Abstract
Background and purpose
New treatment strategies for acute ischemic stroke must be evaluated in the context of effective reperfusion. Minocycline is a neuroprotective agent that inhibits proteolytic enzymes and therefore could potentially both inactivate the clot lysis effect and decrease the damaging effects of tissue plasminogen activator. This study aimed to determine the effect of minocycline on tPA clot lysis and tPA induced hemorrhage formation after ischemia.
Methods
Fibrinolytic and amidolytic activities of tPA were investigated in vitro over a range of clinically relevant minocycline concentrations. A suture occlusion model of three hour temporary cerebral ischemia in rats treated with tPA and two different minocycline regimens was used. Blood brain barrier basal lamina components, MMPs, hemorrhage formation, infarct size, edema and behavior outcome were assessed.
Results
Minocycline did not affect tPA fibrinolysis. However, minocycline 3 mg/kg intravenous treatment decreased total protein expression of both MMP-2 (p=0.0034) and MMP-9 (p = 0.001 for 92 kDa and p=0.0084 for 87 kDa). It also decreased incidence of hemorrhage (p = 0.019), improved neurological outcome (p=0.0001 for Bederson score and p=0.0391 for paw grasp test) and appeared to decrease mortality. MMP inhibition was associated with decreased degradation in collagen IV and laminin-α1 (p=0.0001).
Conclusions
Combination treatment with minocycline is beneficial in tPA-treated animals and does not compromise clot lysis. These results also suggest minocycline neurovascular protection after stroke may involve the direct protection of the blood brain barrier during thrombolysis with tPA.
Keywords: cerebral ischemia, minocycline, tPA, MMP, vascular protection, hemorrhagic transformation
BACKGROUND
The scientific community and regulatory bodies demand that all new acute treatments of ischemic stroke be evaluated for their potential to interact with the only approved therapy, tissue plasminogen activator (tPA). Intravenous treatment with tPA within three hours of onset has been shown to be beneficial in achieving better outcomes [1] and recent information suggests that carefully selected patients may benefit when treated even up to 4.5 hours after the onset of symptoms [2]. However, there is still a great need to develop treatments that complement and enhance the safety and efficacy of tPA.
Recent data have shown that tPA has deleterious effects that are independent of its fibrinolytic activity and that tPA leads to increased chances of hemorrhagic transformation by amplifying the matrix metalloproteinase (MMP) cascade triggered by ischemic damage in the brain [3]. MMPs 2 (72 kDa) and 9 (92 kDa) have been shown to be elevated early after experimental stroke in rats [4, 5], [6] and the formation of edema and hemorrhagic transformation associated with thrombolysis with tPA has been linked to MMPs [7], [8, 9], MMP inhibition might represent a target for decreasing the risks of thrombolysis [10].
Minocycline treatment has been shown to decrease the disruption and leakage of the blood brain barrier [11] and attenuate the enzymatic activity of the proteolytic enzyme MMP-9 following stimulation with vascular endothelial growth factor [12]. Furthermore, minocycline has been shown to decrease microvascular permeability associated with MMP-2 and MMP-9 activity [13]. Our previous studies showed that post – injury minocycline treatment decreases the activation of MMPs after temporary cerebral ischemia [14].
Although minocycline is a promising neuroprotective strategy, a thorough investigation of its interaction with tPA after stroke has not been pursued. Minocycline could reduce tPA's fibrinolytic activity through its enzyme inhibitory effect. However, it could also prevent reperfusion– induced MMP activation and vascular damage. This study aimed to determine whether the interaction of minocycline with tPA was significant in vitro and in an experimental model of stroke.
MATERIALS AND METHODS
tPA in vitro clot lysis and amidolytic activity
tPA activity was measured by in vitro fibrinolytic and amidolytic assays. For the fibrinolytic assay, whole blood was obtained from 4 healthy individuals. This study was approved by the Human Assurance Committee of the Medical College of Georgia. Whole blood (50 μl) from healthy individuals was mixed with trace amounts of 125I-labeled human fibrinogen (100,000 cpm) and clotted . The formed clots were washed, suspended in plasma (1 ml) and placed in a water bath at 37°C. A wide range of clinically relevant concentrations of minocycline (0–30 μg/ml) were added to the supernatant, and clot lysis was immediately initiated by t-PA 2nM (Activase; Genentech). The degree of fibrinolysis was measured at various times of incubation by counting the percent soluble 125I-fibrin degradation products [15, 16] . The kinetic parameters of amidolysis were measured in the presence or absence of minocycline with H-D-isoleucyl-L-prolyl-L-arginine-p-nitroanilide dihydrochloride (S-2288; Chromogenic) as substrate. 100 nM tPA was added to the microtiter plate containing assay buffer (0.1 M Tris-HCl, 0.1 M NaCl, pH 8.4), minocycline (30 μg/ml) and S-2288 (150– 1500 μM) at 37°. The generation of amidolytic activity was measured at 405 nm for 7 minutes in a microplate reader (Synergy HT, Bio-Tech). The data was plotted as velocity of p-nitroanilide release over substrate concentration and analyzed by hyperbolic curve fitting with GraphPad Prism software [17].
Animal procedures and experimental stroke
The Institutional Animal Care and Use Committee (IACUC) of the Veterans Affairs Medical Center approved our study protocol. Male Wistar rats in a range of 270–300g of body weight purchased from Charles River Laboratory (Wilmington, MA) were used. Cerebral ischemia was induced by intraluminal suture occlusion of the middle cerebral artery (MCA) for 3 hours [14]. Immediately before removal of the suture, the jugular vein was exposed and the tip of a rat jugular vein catheter (Braintree Scientific R-JVC) plugged with a catheter port plug (Braintree Scientific 23-PP) was inserted into the lumen. After removing the suture, the jugular catheter was connected to polyethylene 50 tubing, flushed with 1mL of heparinized saline, followed by a bolus injection containing 10 % of the tPA dose, and a 20 minute infusion (Harvard Apparatus Infusion Pump). Animals were assigned to one of 4 groups: control (saline treated), tPA (10 mg/kg) alone, tPA plus minocycline (Sigma Aldrich CO.) intravenously (3 mg/kg IV – jugular vein) and tPA plus minocycline intraperitoneally (IP) groups. Minocycline 45 mg/kg was injected IP 5 minutes after the onset of reperfusion and both IV and IP groups got an IP dose 12 hours later. Sacrifice, with either saline perfusion (infarct size and hemoglobin) or flash freezing (molecular markers) of brain tissue, occurred 24 hours after stroke.
Gelatin Zymography and MMP immunoblotting
After extraction, the brain was washed with ice-cold PBS and placed in a coronal matrix to be sliced and homogenized as previously described by Heo and collaborators [5, 14]. Gelatin Zymography and MMP 2 and 9 immunoblotting were performed as reported in our previous study [14]. The bands were quantified with the use of the GelPro image analysis software.
Collagen type IV and laminin-α1 slot blot analysis
The basal lamina components laminin-α1 and collagen type IV were used to determine the health of the blood brain barrier in the experimental animals. Both collagen IV and laminin proteins were studied with slot blotting and semi quantification by densitometric analysis. The same brain tissue homogenate used for MMP analysis was used. Nitrocellulose membranes were used. Samples were loaded into the Bio – Dot apparatus (Bio-Rad Laboratories, CA) and a slow vacuum was applied. Collagen type IV antibody (rabbit polyclonal anti – collagen IV, Santa Cruz Biotechnology) and laminin-α1 (goat polyclonal anti – laminin-α1, Santa Cruz Biotechnology) were used. Secondary antibodies anti- rabbit IgG and anti – goat IgG horseradish peroxidase conjugated antibodies were used, respectively. The membranes were developed and exposed to autoradiography films (Hyblot CL, Denville Scientific Inc.). The semi quantification of the bands was performed with the use of the GelPro image analysis software.
Infarct size and edema determination
The infarct volume were measured using 2,3,5 – triphenyltetrazolium chloride (TTC) stained brain slices as previously described [18]. The image of the slices were captured and analyzed with the Zeiss KS300 software. The total infarct volume was reported as percent volume to the total ischemic hemisphere. The edema was quantified by the percent difference of volume between the stroke and contralateral hemispheres.
Hemorrhage formation and enzyme linked immunosorbent assay for hemoglobin
The presence of visible hematoma or hemorrhagic transformation was recorded and the quantification of hemorrhage was done by assessing the hemoglobin content in the tissue after complete perfusion of the brain. This was accomplished by an ELISA method for hemoglobin, as has been previously reported [19].
Neurological examination
All animals were examined immediately prior to reperfusion and prior to sacrificing for motor function. The Bederson method [20] and paw grasp test were used. The paw grasp test measures in a scale of 0 to 3, the use and grasping strength of the ipsilateral forelimb. The occurrence of death – or near death, unable to perform tests – was also recorded.
Data analysis
To examine differences in various outcome measures between treatment groups, chi-square tests (if the variable was categorical) and one-way ANOVA (if the variable was continuous) were performed. Because not all post hoc pair wise comparisons were warranted between treatment groups, a Bonferroni adjustment to the overall alpha level for the number of post hoc comparisons were used. All statistical analyses were performed using SAS 9.1.3 and overall statistical significance was assessed using an alpha level of 0.05.
RESULTS
tPA activity
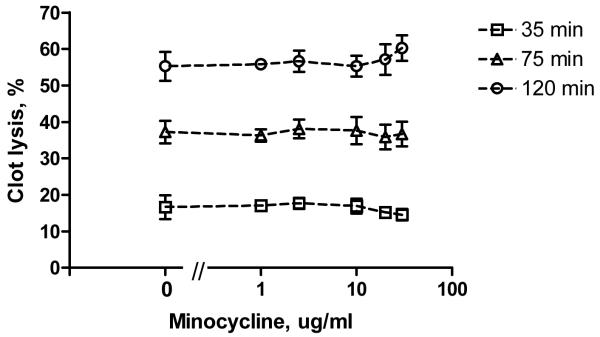
Kinetic studies were performed to determine whether minocycline may directly affect tPA proteolytic activity. The kinetic parameters of cleavage of the tripeptide substrate S-2288 at pH 7.4 and 37°C by tPA are shown in Table 1. Minocycline (30 μg/ml) did not change either the apparent Michaelis-Menten constant (Km) or the catalytic constant (Kcat). Thus, minocycline does not affect amidolytic efficiency of tPA. To test fibrinolytic activity of tPA, in vitro clot lysis assay was used. In the presence of different minocycline concentrations (1–30 μg/ml), the rate of clot lysis by tPA (2 nM) remained at 17%, 38% and 55% after 35, 75 and 120 minutes, respectively, regardless of minocycline concentration (Fig. 1). At no concentrations tested did minocycline decrease fibrinolysis by tPA.
Table 1.
Effect of minocycline on amidolytic parameters of tPA. The hydrolysis of S-2288 was monitored for 7 minutes at 37°C and analyzed by Michaelis-Menten curve fitting. Minocycline did not affect tPA amidolytic efficiency. Values represent the mean ± standard error of the mean (SEM) (n=6).
| Minocycline | Km, μM | kcat, s-1 | Km/kcat, mM-1s-1 |
|---|---|---|---|
| none | 1332 ± 203 | 6.2 ± 0.5 | 4.7 |
| 30 μg/ml | 1330 ± 227 | 6.0 ± 0.6 | 4.5 |
Figure 1.
Impact of minocycline on fibrinolytic activity of tPA. The lysis of blood clots was initiated by 2nM tPA at 37°C. The amount of fibrinolysis was determined by measuring the release of soluble 125I-fibrin degradation products at various time intervals (35, 75 and 120 minutes). The fibrinolytic activity of tPA was not affected by any concentration tested of minocycline (1 through 30 ug/ ml). The means ± SEM (n=4) are shown.
Matrix metalloproteinases 2 and 9
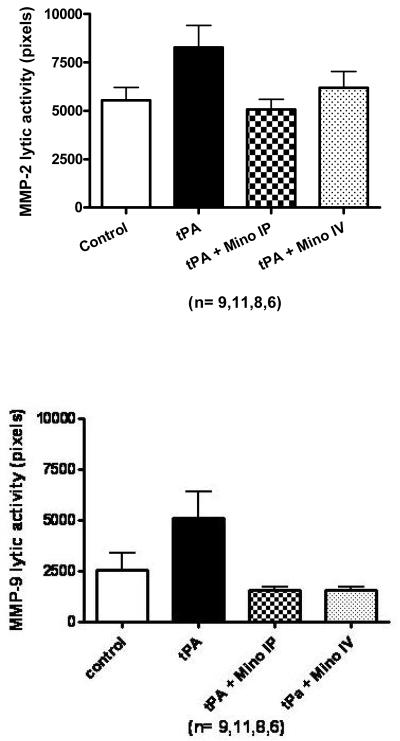
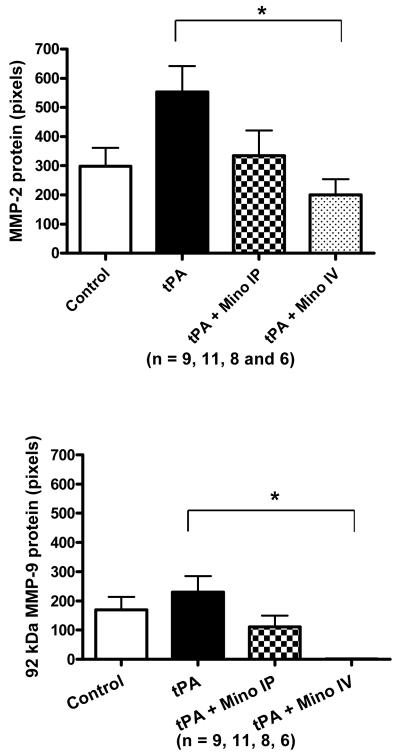
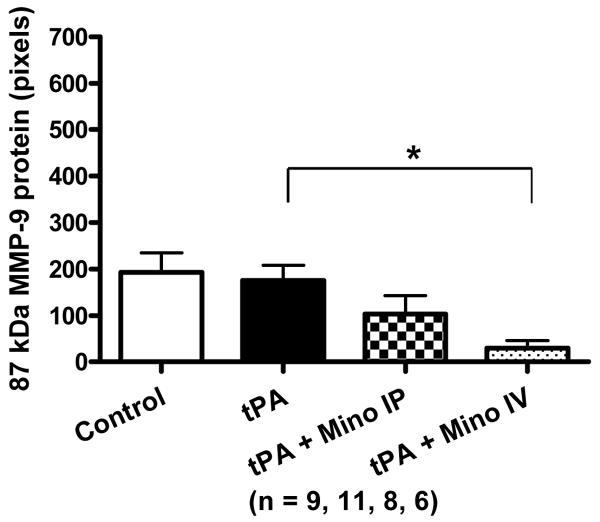
Only two bands were detected by gelatin zymography: 85 kDA and 67 kDa, corresponding to active MMP-9 and active MMP-2 respectively. tPA treatment during reperfusion increased the activity of both MMP-2 and MMP-9 in the brain. The minocycline-treated animals had decreased tPA-induced exacerbation of MMP activity compared to untreated animals but these were not significant (Fig. 2A). IV minocycline treatment however, significantly impacted the protein content of both MMP-2 (detected at 72 kDa – F=3.88; p=0.0185) and MMP-9 (92 kDa; F=4.75; p=0.008 and 87 kDa; F=4.03; p=0.0160 bands) (Fig. 2B). Minocycline IV significantly decreased all the bands detected compared to tPA (p=0.0034 for MMP-2, p=0.0084 for MMP-9 87 kDa and p=0.001 for MMP-9 92 kDa) and MMP-9 87kDa, compared to control (p=0.004).
Figure 2.
(A) Brain MMP-2 and MMP-9 activities after stroke as measured by gelatin zymography. MMP-2 and MMP-9 were elevated by tPA compared to stroked saline controls. Minocycline decreased MMP-9 activity to below control levels (NS). (B) Brain MMP-2 (72 kDa) and MMP-9 (87 kDa and 92kDa) protein content 24 hours after stroke as determined by immunoblotting. IV Minocycline group had significantly lower MMP-2 protein, MMP-9 92 kDa and MMP-9 87 kDa proteins compared to tPA animals (p=0.0034; p=0.001and p=0.0084, respectively). Vertical bars indicate SEM.
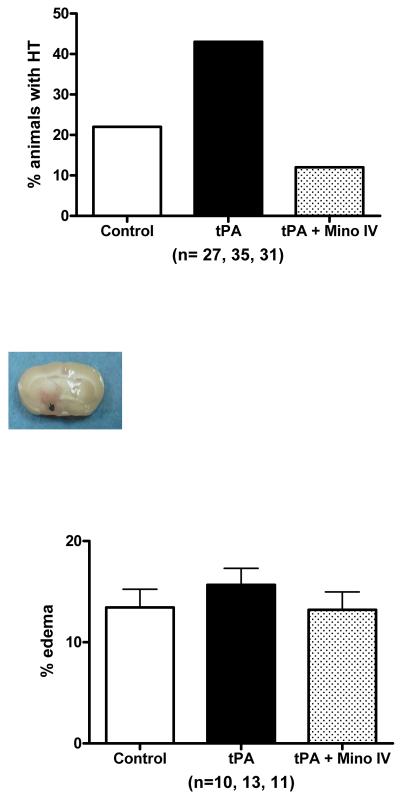
Minocycline plus tPA and vascular outcome
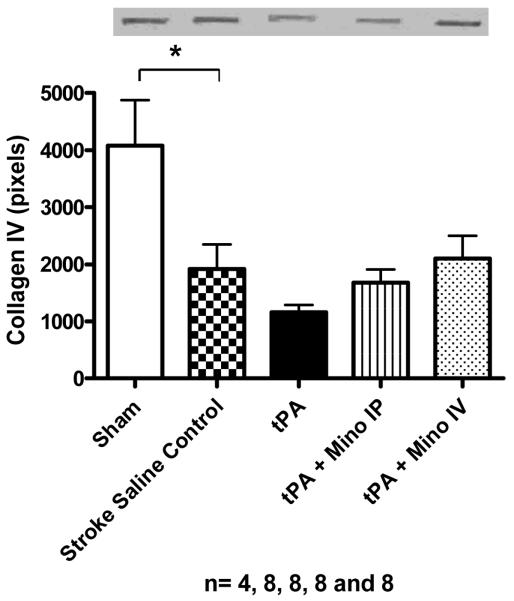
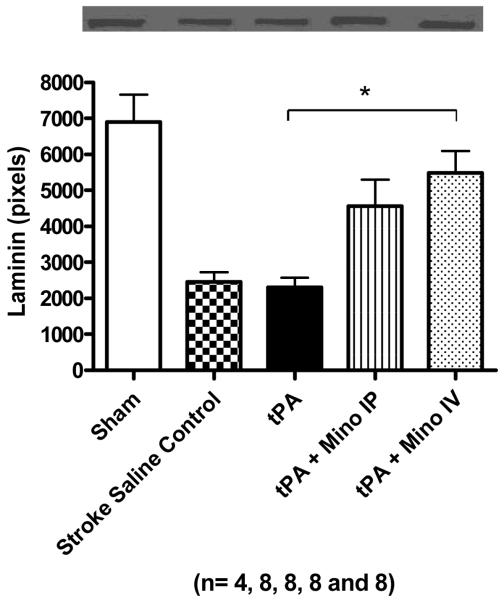
We measured vascular integrity using four different parameters: 1) incidence of visible hemorrhagic transformation, 2) the content of hemoglobin in the brain parenchyma after complete perfusion, 3) the formation of brain edema and 4) basal lamina protein degradation. Although the content of tissue hemoglobin in tPA treated animals was only slightly larger than in untreated animals (not shown), the occurrence of bleeding observed macroscopically in these animal brains was two fold higher than control and minocycline IV treated animals (p = 0.0190) (Fig. 3A). Inspection of animals that died prematurely also revealed that tPA–treated animals had developed large brain hematomas as illustrated by Fig. 3B. Although not significant, edema was slightly elevated in the tPA group and decreased in combination with IV minocycline (Fig. 3C). Stroked brains had significantly reduced collagen type IV compared to sham brains (F=5.96; p=0.0011). tPA treatment further reduced the amount of collagen and combination treatment with 3 mg/ kg IV minocycline preserved this protein to above stroked control animals (Fig. 4A)(NS). Likewise, IV minocycline robustly prevented laminin-α1 degradation in the brain (F=11.28; p<0.0001), compared to both stroke groups (Fig. 4B).
Figure 3.
(A). Hemorrhagic transformation was increased (p = 0.0190) in animals treated with tPA 10 mg/ kg. Adjuvant treatment with minocycline 3 mg/ kg intravenously prevented this increase. (B) Bleeding in an animal in the tPA treated group (10 mg/ kg). Hematomas were seen in the animals that died. (C) Minocycline treatment (3 mg/ kg intravenously) did not significantly decrease edema. Vertical bars indicate SEM.
Figure 4.
(A) Effect of treatment on collagen type IV protein content in the brain. Stroke significantly decreased baseline value (p=0.0011). Treatment with tPA 10 mg/ kg further decreased collagen IV. Treatment with 3 mg/ kg minocycline intravenously diminished collagen degradation. (B) Effect of treatment on laminin-α1 protein content in the brain. Stroke significantly decreased laminin and treatment with 3 mg/ kg minocycline strongly prevented laminin degradation (p = 0.0001). Vertical bars indicate SEM.
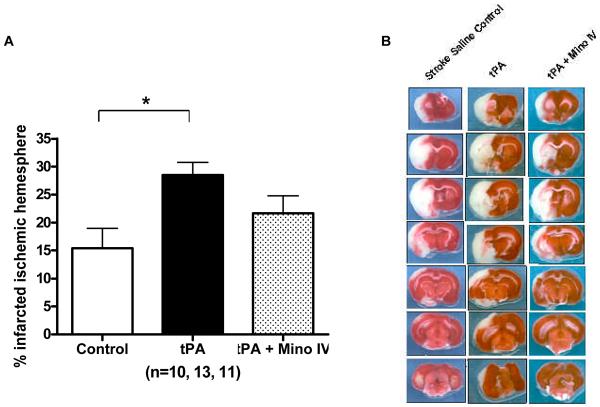
Infarct size and neurological evaluation
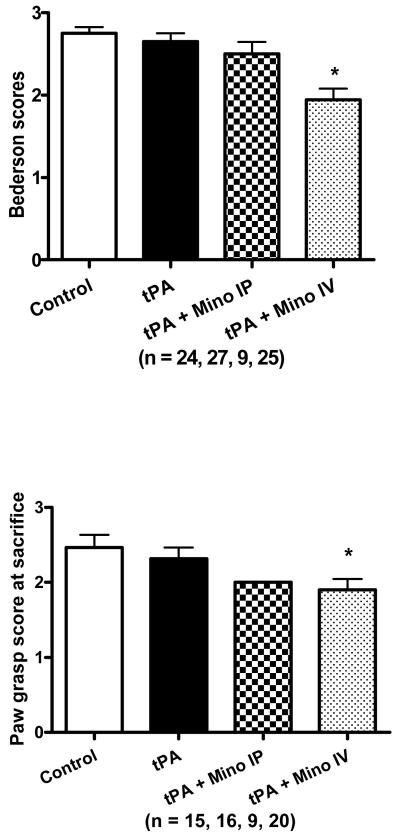
tPA increased infarct volume (F=4.99; p=0.013) when compared to stroked control animals (infused with just saline) (p=0.0035). Intravenous minocycline animals had decreased infarct size compared to tPA alone animals, but it remained above control levels and it was not statistically significant (Fig. 5A). tPA increased mortality and this was ameliorated by minocycline (Table 2). Minocycline acutely (IV) resulted in improvement of the Bederson score (F=11.16; p<0.0001) and paw grasp (F=3.31; p=0.0391), compared to tPA alone (p<0.0001), control (p<0.01) and Minocycline IP group (p<0.01 for Bederson only)(Figs. 6 A and B). Although IP treatment with minocycline seemed to decrease the impairment, the effect was not statistically significant, suggesting early delivery is critical to achieve optimal improved functional outcome.
Figure 5.
(A) Infarct size after correction for edema. tPA significantly increased infarct size (p=0.0035). Adjuvant treatment with 3 mg/ kg of minocycline intravenously decreased infarct size. Vertical bars indicate SEM. (B) Illustration with a representative brain of each of the treatment groups.
Table 2.
Mortality rate. Mortality was three times higher in tPA animals compared to both stroke saline control animals and IV (early delivery) minocycline-treated (3 mg/ kg) animals, although it was not statistically significant. When minocycline was delivered late (through IP injection), the mortality was similar to that of the tPA treated group.
| Group | Stroke Saline Control n = 25 | tPA n = 28 | tPA + Mino IP n = 11 | tPA + Mino IV n = 27 |
|---|---|---|---|---|
| Dead (mortality rate) | 2 (8%) | 7 (25%) | 2 (18%) | 2 (7%) |
Figure 6.
Effect treatment in behavioral outcome. (A) Improvement in the Bederson scale of animals treated with IV minocycline compared to tPA only treated animals (p=0.0001). (B) tPA + Mino IV treatment group performed better in the paw grasp task (p=0.0391) (B). Vertical bars in all graphs represent SEM.
DISCUSSION
This study demonstrated that the MMP inhibition effect of minocycline did not impair the ability of tPA to cleave plasminogen and exert its fibrinolytic effect. This was validated in vivo by Murata and collaborators [21]. In their study, minocycline treatment did not have a significant effect on the cerebral perfusion restored by tPA after embolic stroke in rats. However, the clot lysis effect of the treatment interaction was not studied directly.
We demonstrated in this study that post-reperfusion treatment with both low dose IV minocycline and high dose IP minocycline inhibited MMPs that are upregulated by treatment with tPA. These two drug dosing regimens were based on previous studies demonstrating therapeutic efficacy of minocycline in experimental stroke [18, 22]. Intraperitoneal administration has been shown to achieve a delayed but constant plasma concentration [23]. However, because systemic delivery of minocycline is delayed, we also tested whether acute delivery of minocycline (by IV administration) would achieve improved or comparable results. Both treatments appeared to decrease the tPA induced increase in MMP-9, but only the IV treated group achieved a significant reduction in protein expression. These findings support our previous results demonstrating that ischemia-induced MMPs 2 and 9 are sensitive to minocycline inhibition [14] and that earlier delivery of minocycline may achieve better MMP inhibition.
The major endpoint in our study was the formation of hemorrhage in the brain. Image analysis of macroscopic hemorrhages showed a two fold increase in tPA treated animals when compared to untreated animals. Treatment with intravenous minocycline proved beneficial. A lack of significant reduction in edema may have been due to the insensitivity of the method used. Despite this, the increase in mortality among tPA treated animals appeared to be ameliorated with minocycline.
Our results also showed that minocycline decreased infarct size, even after corrected for edema, and improved overall outcome as shown by the behavior tests. It could be argued that the decreased mortality and improved behavior outcome in the combination therapy group derived from minocycline's ability to decrease lesion volume rather than its direct protection of the vasculature [18, 24]. However, because hemorrhage formation has been shown to be directly related to increased mortality and minocycline prevented basal lamina degradation, it is likely that minocycline MMP inhibition and vascular protection contribute at least partially to the decreased mortality and overall protection after acute ischemic stroke.
Minocycline seems a logical addition to reperfusion therapy with tPA in acute ischemic stroke. Although known as an inhibitor of proteases, minocycline, in a wide range of clinically relevant concentrations, does not negatively impact the ability of tPA to lyse clots in vitro. In addition, minocycline's pleiotropic effects in the brain include structural protection of the vasculature to prevent leakiness and hemorrhagic transformation. Since tPA has the potential to exert multiple negative actions in the brain vasculature – directly or via MMPs or fibrin degradation products [25], combination therapy is likely to at least be additive in benefit.
Acknowledgments
This work was supported by NIH-NINDS-RO1NS044216-01(SCF), VA merit Review (SCF), NIH-NINDS-RO1NS055728-01(DCH and SCF), American Heart Association-SDG-0830309N (IYS) and American Heart Association SDG to ABE.
REFERENCES
- 1.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333(24):1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 3.Ning M, Furie KL, Koroshetz WJ, Lee H, Barron M, Lederer M, Wang X, Zhu M, Sorensen AG, Lo EH, et al. Association between tPA therapy and raised early matrix metalloproteinase-9 in acute stroke. Neurology. 2006;66(10):1550–1555. doi: 10.1212/01.wnl.0000216133.98416.b4. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg GA, Navratil M, Barone F, Feuerstein G. Proteolytic cascade enzymes increase in focal cerebral ischemia in rat. J Cereb Blood Flow Metab. 1996;16(3):360–366. doi: 10.1097/00004647-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Heo JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19(6):624–633. doi: 10.1097/00004647-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Gasche Y, Fujimura M, Morita-Fujimura Y, Copin JC, Kawase M, Massengale J, Chan PH. Early appearance of activated matrix metalloproteinase-9 after focal cerebral ischemia in mice: a possible role in blood-brain barrier dysfunction. J Cereb Blood Flow Metab. 1999;19(9):1020–1028. doi: 10.1097/00004647-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002;33(3):831–836. doi: 10.1161/hs0302.104542. [DOI] [PubMed] [Google Scholar]
- 8.Tsuji K, Aoki T, Tejima E, Arai K, Lee SR, Atochin DN, Huang PL, Wang X, Montaner J, Lo EH. Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke. 2005;36(9):1954–1959. doi: 10.1161/01.STR.0000177517.01203.eb. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, Lo EH. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35(11 Suppl 1):2726–2730. doi: 10.1161/01.STR.0000143219.16695.af. [DOI] [PubMed] [Google Scholar]
- 10.Pfefferkorn T, Rosenberg GA. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke. 2003;34(8):2025–2030. doi: 10.1161/01.STR.0000083051.93319.28. [DOI] [PubMed] [Google Scholar]
- 11.Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke. 2006;37(4):1087–1093. doi: 10.1161/01.STR.0000206281.77178.ac. [DOI] [PubMed] [Google Scholar]
- 12.Yao JS, Shen F, Young WL, Yang GY. Comparison of doxycycline and minocycline in the inhibition of VEGF-induced smooth muscle cell migration. Neurochem Int. 2007;50(3):524–530. doi: 10.1016/j.neuint.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutton TA, Kelly KJ, Mang HE, Plotkin Z, Sandoval RM, Dagher PC. Minocycline reduces renal microvascular leakage in a rat model of ischemic renal injury. Am J Physiol Renal Physiol. 2005;288(1):F91–97. doi: 10.1152/ajprenal.00051.2004. [DOI] [PubMed] [Google Scholar]
- 14.Machado LS, Kozak A, Ergul A, Hess DC, Borlongan CV, Fagan SC. Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC Neurosci. 2006;7:56. doi: 10.1186/1471-2202-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reed GL, Houng AK, Liu L, Parhami-Seren B, Matsueda LH, Wang S, Hedstrom L. A catalytic switch and the conversion of streptokinase to a fibrin-targeted plasminogen activator. Proc Natl Acad Sci U S A. 1999;96(16):8879–8883. doi: 10.1073/pnas.96.16.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colucci M, Scopece S, Gelato AV, Dimonte D, Semeraro N. In vitro clot lysis as a potential indicator of thrombus resistance to fibrinolysis--study in healthy subjects and correlation with blood fibrinolytic parameters. Thromb Haemost. 1997;77(4):725–729. [PubMed] [Google Scholar]
- 17.Gladysheva IP, Turner RB, Sazonova IY, Liu L, Reed GL. Coevolutionary patterns in plasminogen activation. Proc Natl Acad Sci U S A. 2003;100(16):9168–9172. doi: 10.1073/pnas.1631716100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu L, Fagan SC, Waller JL, Edwards D, Borlongan CV, Zheng J, Hill WD, Feuerstein G, Hess DC. Low dose intravenous minocycline is neuroprotective after middle cerebral artery occlusion-reperfusion in rats. BMC Neurol. 2004;4:7. doi: 10.1186/1471-2377-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilali HM, Simpkins AN, Hill WD, Waller JL, Knight RA, Fagan SC. Single slice method for quantification of hemorrhagic transformation using direct ELISA. Neurol Res. 2004;26(1):93–98. doi: 10.1179/016164104773026606. [DOI] [PubMed] [Google Scholar]
- 20.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17(3):472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 21.Murata Y, Rosell A, Scannevin RH, Rhodes KJ, Wang X, Lo EH. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke. 2008 doi: 10.1161/STROKEAHA.108.514026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fagan SC, Edwards DJ, Borlongan CV, Xu L, Arora A, Feuerstein G, Hess DC. Optimal delivery of minocycline to the brain: implication for human studies of acute neuroprotection. Exp Neurol. 2004;186(2):248–251. doi: 10.1016/j.expneurol.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Tsuji M, Wilson MA, Lange MS, Johnston MV. Minocycline worsens hypoxic-ischemic brain injury in a neonatal mouse model. Exp Neurol. 2004;189(1):58–65. doi: 10.1016/j.expneurol.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999;96(23):13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahles T, Foerch C, Sitzer M, Schroeter M, Steinmetz H, Rami A, Neumann-Haefelin T. Tissue plasminogen activator mediated blood-brain barrier damage in transient focal cerebral ischemia in rats: relevance of interactions between thrombotic material and thrombolytic agent. Vascul Pharmacol. 2005;43(4):254–259. doi: 10.1016/j.vph.2005.07.008. [DOI] [PubMed] [Google Scholar]