Abstract
This paper reports the synthesis of compounds formed by two indole systems separated by a heterocycle (pyridine or piperazine). As a primary screening, the new compounds were submitted to the National Cancer Institute for evaluation of antitumor activity in the human cell line screen. The pyridine derivatives were far more active than the piperazine derivatives. For the study of the mechanism of action, the most active compounds were subjected to COMPARE analysis and to further biological tests including proteasome inhibition and inhibition of plasma membrane electron transport. The compound bearing the 5-methoxy-2-indolinone moiety was subjected to the first in vivo experiment (hollow fiber assay) and was active. It was therefore selected for the second in vivo experiment (human tumor xenograft in mice).
In conclusion we demonstrated that this approach was successful since some of the compounds described are much more active than the numerous, so far prepared and tested 3-indolylmethylene-2-indolinones.
Introduction
In 19842 we published the first of a series of papers devoted to the synthesis of compounds with positive inotropic activity. In 19903 we introduced in this project new derivatives arising from the Knoevenagel reaction between an indole aldehyde and a compound containing an active methylene group. Later we found that analogous compounds showed antitumor activity too. In our first paper on 3-(2-chloro-3-indolylmethylene)1,3-dihydroindol-2-ones as antitumor agents,4 we acknowledged that the first synthesis of the unsubstituted 3-indolylmethylene-1,3-dihydroindol-2-one was performed by a German team in 19695 and the first attempt to search an antitumor potential in this class of compounds was made in 1977.6 We also reported that a team at Farmitalia-Carlo Erba was working on the antitumor activity of analog compounds.7 In the subsequent years we developed our project based on 3-(2-chloro-3-indolylmethylene)1,3-dihydroindol-2-ones8–11 whereas the project at Farmitalia-Carlo Erba, passing through Pharmacia, Upjohn, Sugen and finally Pfizer, led to Sutent (see Chart 1 and http://www.sutent.com/) which was approved by FDA in 2006.
Chart 1.
We also prepared compounds similar to 3-(2-chloro-3-indolylmethylene)1,3-dihydroindol-2-ones but with the two indole systems separated by an heterocycle instead of a methine bridge. These compounds were first tested as positive inotropic agents12 and later as antitumor agents but only on HeLa cells.13 In this paper we describe several aspects of the antitumor activity of this class of compounds, reported in Scheme 1, whose structures show similarity to TAS-20214 and RITA15 (Chart 1) for their molecular symmetry.
Scheme 1.
Chemistry
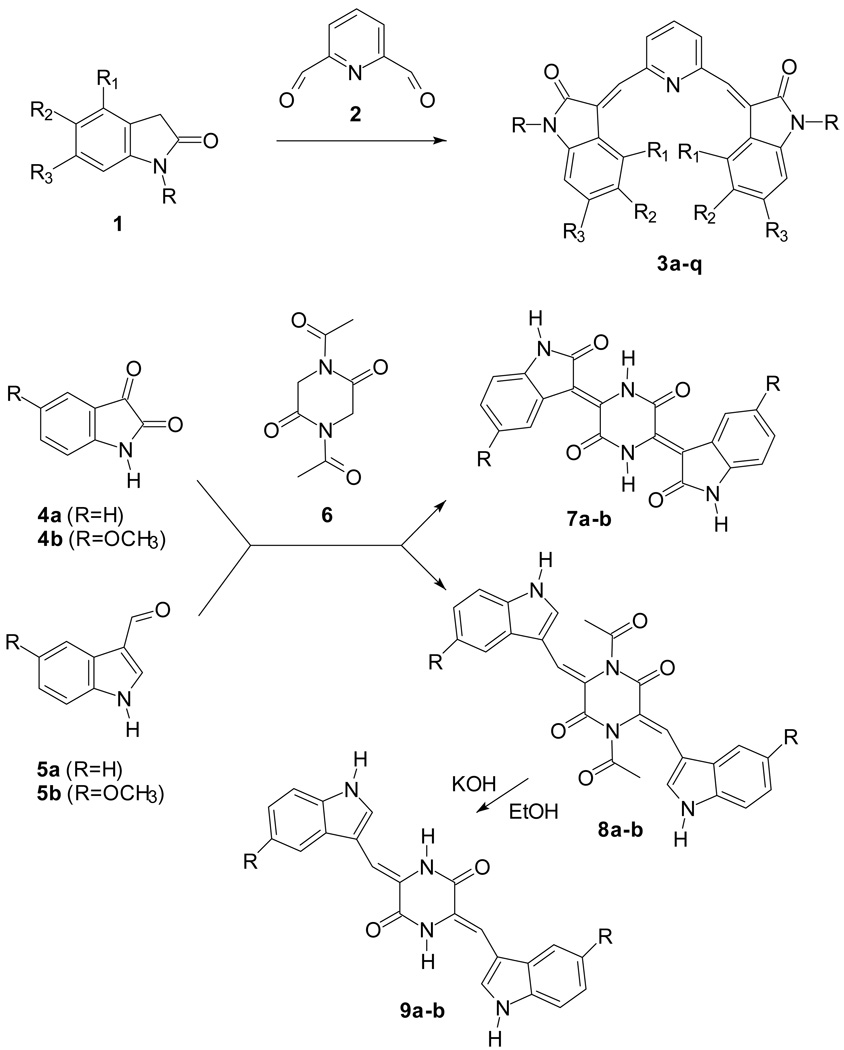
The Knoevenagel reaction we employed in the previous papers involved the condensation of a carbonyl group with a methylene activated by a neighboring electron withdrawing group. The two classes of compounds reported in Scheme 1 present our continued synthetic efforts using the Knoevenagel reaction. In the first case (3a–q) the carbonyl groups were in the core (a pyridine ring) whereas the active methylene groups were in the indole wings. In the second case (7–9) this situation was inverted: the active methylene groups were in the core (a piperazine ring) whereas the carbonyl groups were in the indole wings. Moreover the core may be connected to the wings by a double bond (7a–b) or through a methine bridge (8a–b, 9a–b), as in compounds 3a–q.
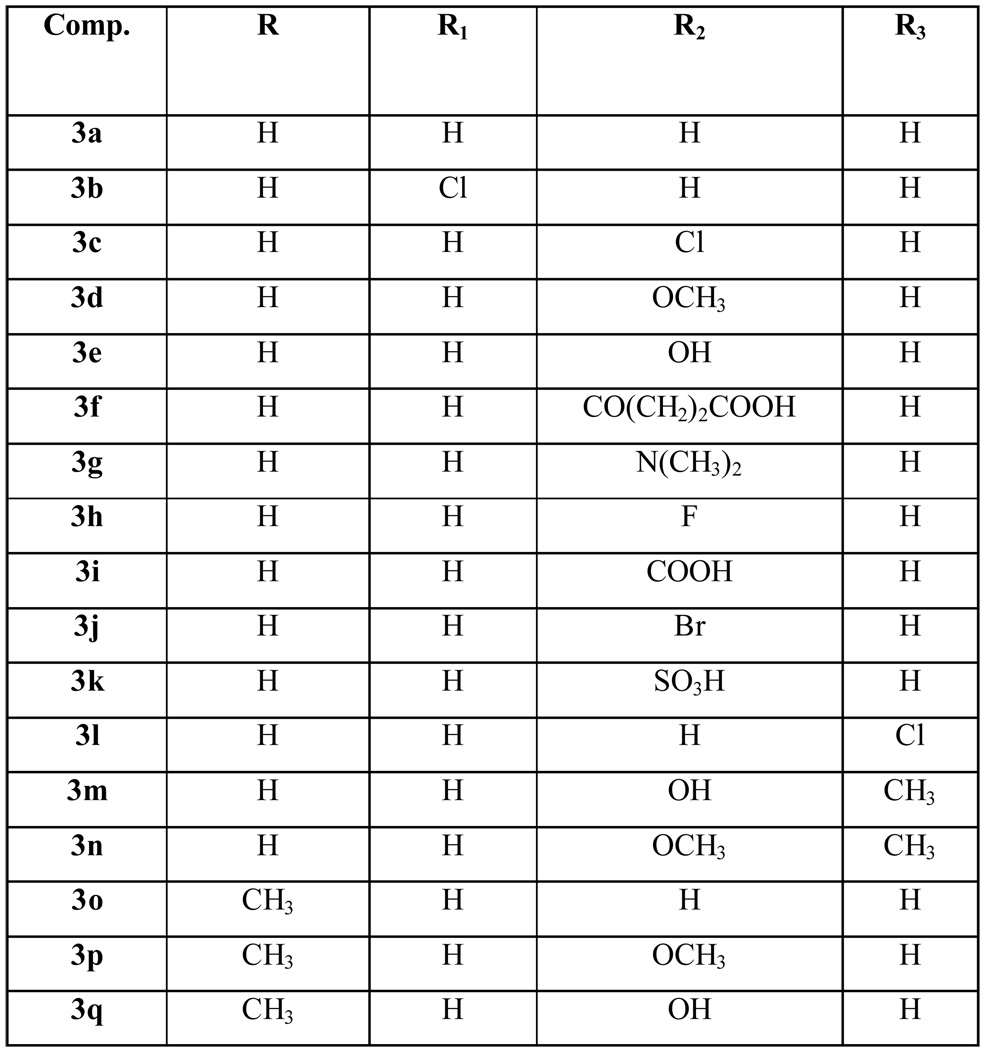
Most of the new derivatives 3 have been prepared with the same procedure described for the previously published compounds:12,13 the appropriate indolinone 1 in methanol has been treated with pyridine-2,6-dicarbaldehyde 2 in the presence of piperidine (see Scheme 1 and Table 1, method 1). Only compounds 3f, 3i–k have been prepared in a mixture of acetic acid/hydrochloric acid (method 2).
Table 1.
Compounds 3, 7–9.
| Comp. | Formula | M.W. | Method | M.p. or ref. | Starting material ref. |
|---|---|---|---|---|---|
| 3a | C23H15N3O2 | 365.39 | 1 | [a] | |
| 3b | C23H13Cl2N3O2 | 434.28 | 1 | [b] | |
| 3c | C23H13Cl2N3O2 | 434.28 | 1 | [b] | |
| 3d | C25H19N3O4 | 425.44 | 1 | [a] | |
| 3e | C23H15N3O4 | 397.39 | 1 | 335–340 | [c] |
| 3f | C31H23N3O8 | 565.53 | 2 | 210–215 dec. | [d] |
| 3g | C27H25N5O2 | 451.52 | 1 | 340–350 | [e] |
| 3h | C23H13F2N3O2 | 401.37 | 1 | 347–350 dec. | [f] |
| 3i | C25H15N3O6 | 453.41 | 2 | >360 | [g] |
| 3j | C23H13Br2N3O2 | 523.18 | 2 | 355 dec. | [h] |
| 3k | C23H15N3O8S2 | 525.52 | 2 | 325–330 | |
| 3l | C23H13Cl2N3O2 | 434.28 | 1 | 332 dec. | |
| 3m | C25H19N3O4 | 425.44 | 1 | >360 | [i] |
| 3n | C27H23N3O4 | 453.49 | 1 | 314–315 dec. | [j] |
| 3o | C25H19N3O2 | 393.44 | 1 | [a] | |
| 3p | C27H23N3O4 | 453.49 | 1 | 218–220 | [k] |
| 3q | C25H19N3O4 | 425.44 | 1 | 260–262 dec. | [k] |
| 7a | C20H12N4O4 | 372.34 | - | >360 | |
| 7b | C22H16N4O6 | 432.39 | - | >360 | |
| 8a | C26H20N4O4 | 452.46 | - | 302–305 dec. | |
| 8b | C28H24N4O6 | 512.52 | - | 344–346 dec. | |
| 9a | C22H16N4O2 | 368.39 | - | 355–358 dec. | |
| 9b | C24H20N4O4 | 428.44 | - | >360 |
For the synthesis of compound 3k, ethyl 2-oxoindoline-5-sulfonate was the starting indolinone. It was prepared by treating 2-oxoindoline-5-sulfonyl chloride16 with ethanol. The condensation with pyridine-2,6-dicarbaldehyde under acidic conditions led to the simultaneous hydrolysis of the ester and to the formation of the expected sulfonic acid 3k.
The synthesis of compounds 7a–b from the appropriate isatine 4 and 1,4-diacetylpiperazine-2,5-dione 6 has been performed in dimethylformamide in the presence of triethylamine.17 In this reaction the condensation took place with contemporaneous hydrolysis of the acetyl group. When the indolaldehyde 5 was employed in place of the isatine 4 for the synthesis of compounds 8a–b, this procedure gave poor yields whereas a better result was obtained with acetic anhydride/sodium acetate. Compounds 9a–b were obtained by alkaline hydrolysis of the corresponding acetyl derivatives 8a–b.
All compounds 3 were obtained as pure geometrical isomers and the configuration was assigned by means of 1H-NMR spectra (Table S1). We performed Nuclear Overhauser Enhancement (NOE) analysis on compound 3n: the spectrum shows that the geometrical configuration of the two possible centers is the same, since the protons at the positions 3 and 5 of the pyridine ring give a single signal. The same happens for the couple of NH groups and for the other couples of indole protons/substituents: H-4, H-7, OCH3-5, CH3-6. The first NOE analysis was devoted to the identification of H-4 and H-7 in the indolinone system. The irradiation of the NH groups (10.39 ppm) and of the 6-methyl groups (2.04 ppm) produced NOE at 6.62 ppm (peak which was assigned to H-7) whereas the irradiation of the 5-methoxy groups (3.33 ppm) produced NOE at 8.01 ppm (H-4). Therefore the remaining singlet of the aromatic region (7.67 ppm) was assigned to the methine groups. When this peak was irradiated, NOE was observed only at the protons in position 3 and 5 of the pyridine ring (7.90 ppm) therefore compound 3n belongs to the E configuration.
Also compounds 7–9 were obtained as pure isomers and for the study of the geometrical configuration of these derivatives, NOE analyses were performed on compound 8a. As observed in the previous case, the 1H-NMR spectrum shows a symmetrical molecule i.e. the two centers have the same configuration. The irradiation of the NH group (10.44 ppm) gave NOE at the indole protons in position 2 (8.39 ppm) thus allowing us to confirm that the other singlet in the aromatic region (6.99 ppm) was due to the methine bridges. When these protons were irradiated, NOE was observed only at 7.74 ppm (which is a doublet of the indole protons at the 4 position: as a consequence the doublet at 8.38 ppm was attributed to the indole protons at the 7-position). This is in agreement with the Z configuration. The irradiation of the CH3CO groups (2.77 ppm) produced NOE only at the indole protons in position 2 (8.39 ppm) and not at the methine bridges, thus confirming the Z configuration.
Antitumor activity
As a primary screening, compounds 3 and 7–9 were submitted to the Developmental Therapeutics Program (DTP) at the National Cancer Institute (NCI) (http://dtp.nci.nih.gov) for evaluation of antitumor activity in the human cell line screen. In a preliminary screen at a single concentration (100 µM) against three human cell lines (NCI-H460 lung cancer, MCF7 breast cancer and SF-268 glioma) a compound is considered active when it reduces the growth of any of the cell lines to 32% or less. Most of the compounds reported in Scheme 1 were active and passed on for evaluation in the full panel of sixty human tumor cell lines. This panel is organized into subpanels representing leukemia, melanoma and cancers of lung, colon, kidney, ovary, breast, prostate and central nervous system.
The test compounds were dissolved in DMSO and evaluated using five concentrations at ten-fold dilutions, the highest being 10−4 M. Table 2 reports the results obtained, expressed as the negative log of the molar concentration at three assay endpoints: the 50% growth inhibitory power (pGI50), the cytostatic effect (pTGI=Total Growth Inhibition) and the cytotoxic effect (pLC50). Some of the compounds were reassayed based on the results of the first screen: compounds 3d, 3h, and 3n were assayed twice, compounds 3b and 3i were assayed three times and all other compounds were assayed once. The pyridine derivatives (3) were far more active than the piperazine derivatives (7–9). Compounds 3a–d, g, h, j, l, o and p were potent growth inhibitors with mean pGI50 ranging from 5.08 to 6.34 and cytotoxicity from 4.04 to 4.85. The difference between the average concentration which caused 50% growth inhibition and the concentration which killed 50% of the cells was around 1.2 log with only 0.78 for 3o and 1.71 for 3b. Within each of the nine cell panels there was a difference of 0.9 between the least-sensitive and the most-sensitive (leukemia) cell lines in response to 3b.
Table 2.
Sixty cell panel (growth inhibition, cytostatic and cytotoxic activity of the selected compounds).
| Comp a | Modes | Leu-kemia | NSCLC | Colon | CNS | Mela-noma | Ova-rian | Renal | Pros-tate | Breast | MG-MIDb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3a | pGI50 | 6.63 | 6.09 | 6.55 | 6.24 | 6.52 | 6.23 | 6.24 | 6.20 | 6.31 | 6.34 |
| pTGI | 5.71 | 5.50 | 6.11 | 5.74 | 6.03 | 5.75 | 5.79 | 5.57 | 5.70 | 5.79 | |
| pLC50 | - | 4.65 | 5.19 | 5.15 | 5.33 | 5.18 | 5.27 | 4.61 | 4.24 | 4.85 | |
| 3b | pGI50 | 7.04 | 6.17 | 6.38 | 6.22 | 6.23 | 6.30 | 6.37 | 6.36 | 6.41 | 6.34 |
| pTGI | 5.72 | 5.57 | 5.30 | 5.55 | 5.50 | 5.55 | 5.82 | 5.68 | 5.48 | 5.55 | |
| pLC50 | 4.35 | 4.46 | 4.72 | 4.63 | 4.80 | 4.51 | 5.12 | 4.80 | 4.40 | 4.63 | |
| 3c | pGI50 | 6.18 | 5.76 | 6.03 | 6.05 | 6.16 | 5.90 | 5.90 | 5.82 | 5.95 | 5.97 |
| pTGI | 5.31 | 5.36 | 5.59 | 5.56 | 5.72 | 5.38 | 5.53 | 5.18 | 5.38 | 5.47 | |
| pLC50 | 4.32 | 4.70 | 4.82 | 4.83 | 5.10 | 4.75 | 5.12 | 4.61 | 4.64 | 4.79 | |
| 3d | pGI50 | 6.21 | 5.77 | 6.10 | 5.79 | 6.05 | 5.95 | 5.84 | 5.78 | 5.83 | 5.92 |
| pTGI | 5.42 | 4.97 | 5.33 | 4.89 | 5.40 | 5.03 | 5.37 | 5.03 | 4.83 | 5.15 | |
| pLC50 | 4.24 | 4.36 | 4.51 | 4.20 | 4.87 | 4.33 | 4.62 | 4.16 | 4.09 | 4.40 | |
| 3e | pGI50 | 4.76 | 4.47 | 4.41 | 4.39 | 4.08 | 4.08 | 4.46 | 4.70 | 4.39 | 4.40 |
| pTGI | 4.26 | - | - | - | - | - | 4.16 | - | 4.07 | 4.06 | |
| pLC50 | 4.03 | - | - | - | - | - | 4.02 | - | - | 4.01 | |
| 3f | pGI50 | 4.81 | - | - | 4.52 | - | - | - | - | - | 4.12 |
| pTGI | - | - | - | 4.21 | - | - | - | - | - | 4.02 | |
| 3g | pGI50 | 5.63 | 5.28 | 5.17 | 5.36 | 5.36 | 5.12 | 5.06 | 5.45 | 5.22 | 5.27 |
| pTGI | 5.02 | 4.36 | 4.41 | 4.35 | 4.52 | - | 4.30 | 4.53 | 4.16 | 4.39 | |
| pLC50 | - | - | - | 4.05 | 4.22 | - | - | - | - | 4.04 | |
| 3h | pGI50 | 5.93 | 5.14 | 5.67 | 5.42 | 5.69 | 5.38 | 5.38 | 5.46 | 5.53 | 5.49 |
| pTGI | 5.11 | 4.58 | 5.27 | 4.94 | 5.31 | 4.60 | 4.89 | 4.91 | 4.84 | 4.93 | |
| pLC50 | - | 4.10 | 4.40 | 4.27 | 4.44 | 4.09 | 4.29 | 4.13 | 4.30 | 4.22 | |
| 3i | pGI50 | 5.21 | - | - | 4.14 | - | - | - | - | - | 4.14 |
| pTGI | 4.67 | - | - | 4.06 | - | - | - | - | - | 4.08 | |
| pLC50 | 4.46 | - | - | - | - | - | - | - | - | 4.05 | |
| 3j | pGI50 | 5.01 | 4.96 | 4.98 | 5.43 | 5.00 | 5.01 | 5.10 | 5.19 | 5.21 | 5.08 |
| pTGI | - | 4.55 | 4.61 | 4.85 | 4.63 | 4.59 | 4.66 | 4.68 | 4.74 | 4.59 | |
| pLC50 | - | 4.20 | 4.28 | 4.39 | 4.28 | 4.23 | 4.29 | 4.26 | 4.27 | 4.25 | |
| 3k | pGI50 | 4.03 | - | - | - | 4.02 | - | 4.07 | - | - | 4.01 |
| 3l | pGI50 | 5.84 | 5.43 | 5.76 | 5.65 | 5.75 | 5.52 | 5.97 | 5.47 | 5.64 | 5.69 |
| pTGI | 5.09 | 4.83 | 5.31 | 5.11 | 5.21 | 4.71 | 5.48 | 4.39 | 4.87 | 5.06 | |
| pLC50 | 4.02 | 4.24 | 4.60 | 4.44 | 4.67 | 4.20 | 4.70 | 4.15 | 4.28 | 4.40 | |
| 3m | pGI50 | 4.56 | 4.07 | 4.14 | - | 4.06 | 4.12 | 4.17 | 4.18 | 4.15 | 4.16 |
| pTGI | - | - | - | - | - | - | - | - | 4.06 | 4.01 | |
| 3n | pGI50 | 5.36 | 4.08 | 4.31 | 4.05 | 4.10 | 4.05 | 4.28 | 4.47 | 4.13 | 4.29 |
| pTGI | 4.22 | - | - | - | - | - | - | - | 4.03 | 4.02 | |
| pLC50 | 4.04 | - | - | - | - | - | - | - | - | - | |
| 3o | pGI50 | 5.51 | 4.92 | 5.00 | 5.12 | 5.31 | 5.00 | 5.00 | 4.87 | 5.05 | 5.09 |
| pTGI | 4.80 | 4.52 | 4.55 | 4.53 | 4.65 | 4.53 | 4.63 | 4.40 | 4.48 | 4.57 | |
| pLC50 | 4.30 | 4.30 | 4.30 | 4.30 | 4.36 | 4.30 | 4.33 | 4.30 | 4.30 | 4.31 | |
| 3p | pGI50 | 5.93 | 5.61 | 5.84 | 5.49 | 5.98 | 5.59 | 5.82 | 5.68 | 5.79 | 5.76 |
| pTGI | 4.42 | 4.72 | 5.47 | 4.83 | 5.42 | 4.50 | 5.28 | - | 4.70 | 4.91 | |
| pLC50 | - | 4.13 | 4.93 | - | 5.05 | - | 4.68 | - | 4.20 | 4.36 | |
| 7a | pGI50 | - | 4.07 | - | 4.13 | - | 4.01 | 4.08 | - | 4.07 | 4.05 |
| 8a | pGI50 | 4.03 | 4.07 | - | 4.12 | 4.06 | 4.10 | 4.53 | - | 4.18 | 4.13 |
| pTGI | - | - | - | - | - | - | 4.30 | - | - | 4.04 | |
| 9a | pGI50 | - | 4.01 | - | 4.19 | 4.26 | - | 4.15 | - | 4.19 | 4.09 |
| 9b | pGI50 | - | - | - | - | 4.14 | 4.05 | - | - | 4.10 | 4.03 |
| Vincrist ine sulfatec |
pGI50 | 7.00 | 6.60 | 7.00 | 6.90 | 6.80 | 6.50 | 6.50 | 6.90 | 6.50 | 6.70 |
| pTGI | 4.80 | 4.80 | 5.40 | 5.20 | 5.10 | 4.70 | 4.70 | 5.20 | 5.10 | 5.00 | |
| pLC50 | 3.20 | 3.60 | 4.10 | 3.70 | 3.60 | 3.50 | 3.60 | 3.50 | 3.50 | 3.60 |
Highest conc. = 10−4M unless otherwise reported; only modes showing a value > 4.00 are reported
Mean Graph MIDpoint i.e. the calculated mean panel
Highest conc. = 10−3M
A comparison of the activities of the compounds reported in this paper shows that the weak activity of 7–9 is not related to the methoxy group in the indole system, to the acetyl group in the piperazine ring or to the methine bridge. Even with this limited number of compounds, it seems clear enough that the core is important since in the pyridyl derivatives 3a-q the antitumor activity is evident and related to the substituents in the indole system. The presence of acidic groups led to loss of activity probably because the resulting compounds (3f, i, k) are too hydrophilic (in particular compounds 3f, i showed their highest activity on leukemia cells). This hypothesis is confirmed by the weak activity of the hydroxy derivatives 3e, m, q. Interesting results were obtained with halogens, methoxy and dimethylamino groups. The compound containing chlorine at the 4 position of the indole (3b) was very active: the activity decreased by shifting the halogen from the 4 to the 5 (3c) and 6 position (3l).
COMPARE analyses
We used the COMPARE tool from the DTP at the NCI to analyze the screening results of the most active compounds for indications of mechanism of action. COMPARE uses the pattern of relative sensitivity/insensitivity of the cell lines in the screen to identify other compounds with similar patterns of activity or to identify molecular targets whose patterns of expression correlate with the pattern of compound activity.
Matrix COMPARE was used to determine the pair-wise correlation of the cell response pattern for every combination of two compounds in the set of nine compounds for which we had data at the GI50 endpoint. In addition, the individual compound screening results at the GI50 endpoint were also submitted for COMPARE analyses against the NCI standard agent compound set, against all of the NCI public compound screening data and against all of the NCI public microarray expression data. COMPARE correlations of 0.6 or greater between two compounds suggest that the compounds affect the same mechanism(s) within cells.18
In the matrix COMPARE analysis of the nine compounds, the highest correlation was 0.74 between 3c and 3h, and the second highest correlation of 0.65 was between 3c to 3o. The direct correlation between 3h and 3o was 0.48. Qualitative inspection of the relative sensitivities in the cell line panels indicates that these correlations are driven by greater-than-average activity against leukemia and melanoma cell lines coupled with less-than-average activity against non-small cell lung and renal cell lines. Within the matrix COMPARE results, correlations other than those above had an average of about 0.19 with a standard deviation of 0.19, and were not considered further.
COMPARE has not identified any single strong candidates for mechanism of action or molecular targets that may be targeted by the bis-indole derivatives, though the correlations among a few compounds in this series, along with high correlations to some related compounds previously submitted, suggest commonalities among them. It may be that the bis-indole derivatives are affecting several different cellular processes at the same time producing cell response patterns which do not correlate with any public compounds previously submitted to COMPARE. It is also possible that these compounds do not satisfy the important assumptions when using COMPARE to search for a mechanism of action: compounds with similar mechanism(s) or the relevant molecular target(s) must already have been assayed and included in the database, and the standard 48-hour exposure time in the screening assay must have been sufficient for a compound's effects to manifest themselves; and, in addition, the cell lines in the screen must have exhibited a range of sensitivities to the compounds or a range of expression of the molecular targets so that there are useful patterns for COMPARE to work with.
Further studies for compounds 3b and 3d
Compounds 3b and 3d, with pGI50 values of 6.34 and 5.92 respectively, were among the most active of the whole series. In a collaboration with Oncotest (Freiburg, Germany) these compounds were subjected to a further in vitro evaluation on a panel of 36 human tumor cell lines (bladder, colon, CNS, gastric, head-neck, lung, breast, melanoma, ovarian, pancreas, prostate, pleuramesothelioma, renal, uterus body) and the result was subjected to COMPARE analysis against the company's own database of compound screening data. This is a similar methodology to the NCI COMPARE above, but used a separate set of screening data obtained from a distinct set of cell lines.
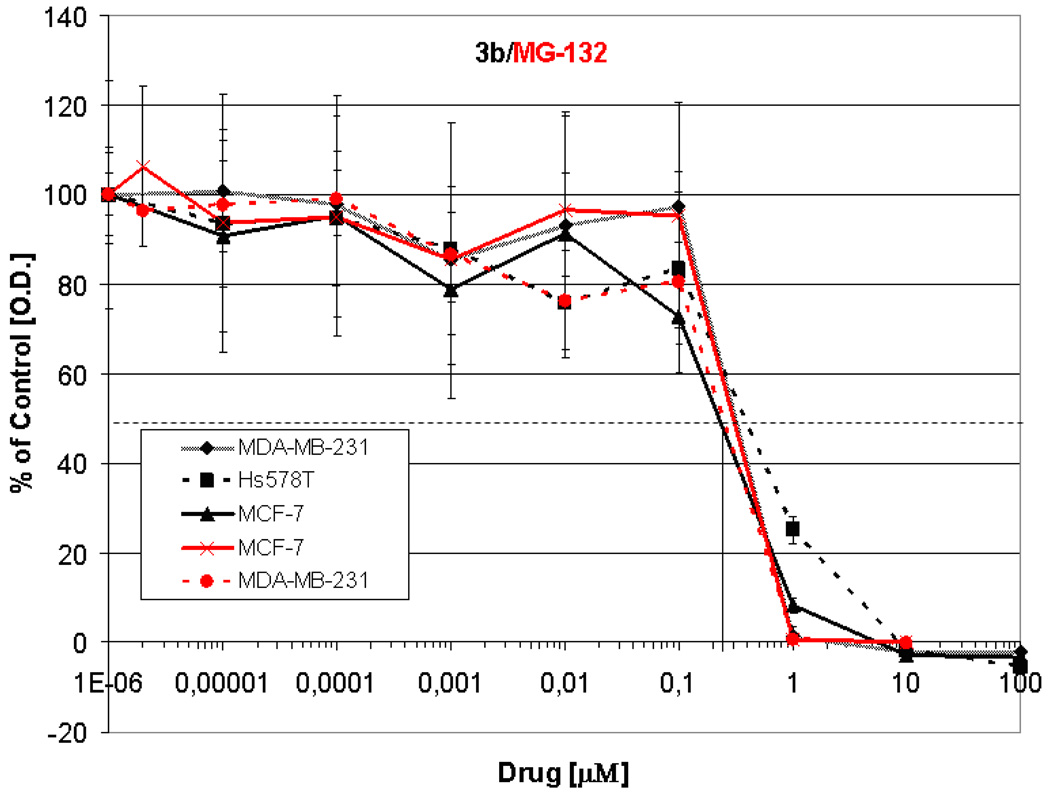
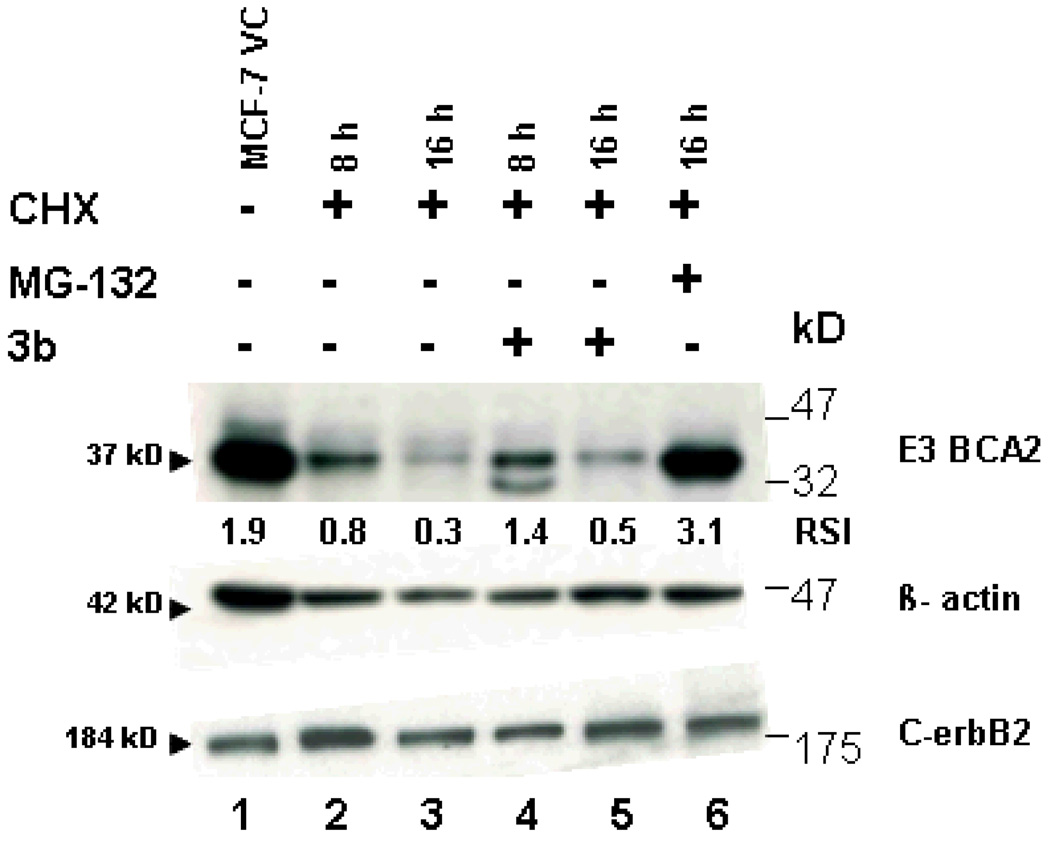
Compound 3b was the closest match with 3d. Overall, 3b was more potent than 3d (mean IC70 0.35 versus 0.56 µg/ml) and has a more pronounced selectivity. Compounds 3b and 3d were similar in the COMPARE analyses (r=0.60) and 3b was correlated with Tyropeptin A, a proteasome inhibitor, with a correlation of r=0.54. This result prompted us to test whether compound 3b was a proteasome inhibitor. The study was performed at the University of Maryland after taking into account the high growth inhibitory effect of compound 3b against breast cancer cells and its comparable in vitro growth inhibitory activity to the known proteasome inhibitor MG-132 (Z-Leu-Leu-Leu-al) as shown in Figure 1. The ubiquitin-proteasome system regulates cellular protein degradation and homeostasis. In fact, many of the known E3 ligases that tag a given protein with ubiquitin targeting it for degradation in the proteasome play a role in various diseases, including cancer development and progression. In particular, breast cancer seems to be associated with the aberrant expression of several RING E3 ligases including BCA2.19 E3 ligases are very unstable because of autoubiquitination, a hallmark of E3 activity, and their rapid degradation in the proteasome.19 Although, compound 3b and MG-132 had very similar activity in the considered breast cancer cell line panel (Figure 1 and Table 3), their behavior was very different when we examined the compounds ability to influence the stability of endogenous BCA2, via inhibition of the proteasome. While MG-132 can stabilize the protein, in the presence of the protein synthesis inhibitor cycloheximide (CHX), 3b had only very limited effects. However some potential to stabilize BCA2 was seen at 16 h (lanes 3 and 5, Figure 2) in that 3b treatment led to an approximately 2-fold increase of BCA2 protein compared to CHX treatment. However, MG-132 was able to stabilize BCA2 more than 10-fold compared to the CHX treatment (Figure 2). In particular at 8 h a second, smaller band appeared in the Western blot. This band is likely a form of BCA2 that is phosphorylated by AKT as shown before, suggesting that 3b might modulate the stability of kinases such as AKT.20
Figure 1. Antiproliferative activity of 3b (black lines) in a panel of breast cancer cell lines in comparison to the proteasome inhibitor MG-132 (red lines).
Growth curves are shown for effects of drugs after 5 days of continuous exposure. Proliferation was measured by MTT assay and data are displayed as percent growth inhibition compared to the growth of vehicle treated control cells.
Table 3.
Summary of In Vitro Growth Data
| Breast Cancer Cell Line | 3b IC50 | MG-132 IC50 |
|---|---|---|
| MCF-7 | 0.23 µM | 0.3 µM |
| MDA-MB-231 | 0.3 µM | 0.25 µM |
| Hs578T | 0.4 µM | not done |
Figure 2. (VC, vehicle control).
Effects of 3b on the stability of the ubiqutin E3 ligase BCA2 in MCF-7 breast cancer cells. The protein synthesis inhibitor CHX alone and in combination with the proteasome inhibitor MG-132 and 3b were evaluated for effects on the protein stability of BCA2 (which is degraded in the proteasome), the stability of C-erbB2 (a receptor tyrosine kinase that is degraded in the lysosome) and the stable housekeeping gene β-actin. Expression levels of BCA2 relative to β-actin are shown below each lane of the BCA2 blot. Compound 3b treated MCF-7 cells lysates exhibit approximately double the amount of BCA2 compared to CHX alone treated cells. The expression levels of C-erbB2 relative to β-actin ranged from 1.1 to 0.8 RSI (relative signal intensity) as determined by ImageJ software.
No effects of 3b were found on β-actin (used as loading control) or the oncogenic receptor C-erbB2, a protein that is degraded via the lysosomal pathway (Figure 2).
PMET inhibition
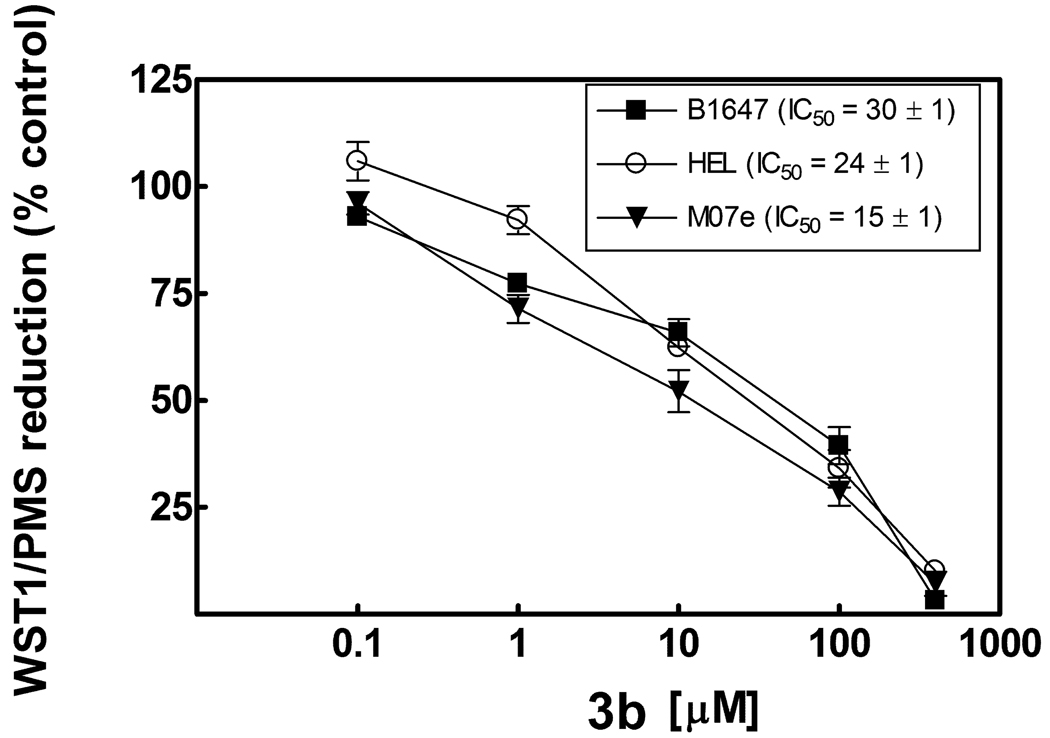
Another potential mechanism of action of anticancer agents is the inhibition of plasma membrane electron transport (PMET), a stress adaptation pathway that has been associated with tumor activity.21,22 Since these compounds could exhibit a potential redox activity due to the presence of ketone groups, we thought it was useful to determine whether the in vitro activity was associated with the ability to inhibit PMET. Therefore compounds active against HL60 cells and some inactive compounds were selected (3b–e, g–i, l–o, 8a,9a) and tested at the Malaghan Institute of Medical Research (New Zealand) in human leukemic HL60 cells and mitochondrial gene-knockout HL60ρ0 cells which have defective mitochondrial electron transport and therefore exclude possible respiratory effects. PMET was assayed by measuring the extracellular reduction of the tetrazolium dye, WST-1, in the presence of the intermediate electron acceptor, 1-methoxyphenazinemethosulfate (mPMS). Compounds 3g, 3i, 3o, 8a and 9a were inactive at 200 µM while IC50s for the other compounds were 117 µM or greater in both cell lines. In contrast, the IC50 values for cell proliferation as determined in a 2-day MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] cell proliferation assay for compounds 3b and 3c were 3–8 µM for both HL60 and HL60 ρ0 cells. Other experiments, performed at the Biochemistry Department - University of Bologna, showed that inhibition of PMET occurred in different human leukemic cell lines, such as B1647, HEL and M07e, only in the presence of 3b (IC50 15–30 µM) (Figure 3), 3d (IC50 30–45 µM) and, to a lesser extent, 3e (IC50 40–50 µM). These data correlate with effects on mitochondrial Complex I and on total respiratory chain activity. In these cell lines the MTT assay confirmed the antiproliferative activity of all tested compounds. Results here reported underline that PMET inhibitory activity of compounds 3b, 3d and 3e may contribute to their antiproliferative effect in some leukemic cell lines but, in general, PMET inhibitory activity does not correlate closely with antiproliferative effect in the leukemic cells tested.
Figure 3. Effect of compound 3b on PMET activity in acute leukemic cell lines.
Cells (105cells per well in 0.1 ml) were pretreated for 30 min. with 3b compound dissolved in DMSO prior to adding WST-1/mPMS (final concentration of 500 µM and 20 µM, respectively). WST-1 reduction measured in real time at 450 nm in a BMG Fluostar microplate reader. Results are averages ± S.E.M. of four independent experiments.
In vivo experiments
Further studies continued at the NCI where the maximum tolerated dose (MTD) was determined for compounds 3b and 3d and it was found high enough (400 mg/Kg) to warrant further testing which started with 3d only. The first in vivo experiment was the "hollow fiber assay".23 Compound 3d was tested against a panel of six tumor cell lines consisting of the leukemia cell lines HL-60, K562, MOLT-4, CCRF-CEM, RPMI-8226, SR. A compound is considered for xenograft testing if it has a combined i.p. + s.c. score of 10 or greater, a s.c. score of 4 or greater, or produces cell kill of any cell line at either dose level evaluated. Compound 3d resulted in a i.p. + s.c. score of 22 and it was therefore examined for distal site antitumor activity in an appropriate human tumor xenograft model in nude mice24,25 (Table S2). As shown in Table S3 it did not show significant activity in the tumor employed (HL-60-TB leukemia).
In conclusion we demonstrated that this new approach was successful since some of the compounds here described are much more active than the numerous, so far prepared and tested 3-indolylmethylene-2-indolinones which have been mentioned in the introduction as the rationale for this search.
Experimental section
A) Chemistry
The melting points are uncorrected. Elemental analyses were performed with a Fisons Carlo Erba Instrument EA1108 and resulted within 0.4% of the theoretical values (Table S4). Bakerflex plates (silica gel IB2-F) were used for TLC: the eluent was petroleum ether/acetone in various proportions. The IR spectra were recorded in nujol on a Nicolet Avatar 320 E.S.P.; νmax is expressed in cm−1. The 1H-NMR spectra were recorded in (CD3)2SO or CF3COOD on a Varian Gemini (300 MHz); the chemical shift (referenced to solvent signal) is expressed in δ (ppm) and J in Hz. For the spectra which are not reported here see Table S1.
Pyridine-2,6-dicarbaldehyde and all the starting indolinones 1, except ethyl 2-oxoindoline-5-sulfonate, are commercially available or have been prepared as described in the literature (see Table 1).9,12,13,26–33 Compounds 7–9 were prepared from commercially available starting material.
Synthesis of ethyl 2-oxoindoline-5-sulfonate
2-Oxoindoline-5-sulfonyl chloride (6 mmol) was refluxed for 30 min. with 30 ml of ethanol. The white crystalline precipitate formed after cooling was separated by filtration with a yield of 90% and used without further purification.
C10H11NO4S Mw 241.27 mp 150°C. IR: 1716, 1614, 1004, 922, 784. NMR: 1.19 (3H, t, CH3-CH2, J=7.2) 3.61 (2H, s, ind-3), 4.04 (2H, q, CH3-CH2), 7.01 (1H, d, ind-7, J=8), 7.69 (1H, s, ind-4), 7.73 (1H, d, ind-6, J=8), 10.90 (1H, s, NH).
Synthesis of 3,3'-[pyridine-2,6-diylbis(methylene)]bis(1,3-dihydroindol-2-ones) (3a–q)
Two different methods were employed according to the substituents in the indole system (Table 1).
Method 1 (compounds 3e, g, h, l-n, p, q). This method was emplyed even for the published compounds 3a–d, o.12,13 The appropriate indolinone 1 (10 mmol) was dissolved in methanol and treated with pyridine-2,6-dicarbaldehyde 2 (5 mmol) and piperidine (2 ml). The reaction mixture was refluxed for 3–5 h (according to a TLC test), cooled and, if necessary, concentrated at reduced pressure. The yellow to orange precipitate thus formed was collected by filtration with a yield of 80% for compounds 3n and 50–60% for the others. They were used as such without further purification.
Data for 3g. IR: 3283, 1710, 1677, 1214, 1091. 1H-NMR: 2.52 (12H, s, CH3), 6.60 (2H, dd, ind-6, J=8.4 Hz, J=2.2 Hz), 6.66 (2H, d, ind-7, J=8.4 Hz), 7.67 (2H, s, -CH=), 7.87(2H, d, ind-4, J=2.2 Hz), 7.89 (2H, d, py-3,5, J=7.8 Hz), 8.09 (1H, t, py-4, J=7.8 Hz), 10.28 (2H, s, NH). Anal. C27H25N5O2 (C, H, N).
Data for 3h. IR: 3100, 1695, 1617, 1194, 912. 1H-NMR: 6.76 (2H, m, ind), 6.91 (2H, m, ind), 7.81 (2H, s, -CH=), 7.98 (4H, m, ind+py-3,5), 8.15 (1H, t, py-4, J=7 Hz), 10.65 (2H, s, NH). Anal. C23H13F2N3O2 (C, H, N).
Data for 3l. IR: 3201, 1721, 1611, 1070, 917. 1H-NMR: 6.48 (2H, dd, ind-5, J=8.1 Hz, J=1.5 Hz), 6.81 (2H, d, ind-7, J=1.5 Hz), 7.44 (2H, s, -CH=), 7.94 (2H, d, py-3,5, J=7.8 Hz), 8.11 (1H, t, py-4, J=7.8 Hz), 8.12 (2H. d, ind-4, J=8.1 Hz), 10.78 (2H, s, NH). Anal. C23H13Cl2N3O2 (C, H, N).
Method 2 (compounds 3f, i–k). Pyridine-2,6-dicarbaldehyde 2 (10 mmol) was dissolved in acetic acid (50 ml) and treated with the appropriate indolinone 1 (20 mmol) and 37% hydrochloric acid (1 ml). The reaction mixture was refluxed for 3–5 h (according to a TLC test) and cooled. The yellow to orange precipitate thus formed was collected by filtration with a yield of 80% for 3k and 50–60% for the others. They were used as such without further purification.
Data for 3j. IR: 3170, 1706, 1609,1301, 717. 1H-NMR: 6.86 (2H, d, ind-7, J=8.5 Hz), 7.49 (2H, dd, ind-6, J=8.5 Hz, J=1.2 Hz), 8.01 (2H, s, -CH=), 8.07 (2H, d, ind-4, J= 1.2 Hz), 8.27 (1H, t, py-4, J=7.5 Hz), 8.65 (2H, d, py-3+5, J=7.5 Hz), 11.11 (2H, s, NH). Anal. C23H13Br2N3O2 (C, H, N).
Synthesis of 3,6-bis(2-oxo-1,2-dihydroindol-3-ylidene)piperazine-2,5-diones (7a–b)
The appropriate isatine (4a–b, 5 mmol) was added to a solution of 1,4-diacetylpiperazine-2,5-dione 6 (2.5 mmol) in DMF (10 ml). After the addition of triethylamine (5.5 mmol), the reaction mixture was stirred at room temperature for 3–5 h. The brown precipitate thus formed was collected by filtration with a yield of 90% and used without further purification.
Synthesis of 1,4-diacetyl-3,6-bis(indol-3-ylmethylene)piperazine-2,5-diones (8a–b)
The appropriate indolealdehyde 5 (10 mmol) was treated with 1,4-diacetylpiperazine-2,5-dione 6 (5 mmol), anhydrous sodium acetate (20 mmol) and acetic anhydride (10 ml). The reaction mixture was refluxed for 3–5 h (according to a TLC test) and acetic anhydride was removed under reduced pressure. The residue was treated with ice water and the resulting yellow to orange precipitate was collected by filtration with a yield of 70% and used without further purification.
Synthesis of 3,6-bis(indol-3-ylmethylene)piperazine-2,5-diones (9a–b)
The appropriate compound 8 (1 mmol) was dissolved in ethanol (250 ml) and refluxed for 5 h with 2N KOH (3 ml). The yellow to orange precipitate formed after cooling was collected by filtration and used without further purification. The yield was 60% for 9a and 30% for 9b.
B) Biology
a) In Vitro Growth Inhibition and Cytotoxicity
It was determined on the 3 and on the 60 cell line panel by the NCI according to standard procedures.34
b) 36 Cell line assay
The 36 cell line assay was performed at Oncotest: 24 of the 36 cell lines were established from patient-derived tumor xenografts passaged subcutaneously in nude mice.35 The origin of the donor xenografts has been already described.36,37 The other 12 cell lines were commercially available. All cells were grown at 37°C in a humidified atmosphere (95% air, 5% CO2) in RPMI 1640 medium (PAA, Cölbe, Germany) supplemented with 10% fetal calf serum (PAA) and 0.1 mg/ml gentamicin (PAA). A modified propidium iodide assay38 was used to assess the effects of compounds. Tumor derived cell lines were incubated in 96 multi-well plates. After one day, the compounds under test were added to the plates at 5 concentrations in the range from 0.001 mg up to 10 mg and left for further four days. The inhibition of proliferation was determined by measuring the DNA content using an aqueous propidium iodide solution (7 mg/ml). Fluorescence was measured using the Cytofluor 4000. All compounds were tested in three independent experiments. In each experiment, all data points were determined in triplicate.
The COMPARE Algorithm uses in vitro activity data to obtain clues as to the mechanism of action of a test compound. The individual IC70-values of the test compounds in 36 test cell lines obtained in the monolayer assay were correlated to the corresponding IC70-values for more than 100 standard agents determined in exactly these 36 cell lines. Data for these standard agents are available in the Oncotest database.
c) Analysis of BCA2 protein stability
To determine the stability of endogenous BCA2, 1×106 MCF7 cells were seeded into six-well plates and grown overnight. The protein synthesis inhibitor cyclohexamide (Sigma) was added alone (100 µmol/L) or in combination with the proteasome inhibitor MG-132 (10 µmol/L, Calbiochem) or compound 3b for a total of 8 and 16 hours. Cell lysates were generated and Western blots performed as described before.39 We used the polyclonal anti-BCA2 antibody (dil. 1:800) that has been generated,19 the β-actin monoclonal mouse antibody was from Sigma (St. Louis, MO, dil. 1:5000) and the monoclonal anti-C-erbB2 antibody (dil. 1:2000) from EMD Bioscienes, La Jolla, CA.
Signals were quantified using the NIH ImageJ software by measuring the mean signal intensity (grey scale) value and its integrated density. Resulting absolute intensities were determined and a relative intensity value obtained by dividing the absolute intensity by the absolute intensity of the control sample. MTT cytotoxicity assays were performed with the breast cancer cell lines in Table 3 as described before.39
d) PMET and MTT cell proliferation assays
Inhibition of PMET was determined essentially as described 22 using a microplate format with 100,000 cells per well in 0.1 ml. Cells were pretreated for 30 min with compound dissolved in DMSO (<0.1% final concentration) prior to adding WST-1/mPMS and WST-1 reduction measured in real time at 450 nm in a BMG Fluostar microplate reader. MTT reduction was measured following incubation of cells with compound for 48 h.
e) MTD assay (http://dtp.nci.nih.gov)
Basically what is done is to dose a mouse with a single injection of 400 mg/kg, i.p.. If the animal survives 14 days, the MTD is 400. If the animal dies, the dose is lowered to 200 mg/kg. The dose keeps being lowered by 50% until a tolerated dosage is determined and that will be the MTD.
f) Hollow fiber assay and xenograft
These tests were performed by the NCI according to standard procedures (http://dtp.nci.nih.gov/branches/btb/hfa.html).24,25
Supplementary Material
IR and 1H-NMR of the new compounds (Table S1), Hollow Fiber Data for compound 3d (Table S2), Response of advanced stage SC HL-60(TB)Leukemia xenografts to 3d (Table S3), Elemental analyses (Table S4), NSC numbers (Table S5). This material is available free of charge at http://pubs.acs.org.
Acknowledgements
This work has been supported by a grant from MIUR-COFIN 2006. We are grateful to the National Cancer Institute (Bethesda, MD) for the anticancer tests and to Professor Romana Fato for the studies on mitochondrial Complex I and on total respiratory chain.
Abbreviations
- NOE
Nuclear Overhauser Enhancement
- DTP
Developmental Therapeutics Program
- NCI
National Cancer Institute
- GI
Growth Inhibition
- TGI
Total Growth Inhibition
- LC
Lethal Concentration
- DAVID
Database for Annotation, Visualization and Integrated Discovery
- PMET
plasma membrane electron transport
- mPMS
1-methoxyphenazinemethosulfate
- WST-1
water soluble tetrazolium salt-1
- MTT 3-(4,5-Dimethylthiazol-2-yl)-2
5-diphenyltetrazolium bromide
- CHX
cycloheximide
- RSI
relative signal intensity
- MTD
maximum tolerated dose
References
- 1.Potential Antitumor Agents. 43 (hawks-1). For part 42, seeAndreani A, Burnelli S, Granaiola M, Leoni A, Locatelli A, Morigi R, Rambaldi M, Varoli L, Calonghi N, Cappadone C, Farruggia G, Zini M, Stefanelli C, Masotti L, Radin NS, Shoemaker RH. New Antitumor Imidazo[2,1-b]thiazole Guanylhydrazones and Analogues. J. Med. Chem. In press doi: 10.1021/jm701246g.
- 2.Andreani A, Rambaldi M, Bonazzi D, Lelli G, Bossa R, Galatulas I. Substituted Imidazo[2,1-b]thiazoles and Thiazolines as Potential Cardiotonic Agents. Eur. J. Med. Chem. 1984;19:219–222. [Google Scholar]
- 3.Andreani A, Rambaldi M, Locatelli A, Bossa R, Galatulas I, Ninci M. Synthesis and Cardiotonic Activity of 2-Indolinones. Eur. J. Med. Chem. 1990;25:187–190. [Google Scholar]
- 4.Andreani A, Locatelli A, Rambaldi M, Leoni A, Bossa R, Fraccari A, Galatulas I. Potential Antitumor Agents. 25. Synthesis and Cytotoxic Activity of 3-(2-Chloro-3-indolylmethylene)1,3-dihydroindol-2-ones. Anticancer Res. 1996;16:3585–3588. [PubMed] [Google Scholar]
- 5.von Dobeneck H, Wolkenstein D, Blankenstein G. α,β’-Diindolylmethane und – methene Der Urorosein-Chromophor. Chemische Berichte. 1969;102:1347–1356. [Google Scholar]
- 6.Kobayashi G, Matsuda Y, Tominaga Y, Ohkuma M, Shinoda H, Kohno M, Mizuno D. Antitumor Activity of Indole Derivatives. Yakugaku Zasshi. 1977;97:1033–1039. doi: 10.1248/yakushi1947.97.9_1033. [DOI] [PubMed] [Google Scholar]
- 7.Buzzetti F, Pinciroli V, Brasca MG, Crugnola A, Fustinoni S, Longo A. Synthesis and Configuration of Some New Bicyclic 3-Arylidene-and 3-Heteroarylidene-2-oxindoles. Gazz. Chim. Ital. 1995;125:69–75. [Google Scholar]
- 8.Andreani A, Locatelli A, Leoni A, Morigi R, Chiericozzi M, Fraccari A, Galatulas I, Salvatore G. Synthesis and Potential Coanthracyclinic Activity of Pyridylmethylene and Indolylmethylene Lactams. Eur. J. Med. Chem. 1998;33:905–909. [Google Scholar]
- 9.Andreani A, Granaiola M, Leoni A, Locatelli A, Morigi R, Rambaldi M, Garaliene V. Synthesis and Antitumor Activity of 1,5,6-Substituted 3-(2-chloro-3-indolylmethylene)1,3-dihydroindol-2-ones. J. Med. Chem. 2002;45:2666–2669. doi: 10.1021/jm011123c. [DOI] [PubMed] [Google Scholar]
- 10.Andreani A, Granaiola M, Leoni A, Locatelli A, Morigi R, Rambaldi M, Garaliene V, Farruggia G, Masotti L. Substituted E-3-(2-Chloro-3-indolylmethylene)1,3-dihydroindol-2-ones With Antitumor Activity. Bioorg. Med. Chem. 2004;12:1121–1128. doi: 10.1016/j.bmc.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 11.Andreani A, Burnelli S, Granaiola M, Leoni A, Locatelli A, Morigi R, Rambaldi M, Varoli L, Calonghi N, Cappadone C, Farruggia G, Zini M, Stefanelli C, Masotti L. Substituted E-3-(2-Chloro-3-indolylmethylene)1,3-dihydroindol-2-ones with Antitumor Activity. Effect on the Cell Cycle and Apoptosis. J. Med. Chem. 2007;50:3167–3172. doi: 10.1021/jm070235m. [DOI] [PubMed] [Google Scholar]
- 12.Andreani A, Rambaldi M, Leoni A, Locatelli A, Bossa R, Chiericozzi M, Dambrosio M, Galatulas I. Synthesis and Cardiotonic Activity of 2-Indolinones Bearing Pyridyl groups. Eur. J. Med. Chem. 1993;28:653–657. [Google Scholar]
- 13.Andreani A, Leoni A, Locatelli A, Morigi R, Chiericozzi M, Fraccari A, Galatulas I. Potential Coanthracyclinic Activity of Pyridylmethylene-2-indolinones. Anticancer Res. 1998;18:3407–3410. [PubMed] [Google Scholar]
- 14.Tanaka K, Konno Y, Kuraishi Y, Kimura I, Suzuki T, Kiniwa M. Synthesis of a Magnosalin Derivative, 4-(3,4,5-Trimethoxyphenyl)-6-(2,4,5-trimethoxyphenyl)-2-diethylaminopyrimidine, and the Anti-Angiogenic and Anti-Rheumatic Effect on Mice by Oral Administration. Bioorg. Med. Chem. Lett. 2002;12:623–627. doi: 10.1016/s0960-894x(01)00810-1. [DOI] [PubMed] [Google Scholar]
- 15.Doggrell SA. RITA-A Small-molecule Anticancer Drug that Targets p53. Expert Opin. Investig. Drugs. 2005;14:739–742. doi: 10.1517/13543784.14.6.739. [DOI] [PubMed] [Google Scholar]
- 16.Barlaam B, Bird TG, Lambert-van der Brempt C, Campbell D, Foster SJ, Maciewicz R. New r-Substituted Succinate-Based Hydroxamic Acids as TNFr Convertasi Inhibitors. J. Med. Chem. 1999;42:4890–4908. doi: 10.1021/jm990377j. [DOI] [PubMed] [Google Scholar]
- 17.Katritzky AR, Fan W-Q, Szajda M, Li Q-L, Caster KC. Conjugated Systems Derived from Piperazine-2,5-dione. J. Heterocyclic Chem. 1988;25:591–597. [Google Scholar]
- 18.Paull KD, Lin CM, Malspeis L, Hammel E. Identification of Novel Antimitotic Agents Acting at the Tubulin Level by Computer-assisted Evaluation of Differential Cytotoxicity Data. Cancer Res. 1992;52:3892–3900. [PubMed] [Google Scholar]
- 19.Burger A, Amemiya Y, Kitching R, Seth AK. Novel RING E3 Ubiquitin Ligases in Breast Cancer. Neoplasia. 2006;8:689–695. doi: 10.1593/neo.06469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connor MK, Azmi PB, Subramaniam V, Li H, Seth A. Molecular Characterization of Ring Finger Protein 11. Molecular Cancer Research. 2005;3:453–461. doi: 10.1158/1541-7786.MCR-04-0166. [DOI] [PubMed] [Google Scholar]
- 21.Herst PM, Berridge MV. Plasma Membrane Electron Transport: a New Target for Cancer Drug Development. Curr. Mol. Med. 2006;6:895–904. doi: 10.2174/156652406779010777. [DOI] [PubMed] [Google Scholar]
- 22.Herst PM, Petersen T, Jerram P, Baty J, Berridge MV. The Antiproliferative Effects of Phenoxodiol are Associated with Inhibition of Plasma Membrane Electron Transport in Tumour Cell Lines and Primary Immune Cells. Biochem. Pharm. 2007;74:1587–1595. doi: 10.1016/j.bcp.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Hollingshead M, Alley MC, Camalier RF, Abbott BJ, Mayo JG, Malspeis L, Grever MR. In Vivo Cultivation of Tumor Cells in Hollow Fibers. Life Sciences. 1995;57:131–141. doi: 10.1016/0024-3205(95)00254-4. [DOI] [PubMed] [Google Scholar]
- 24.Alley MC, Hollingshead MG, Dykes DJ, Waud WR. Human Tumor Xenograft Models in NCI Drug Development. In: Teicher BA, Andrews PA, editors. Cancer Drug Discovery and Development: Anticancer Drug Development Guide: Preclinical Screening, Clinical Trials, and Approval. Chapter 7. 2nd Ed. Totowa, NJ: Humana Press Inc; 2004. [Google Scholar]
- 25.Plowman J, Dykes DJ, Hollingshead M, Simpson-Herren L, Alley MC. Human Tumor Xenograft Models in NCI Drug Development. In: Teicher B, editor. Anticancer Drug Development Guide: Preclinical Screening, Clinical Trials, and Approval. Chapter 6. Totowa, NJ: Humana Press Inc; 1997. [Google Scholar]
- 26.Beer RJS, Davenport HF, Robertson A. Extensions of the Synthesis of Hydroxyindoles from p-Benzoquinones. J. Chem. Soc. 1953:1262–1264. [Google Scholar]
- 27.Nakagawa K, Sato T, Nishi T, Oshiro Y, Yamamoto K Carbostyril and Oxindole Derivatives. Jpn. Kokai Tokkyo Koho. 1977:5. CODEN: JKXXAF JP 52073866 19770621 Showa. Patent written in Japanese. Application: JP 75-150935 19751216. CAN 87:167899 AN 1977:567899 CAPLUS. [Google Scholar]
- 28.Minisci F, Galli R, Cecere M. Amminazione Radicalica di Composti Aromatici Attivati: Acetammidi. Nuovo Processo per la Sintesi di para-Ammino-N,N-dialchilaniline. La Chimica e l’Industria. 1966;48:1324–1326. [Google Scholar]
- 29.Zakrzewska A, Kolehmainen E, Osmialowski B, Gawinecki R. 4-Fluoroanilines: Synthesis and Decomposition. J. Fluorine Chem. 2001;111:1–10. [Google Scholar]
- 30.Hidenori O, Shigeharu T, Takafumi F, Shuji T, Kazumi K, Shuji Y, Youichi Y, Michiaki T, Kazuyuki N. Studies on Positive Inotropic Agents. Synthesis of 1-Heteroaroylpiperazine Derivatives. Chem. Pharm. Bull. 1988;36:2253–2258. doi: 10.1248/cpb.36.2253. [DOI] [PubMed] [Google Scholar]
- 31.Sun L, Tran N, Tang F, App H, Hirth P, McMahon G, Tang C. Synthesis and Biological Evaluations of 3-Substituted Indolin-2-ones: A Novel Class of Tyrosine Kinase Inhibitors that Exhibit Selectivity toward Particular Receptor Tyrosine Kinases. J. Med. Chem. 1998;41:2588–2603. doi: 10.1021/jm980123i. [DOI] [PubMed] [Google Scholar]
- 32.Andreani A, Rambaldi M, Bonazzi D, Greci L, Andreani F. Potential Antitumor Agents. III. Hydrazone Derivatives of 5-Substituted 2-Chloro-3-formyl-6-methylindole. Farmaco. 1979;34:132–138. doi: 10.1002/chin.197924211. [DOI] [PubMed] [Google Scholar]
- 33.Porter JC, Robinson R, Wyler M. Monothiophthalimide and Some Derivatives of Oxindole. J. Chem. Soc. 1941:620–624. [Google Scholar]
- 34.Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A. Feasibility of a High-flux Anticancer Drug Screen Using a Diverse Panel of Cultured Human Tumor Cell Lines. J. Natl. Cancer Inst. 1991;83:757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 35.Roth T, Burger AM, Dengler W, Willmann H, Fiebig HH. Fiebig HH, Burger AM. Human Tumor Cell Lines Demonstrating the Characteristics of Patient Tumors as Useful Models for Anticancer Drug Screening. In Relevance of Tumor Models for Anticancer Drug Development. Contrib. Oncol. 1999;54:145–156. [Google Scholar]
- 36.Fiebig HH, Dengler WA, Roth T. Human Tumor Xenografts: Predictivity, Characterization, and Discovery of New Anticancer Agents. In Relevance of Tumor Models for Anticancer Drug Development. In: Fiebig HH, Burger AM, editors. Contrib. Oncol. Vol. 54. 1999. pp. 29–50. [Google Scholar]
- 37.Fiebig HH, Berger DP, Dengler WA, Wallbrecher E, Winterhalter BR. Combined in vitro/in vivo Test Procedure with Human Tumor Xenografts. In: Fiebig HH, Berger DP, editors. Immunodeficient Mice in Oncology. Vol. 42. 1992. pp. 321–351. Karger, Basel. [Google Scholar]
- 38.Dengler WA, Schulte J, Berger DP, Mertelsmann R, Fiebig HH. Development of a Propidium Iodide Fluorescence Assay for Proliferation and Cytotoxicity Assay. Anti-Cancer Drugs. 1995;6:522–532. doi: 10.1097/00001813-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Burger AM, Gao Y, Amemiya Y, Kahn HJ, Kitching R, Yang Y, Sun P, Narod SA, Hanna WM, Seth AK. A Novel RING-Type Ubiquitin Ligase Breast Cancer-Associated Gene 2 Correlates with Outcome in Invasive Breast Cancer. Cancer Res. 2005;65:10401–10412. doi: 10.1158/0008-5472.CAN-05-2103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
IR and 1H-NMR of the new compounds (Table S1), Hollow Fiber Data for compound 3d (Table S2), Response of advanced stage SC HL-60(TB)Leukemia xenografts to 3d (Table S3), Elemental analyses (Table S4), NSC numbers (Table S5). This material is available free of charge at http://pubs.acs.org.