Abstract
We measured ambulatory blood pressure using the AM5600 in children and adolescents participating in a research study to assess the relationship of BP to risk factors for cardiovascular disease. Although this use of this monitor has been previously reported in adults, it has not been validated in pediatric subjects. In this study, we assess the accuracy of the monitor as compared to the mercury sphygmomanometer in children ages 7-18 years of age. We found that the mean of the difference between the monitor and the mercury device was 0.29 ± 3.5 and 0.045 ± 3.7 mmHg for systolic and diastolic BP respectively, which fulfills the AAMI standard for use of a device. The cumulative percent of readings between the two devices which differed assigned the device a grade of A according to the British Hypertension Society..
Keywords: hypertension, ambulatory blood pressure monitoring, Blood pressure measuring device
Introduction
Characterization of ambulatory BP is superior to measurement of casual BP in the assessment of cardiovascular risk in adults. The technique has gained popularity for use in children and adolescents as it provides a more representative sample of the individual BP profile. Validation of devices which accurately measure ambulatory BP in children is particularly challenging. We have conducted studies in children and adolescents using the AM5600 ambulatory BP monitor [1], which employs an auscultatory method of measurement as the primary measurement technique but also has an oscillometric method of measurement as the alternative mode. In this manuscript we report the validation of the monitor for measurement of ABP in children and adolescents using both the British Hypertension Society (BHS) and the American Association for the Advancement of Medical Instrumentation (AAMI) criteria for validation of test device accuracy. The BHS grades devices based upon the absolute difference between the simultaneously obtained values made with the device as compared to a mercury standard. Grades are given according to the cumulative percentage of readings within 5, 10 and 15 mmHg. [2] The AAMI standard requires that the mean BP of the test device not differ more than 5mmHg from the mercury standard and that the standard deviation of that difference not be > 8 mmHg. We used both criteria to assess the accuracy of the measurement made in 111 subjects.
Methods
Subjects were recruited from primary care clinics and from children referred to University of Tennessee Medical Group nephrology or cardiology clinics for evaluation of elevated blood pressure. Children ages 6-18 years with a casual BP ≥ 90th percentile or a first-degree relative on anti-hypertensive therapy were eligible for the study. A small group of healthy normal children were also enrolled. Informed consent was obtained from the parent and assent obtained from the study subject if the subject was a minor. The research protocol was approved by the University of Tennessee Health Science Center Institutional Review Board and followed the guidelines for good clinical practice.
Study Procedures
Subject height and weight were measured using a balance beam scale and pediatric wall-mounted stadiometer. Height percentile was calculated using the CDC NHANES III data tables by age in months. [3] Body mass index (BMI) was calculated and assigned a percentile using the CDC tables from NHANES III. [3] Ethnicity was categorized as that reported by the subject.
Ambulatory blood pressure monitoring was performed using the AM5600 ambulatory blood pressure monitor (Advanced Biosensor, Columbia, SC). Information regarding the use of this device in adults has previously been published. [4] The monitors use an auscultatoory technique to detect systolic BP at Korotkoff phase I and diastolic BP at Korotkoff phase V. After selection of the appropriate cuff size, the brachial artery of the non-dominant arm at the anticubital fossa was located. The microphone was taped to the subject's arm over the strongest impulse followed by placement of the cuff and electrodes. During the AM5600 “office check period”, with the subject in a seated position, a minimum of three readings were taken simultaneously in the same arm via a 3-way stopcock, with a mercury sphygmomanometer and the AM5600, using the recorder's BP cuff. Values for systolic and diastolic BP were obtained in the same arm for a total of three each for the mercury manometer and the ABPM device. Two trained research coordinators performed all measurements according to the American Heart Association protocol for measurement of BP {Perloff, 1993 #180}. Each manual reading was recorded prior to recording of the ABPM device reading.
Statistical Analysis
SAS software was used for statistical analyses to calculate the mean, standard deviation, distribution and normal plots of variables studied. Inter-method reliability between the baseline readings obtained using the mercury sphygmomanometer and the AM5600 ABPM system was assessed using ANOVA, mixed effects model. Three calibration readings taken simultaneously by each method were averaged for calculation of the mean difference between each method and the mean of the two methods for Bland-Altman analysis. The mean of the differences and standard deviation of the mean differences and 95% confidence intervals were also calculated for SBP and DBP. The cumulative percent of AM5600 readings which differed from the mercury readings by ≤ 5, ≤ 10 and ≤ 15 mmHg was also calculated for both SBP and DBP.
Methods
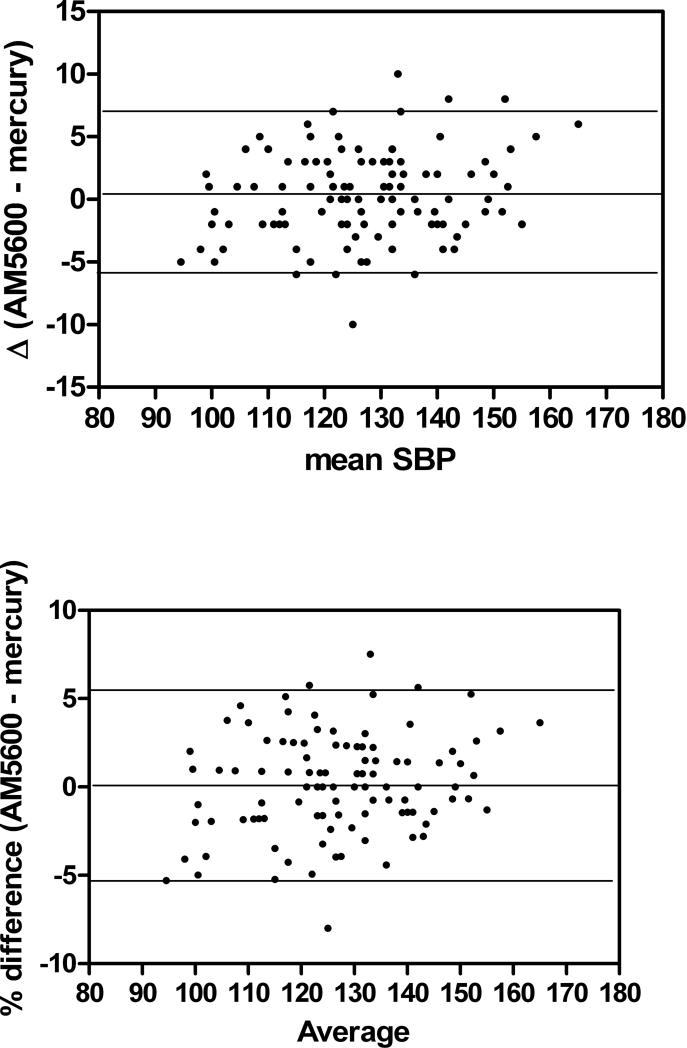
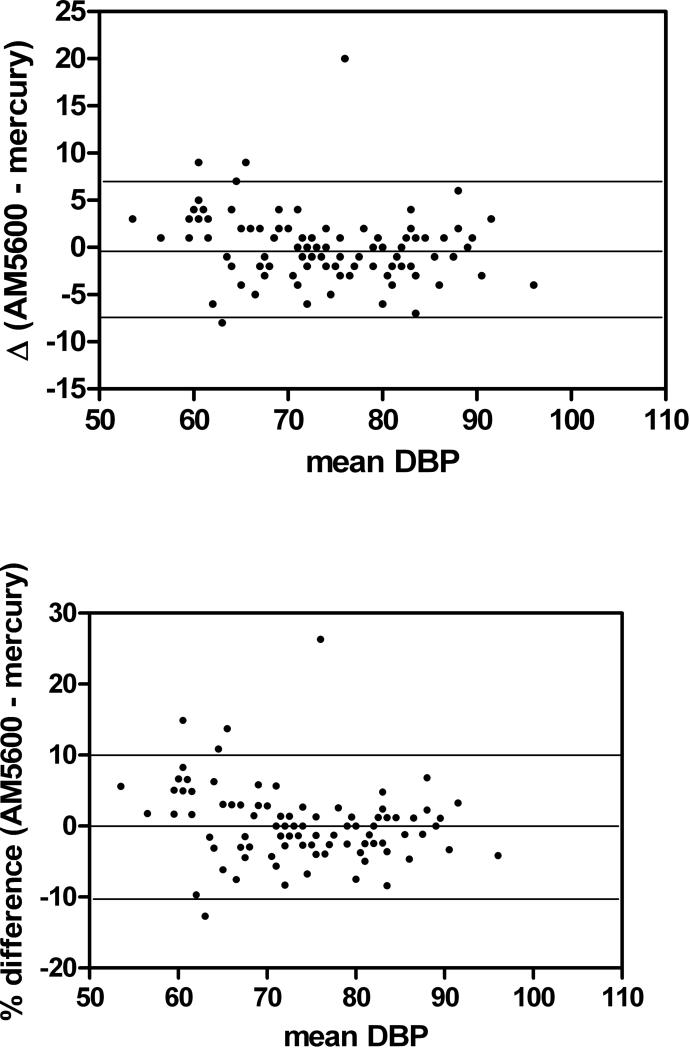
The characteristics of the subjects are found in Table 1. There was a predominance of African-American children as well as males. There were no significant differences between the measurement by the AM5600 and the mercury manometer for either SBP or DBP. The Pearson correlation coefficients were 0.992 and 0.979 for systolic and diastolic BP, respectively, with coefficients of variation of 1.5 and 3.5%. There were no statistical differences between the two methods for SBP or DBP. BP data are summarized in Table 2.
Table 1.
Characteristics of subjects (n = 111)
| Age (years) | 13.5 ± 2.7 (7 - 18) |
| Gender | M/F (63/48) |
| Ethnic group | AA/W/As (71/39/1) |
| Height (cm) | 161.9 ± 16.1 (118.7 – 190.8) |
| Weight (kg) | 72.2 ± 27.2 (22.0 – 156.3) |
| BMI (wt/ht2) | 26.8 ± 7.7 (14.6 – 47.1)) |
| BMI percentile | 75.6 ± 29.3 (5 – 97) |
Continuous data expressed as mean ± SD, M = male, F = female, AA = African American, W = white, As = Asian.
Table 2.
Mercury manometer and AM5600 BP levels (mmHg)
| Systolic | Diastolic | |
|---|---|---|
| AM5600 | ||
| Mean | 126.9 ± 14.8 | 73.5 ± 9.5 |
| Range | (97 – 162.3) | (52.3 – 98.3) |
| Mercury | ||
| Mean | 127.1 ± 15.5 | 73.5 ± 8.7 |
| Range | (92.0 – 168) | (55.3 – 94) |
| Mean of difference | 0.29 ± 3.48 | 0.045 ± 3.71 |
| 95% confidence interval | − 6.5, 7.1 | − 7.3, 7.2 |
| Difference in methods n, (%) | ||
| ≤ 5 mmHg | 100 (90) | 100 (90) |
| ≤ 10 mmHg | 111 (100) | 110 (99) |
| ≤ 15 mmHg | 111 (100) | 110 (99) |
Values are mean ± standard deviation.
The mean of the difference between the AM5600 and the mercury manometer was 0.29 ± 3.5 and 0.15 ± 2.8 mmHg for SBP and DBP, respectively, thus fulfilling the AAMI criteria. The cumulative percentage of readings between the two devices which differed by ≤ 5, ≤ 10 and ≤ 15 mmHg was within the grade of A according to the BSH (Table 2).
Data are represented as Bland-Altman plots which show the mean of each subject's BP as measured by the AM5600 and the mercury manometer plotted against the difference of the two readings as well as the % difference between the two techniques (Figure 1).
Figure 1.
Bland-Altman plots of absolute difference in mmHg in systolic BP and diastolic BP as measured by the AM5600 and the mercury sphygmomanometer.
Discussion
We assessed the accuracy of the AM5600 device as compared to the best available standard, the mercury manometer. Baseline readings during the calibration period were not statistically different. Using the BHS grading criteria, the device received a grade of A for both SBP and DBP. Using the AAMI standards, the device fulfilled the criteria for validation. Therefore, we conclude that this device is accurate for measurement of ABP in children and adolescents. The disadvantage of the auscultatory technique includes a greater percentage of missed readings due to the potential for movement error. This study did not compare the auscultatory to the oscillometric technique, rather the accuracy of the AM5600 device compared to mercury. The overall performance of this device has been previously described. [4] This device was used for our studies employing ABPM because of the experience of the investigators (PAR and BSA) with the device and the ready availability of the device for use in the pilot study. Very few studies have reported validation of an ambulatory device in children; this problem is discussed in two recent reviews on ambulatory BP monitoring in children {Lurbe, 2004 #8} {Graves, 2006 #46}. Belsha et al reported that the Spacelabs 90207 device achieved a grade of C for systolic and a grade of D for diastolic BP, and therefore, it was not recommended {Belsha, 1996 #181}. Whereas the Spacelabs 20217 device is the most commonly used device in children based upon published studies, and the device has been recommended in adults, it has not been validated in children. The QuietTrak device, which is an auscultatory monitor, was validated and recommended for use in children, but is no longer manufactured {Modesti, 1996 #183}. The oscillometric device, TM-2421, achieved a grade of A for systolic and B for diastolic but has a questionable recommendation {O'Sullivan, 1998 #184} but this device is also no longer available.
We conclude that the AM5600 fulfills both the AAMI and the BHS criteria for validation of ambulatory BP measurement devices in children and adolescents over a wide range of sizes.
Figure 2.
Acknowledgments
Supported by a grant from the Children's Foundation Research Center at Le Bonheur Children's Medical Center, Memphis, TN, and the University of Tennessee Health Science Center GCRC NCRR #000211 and NIH NHLBI #5K23HL83910-2.
References
- 1.Richey PA, DiSessa TG, Hastings MC, Somes GW, Alpert BS, Jones DP. Ambulatory blood pressure is associated with increased left ventricular mass in children at risk for hypertension. J Pediatr. 2008;152:343–348. doi: 10.1016/j.jpeds.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Brien E, Waeber B, Parati G, Staessen J, Myers MG. Blood pressure measuring devices: recommendations of the European Society of Hypertension. Bmj. 2001;322:531–536. doi: 10.1136/bmj.322.7285.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 4.Richey PA, Jones CL, Harshfield GA, Somes GW, Johnson KC, Bailey JE, Soberman JE. The AM5600 ambulatory blood pressure recording system. Blood Press Monit. 1997;2:193–195. [PubMed] [Google Scholar]