cdc25 was retrieved from the extract at the indicated times using glutathione–Sepharose beads. Beads were washed extensively with egg lysis buffer (ELB) and boiled in sample buffer for SDS–PAGE and immunoblotting with the indicated antibodies. (D–F) Top: radiolabeled phosphorylase A was incubated in mitotic egg extract in the presence of increasing concentrations of fostriecin (D), okadaic acid (E) or thiophosphorylated I-1 (F). The percentage of phosphorylase A which was dephosphorylated is shown. Inhibition of PP2A by fostriecin allows 60% dephosphorylation of phosphorylase A by PP1, while PP1 inhibition by thio-I-1 allows 40% dephosphorylation by PP2A. Similar ratios of PP1 and PP2A-dependent dephosphorylation are observed with okadaic acid titration. Lower panels: GST–Cdc25 (amino acids 1–322) was pre-phosphorylated with Chk1 in kinase buffer with ATP at 30°C for 1.5 h, washed and then incubated with mitotic egg extract and increasing amounts of fostriecin, okadaic acid or thio-I-1, as indicated. Samples were processed for anti-pS287 or anti-GST immunoblotting. (G) PP1 was depleted from mitotic egg extracts using GST–I-2–Sepharose or mock depleted using GST–Sepharose. Depleted extracts were resolved by SDS–PAGE and immunoblotted with anti-PP1 sera. (H) Full-length GST–Cdc25 was incubated in interphase extract, retrieved on glutathione–beads, washed with ELB and placed into either GST or GST–I-2-depleted extracts. Samples were collected at the indicated times, washed, boiled in sample buffer for SDS–PAGE and then immunoblotted with anti-pS287 antibodies. (I) Left: extracts depleted as in (G) were immunoblotted with anti-PP1 before and after re-addition of pure PP1. Right: samples were processed as in (H) in the presence and absence of reconstituted pure PP1. (J) Stage VI oocytes were injected with either GST or thio-I-1 followed by progesterone treatment. Twenty oocytes were taken at the indicated times and frozen in liquid nitrogen. Samples were lysed, and endogenous Cdc25 was detected by anti-pS287 and anti-Cdc25 immunoblotting.
To address the role of cellular phosphatases in regulating S287 dephosphorylation, we utilized three distinct phosphatase inhibitors with differing selectivity for the two major eukaryotic phosphatases, PP1 and PP2A. As prior studies by Hutchins et al. (2002) suggested that PP2A might be the major Cdc25 phosphatase, our initial experiments utilized fostriecin, which shows >10 000-fold selectivity for PP2A over PP1 (Walsh et al., 1997). Dephosphorylation of the standard PP1/PP2A substrate, phosphorylase A, by mitotic extracts was inhibited in a dose-dependent manner, plateauing with 60% of the substrate being dephosphorylated in the presence of micromolar concentrations of fostriecin (Figure 1D). This suggested that ∼40% of the total phosphorylase-directed phosphatase activity in the egg extracts represented PP2A and other type-2 phosphatases. In contrast, over this range of concentrations, fostriecin had no effect on dephosphorylation of S287 by the mitotic extracts, suggesting that type-2 phosphatases played little or no role in dephosphorylating S287. Okadaic acid inhibits both PP1 and PP2A (reviewed in Sheppeck et al., 1997), albeit at differing concentrations. Consistent with this, we observed a biphasic dose response for inhibition of phosphorylase phosphatase activity by mitotic extracts, with PP2A being inhibited at low nanomolar concentrations of okadaic acid and higher concentrations required to inhibit PP1 (Figure 1E). These data confirmed that ∼40% of the phosphatase activity in egg extracts was PP2A and 60% represented PP1. The dose response for inhibition of S287 dephosphorylation was monophasic and best reflected PP1 as the S287 phosphatase in these extracts (Figure 1E). To confirm this, we examined the effects of I-1, a phosphorylation-dependent PP1-specific inhibitor (Connor et al., 1998), on the dephosphorylation of phosphorylase A and Cdc25 by mitotic extracts. As shown in Figure 1F, thiophosphorylated I-1 inhibited ∼60% of the phosphorylase phosphatase activity in these extracts. Most importantly, I-1 abolished S287 dephosphorylation by mitotic extracts, strongly suggesting that PP1 represented the major Xenopus Cdc25 S287-directed phosphatase. To strengthen this finding further, we depleted PP1 from mitotic extracts, using Sepharose-immobilized GST–I-2, another mammalian PP1 inhibitor, and analyzed dephosphorylation of S287 by the PP1-depleted extracts (Figure 1G and H). As shown in Figure 1H, PP1 depletion prevented S287 dephosphorylation. Moreover, re-addition of purified PP1 to depleted extracts restored S287 dephosphorylation (Figure 1I), confirming that this dephosphorylation of Cdc25 is catalyzed by PP1.
It has been reported that injection of I-1 into oocytes prevents maturation, though the relevant target of this inhibition has been elusive (Huchon et al., 1981a). We repeated these experiments, confirming the inhibition of oocyte maturation by I-1 (data not shown). Importantly, this injection not only stopped germinal vesicle breakdown (GVBD), but, as shown in Figure 1J, effectively interfered with S287 dephosphorylation. These data strongly suggest that the I-1 effect on oocyte maturation stems, at least in part, from a failure to dephosphorylate S287, and thereby identify Cdc25 as a PP1 target critical for mitotic entry.
PP1 binding to Cdc25 is required for S287 dephosphorylation
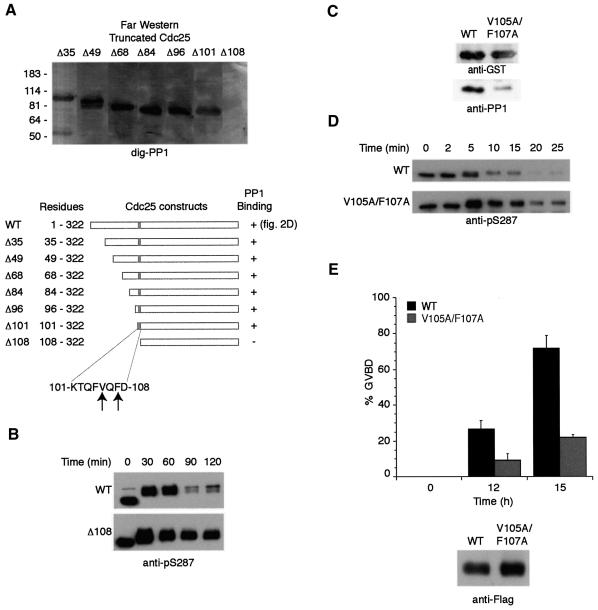
Recent evidence suggests that protein Ser/Thr phosphatases derive their specificity by either localizing in close proximity to their substrates or existing in complexes with these proteins, often through targeting subunits (reviewed in Cohen, 2002). Consistent with a role for PP1 in dephosphorylating S287, we found that glutathione–Sepharose-bound GST–Cdc25 could retrieve PP1 efficiently from mitotic egg extract (Figure 2A; note that 1–322 Cdc25 was used here because it was easier to produce large amounts of protein). Moreover, endogenous Cdc25 and PP1 could be co-immunoprecipitated from these extracts using either anti-Cdc25 or anti-PP1 antibodies (Figure 2B and C). In an attempt to identify a targeting subunit that might confer PP1 recognition of Cdc25, we dipped Sepharose-bound GST–Cdc25 or control GST protein into mitotic egg extracts, retrieved the beads by centrifugation, washed the beads, and resolved the bead-bound material by SDS–PAGE. Following transfer to Immobilon, Cdc25 and its associated proteins were probed by far western using digoxigenin-labeled PP1 (dig-PP1). Surprisingly, the only prominent band recognized by PP1 was Cdc25 itself (Figure 2D). Consistent with these data, we found that recombinant Cdc25 could interact directly with pure PP1 in the absence of other cellular factors (Figure 2E).
Fig. 2. PP1 binds directly to Cdc25. (A) GST or GST–Cdc25 linked to glutathione–Sepharose was dipped in mitotic egg extracts, retrieved by centrifugation, washed and immunoblotted with anti-PP1 antibody. Coomassie-stained gel of pull-downs showed equal loading of GST and GST–Cdc25. The same protein preparations were used in (E), below. (B) Immunoprecipitates formed from mitotic egg extracts using either anti-Cdc25 or pre-immune sera were resolved by SDS–PAGE and immunoblotted with either anti-Cdc25 or anti-PP1 antibodies. The small arrowhead denotes the IgG heavy chain. (C) Mitotic extract samples were immunoprecipitated with anti-PP1 monoclonal or control IgG and blotted with either anti-Cdc25 or anti-PP1 antibodies. The small arrowhead denotes the IgG light chain. (D) Sepharose beads linked to 0.1 µg of GST–Cdc25 (amino acids 1–322) or GST were incubated in 100 µl egg extracts supplemented with non-degradable cyclin. After 30 min, samples were retrieved by centrifugation, washed, resolved by SDS–PAGE, transferred to PVDF membrane and probed with dig-PP1 (as in Terry-Lorenzo et al., 2000). (E) GST or GST–Cdc25 linked to glutathione–Sepharose was incubated with purified PP1 in vitro. Samples were collected by centrifugation, washed, and prepared for anti-PP1 immunoblotting.
To better define the association of PP1 with the Cdc25 N-terminus, we created a deletion panel of recombinant Cdc25 proteins with increasingly shortened N-termini, all ending at residue 322. The recombinant proteins were resolved by SDS–PAGE and analyzed by dig-PP1 far western. PP1 binding was observed with most polypeptides (Δ35, Δ49, Δ68, Δ84, Δ96 and Δ101), but not Δ108 (Figure 3A). This suggested that the sequence KTQFVQFD between residues 101 and 108 played a key role in PP1 binding. Consistent with this observation, the Chk1-phosphorylated Δ108 Cdc25 mutant was not dephosphorylated effectively at S287 upon mitotic entry (Figure 3B). Previous reports have suggested a consensus PP1-binding motif, RVXF or KIXF, wherein substitution of alanines in place of either V/I or F disrupted PP1 binding (Egloff et al., 1997; Wakula et al., 2003). Interestingly, the sequence between residues 101 and 108, shown to be necessary for PP1 binding, contained a VxF motif. To determine if this sequence might be part of a core PP1-binding site, we substituted alanine in place of V105 and F107 in the full-length Cdc25. As shown in Figure 3C, mutation of the VxF sequence markedly diminished the Cdc25–PP1 interaction. Moreover, in comparison with WT Cdc25, the V105A/F107A was poorly dephosphorylated at S287 (Figure 3D). These data indicate that efficient S287 dephosphorylation is mediated by PP1 bound to Cdc25 and strongly suggest that the VxF motif at amino acids 105–107 comprises part of the PP1-binding site. Consistent with a role for the VxF motif in mediating PP1 docking, V105A/F107A Cdc25 was considerably less potent than WT Cdc25 in inducing oocyte maturation (GVBD) (Figure 3E).
Fig. 3. Cdc25 contains a consensus PP1-binding site important for efficient S287 dephosphorylation. (A) An N-terminal truncation series of GST–Cdc25 proteins was constructed and probed for PP1 binding by far western blotting with dig-PP1. (B) The GST–Δ108 mutant and WT GST–Cdc25 proteins were incubated in Xenopus egg extracts followed by addition of non-degradable cyclin. Proteins were retrieved from extracts with glutathione–Sepharose and immunoblotted with anti-pS287 antibody. (C) WT and V105A/F107A Cdc25 proteins were assayed for their ability to bind PP1 from egg extract as in Figure 2A. Samples were also blotted with anti-GST to show equal loading of baits. (D) GST–WT Cdc25 and V105A/F107A proteins were treated as in (B). (E) mRNA encoding Flag-tagged WT or V105A/F107A Cdc25 was injected into oocytes treated with the nuclear export inhibitor leptomycin B (200 nM), which enhances Cdc25 potency. Oocytes were monitored for GVBD over time. At 15 h incubation, 30 oocytes were collected, lysed, mixed with sample buffer, and resolved by SDS–PAGE for immunoblotting with anti-Flag antibodies.
PP1-mediated Cdc25 dephosphorylation is regulated by phosphorylation at Thr138 and 14-3-3 binding
Concomitant with S287 dephosphorylation during mitotic entry, Cdc25 undergoes increased phosphorylation at multiple other mitosis-specific sites (Peng et al., 1997). This temporal coincidence raised the possibility that mitotic phosphorylations might contribute to controlling the susceptibility of Cdc25 to S287 dephosphorylation. To address this, we wished to monitor the S287 dephosphorylation of Cdc25 proteins lacking specific mitotic phosphorylations.
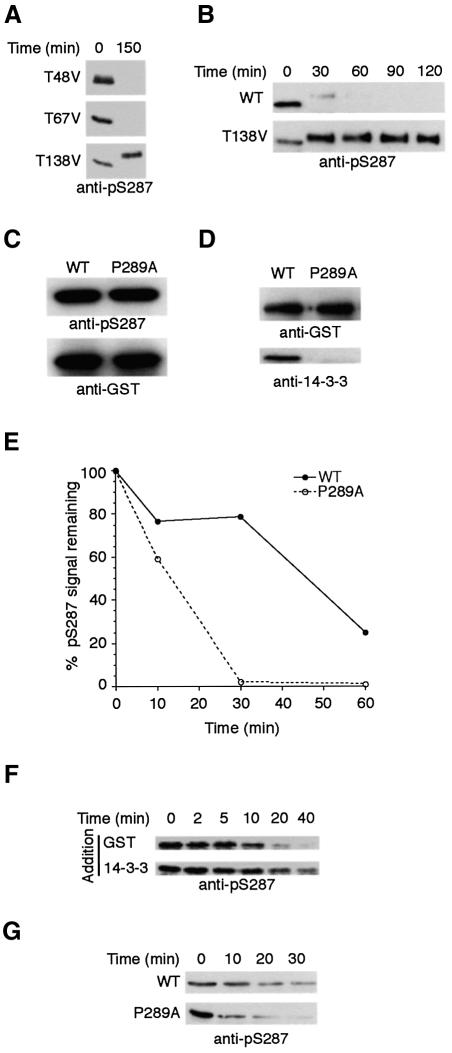
Three different threonine residues in the N-terminal region of Xenopus Cdc25 have been mapped as bona fide mitotic phosphorylation sites (Izumi and Maller, 1993). To address the importance of these sites for S287 dephosphorylation, we obtained a series of Cdc25 constructs in which the known threonine phosphorylation sites had been mutated to valine. GST–Cdc25 (1–322) or full-length GST–Cdc25 proteins containing these mutations were incubated in interphase extracts, where they could be phosphorylated on S287 and bound to 14-3-3 protein, then transferred to mitotic extracts to monitor their rates of S287 dephosphorylation. Mutation of Thr138 (T138V), but not Thr48 (T48V) or Thr67 (T67V), severely abrogated S287 dephosphorylation (Figure 4A). This effect was highlighted in the time course shown in Figure 4B, where WT Cdc25 was almost completely dephosphorylated at S287 in 30 min, while S287 phosphorylation of the T138V mutant was essentially intact over 120 min.
Fig. 4. S287 dephosphorylation is regulated by 14-3-3 binding. (A) T138V, T48V and T67V GST–Cdc25 (1–322) proteins were incubated in interphase extract supplemented with non-degradable cyclin. Cdc25 proteins were retrieved on glutathione–Sepharose at the indicated times and immunoblotted with anti-pS287 antibody. (B) Full-length WT or T138V mutant GST–Cdc25 proteins were tested as in (A). (C) GST–P289A and WT Cdc25 proteins were incubated with Chk1 in kinase buffer and ATP to assess comparative abilities of the P289A and WT Cdc25 proteins to be phosphorylated at S287. Samples were immunoblotted with anti-pS287 or anti-GST antibodies. (D) GST–P289A and WT Cdc25 proteins were incubated in egg extract for 1 h at 4°C. Samples were retrieved on glutathione–Sepharose, washed with ELB plus 0.1% Triton X-100 and 300 mM NaCl, and resolved by SDS–PAGE to blot for 14-3-3 or GST. (E) GST–P289A and WT Cdc25 proteins were incubated in interphase extract followed by addition of non-degradable cyclin. Cdc25 proteins were retrieved on glutathione–Sepharose at the indicated times and immunoblotted with anti-pSer287 antibodies. Signals quantitated by densitometry are represented as percentage pS287 signal remaining. (F) GST–Cdc25 (amino acids 1–322) was pre-phosphorylated using Chk1 and then incubated with PP1 in vitro in the presence of GST or 14-3-3 at 37°C. Samples were taken at the indicated times, washed, and immunoblotted with anti-pS287. (G) Samples were processed as in (F), but P289A and WT proteins were compared in the presence of recombinant 14-3-3.
The other notable distinction between interphase and mitotic Cdc25, which might render them differentially susceptible to phosphatase action, is the absence of 14-3-3 binding on the latter. Specifically, we hypothesized that 14-3-3 might mask the S287 phosphate, protecting it from dephosphorylation. To test this supposition, we mutated Pro289 of Cdc25 to alanine (P289A), predicted to form part of the consensus 14-3-3-binding site (Yaffe et al., 1997). Indeed, this protein could be phosphorylated on S287 but could not bind 14-3-3 (Figure 4C and D). When S287 dephosphorylation rates of the P289A mutant were compared with that of WT Cdc25 (in extract containing 14-3-3), we found, as hypothesized, that the inability to bind 14-3-3 protein rendered Cdc25 more susceptible to S287 dephosphorylation (Figure 4E). Moreover, addition of recombinant 14-3-3 protein appeared to retard in vitro dephosphorylation of Chk1-phosphorylated WT Cdc25 by purified PP1, without impeding dephosphorylation of the P289A Cdc25 (Figure 4F and G).
14-3-3 release precedes S287 dephosphorylation of WT Cdc25 and is defective in the T138V mutant
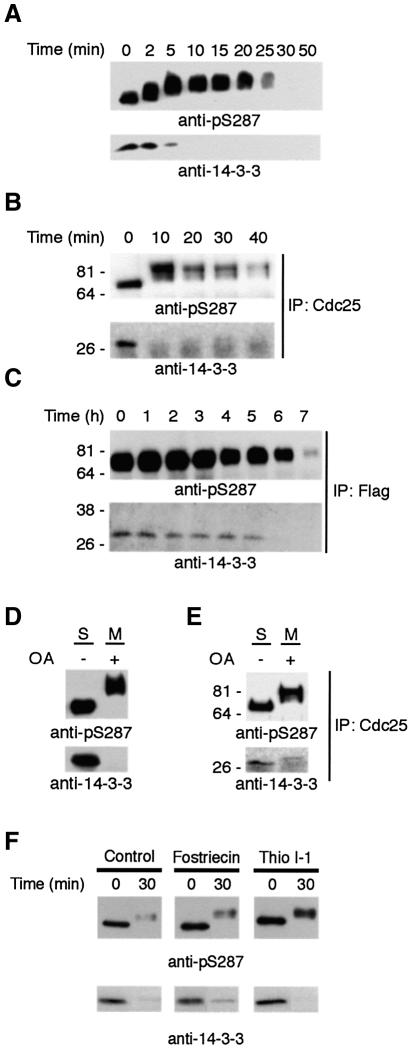
If 14-3-3 binding to Cdc25 could regulate the rate of S287 dephosphorylation, this suggested that removal of 14-3-3 from Cdc25 might be an important step in regulating mitotic entry. Given this, we reasoned that 14-3-3 removal must normally precede S287 dephosphorylation. Moreover, 14-3-3 dissociation from Cdc25 might not result simply from the lack of a phosphorylated docking site (following S287 desphosphorylation), but, rather, from an active 14-3-3 removal process. To address these issues, we monitored dephosphorylation and 14-3-3 binding of GST–Cdc25 that had been incubated in interphase extract (and thereby phosphorylated on S287 and bound to 14-3-3) and subsequently transferred to mitotic extract. Consistent with the existence of a distinct 14-3-3 removal mechanism, we found that 14-3-3 removal significantly preceded S287 dephosphorylation (Figure 5A). Similar results were obtained with endogenous Cdc25 immunoprecipitated from interphase extracts and incubated with mitotic extracts (Figure 5B). When oocytes were injected with mRNA encoding Flag-tagged WT Cdc25, treated with progesterone, lysed, and immunoprecipitated with anti-Flag for immunoblotting with anti-pSer 287 and 14-3-3 antibodies, 14-3-3 was also found to be removed before S287 dephosphorylation (Figure 5C). These data suggested that 14-3-3 removal did not depend on S287 dephosphorylation. Indeed, 14-3-3 was still removed from Cdc25 even when S287 dephosphorylation was inhibited by addition of okadaic acid to mitotic extracts (Figure 5D and E). Additionally, neither fostriecin nor I-1 prevented 14-3-3 release (Figure 5F). Taken together, these data suggest that 14-3-3 is removed from Cdc25 by a distinct process which then facilitates S287 dephosphorylation.
Fig. 5. 14-3-3 removal occurs prior to and independently of S287 dephosphorylation. (A) GST–Cdc25 phosphorylated by Chk1 and bound to 14-3-3 was added to M phase egg extract. Cdc25 was retrieved from the extract at the times shown, resolved by SDS–PAGE, and immunoblotted with either anti-14-3-3 antibody or anti-pSer 287 antibody. (B) Endogenous Cdc25 was immunoprecipitated from interphase extract and transferred into mitotic extracts. At the times indicated, the precipitates were then re-collected by centrifugation and processed for immunoblotting with either anti-pS287 or anti-14-3-3 antibody. (C) Stage VI oocytes were injected with mRNA encoding Flag-tagged Cdc25 and treated with progesterone. At the times indicated, 30 oocytes were collected, lysed, immunoprecipitated with anti-Flag, resolved by SDS–PAGE, and immunoblotted with anti-pS287 and anti-14-3-3 antibodies. (D) Cdc25 phosphorylated on S287 and loaded with 14-3-3 was transferred into M extract in the presence or absence of 5 µM okadaic acid. Samples were removed and immunoblotted with either 14-3-3 antibody or anti-pS287. Note that 14-3-3 was removed despite the maintenance of S287 phosphorylation. (E) Endogenous Cdc25 immunoprecipitated from interphase extract was transferred into mitotic extract supplemented with 5 µM okadaic acid and processed as in (D). (F) Samples were processed as in (D), but in the presence of fostriecin or thio I-1.
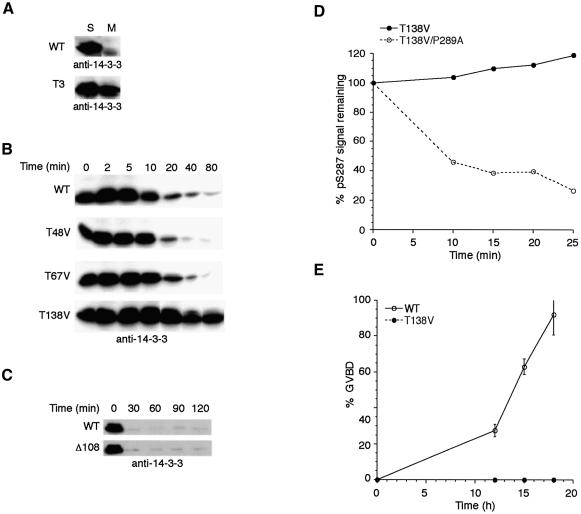
The above results led us to question whether the failure of the T138V Cdc25 mutant to be dephosphorylated at S287 reflected a fundamental deficiency in its recognition by PP1 or might arise as a secondary consequence of an inability to dissociate from 14-3-3. To test this, we monitored dissociation of 14-3-3 from WT and T3 Cdc25, which lacks all three mitotically phosphorylated threonines (T48V, T67V and T138V). Like WT Cdc25, T3 was phosphorylated efficiently at S287 and bound 14-3-3 in interphase extracts. However, when this protein was transferred into mitotic extracts, 14-3-3 remained bound to Cdc25, even after prolonged incubation, suggesting that at least one of these sites must be important for 14-3-3 dissociation (Figure 6A). Analysis of time-dependent dissociation of 14-3-3 from WT and mutant Cdc25 proteins lacking individual mitotically phosphorylated threonines showed that compared with WT Cdc25, T138V, but not T48V or T67V, exhibited prolonged 14-3-3 binding (Figure 6B). Consistent with these data, the Δ108 Cdc25 mutant, which lacked T48 and T67, but retained T138, released 14-3-3 at similar rates to WT Cdc25 (Figure 6C).
Fig. 6. T138 phosphorylation is required for 14-3-3 removal from Cdc25. (A) WT GST–Cdc25 or GST–Cdc25 in which Thr48, Thr67 and Thr138 had all been changed to valine were incubated in interphase extract to acquire S287 phosphorylation and bind 14-3-3. These samples were retrieved on glutathione–Sepharose and transferred to either interphase or mitotic egg extract. Samples were then re-precipitated and processed for anti-14-3-3 immunoblotting. (B) Individual mutant Cdc25 proteins were incubated in interphase extract, captured on glutathione–Sepharose and transferred to mitotic extract. Beads containing Cdc25 were pelleted at the times indicated, washed, and processed for anti-14-3-3 immunoblotting. (C) The samples shown in Figure 3B were immunoblotted with anti-14-3-3 antibodies. (D) The T138V and T138V/P289A double mutant Cdc25 proteins were incubated in interphase extract followed by addition of non-degradable cyclin. Cdc25 proteins were retrieved on glutathione–Sepharose at the indicated times and immunoblotted with anti-pS287 antibodies. Signals were quantitated by densitometry and are represented as percentage pS287 signal remaining. (E) Oocytes were injected with mRNA encoding WT or T138V Flag-tagged Cdc25 in the presence of 200 nM leptomycin B. Samples were monitored for GVBD over time.
To address further the idea that the observed defect in S287 dephosphorylation of the T138V Cdc25 arose from its inability to dissociate from 14-3-3, we introduced the T138V mutation into the P289A background, which prevents 14-3-3 binding. As shown in Figure 6D, the T138V protein remained phosphorylated at S287, while the T138V/P289A double mutant was rendered susceptible to S287 dephosphorylation. Taken together, these data demonstrate that 14-3-3 removal facilitates S287 dephosphorylation and that preventing this removal (e.g. by mutation of T138) interferes with dephosphorylation of that site. Furthermore, T138 is the only one of the N-terminally phosphorylated threonine sites required for 14-3-3 removal during mitosis.
Based on the above data, we predicted that the T138V protein would be a poor inducer of M phase entry, as neither 14-3-3 removal nor S287 dephosphorylation would occur in a timely fashion. To address this, we injected WT or T138V Cdc25-encoding mRNA into Xenopus oocytes (in the presence of the nuclear export inhibitor, leptomycin B, to enhance Cdc25 potency; Yang et al., 1999). As shown in Figure 6E, WT Cdc25 was markedly more efficient than the T138V mutant at promoting GVBD, consistent with the T138V mutant being essentially ‘locked’ in an interphase configuration.
Cdk2 regulation of Cdc25
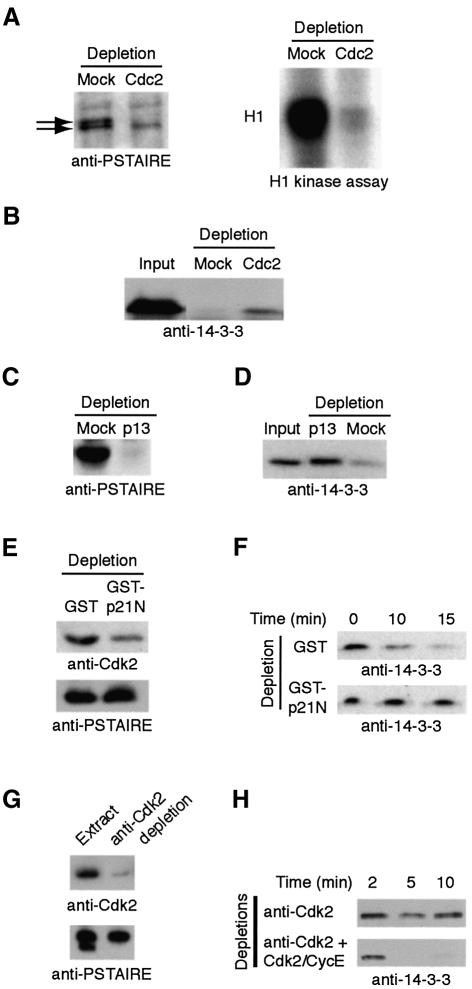
The apparent importance of T138 phosphorylation for the biological activity of Cdc25 suggested that the kinase(s) acting on that site would be important for 14-3-3 removal, S287 dephosphorylation and consequent Cdc25 activation and mitotic entry. Prior studies had shown that Cdc2 could phosphorylate T138 (Izumi and Maller, 1993). However, as Cdc2 activation would be expected to follow, rather than precede, Cdc25 activation, Cdc2 seemed an unlikely candidate for the enzyme promoting 14-3-3 release. Indeed, immunodepletion of Cdc2 from extracts, confirmed by immunoblotting and H1 kinase assays, could not prevent 14-3-3 removal from Cdc25 (Figure 7A and B). However, since Cdc2 and Cdk2 share similar specificity determinants, we were intrigued by published data showing that Cdk2–cyclin E was required for mitotic entry in Xenopus egg extracts (Guadagno and Newport, 1996). Although the basis for this requirement has been elusive, our data raised the possibility that Cdk2 might be required for 14-3-3 removal. Indeed, we had no difficulty demonstrating phosphorylation of T138 by Cdk2 in vitro (data not shown). To remove Cdk2 from the extract, we first depleted extracts using p13 Sepharose. This depletion removed both Cdc2 and Cdk2, while the anti-Cdc2 depletion had removed only Cdc2 (Figure 7A and C). As shown in Figure 7D, the p13 Sepharose depletion completely abrogated 14-3-3 dissociation from Cdc25. These data suggested that Cdk2 might play a role in regulating 14-3-3 release from Cdc25. To establish this, we first took advantage of the previously reported ability of the N-terminus of the Cdk inhibitor, p21, to deplete Cdk2 selectively (Moore et al., 2003). As shown in Figure 7E and F, removal of ∼70% of Cdk2 from mitotic extracts resulted in a nearly complete inhibition of 14-3-3 removal from Cdc25. Furthermore, immunodepletion of extracts using an affinity-purified Cdk2-directed antibody also prevented 14-3-3 removal from Cdc25, and 14-3-3 release was restored by re-addition of Cdk2–cyclin E to the depleted extracts (Figure 7G and H). These data strongly support a role for Cdk2 in this step in mitotic entry.
Fig. 7. Cdk2 participates in 14-3-3 removal from Cdc25. (A) Mitotic extracts were immunodepleted using Sepharose linked to Xenopus Cdc2 antibodies and immunoblotted with anti-PSTAIRE monoclonal antibody [able to recognize both Cdc2 (upper band) and Cdk2 (lower band)]. Histone H1 kinase assays were performed on mock-depleted and Cdc2-depleted samples. (B) GST–Cdc25 bound to 14-3-3 was added to the anti-Cdc2 or mock-depleted extracts, retrieved on glutathione–Sepharose, washed, resolved by SDS–PAGE and immunoblotted with anti-14-3-3 antibody. (C) Mitotic egg extract was depleted on p13 Sepharose or mock depleted. Depleted extracts resolved by SDS–PAGE were immunoblotted with anti-PSTAIRE. (D) The extracts from (C) were processed for Cdc25–14-3-3 interactions as in (B). (E) GST linked to a N-terminal fragment of p21 protein or GST alone was bound to glutathione–Sepharose and used as a resin to deplete mitotic extracts. Depleted samples were immunoblotted with Cdk2 antibody. (F) Samples depleted as in (E) were supplemented with GST–Cdc25 bound to 14-3-3. The Cdc25 protein was retrieved from the extracts at the indicated times, washed, resolved by SDS–PAGE and immunoblotted with anti-14-3-3 antibody. (G) Mitotic extracts were immunodepleted using anti-Cdk2 antibodies linked to protein A–Sepharose. Depleted extracts were immunoblotted with anti-Cdk2 or anti-PSTAIRE (note Cdk2, but not Cdc2, is removed). (H) Cdk2-depleted samples from (G) in the presence or absence of reconstituted Cdk2–cyclin E were assayed for 14-3-3 release as in (F).
Discussion
Both G2/M checkpoints and PKA inhibit M phase entry, at least in part, through Cdc25 suppression. This regulation depends on phosphorylation of Cdc25 at S287, thereby creating a docking site for 14-3-3 proteins. In this report, we show that this inhibition of Cdc25 is reversed first through 14-3-3 removal, then by PP1-mediated S287 dephosphorylation. Moreover, we have implicated Cdk2 in the process of 14-3-3 removal, helping to explain its reported role in promoting mitotic entry.
PP1 is the primary S287-directed phosphatase
It has been known for over a decade that PP1 inhibition can prevent Xenopus oocyte maturation, though the phosphorylated substrate critical for this effect has not been identified. Our data demonstrate that one such PP1 target important for G2/M regulation is Cdc25. It has been reported recently that Cdc2-mediated phosphorylation of S285 (human Cdc25C S214) in mitosis prevents Chk1-mediated re-phosphorylation of S287 during mitosis (Bulavin et al., 2003). Our data show that the initial removal of the phosphate from S287 at the time of mitotic entry is PP1 catalyzed.
Although both PP2A and PP1 can dephosphorylate fragments of Cdc25 in vitro, it is not unusual for phosphatases to catalyze dephosphorylation of an isolated peptide, yet be uninvolved in dephosphorylation of the full-length protein in vivo. We have shown here that PP1 can act on full-length Cdc25 and that this reaction depended on a PP1 docking site. It is the VXF motif, rather than an auxiliary targeting subunit, that appears to target PP1 to the Cdc25 substrate. Mutation of this sequence abrogated PP1–Cdc25 binding, impaired S287 dephosphorylation and markedly interfered with the ability of Cdc25 to induce oocyte maturation. When taken together with the fact that specific depletion of PP1 from the egg extract greatly impeded Cdc25 dephos phorylation, these data indicate that PP1 is primarily responsible for Cdc25 S287 dephosphorylation.
14-3-3 release precedes S287 dephosphorylation
In examining the sequence of events that occurred when Cdc25 switched from an ‘interphase state’ to a ‘mitotic state’, we noted that both 14-3-3 removal and increased PP1 binding to Cdc25 precedes S287 dephosphorylation (see Supplementary data B). These data suggest that while 14-3-3 binding might regulate dephosphorylation additional mechanisms are present to mediate PP1 binding to Cdc25. Indeed, Clarke and colleagues (Hutchins et al., 2002) found that PP2A-mediated dephosphorylation of the Cdc25 peptide used in their studies could be inhibited by 14-3-3 binding, suggesting that masking of the phosphoserine could impede phosphatase access. To confirm this, we engineered a Cdc25 mutant (P289A) unable to bind 14-3-3, yet susceptible to Chk1-mediated phosphorylation. This mutant protein was dephosphorylated more rapidly than WT Cdc25, consistent with the idea that 14-3-3 binding can retard S287 dephosphorylation. Compared with WT, the P289A mutant protein is also more susceptible to dephosphorylation in interphase extract (S.S.Margolis, unpublished), suggesting that differential 14-3-3 binding in interphase and mitosis provides for cell cycle-specific dephosphorylation of WT Cdc25. Indeed, Cdc25 altered at T138 could not be dephosphorylated precisely because 14-3-3 could not be removed. Even if 14-3-3 were not ‘locked’ onto WT Cdc25 to the same extent as observed with the T138 mutant, we suspect that steric protection of phospho-S287 by interphase 14-3-3 binding would effectively retard S287 dephosphorylation. This is because any Cdc25 protein left unprotected by transient 14-3-3 dissociation could be re-phosphorylated by Chk1 and Cds1 kinases. In mitosis, both 14-3-3 binding and Chk1/Cds1-mediated phosphorylation are lost, enabling efficient PP1-mediated dephosphorylation.
14-3-3 removal from Cdc25 occurs independently of S287 dephosphorylation
Because 14-3-3 release occurred prior to S287 dephosphorylation, and preventing dephosphorylation did not inhibit this release, we conclude that a distinct mechanism must exist for 14-3-3 removal from Cdc25. Our data demonstrate that one element of such a signaling pathway is likely to be Cdk2. As mutation of a candidate Cdk2 phosphorylation site (T138) and selective depletion of Cdk2 from extracts both abrogated 14-3-3 release, we propose that the requirement for Cdk2 in promoting mitotic entry stems from its role in 14-3-3 release. Although our data show that Cdk2 is required for 14-3-3 release from Cdc25, we do not believe it is sufficient for this release as neither Cdk2–cyclin E nor Cdk2–cyclin A complexes could promote release of 14-3-3 from Chk1-phosphorylated Cdc25 in vitro (S.S.Margolis, unpublished). We therefore hypothesize that Cdk2 activity during interphase ‘primes’ Cdc25 for a subsequent 14-3-3-releasing signal (perhaps phosphorylation by another kinase) at G2/M. Although we considered the phospho-Ser/Pro-directed prolyl isomerase, Pin1, as a candidate for promoting Cdc25/14-3-3 release, addition of Pin1 to the in vitro reaction did not allow purified Cdk2–cyclin E to trigger release (S.S.Margolis, unpublished). Future experiments will therefore be directed at identification of critical 14-3-3 releasing factors.
There has been some controversy concerning the precise roles of phosphorylation and 14-3-3 in suppressing Cdc25. In this regard, it is interesting to note that we have found the P289A mutant protein to be more potent than WT, but less potent than S287A Cdc25 in inducing oocyte maturation (S.S.Margolis, unpublished). Futhermore, WT Cdc25 is imported into nuclei more rapidly than a mutant of Cdc25 that cannot bind PP1 (see Supplementary data A). While this issue requires further examination, it is interesting to speculate that dephosphorylation of S287 and not just 14-3-3 removal is required. Whether it is the suppression of Cdc25 enzymatic activity, changes in subcellular localization or both that contribute to Cdc25 inhibition during interphase, this observation suggests that 14-3-3 binding and S287 phosphorylation cooperate to inhibit Cdc25. Although 14-3-3 may play some independent role in inhibiting Cdc25, data presented here show that 14-3-3 can protect Cdc25 from PP1-mediated S287 dephosphorylation. Moreover, mechanisms distinct from dephosphorylation and involving the kinase Cdk2 can regulate 14-3-3–Cdc25 dissociation.
Materials and methods
Protein expression clones
WT, T48V, T67V and T138V mutant Cdc25 cDNAs, generously provided by J.Maller, were used as templates to generate N-terminal clones in pGEXKG using the primers described in Yang et al. (1999). Full-length WT Cdc25 or S287A mutant Cdc25 cDNAs were PCR amplified using the Xenopus Cdc25 clones previously described (Yang et al., 1999) as template with the primers 5′-CATGCCAT GGGAATGGCAGAGAGTCACATAATG-3′ and 5′-CTAGTCTAGA TTAAAGCTTCATTATGCGGGC-3′. PCR products were purified, digested, and cloned into the NcoI and XhoI sites of pGEXKG.
The Quikchange kit (Stratagene) was used to generate V105A/F107A and P289A mutants in full-length Cdc25. V105A/F107A primers were 5′-CTCCAAAGACACAATTTGCCCAGGCCGATGGCCTATTTACACCTG-3′ and its complement, and P289A primers were 5′-CCGCTCACCTTCTATGGCAGAGAAACTTGACAGGC-3′ and its complement. His-tagged 14-3-3 ε was expressed as in Kumagai et al. (1998b).
To generate Flag-tagged Cdc25 for oocyte expression, a 5′ primer for PCR was designed containing a BglII site and the Flag sequence in-frame with the Cdc25 start codon. The 3′ primer contained a BglII site after the Cdc25 stop codon. WT, V105A/F107A and T138V Cdc25s in pGEXKG were used as templates to generate PCR products which were cloned into pSP64T. mRNA was generated from linearized SP64T clones using the Stratagene mRNA capping kit. His-Chk1 was produced using the Invitrogen baculovirus expression system and purified on a nickel chelate column. Cyclin B1Δ13 was made as described previously (Walsh et al., 2003).
Kinase and phosphatase assays
To generate phosphorylated GST–Cdc25, recombinant His-Chk1 and 1–2 µg of Cdc25 were incubated in kinase buffer [10 mM Tris–HCl pH 7.5, 10 mM MgCl2, 1 mM dithiothreitol (DTT), 100 µM ATP] for 30 min at 30°C.
A 1 µg aliquot of Chk1-phosphorylated Cdc25 was incubated in phosphatase buffer (50 mM Tris–HCl pH 7.5, 1 mM EDTA, 0.1% β-mercaptoethanol) with purified PP1 in the presence of 4 µg of either GST or His-14-3-3 at 37°C. Samples were washed with phosphatase buffer and resolved by SDS–PAGE.
The rate of S287 dephosphorylation was measured by incubation of GST–Cdc25 in CSF extracts (1 µg of Cdc25/100 µl of extract) or in interphase egg extracts supplemented with Cyclin B1Δ13. Samples were incubated at room temperature, withdrawn at the indicated times, and washed 10 times with egg lysis buffer (ELB: 250 mM sucrose, 2.5 mM MgCl2, 1 mM DTT, 50 mM KCl, 10 mM HEPES pH 7.7).
Fostriecin or thio-phosphorylated I-1 was incubated with egg extracts for 15 min prior to addition of Chk1-phosphorylated Cdc25. The reaction was then incubated for 1 h at 37°C. Samples were washed 10 times with ELB, resolved by SDS–PAGE and immunoblotted with anti-pSer 287 (Cell Signaling Technology).
Preparation of Xenopus oocytes and extracts
Egg extracts were prepared as described by Smythe and Newport (1991). Stage VI oocytes were prepared for microinjection and, where indicated, treated with progesterone as described previously (Walsh et al., 2003). For western blotting, oocytes were lysed manually in 10 µl of lysis buffer [20 mM HEPES pH 7.5, 20 mM β-glycerophosphate, 15 mM MgCl2, 20 mM EGTA, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 µg aprotenin/leupeptin] per oocyte and spun at 14 000 g for 5 min.
Co-precipitation and immunodepletion experiments
Flag-Cdc25 proteins expressed in microinjected oocytes were immunoprecipitated using anti-Flag Sepharose, washed with ELB plus 0.1% Triton X-100, and analyzed by SDS–PAGE and western blotting. To analyze Flag-Cdc25–14-3-3 co-immunopreciptiation, anti-Flag precipitates were washed with ELB containing 0.1% Triton X-100 and 300 mM NaCl and immunoblotted with 14-3-3 antibody (Santa Cruz). For precipitation of endogenous Cdc25, anti-Cdc25 polyclonal sera (a kind gift of Dr M.Doree) was coupled to cyanogen bromide Sepharose, washed, and incubated with Xenopus egg extract for 1–2 h at 4°C. Samples were then washed with 5 ml of ELB for anti-PP1 immunoblotting (Transduction Laboratories) or washed in ELB containing 0.1% Triton X-100 and 300 mM NaCl for 14-3-3 immunoblotting.
To analyze interactions between purified proteins, N-terminal GST–Cdc25 or GST was incubated with purified PP1 in ELB and 0.1% bovine serum albumin (BSA). Proteins were incubated at 4°C for 1–2 h and washed in a column with 5 ml of ELB. Samples were analyzed by SDS–PAGE and western blotting.
Xenopus mitotic egg extract was depleted of PP1 by four 30 min incubations with GST or GST–I-2 bound to glutathione–Sepharose. Cdk2 was depleted from Xenopus mitotic extract by four rounds of depletion with GST–p21N bound to glutathione–Sepharose beads at 4°C or with anti-Cdk2 sera.
14-3-3 release
GST–Cdc25 proteins were either incubated in interphase extract (1 µg of Cdc25/100 µl extract) to acquire 14-3-3 or phosphorylated in vitro with Chk1 and bound to recombinant 14-3-3 (1 µg of Cdc25/4 µg of 14-3-3). Cdc25–14-3-3 complexes bound to glutathione–Sepharose were transferred to mitotic egg extracts (1 µg of 14-3-3-Cdc25/100 µl of extract) and retrieved at various times by centrifugation. Samples were washed in ELB containing 0.1% Triton X-100 and 300 mM NaCl, resolved by SDS–PAGE, and immunoblotted with anti-14-3-3.
Supplementary data
Supplementray data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We are grateful to M.Doree, T.Stukenberg and M.Kirschner for generously providing anti-Cdc25 antibodies, B.-B.Zhou for providing anti-pSer 287 sera, J.Maller for Cdc25 mutants, A.Dutta and J.Pines for p21 constructs, and C.Holley and A.Yamada for assistance with oocyte injections. This work was supported by NIH grants to S.K. (NIH RO1 GM067225) and S.S. (NIH RO1 DK52054).
References
- Bulavin D.V. et al. (2003) Dual phosphorylation controls Cdc25 phosphatases and mitotic entry. Nat. Cell Biol., 5, 545–551. [DOI] [PubMed] [Google Scholar]
- Cohen P.T. (2002) Protein phosphatase 1-targeted in many directions. J. Cell Sci., 115, 241–256. [DOI] [PubMed] [Google Scholar]
- Coleman T.R. and Dunphy,W.G. (1994) Cdc2 regulatory factors. Curr. Opin. Cell Biol., 6, 877–882. [DOI] [PubMed] [Google Scholar]
- Connor J.H., Quan,H., Oliver,C. and Shenolikar,S. (1998) Inhibitor-1, a regulator of protein phosphatase 1 function. Methods Mol. Biol., 93, 41–58. [DOI] [PubMed] [Google Scholar]
- Duckworth B.C., Weaver,J.S. and Ruderman,J.V. (2002) G2 arrest in Xenopus oocytes depends on phosphorylation of cdc25 by protein kinase A. Proc. Natl Acad. Sci. USA, 99, 16794–16799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff M.P., Johnson,D.F., Moorhead,G., Cohen,P.T., Cohen,P. and Barford,D. (1997) Structural basis for the recognition of regulatory subunits by the catalytic subunit of protein phosphatase 1. EMBO J., 16, 1876–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finidori-Lepicard J., Schorderet-Slatkine,S., Hanoune,J. and Baulieu,E.E. (1981) Progesterone inhibits membrane-bound adenylate cyclase in Xenopus laevis oocytes. Nature, 292, 255–257. [DOI] [PubMed] [Google Scholar]
- Furnari B., Rhind,N. and Russell,P. (1997) Cdc25 mitotic inducer targeted by chk1 DNA damage checkpoint kinase. Science, 277, 1495–1497. [DOI] [PubMed] [Google Scholar]
- Furnari B., Blasina,A., Boddy,M.N., McGowan,C.H. and Russell,P. (1999) Cdc25 inhibited in vivo and in vitro by checkpoint kinases Cds1 and Chk1. Mol. Biol. Cell, 10, 833–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves P.R., Lovly,C.M., Uy,G.L. and Piwnica-Worms,H. (2001) Localization of human Cdc25C is regulated both by nuclear export and 14-3-3 protein binding. Oncogene, 20, 1839–1851. [DOI] [PubMed] [Google Scholar]
- Guadagno T.M. and Newport,J.W. (1996) Cdk2 kinase is required for entry into mitosis as a positive regulator of Cdc2–cyclin B kinase activity. Cell, 84, 73–82. [DOI] [PubMed] [Google Scholar]
- Hoffmann I., Clarke,P.R., Marcote,M.J., Karsenti,E. and Draetta,G. (1993) Phosphorylation and activation of human cdc25-C by cdc2–cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO J., 12, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huchon D., Ozon,R. and Demaille,J.G. (1981a) Protein phosphatase-1 is involved in Xenopus oocyte maturation. Nature, 294, 358–359. [DOI] [PubMed] [Google Scholar]
- Huchon D., Ozon,R., Fischer,E.H. and Demaille,J.G. (1981b) The pure inhibitor of cAMP-dependent protein kinase initiates Xenopus laevis meiotic maturation. A 4-step scheme for meiotic maturation. Mol. Cell. Endocrinol., 22, 211–222. [DOI] [PubMed] [Google Scholar]
- Hutchins J.R., Dikovskaya,D. and Clarke,P.R. (2002) Dephosphorylation of the inhibitory phosphorylation site S287 in Xenopus Cdc25C by protein phosphatase-2A is inhibited by 14-3-3 binding. FEBS Lett., 528, 267–271. [DOI] [PubMed] [Google Scholar]
- Izumi T. and Maller,J.L. (1993) Elimination of cdc2 phosphorylation sites in the cdc25 phosphatase blocks initiation of M-phase. Mol. Biol. Cell, 4, 1337–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T., Walker,D.H. and Maller,J.L. (1992) Periodic changes in phosphorylation of the Xenopus cdc25 phosphatase regulate its activity. Mol. Biol. Cell, 3, 927–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaiskou A., Cayla,X., Haccard,O., Jessus,C. and Ozon,R. (1998) MPF amplification in Xenopus oocyte extracts depends on a two-step activation of cdc25 phosphatase. Exp. Cell Res., 244, 491–500. [DOI] [PubMed] [Google Scholar]
- Kumagai A. and Dunphy,W.G. (1996) Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science, 273, 1377–1380. [DOI] [PubMed] [Google Scholar]
- Kumagai A., Guo,Z., Emami,K.H., Wang,S.X. and Dunphy,W.G. (1998a) The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J. Cell Biol., 142, 1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A., Yakowec,P.S. and Dunphy,W.G. (1998b) 14-3-3 proteins act as negative regulators of the mitotic inducer Cdc25 in Xenopus egg extracts. Mol. Biol. Cell, 9, 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A. and Dunphy,W.G. (1999) Binding of 14-3-3 proteins and nuclear export control the intracellular localization of the mitotic inducer Cdc25. Genes Dev., 13, 1067–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew D.J. and Kornbluth,S. (1996) Regulatory roles of cyclin dependent kinase phosphorylation in cell cycle control. Curr. Opin. Cell Biol., 8, 795–804. [DOI] [PubMed] [Google Scholar]
- Maller J.L., Butcher,F.R. and Krebs,E.G. (1979) Early effect of progesterone on levels of cyclic adenosine 3′:5′-monophosphate in Xenopus oocytes. J. Biol. Chem., 254, 579–582. [PubMed] [Google Scholar]
- Moore J.D., Kirk,J.A. and Hunt T. (2003) Unmasking the S-phase-promoting potential of cyclin B1. Science, 300, 987–990. [DOI] [PubMed] [Google Scholar]
- Mulner O., Huchon,D., Thibier,C. and Ozon,R. (1979) Cyclic AMP synthesis in Xenopus laevis oocytes: inhibition by progesterone. Biochim. Biophys Acta, 582, 179–184. [DOI] [PubMed] [Google Scholar]
- Peng C.-Y., Graves,P.R., Thoma,R.S., Wu,Z., Shaw,A.S. and Piwnica-Worms,H. (1997) Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25c on serine-216. Science, 277, 1501–1504. [DOI] [PubMed] [Google Scholar]
- Peng C.Y., Graves,P.R., Ogg,S., Thoma,R.S., Byrnes,M.J.,3rd, Wu,Z., Stephenson,M.T. and Piwnica-Worms,H. (1998) C-TAK1 protein kinase phosphorylates human Cdc25C on serine 216 and promotes 14-3-3 protein binding. Cell Growth Differ., 9, 197–208. [PubMed] [Google Scholar]
- Pinna L.A. and Donella-Deana,A. (1994) Phosphorylated synthetic peptides as tools for studying protein phosphatases. Biochim. Biophys Acta, 1222, 415–431. [DOI] [PubMed] [Google Scholar]
- Qian Y.W., Erikson,E., Taieb,F.E. and Maller,J.L. (2001) The polo-like kinase Plx1 is required for activation of the phosphatase Cdc25C and cyclin B–Cdc2 in Xenopus oocytes. Mol. Biol. Cell, 12, 1791–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler S.E. and Maller,J.L. (1981) Progesterone inhibits adenylate cyclase in Xenopus oocytes. Action on the guanine nucleotide regulatory protein. J. Biol. Chem., 256, 6368–6373. [PubMed] [Google Scholar]
- Sanchez Y., Wong,C., Thoma,R.S., Richman,R., Wu,Z., Piwnica-Worms,H. and Elledge,S.J. (1997) Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science, 277, 1497–1501. [DOI] [PubMed] [Google Scholar]
- Sheppeck J.E. 2nd, Gauss,C.M. and Chamberlin,A.R. (1997) Inhibition of the Ser–Thr phosphatases PP1 and PP2A by naturally occurring toxins. Bioorg. Med. Chem., 5, 1739–1750. [DOI] [PubMed] [Google Scholar]
- Smythe C. and Newport,J.W. (1991) Systems for the study of nuclear assembly, DNA replication and nuclear breakdown in Xenopus laevis egg extracts. Methods Cell Biol., 35, 449–468. [DOI] [PubMed] [Google Scholar]
- Terry-Lorenzo R.T., Inoue,M., Connor,J.H., Haystead,T.A., Armbruster,B.N., Gupta,R.P., Oliver,C.J. and Shenolikar,S. (2000) Neurofilament-L is a protein phosphatase-1-binding protein associated with neuronal plasma membrane and post-synaptic density. J. Biol. Chem., 275, 2439–2446. [DOI] [PubMed] [Google Scholar]
- Wakula P., Beullens,M., Ceulemans,H., Stalmans,W. and Bollen,M. (2003) Degeneracy and function of the ubiquitous RVXF motif that mediates binding to protein phosphatase-1. J. Biol. Chem., 278, 18817–18823. [DOI] [PubMed] [Google Scholar]
- Walsh A.H., Cheng,A. and Honkanen,R.E. (1997) Fostriecin, an antitumor antibiotic with inhibitory activity against serine/threonine protein phosphatases types 1 (PP1) and 2A (PP2A), is highly selective for PP2A. FEBS Lett., 416, 230–234. [DOI] [PubMed] [Google Scholar]
- Walsh S., Margolis,S.S. and Kornbluth,S. (2003) Phosphorylation of the cyclin b1 cytoplasmic retention sequence by mitogen-activated protein kinase and plx. Mol. Cancer Res., 1, 280–289. [PubMed] [Google Scholar]
- Yaffe M.B., Rittinger,K., Volinia,S., Caron,P.R., Aitken,A., Leffers,H., Gamblin,S.J., Smerdon,S.J. and Cantley,L.C. (1997) The structural basis for 14-3-3: phosphopeptide binding specificity. Cell, 91, 961–971. [DOI] [PubMed] [Google Scholar]
- Yang J., Winkler,K., Yoshida,M. and Kornbluth,S. (1999) Maintenance of G2 arrest in the Xenopus oocyte: a role for 14-3-3-mediated inhibition of Cdc25 nuclear import. EMBO J., 18, 2174–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y., Forbes,K.C., Wu,Z., Moreno,S., Piwnica-Worms,H. and Enoch,T. (1998) Replication checkpoint requires phosphorylation of the phosphatase Cdc25 by Cds1 or Chk1. Nature, 395, 507–510. [DOI] [PubMed] [Google Scholar]