Abstract
Opiates, like morphine, are the most effective analgesics for treating acute and chronic severe pain, but their use is limited by the development of analgesic tolerance and hypersensitivity to innocuous and noxious stimuli. Because opioids are a mainstay of pain management, restoring their efficacy has great clinical importance. We have recently demonstrated that spinal ceramide, a sphingolipid signaling molecule plays a central role in the development of morphine antinociceptive tolerance. We now report that ceramide up-regulation in dorsal horn tissues in response to chronic morphine administration is associated with significant neuronal apoptosis. Inhibition of ceramide biosynthesis attenuated both the increase in neuronal apoptosis and the development of antinociceptive tolerance. These findings indicate that spinal ceramide upregulation is a key pro-apoptotic event that occurs upstream of the development of morphine antinociceptive tolerance and support the rationale for development of inhibitors of ceramide biosynthesis as adjuncts to opiates for the management of chronic pain.
Keywords: ceramide, nitroxidative stress, neuronal apoptosis, peroxynitrite, morphine tolerance
The mechanisms by which prolonged opiate exposure induce hypersensitivity and tolerance remain unclear. Several pathogenic processes that occur at the level of the spinal cord have been implicated in the development of opiate tolerance. These include nitric oxide and superoxide-derived peroxynitrite (ONOO-, PN) production and PN induced nitroxidative stress [3, 27], neuroimmune activation [herein defined as glial cell activation and release of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α)] [35, 38], and neuronal apoptosis [20, 23]. We have recently shown that the potent pro-inflammatory sphingolipid ceramide, generated by either the de novo pathway or the sphingomyelinase pathway is an important signaling mediator in the development of antinociceptive tolerance [28] (Fig. 1). The mechanisms whereby chronic administration of morphine increase ceramide are not known but may involve activation of the μ-receptor and/or neuroimmune activation. To this end, it is well established that cytokines such as TNF-α can increase ceramide production [16]. Ceramide is one of the most potent endogenous pro-apoptotic mediators discovered to date [17, 18]. The involvement of ceramide in apoptosis has in fact been implicated in radiation-induced injury [17], sepsis [8], emphysema [31] and asthma [24] which share with antinociceptive tolerance roles of apoptosis in their pathogenesis. That ceramide may modulate nociceptive processing through apoptosis is supported by studies of hereditary sensory neuropathy, an autosomal dominant disorder traced to certain missense mutations in serine palmitoyltransferase, the rate-limiting enzyme in generation of ceramide from the de novo pathway. Such mutations increase this enzyme's activity and the levels of ceramide, triggering apoptosis in peripheral sensory neurons and progressive degeneration of dorsal root ganglia and motor neurons [5]. We have recently shown that spinal formation of ceramide fosters the development of antinociceptive tolerance through formation of PN and neuroimmune activation [28]. We now hypothesize and show for the first time that spinal formation of ceramide contributes to the development of antinociceptive tolerance at least in part by engaging spinal pathways leading to neuronal apoptosis.
Fig. 1. Schematic of the ceramide's mechanism of action in antinociceptive tolerance.
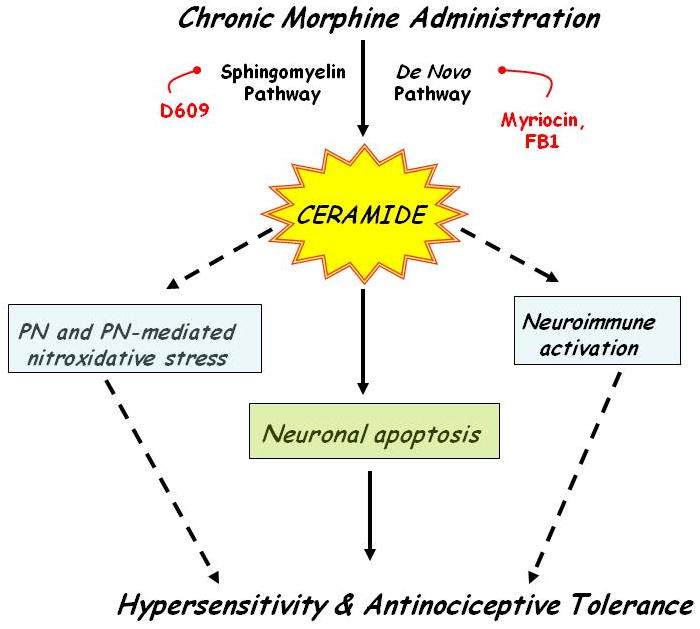
Chronic administration of morphine leads to increased formation of ceramide via the de novo and SMase pathways leading to antinociceptive tolerance; this is blocked by inhibitors of ceramide biosynthesis (this study and [28]). Ceramide fosters the development of tolerance at least in part by neuroimmune activation, formation of PN, PN-mediated inactivation of MnSOD [28] and neuronal apopotosis.
Male CD-1 mice (24-30g; Charles River Laboratory) were housed and cared for in accordance and guidelines of the Institutional Animal Care and Use Committee (IACUC) of the Saint Louis University Medical Center and in accordance with the NIH Guidelines on Laboratory Animal Welfare and the University of Messina in compliance with Italian regulations on protection of animals used for experimental and other scientific purpose (D.M. 116192) as well as with European Economic Community regulations. The IACUC of Saint Louis University Medical Center and the University of Messina approved all studies. Mice were housed 4-5 per cage and maintained under identical conditions of temperature (21 ± 1°C) and humidity (65% ± 5%) with a 12-hour light/12-hour dark cycle and allowed food ad libitum. The tail flick test, which measures withdrawal latencies of the tail from a noxious radiant heat source, was used to measure thermal nociceptive sensitivity [4]. The intensity of the heat stimulus was adjusted so that the mouse flicked its tail after 2-4 s. A cut-off time of 15 s was imposed to prevent tissue damage. Mice received a subcutaneous (sc, 0.2 ml) injection of morphine (20 mg/kg) or its vehicle sterile saline twice daily at 0800-0900 h and 1600-1700 h for 4 days. Fumonisin B1 (FB1) or its vehicle were given by intraperitoneal (i.p) injections (0.2 ml) twice a day and 15 minutes before each morphine injection over the 4 days for the drugs to be distributed more constantly. On day 5, and approximately 16 h after the last morphine injection, each mouse was tested twice on the hot plate and the latency (s) reaction times averaged to obtain a baseline. Mice were then treated with acute subcutaneous (s.c) injection of morphine (10 mg/kg) to study the expression of tolerance. Data obtained were then converted to percentage maximal possible antinociceptive effect (%MPE) as equal to: (response latency–baseline latency)/(cut off latency–baseline latency) × 100. Ten mice per group were used and all experiments were conducted with the experimenters blinded to treatment conditions. Statistical analysis was performed by one-way ANOVA, followed by multiple Student-Newman-Keuls post hoc test. A significant difference was defined as a P <0.05.
On day 5, the % MPE in response to an acute injection of morphine in mice that received saline twice a day for 4 days (Veh-Sal group) was 95±5% (n=10). On the other hand, the %MPE of acute morphine on day 5 in mice that received chronic morphine over the same time frame (Veh-Mor group) was 12±3% (P<0.001; n=10) indicative of the induction of antinociceptive tolerance. This antinociceptive tolerance was associated with increased enzymatic activity in dorsal horn tissues of ceramide synthase, enzyme involved in the biosynthesis of ceramide from the de novo pathway (Fig. 2). Co-administration of morphine over 4 days with FB1 (0.25-1 mg/kg/day, n=10) a competitive and reversible inhibitor of ceramide synthase [7] blocked, as expected, the increased enzymatic activity of ceramide synthase [measured as described previously [28]] and the development of antinociceptive tolerance (Fig. 2), at doses previously shown to be devoid of motor function impairment [28].
Fig. 2. The development of morphine antinociceptive tolerance and activation of ceramide synthase in dorsal horn tissues are attenuated by FB1.
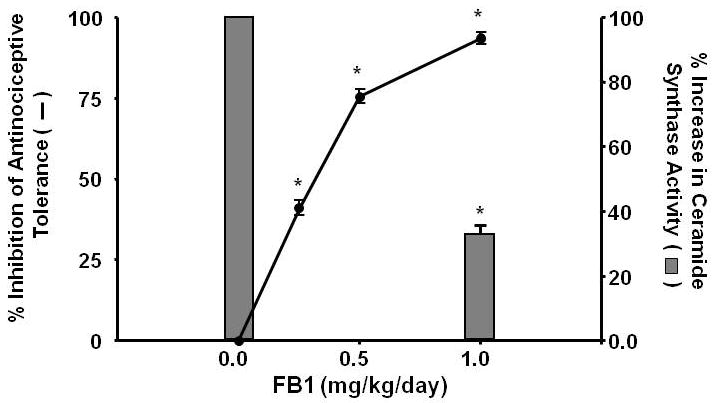
Co-administration of morphine with the ceramide synthesis inhibitor FB1 (1 mg/kg/day, n=10) inhibited both the ceramide synthase activity (bars) and the development of morphine antinociceptive tolerance (closed circle line) in a dose-dependent manner (0.25-1 mg/kg/day, n=10). Results are expressed as mean ± SEM for 10 animals. *P<0.001 for FB1-Mor vs Veh-Mor.
Ceramide fosters morphine antinociceptive tolerance through PN mediated nitroxidative stress in dorsal horn tissues [28]. Under such nitroxidative stress situations, excessive DNA damage causes overt activation of the nuclear enzyme poly-ADP ribose-polymerase (PARP) [3, 27]. PARP is a critical intracellular mechanism of cell death through both necrotic and apoptotic pathways [14]. In fact, PARP activation induces excitotoxic transynaptic morphological changes in superficial dorsal horn “dark neurons” in morphine-induced antinociceptive tolerance and hyperalgesia as well as in neuropathic pain [23, 25]. Inhibition of PARP activation blocks neuronal apoptosis and tolerance and is protective in models of neuropathic pain [23, 25]. As can be seen in Fig. 3 A and B, when compared to mice receiving saline over 4 days, chronic administration of morphine over the same time frame increased the levels of 8OHdG, a marker of oxidative DNA damage, (Fig. 3A) and PARP activity (Fig. 3B) in dorsal horn tissues, measured as previously described [21]. Co-administration of morphine with FB1 (0.25-1 mg/kg/day, n=10) dose-dependently inhibited oxidative DNA damage and PARP activation (Fig. 3A, B). Furthermore, when compared to mice receiving saline over 4 days, chronic administration of morphine over the same time frame increased the activity of caspase-3, a central executioner protease in apoptosis (Fig. 4A); up-regulated pro-apoptotic Bax (Fig. 4B,B1); and concomitantly down-regulated anti-apoptotic Bcl-2 protein (Fig. 4C,C1) as detected by Western blotting analysis. These events were blocked by FB1 (Fig. 4A-C). The relative expression of the anti-apoptotic Bcl-2 protein and the pro-apoptotic Bax protein is a marker of cellular fate, with an increased Bax/Bcl-2 predicting apoptotic cell death, whereas a reverse effect predicts survival [19]. Indeed, the upregulation of Bax predicts apoptotic neuronal injury in several models of neurotoxiciy [32, 40]. The downregulation of the Bax/Bcl2 ratio by FB1 suggests ceramide synthesis lies upstream of Bax upregulation in spinal neurons challenged with repeated morphine administration. Although the proximal positioning of ceramide in the intrinsic apoptosis pathway has been previously described [16], this is, to our knowledge, the first report of such pro-apoptotic signaling underlying the pathogenesis of antinociceptive tolerance.
Fig. 3. Ceramide synthesis was necessary for the development of oxidative DNA damage and PARP activation.
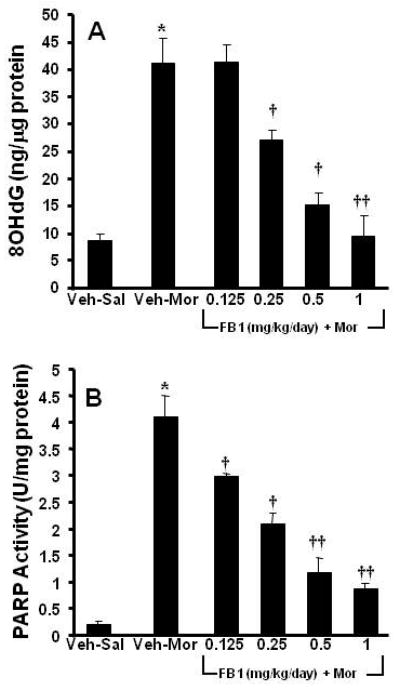
When compared to Veh-Sal, chronic administration of morphine (Veh-Mor) led to oxidative DNA damage as evidenced by a significant increase in 8OHdG (A) and a substantial activation of PARP (B). These events were blocked in a dose-dependent manner by co-administration of morphine with FB1 (0.25-1 mg/kg/d, n=10) (A, B). Results are expressed as mean ± SEM for n=10 animals. * P<0.01 for Veh-Mor versus Veh-Sal; †P<0.01 and ††P<0.001 for FB1-Mor versus Veh-Mor.
Fig. 4. Biochemical indices of spinal cord apoptosis were dependent on ceramide synthesis.
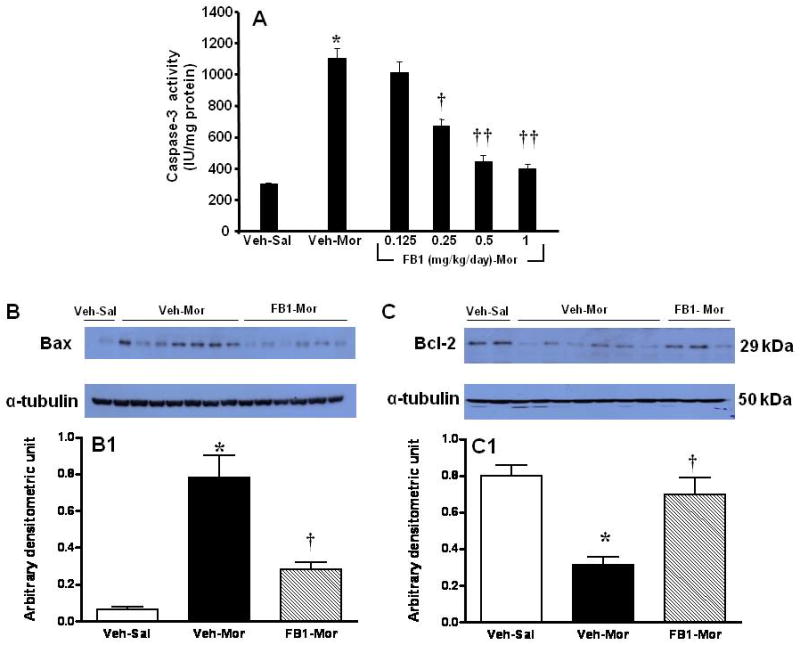
When compared to Veh-Sal, chronic administration of morphine (Veh-Mor) increased the activity of caspase-3 (A) in dorsal horn tissues which was inhibited in a dose-dependent manner by FB1 (n=10). Furthermore, chronic administration of morphine increased Bax (B,B1) and concomitantly decreased Bcl-2 (C,C1) as determined by Western blot in dorsal horn tissues. These events were blocked by FB1 (1 mg/kg/d; B,B1,C,C1). Results are expressed as mean ± SEM for n=5 (B1,C1) and n=10 (A) animals. * P<0.01 for Veh-Mor versus Veh-Sal; †P<0.01 and ††P<0.001 for FB1-Mor versus Veh-Mor.
To further validate that increased formation of ceramide led to cell death by apoptosis, we measured cells progressing through apoptosis according to their Annexin V and propidium iodide (PI) staining pattern. In particular, early apoptotic cells will bind Annexin V but are not sensitive to intracellular staining with PI. As cells progress through apoptosis the integrity of the plasma membrane is lost, allowing PI to penetrate and label the cells with a strong yellow-red fluorescence. In non-tolerant mice there were virtually no apoptotic cells detected in the spinal cord tissue (Fig. 5A,A1). On the other hand, chronic treatment with morphine led to a marked appearance of positive staining for Annexin-V FITC (Fig. 5B,B1). Moreover, some cells showed a positive intracellular staining to PI (Fig. 5B1). On the contrary, no cells in the earlier stage (Fig. 5C) as well as in the later stages (Fig. 5C1; positive to PI) of apoptosis were present in the spinal cord tissues section from mice that received morphine and FB1 (1 mg/kg/day) over the 4 days period. Co-administration of morphine with FB1 consistently attenuated all parameters indicative of neuronal apoptosis (Fig. 4 and 5).
Fig. 5. Visualization of ceramide-dependent spinal cord apoptosis.
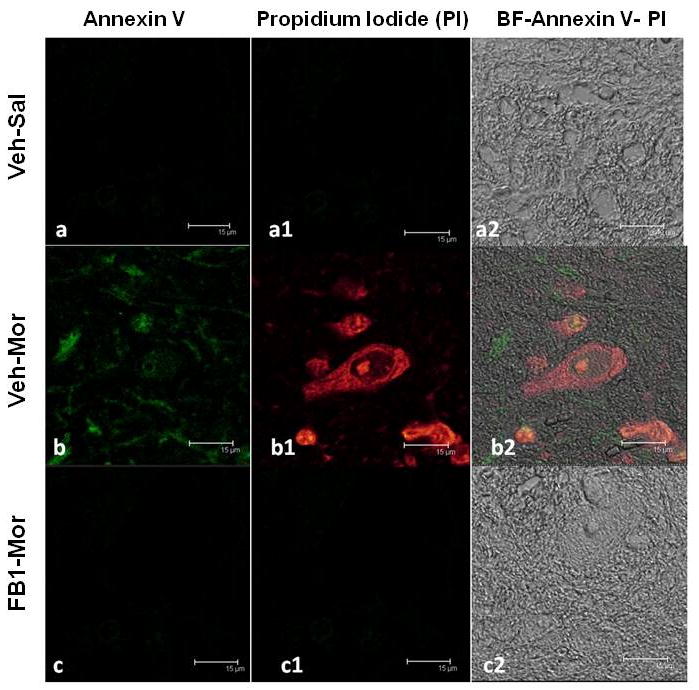
No apoptotic cells were detectable using Annexin-V FITC coloration in the spinal cord tissue of non-tolerant mice (A,A1). In tolerant animals a marked appearance of stain positive for Annexin-V FITC was observed (B). In addition, several cells showed positive staining with PI (B1). Spinal cord section from FB1-treated mice demonstrated a marked reduction in the number of apoptotic cells (C,C1). Figs A2, B2 and C2 represent the overlap images of panels A-A1, B-B1 and C-C1 respectively, superimposed on transmission light images of identical fields, depicting a significant number of cells captured in each field. Figure is representative of at least 3 independent experiments.
Collectively, our findings confirm the role of neuronal apoptosis in development of tolerance [20, 23] and extend these earlier observations by establishing a role of ceramide in this setting.
We propose that an early and likely mechanism through which ceramide activates apoptosis is via PN, a potent pro-apoptotic nitroxidative species and PN-mediated inactivation of mitochondrial manganese superoxide dismutase (MnSOD) the enzyme that keeps [superoxide] in check [26] (Fig. 1). This is supported by the fact that: 1) ceramide increases the formation of superoxide and nitric oxide (precursors of PN) by activating NADPH oxidase [1, 11, 29, 41], inhibiting superoxide dismutases [24, 28, 30], and stimulating nitric oxide synthases [2, 15, 37, 39] respectively and 2) spinal ceramide fosters development of morphine tolerance through PN and PN-mediated inactivation of MnSOD, events blocked by inhibitors of ceramide biosynthesis [28]. The inactivation of MnSOD which increases steady-state concentrations of PN results in positive feedback processes that promote mitochondrial dysfunction and trigger apoptotic signaling at least in part via PARP and caspase activation [34] (Fig. 1).
A recent series of exciting experimental observations highlighted an important reciprocal interaction between the nitroxidative pathway and the ceramide metabolic pathway (see for excellent review [39]). In this context, ceramide increases the formation of superoxide and nitric oxide by mechanisms already discussed in the paragraph above. Other potential causes of ceramide-derived PN formation include mitochondrial dysfunction and downregulation of intracellular antioxidant enzymes [39]. Conversely, ample evidence suggests that superoxide, nitric oxide and PN increase ceramide biosynthesis by activating enzymes in the sphingomyelin and de novo pathway [6, 13, 22, 33, 36] or by blocking ceramidases that degrade ceramide [9, 10, 12]. Given this intimate reciprocity, the interregulation of the ceramide metabolic pathway and the nitroxidative pathway (we will refer to this as the sphingo-nitroxidative pathway in future studies) must be a crucial factor dictating the outcome of neuronal apoptosis in opiate tolerance.
Taken together with our previous results, these data validate the approach of developing inhibitors of the sphingo-nitroxidative pathway as adjuncts to opiates in the management of pain.
Acknowledgments
Supported by 1 R01 DA024074-01A1 and 1 R21 DA023056-01A2 (DS), RO1 HL077328 (IP) and AHA Fellowship grant (DP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arai T, Bhunia AK, Chatterjee S, Bulkley GB. Lactosylceramide stimulates human neutrophils to upregulate Mac-1, adhere to endothelium, and generate reactive oxygen metabolites in vitro. Circulation research. 1998;82:540–547. doi: 10.1161/01.res.82.5.540. [DOI] [PubMed] [Google Scholar]
- 2.Arena S, Pattarozzi A, Thellung S, Villa V, Corsaro A, Massa A, Diana F, Spoto G, Forcella S, Damonte G, Filocamo M, Benatti U, Schettini G, Florioa T. Nitric oxide production stimulated by the basic fibroblast growth factor requires the synthesis of ceramide. Ann N Y Acad Sci. 2002;973:94–104. doi: 10.1111/j.1749-6632.2002.tb04614.x. [DOI] [PubMed] [Google Scholar]
- 3.Batinic-Haberle I, Ndengele MM, Cuzzocrea S, Reboucas JS, Spasojevic I, Salvemini D. Lipophilicity is a critical parameter that dominates the efficacy of metalloporphyrins in blocking the development of morphine antinociceptive tolerance through peroxynitrite-mediated pathways. Free Radic Biol Med. 2009;46:212–219. doi: 10.1016/j.freeradbiomed.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Amour F. A method for determining loss of pain sensation. J Pharmacol xp Ther. 1941;72:74–79. [Google Scholar]
- 5.Dawkins JL, Hulme DJ, Brahmbhatt SB, Auer-Grumbach M, Nicholson GA. Mutations in SPTLC1, encoding serine palmitoyltransferase, long chain base subunit-1, cause hereditary sensory neuropathy type I. Nat Genet. 2001;27:309–312. doi: 10.1038/85879. [DOI] [PubMed] [Google Scholar]
- 6.De Nadai C, Sestili P, Cantoni O, Lievremont JP, Sciorati C, Barsacchi R, Moncada S, Meldolesi J, Clementi E. Nitric oxide inhibits tumor necrosis factor-alpha-induced apoptosis by reducing the generation of ceramide. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5480–5485. doi: 10.1073/pnas.070062397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delgado A, Casas J, Llebaria A, Abad JL, Fabrias G. Inhibitors of sphingolipid metabolism enzymes. Biochimica et biophysica acta. 2006;1758:1957–1977. doi: 10.1016/j.bbamem.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Delogu G, Famularo G, Amati F, Signore L, Antonucci A, Trinchieri V, Di Marzio L, Cifone MG. Ceramide concentrations in septic patients: a possible marker of multiple organ dysfunction syndrome. Crit Care Med. 1999;27:2413–2417. doi: 10.1097/00003246-199911000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Franzen R, Pautz A, Brautigam L, Geisslinger G, Pfeilschifter J, Huwiler A. Interleukin-1beta induces chronic activation and de novo synthesis of neutral ceramidase in renal mesangial cells. J Biol Chem. 2001;276:35382–35389. doi: 10.1074/jbc.M102153200. [DOI] [PubMed] [Google Scholar]
- 10.Franzen R, Pfeilschifter J, Huwiler A. Nitric oxide induces neutral ceramidase degradation by the ubiquitin/proteasome complex in renal mesangial cell cultures. FEBS Lett. 2002;532:441–444. doi: 10.1016/s0014-5793(02)03727-4. [DOI] [PubMed] [Google Scholar]
- 11.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huwiler A, Dorsch S, Briner VA, van den Bosch H, Pfeilschifter J. Nitric oxide stimulates chronic ceramide formation in glomerular endothelial cells. Biochem Biophys Res Commun. 1999;258:60–65. doi: 10.1006/bbrc.1999.0582. [DOI] [PubMed] [Google Scholar]
- 13.Huwiler A, Pfeilschifter J, van den Bosch H. Nitric oxide donors induce stress signaling via ceramide formation in rat renal mesangial cells. J Biol Chem. 1999;274:7190–7195. doi: 10.1074/jbc.274.11.7190. [DOI] [PubMed] [Google Scholar]
- 14.Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–440. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- 15.Johns DG, Osborn H, Webb RC. Ceramide: a novel cell signaling mechanism for vasodilation. Biochem Biophys Res Commun. 1997;237:95–97. doi: 10.1006/bbrc.1997.7084. [DOI] [PubMed] [Google Scholar]
- 16.Kolesnick R. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J Clin Invest. 2002;110:3–8. doi: 10.1172/JCI16127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene. 2003;22:5897–5906. doi: 10.1038/sj.onc.1206702. [DOI] [PubMed] [Google Scholar]
- 18.Kolesnick R, Golde DW. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 1994;77:325–328. doi: 10.1016/0092-8674(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 19.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 20.Lim G, Wang S, Lim JA, Mao J. Activity of adenylyl cyclase and protein kinase A contributes to morphine-induced spinal apoptosis. Neurosci Lett. 2005;389:104–108. doi: 10.1016/j.neulet.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 21.Lodovici M, Casalini C, Cariaggi R, Michelucci L, Dolara P. Levels of 8-hydroxydeoxyguanosine as a marker of DNA damage in human leukocytes. Free Radic Biol Med. 2000;28:13–17. doi: 10.1016/s0891-5849(99)00194-x. [DOI] [PubMed] [Google Scholar]
- 22.Mansat-de Mas V, Bezombes C, Quillet-Mary A, Bettaieb A, D'Orgeix AD, Laurent G, Jaffrezou JP. Implication of radical oxygen species in ceramide generation, c-Jun N-terminal kinase activation and apoptosis induced by daunorubicin. Mol Pharmacol. 1999;56:867–874. doi: 10.1124/mol.56.5.867. [DOI] [PubMed] [Google Scholar]
- 23.Mao J, Price DD, Zhu J, Lu J, Mayer DJ. The inhibition of nitric oxide-activated poly(ADP-ribose) synthetase attenuates transsynaptic alteration of spinal cord dorsal horn neurons and neuropathic pain in the rat. Pain. 1997;72:355–366. doi: 10.1016/s0304-3959(97)00063-8. [DOI] [PubMed] [Google Scholar]
- 24.Masini E, Giannini L, Nistri S, Cinci L, Mastroianni R, Xu W, Comhair SA, Li D, Cuzzocrea S, Matuschak GM, Salvemini D. Ceramide: a key signaling molecule in a Guinea pig model of allergic asthmatic response and airway inflammation. The Journal of pharmacology and experimental therapeutics. 2008;324:548–557. doi: 10.1124/jpet.107.131565. [DOI] [PubMed] [Google Scholar]
- 25.Mayer DJ, Mao J, Holt J, Price DD. Cellular mechanisms of neuropathic pain, morphine tolerance, and their interactions. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7731–7736. doi: 10.1073/pnas.96.14.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 27.Muscoli C, Cuzzocrea S, Ndengele MM, Mollace V, Porreca F, Fabrizi F, Esposito E, Masini E, Matuschak GM, Salvemini D. Therapeutic manipulation of peroxynitrite attenuates the development of opiate-induced antinociceptive tolerance in mice. J Clin Invest. 2007;117:3530–3539. doi: 10.1172/JCI32420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ndengele MM, Cuzzocrea S, Masini E, Vinci MC, Esposito E, Muscoli C, Petrusca DN, Mollace V, Mazzon E, Li D, Petrache I, Matuschak GM, Salvemini D. Spinal ceramide modulates the development of morphine antinociceptive tolerance via peroxynitrite-mediated nitroxidative stress and neuroimmune activation. The Journal of pharmacology and experimental therapeutics. 2009;329:64–75. doi: 10.1124/jpet.108.146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pannu R, Won JS, Khan M, Singh AK, Singh I. A novel role of lactosylceramide in the regulation of lipopolysaccharide/interferon-gamma-mediated inducible nitric oxide synthase gene expression: implications for neuroinflammatory diseases. J Neurosci. 2004;24:5942–5954. doi: 10.1523/JNEUROSCI.1271-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrache I, Medler TR, Richter AT, Kamocki K, Chukwueke U, Zhen L, Gu Y, Adamowicz J, Schweitzer KS, Hubbard WC, Berdyshev EV, Lungarella G, Tuder RM. Superoxide dismutase protects against apoptosis and alveolar enlargement induced by ceramide. Am J Physiol Lung Cell Mol Physiol. 2008;295:L44–53. doi: 10.1152/ajplung.00448.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med. 2005;11:491–498. doi: 10.1038/nm1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Putcha GV, Deshmukh M, Johnson EM., Jr BAX translocation is a critical event in neuronal apoptosis: regulation by neuroprotectants, BCL-2, and caspases. J Neurosci. 1999;19:7476–7485. doi: 10.1523/JNEUROSCI.19-17-07476.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu H, Edmunds T, Baker-Malcolm J, Karey KP, Estes S, Schwarz C, Hughes H, Van Patten SM. Activation of human acid sphingomyelinase through modification or deletion of C-terminal cysteine. J Biol Chem. 2003;278:32744–32752. doi: 10.1074/jbc.M303022200. [DOI] [PubMed] [Google Scholar]
- 34.Radi R, Rodriguez M, Castro L, Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Archives of biochemistry and biophysics. 1994;308:89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- 35.Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001;39:281–286. doi: 10.1016/s0168-0102(00)00226-1. [DOI] [PubMed] [Google Scholar]
- 36.Takeda Y, Tashima M, Takahashi A, Uchiyama T, Okazaki T. Ceramide generation in nitric oxide-induced apoptosis. Activation of magnesium-dependent neutral sphingomyelinase via caspase-3. J Biol Chem. 1999;274:10654–10660. doi: 10.1074/jbc.274.15.10654. [DOI] [PubMed] [Google Scholar]
- 37.Viani P, Giussani P, Ferraretto A, Signorile A, Riboni L, Tettamanti G. Nitric oxide production in living neurons is modulated by sphingosine: a fluorescence microscopy study. FEBS Lett. 2001;506:185–190. doi: 10.1016/s0014-5793(01)02880-0. [DOI] [PubMed] [Google Scholar]
- 38.Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Norman Cousins Lecture. Glia as the “bad guys”: implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun. 2007;21:131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Won JS, Im YB, Khan M, Singh AK, Singh I. The role of neutral sphingomyelinase produced ceramide in lipopolysaccharide-mediated expression of inducible nitric oxide synthase. J Neurochem. 2004;88:583–593. doi: 10.1046/j.1471-4159.2003.02165.x. [DOI] [PubMed] [Google Scholar]
- 40.Xiang H, Kinoshita Y, Knudson CM, Korsmeyer SJ, Schwartzkroin PA, Morrison RS. Bax involvement in p53-mediated neuronal cell death. J Neurosci. 1998;18:1363–1373. doi: 10.1523/JNEUROSCI.18-04-01363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang DX, Zou AP, Li PL. Ceramide-induced activation of NADPH oxidase and endothelial dysfunction in small coronary arteries. Am J Physiol Heart Circ Physiol. 2003;284:H605–612. doi: 10.1152/ajpheart.00697.2002. [DOI] [PubMed] [Google Scholar]


