Abstract
Objective
To report 3-year outcomes of patients who participated in a randomized trial evaluating 1 mg and 4 mg doses of preservative-free intravitreal triamcinolone compared with focal/grid photocoagulation for treatment of diabetic macular edema (DME).
Methods
Eyes with DME and visual acuity 20/40 to 20/320 were randomly assigned to focal/grid photocoagulation, 1 mg triamcinolone, or 4 mg triamcinolone. At the conclusion of the trial, 3-year follow up was available for 306 eyes.
Results
Between two and three years, more eyes in all treatment groups improved than worsened. Change in visual acuity letter score from baseline to 3 years was +5 in the laser group and 0 in each triamcinolone group. The cumulative probability of cataract surgery by three years was 31%, 46%, and 83% in the laser, 1 mg, and 4 mg groups, respectively.
Conclusions
Results in the subset of the randomized subjects who completed 3-year follow up are consistent with the previously published 2 year results, and do not indicate a long-term benefit of intravitreal triamcinolone relative to focal/grid photocoagulation for patients with DME similar to those studied in this clinical trial. Most eyes receiving 4 mg triamcinolone as given in this study are likely to require cataract surgery.
Introduction
Macular edema is a frequent manifestation of diabetic retinopathy and an important cause of impaired vision in individuals with diabetes.1–3 The Diabetic Retinopathy Clinical Research Network (DRCR.net) conducted a trial in 840 eyes of 693 subjects to evaluate intravitreal triamcinolone (1 mg and 4 mg doses) compared with focal/grid photocoagulation for treatment of diabetic macular edema (DME).4 The study found that there was an initial beneficial effect of 4 mg triamcinolone compared with a 1 mg dose or with focal/grid photocoagulation on retinal thickening and visual acuity at 4 months; however, the benefit diminished thereafter, and at two years, mean visual acuity was better in the laser group than in either of the other two groups (P=0.02 comparing the laser and 1 mg groups and P=0.002 comparing the laser and 4 mg groups). OCT results paralleled the visual acuity results. Both triamcinolone doses, and especially the 4 mg dose, were associated with an increased incidence of elevated intraocular pressure and cataract surgery.4
Although the primary trial outcome was assessed at two years, a substantial number of eyes had three-year follow up at the time the trial was stopped. These data provide the opportunity to evaluate change in visual acuity and retinal thickening between 2 and 3 years and to determine whether the treatment group differences seen after two years of follow up were sustained at three years.
Methods
Details of the protocol have been published 5, 4 and the protocol is available on the DRCR.net website (www.drcr.net, date accessed June 5, 2008). Key aspects of the protocol pertinent to this manuscript are summarized below.
Synopsis of Protocol
Eligible subjects were at least 18 years old with type 1 or type 2 diabetes and had at least one eye meeting the following criteria: (1) best corrected electronic-ETDRS visual acuity letter score between 73 (approximately 20/40) and 24 (approximately 20/320), (2) definite retinal thickening due to DME involving the center of the macula assessed to be the main cause of visual loss, and (3) retinal thickness measured on optical coherence tomography (OCT) ≥ 250 microns in the central subfield using a Stratus OCT (Carl Zeiss Meditec, Dublin, CA).
Each study eye was randomly assigned to one of the three treatment groups: (1) focal/grid photocoagulation (referred to as the laser group), (2) 1 mg intravitreal triamcinolone (referred to as the 1 mg triamcinolone group), or (3) 4 mg intravitreal triamcinolone (referred to as the 4 mg triamcinolone group). Subjects with two study eyes had one assigned to the laser group and the other to one of the triamcinolone groups. The triamcinolone study drug was a preservative-free preparation (1 mg or 4 mg) in a prefilled syringe (manufactured by Allergan, Inc., Irvine, CA; 4 mg brand name TRIVARIS). The focal/grid photocoagulation technique was modified from the original ETDRS protocol as described previously and used in prior DRCR.net protocols.6
Follow-up visits occurred every 4 months. Testing at each visit included measurement of best corrected visual acuity using an electronic procedure based on the ETDRS method7 and measurement of retinal thickness on OCT. At each visit, study eyes were evaluated for retreatment according to guidelines previously published.4
Fifty-one subjects (with 28, 18, and 16 study eyes in the laser, 1 mg triamcinolone, and 4 mg triamcinolone treatment groups, respectively) died within three years of entering the study. Among the remaining eyes without three years of follow up, 159, 122, and 122 were from subjects who were enrolled less than 34 months (the beginning of the time window for the 3-year visit) from the close out date of the trial and thus did not have the ability to complete the 3-year visit. Thus, there was the potential for three-year follow up of 143 (43%), 116 (45%), and 116 (46%) of the randomized eyes in the three groups, respectively. Among eyes with the potential to have three-year follow up, follow up was completed for 115 (80%) eyes in the laser group, 93 (80%) eyes in the 1 mg triamcinolone group, and 98 (84%) eyes in the 4 mg triamcinolone group (referred to as “completers”), with follow up being incomplete for the other eyes due to subject withdrawal or loss to follow up (referred to as “non-completers”).
Statistical Methods
Visual acuity was the primary outcome measure and OCT-measured central retinal thickness a secondary outcome. Results were tabulated to assess consistency with results reported at the 2-year primary outcome. Statistical analyses, where performed, paralleled those reported for the two-year analysis.4 In addition, the cumulative probability of a cataract extraction was calculated for each treatment group with the Kaplan-Meier product-limit method and pairwise comparisons were made using a proportional hazards model, adjusted for the factors used to stratify the randomization (baseline visual acuity and prior macular photocoagulation) and accounting for correlation within subjects who had 2 study eyes with a robust sandwich estimate of the covariance matrix.8 For subjects who did not complete the 3-year visit, visual acuity scores from earlier completed visits were compared with those from subjects who did complete the 3 year visit in a repeated measures regression model, adjusted for a treatment group by time interaction and the factors used to stratify the randomization (baseline visual acuity and prior macular photocoagulation).
Results
Completers and non-completers differed in racial/ethnicity distribution, with a higher proportion of the completers being white and a higher proportion of non-completers (who had the potential to complete the study before it was closed) being Hispanic (Table 1, available at www.archophthalmol.com). In all three treatment groups, baseline visual acuity was similar in completers and non-completers. However, visual acuity during follow up on average was about 4 letters worse in non-completers (who had the potential for 3 year follow up) through their last completed visit compared with the completers (P=0.01). Visual acuity during follow up appeared to be similar in completers and the non-completers who did not have the potential for 3-year follow up (P=0.15).
Table 1.
Baseline Characteristics Comparing Completers and Non-completers*
| Completers | Non-completers with potential to complete | Non-completers without potential to complete** | |
|---|---|---|---|
| N=306 eyes | N=69 eyes | N=465 eyes | |
| Treatment Group - N (%) | |||
| Laser | 115(38%) | 28 (41 %) | 187(40%) |
| 1mg | 93 (30 %) | 23 (33 %) | 140 (30 %) |
| 4mg | 98 (32 %) | 18 (26 %) | 138 (30 %) |
| Gender: Women - N (%) | 144 (47 %) | 24 (35 %) | 243 (52 %) |
| Age (years) - median (quartiles) | 63 (58 , 69 ) | 63 (56 , 67 ) | 64 (57 , 70 ) |
| Race - N (%) | |||
| White | 235 (77 %) | 46 (67 %) | 331 (71 %) |
| Black/African American | 32 (10 %) | 5(7%) | 42 (9 %) |
| Hispanic or Latino | 23 (8 %) | 16 (23 %) | 67 (14 %) |
| Asian | 9 (3 %) | 1 (1 %) | 10 (2 %) |
| American Indian/Alaskan Native | 4(1%) | 0 | 2 (<1 %) |
| Asian | 9 (3 %) | 1 (1 %) | 10 (2 %) |
| Native Hawaiian/Other Pacific Islander | 0 | 0 | 2 (<1 %) |
| More than one race | 0 | 0 | 2 (<1 %) |
| Unknown/not reported | 3 (1 %) | 1 (1 %) | 9 (2 %) |
| Diabetes Type - N (%) | |||
| Type 2 | 293 (96 %) | 64 (93 %) | 445 (96 %) |
| Duration of Diabetes (years) - median (quartiles) | 15(9,21) | 14(10,19) | 16(10,22) |
| HbA1c - median (quartiles) | 7.4 (6.7 , 8.4 ) | 8.1(6.8,9.8) | 7.6 (6.8 , 8.6 ) |
| Prior Panretinal Scatter Photocoagulation - N (%) | 57 (19 %) | 9 (13 %) | 69 (15 %) |
| Prior Photocoagulation for DME - N (%) | 198 (65 %) | 44 (64 %) | 268 (58 %) |
| IOP - median (quartiles) | 15(14,18) | 15(13,17) | 16(14,18) |
| Lens Status Phakic (clinical exam)- N (%) | 255 (83 %) | 52 (75 %) | 355 (76 %) |
| E-ETDRS Visual Acuity (letter score) – median (quartiles) | 62 (54, 67) | 61 (53, 66) | 62 (53, 68) |
| Approximate Snellen equivalent | 20/63 | 20/63 | 20/63 |
| Central Subfield Thickness (microns) on OCT - median (quartiles) | 398 (330, 500) | 422 (350 , 486 ) | 400(318,502) |
| Retinal Volume (mm3) on OCT - median (quartiles) | 9.0 (8.0 , 10.4 ) | 9.4 (8.4 , 10.3 ) | 8.9 (7.8 ,10.3 ) |
| Retinopathy Severity Level (ETDRS Severity Scale) - N (%) | |||
| MA/Mild/Moderate NPDR | 46 (16 %) | 9 (13 %) | 106 (24 %) |
| ModeratelySevere/Severe NPDR | 172 (59 %) | 34 (50 %) | 223 (50 %) |
| Mild/Moderate/High-risk PDR | 76 (26 %) | 25 (37 %) | 119(27%) |
Non-completers are partitioned into subjects who did vs did not have the potential for 3year follow up
Includes 51 subjects who died and 331 subjects who were randomized less than 34 months from the study closeout ( 70 who withdrew or were lost to follow up, and 261 who were active at the time of study closeout)
Among the eyes with three-year follow up, the mean number of treatments with the assigned treatment regimen during the three years of follow up were 3.1 in the laser group, 4.2 in the 1 mg triamcinolone group, and 4.1 in the 4 mg triamcinolone group. There were no cases of endophthalmitis following any of the 1898 injections during the entire study. During the third year of follow up, 20 (17%), 22 (24%), and 28 (29%) of eyes in the three treatment groups, respectively, were treated once with the assigned treatment regimen, 8 (7%), 9 (10%), and 21 (21%) were treated twice, and 1 (1%), 8 (9%), and 4 (4%) were treated three times.
Among the 3-year completers, 7 (6%) in the laser group received the 4 mg triamcinolone study drug at some point during the 3 years of follow up, 21 (23%) in the 1 mg triamcinolone group received focal/grid photocoagulation, and 20 (20%) in the 4 mg triamcinolone group received focal/grid photocoagulation. Other treatments for DME (primarily vitrectomy, nonstudy triamcinolone [Kenalog], and bevacizumab) were received by 15 (13%), 16 (17%), and 11 (11%) of eyes in the three treatment groups, respectively.
Effect of Treatment on Visual Acuity
Between two years and three years of follow up, visual acuity improved more often than it worsened in all three treatment groups. Among eyes with visual acuity at two years that was worse than 20/32, about twice as many in each treatment group improved 10 or more letters than worsened 10 or more letters from 2 to 3 years (Table 2, available at www.archophthalmol.com).
Table 2.
Change in Visual Acuity from 2 Years to 3 Years Stratified by 2 Year Visual Acuity*
| 2 Year Visual Acuity >= 74 (>=20/32) | 2 Year Visual Acuity < 74 (<20/32) | |||||
|---|---|---|---|---|---|---|
| Laser N=26 | 1 mg N=13 | 4 mg N=24 | Laser N=89 | 1 mg N=80 | 4 mg N=74 | |
| Change in Visual Acuity from 2 year to 3 year exam letter score | ||||||
| Mean Change ± Standard Deviation (SD) | −2 ± 8 | 0 ± 6 | −4 ± 7 | 2 ± 13 | 3 ± 15 | 4 ± 21 |
| Median Change (25th, 75th percentile) | −2 (−5, 5) | 2 (−3, 3) | −2 (−7, 1) | 3 (−2, 9) | 2 (−4, 12) | 3 (−4, 13) |
| Distribution of Change – N (%) | ||||||
| ≥ 15 letter improvement | 0 | 0 | 0 | 10 (11%) | 15 (19%) | 17 (23%) |
| 14–10 letter improvement | 0 | 0 | 0 | 11 (12%) | 6 (8%) | 3 (4%) |
| 9–5 letter improvement | 7 (27%) | 2 (15%) | 1 (4%) | 15 (17%) | 8 (10%) | 13 (18%) |
| Same ± 4 letters | 11 (42%) | 9 (69%) | 13 (54%) | 37 (42%) | 33 (41%) | 23 (31%) |
| 5–9 letters worse | 5 (19%) | 1 (8%) | 6 (25%) | 8 (9%) | 10 (13%) | 7 (9%) |
| 10–14 letters worse | 2 (8%) | 0 | 1 (4%) | 3 (3%) | 2 (3%) | 0 |
| ≥ 15 letters worse | 1 (4%) | 1 (8%) | 3 (13%) | 5 (6%) | 6 (8%) | 11 (15%) |
includes only eyes with visual acuity measurements at 2 years and 3 years.
Visits occurring between 609 and 852 days from randomization were used as completed 2-year visits.
Visits occurring between 1035 and 1156 days from randomization were used as completed 3-year visits.
When more than one visit occurred in either of these windows, data from the visit closest to the target date was used.
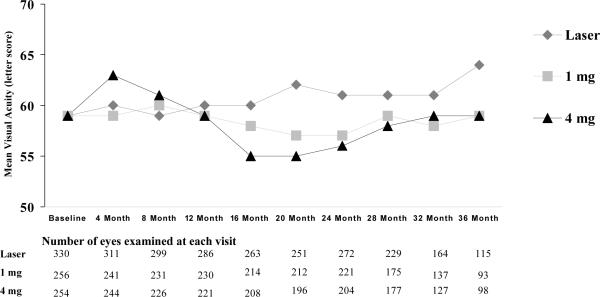
At 3 years, visual acuity outcomes slightly favored the laser group compared with the two triamcinolone groups (Table 3), with the differences between groups at 3 years being of similar magnitude to the differences at 2 years (Figure 1). The mean change in the visual acuity letter score from baseline to 3 years was +5 in the laser group and 0 in the two triamcinolone groups (for the 3 2-group comparisons, mean difference adjusted for baseline visual acuity and prior macular photocoagulation: laser-1mg = +5.6 [95% confidence interval +0.8 to +10.4], laser-4mg = +4.7 [95% confidence interval 0.0 to +9.5] 1mg-4mg = −0.8 [95% confidence interval −6.0 to +4.3]). Using multiple imputation to handle missing data for eyes without 3-year follow up, mean change in the letter score was +2, 0, and −1, respectively and using the last observation carried forward method, mean change in the letter score was +1, −1, and −2, respectively. For the subjects with two study eyes, the mean paired difference in the change in the visual acuity letter score at 3 years was +9.3 (95% confidence interval +2.1 to +16.4) for the laser-1 mg subjects (N=29) and +4.6 (95% confidence interval −6.2 to +15.5) for the laser-4 mg triamcinolone subjects (N=27), in each case favoring the laser group.
Table 3.
Change in Visual Acuity and Retinal Thickness from Baseline to 3 Years*
| Laser | 1 mg | 4 mg | |
|---|---|---|---|
| Change in Visual Acuity from Baseline to 3 Years letter score | N=115 | N=93 | N=98 |
| Mean Change ± Standard Deviation (SD)* | 5 ± 17 | 0 ± 16 | 0 ± 21 |
| Median Change (25th, 75th percentile) | 8 (−2, 15) | 2 (−11, 9) | 4 (−8, 14) |
| Distribution of Change - N (%) | |||
| ≥ 15 letter improvement | 30 (26%) | 19 (20%) | 21 (21%) |
| 14–10 letter improvement | 21 (18%) | 4 (4%) | 16 (16%) |
| 9–5 letter improvement | 21 (18%) | 16 (17%) | 9 (9%) |
| Same ± 4 letters | 24 (21%) | 21 (23%) | 24 (24%) |
| 5–9 letters worse | 5 (4%) | 9 (10%) | 6 (6%) |
| 10–14 letters worse | 5 (4%) | 8 (9%) | 6 (6%) |
| ≥ 15 letters worse | 9 (8%) | 16 (17%) | 16 (16%) |
| Central Subfield on OCT microns | N=111 | N=87 | N=89 |
| Median Thickness at 3 Years (25th, 75th percentile) | 211 (175, 271) | 269 (210, 388) | 248 (195, 342) |
| Mean Change from Baseline ± SD | −175 ±149 | −124 ±184 | −126 ±159 |
| Median Change from Baseline (25th, 75th percentile) | −158 (−273, −75) | −103 (−248, 4) | −114 (−224, −50) |
| < 250 microns at 3 years - N (%) | 75 (68%) | 37 (43%) | 45 (51%) |
| Change in Retinal Volume from Baseline on OCT mm3 | N=54 | N=53 | N=51 |
| Mean Change ± SD | −2.0 ±1.7 | −1.6 ±2.1 | −0.7 ±1.8 |
| Median Change (25th, 75th percentile) | −1.6 (−2.6, −0.7) | −1.1 (−3.0, −0.3) | −0.9 (−1.9, 0.1) |
Visual acuity results includes only eyes with baseline and 3 year visual acuity measurements and OCT results include only eyes with baseline and 3 year OCT measurements
Visits occurring between 1035 and 1156 days from randomization were used as completed 3-year visits.
When more than one visit occurred in this window, data from the visit closest to the 3-year target date were used.
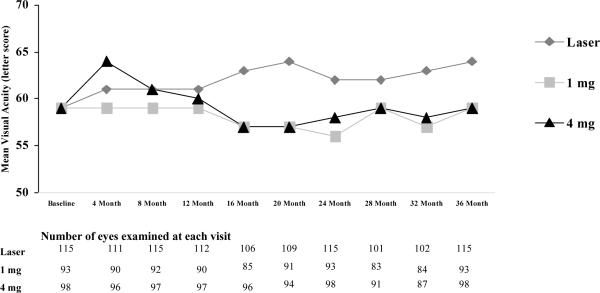
Figure 1.
Mean visual acuity at each visit according to treatment group. Figure 1A: Includes all available data from all randomized eyes. Figure 1B: Includes all available data from the cohort of eyes that completed the 3 year visit.
Among the completers of the 3-year visit, 51 (44%) in the laser group, 23 (25%) in the 1 mg group, and 37 (38%) in the 4 mg group had improvement in the visual acuity letter score of 10 or more from baseline to 3 years and 14 (12%), 24 (26%), and 22 (22%), respectively had worsening of 10 or more letters. For comparison, from baseline to 2 years among the completers of the 3-year visit, the percentages were 33%, 18%, and 32%, respectively improving and 12%, 29%, and 27%, respectively, worsening.
Results of treatment group comparisons were similar when limited to eyes that were either pseudophakic or had minimal lens changes by clinician assessment at 3 years. The mean change in the visual acuity letter score from baseline to 3 years was +5 in the laser group (N=79), +2 in the 1 mg triamcinolone group (N=61), and 0 in the 4 mg triamcinolone group (N=90).
Effect of Treatment on Retinal Thickening
Similar to the visual acuity results, more eyes in all three treatment groups had a decrease in OCT central subfield thickness from year two to year three than had an increase (Table 4, available at www.archophthalmol.com). At three years, central subfield thickness was <250 microns in 75 (67%) eyes in the laser group, 37 (43%) in the 1 mg triamcinolone group, and 45 (51%) in the 4 mg triamcinolone group (Table 3).
Table 4.
Change in Central Subfield Thickness from 2 Years to 3 Years Stratified by 2 Year Central Subfield Thickness*
| 2 Year CSF < 250 microns | 2 Year CSF >= 250 microns | |||||
|---|---|---|---|---|---|---|
| Laser N=61 | 1 mg N=27 | 4 mg N=31 | Laser N=50 | 1 mg N=59 | 4 mg N=54 | |
| <250 microns at 3 years -N (%) | 57 (93%) | 18 (67%) | 23 (74%) | 18 (36%) | 18(31%) | 21 (39%) |
| Change from 2 year to 3 year visit microns | ||||||
| Mean Change ± Standard Deviation (SD) | −3 ± 53 | 12 ± 50 | 27 ± 119 | −79 ±115 | −44 ± 133 | −84 ± 164 |
| Median Change (25th, 75th percentile) | −10 (−26, 3) | −2 (−26, 41) | −3 (−24, 30) | −77 (−155, 2) | −45 (−91, 32) | −53 (−145, −7) |
| Decreased ≥10% and ≥25 microns–N(%) | 17 (28%) | 7 (26%) | 7 (23%) | 30 (60%) | 32 (54%) | 31 (57%) |
| Decreased ≥20% and ≥50 microns–N(%) | 7 (7%) | 1 (4%) | 4 (13%) | 27 (54%) | 20 (34%) | 25 (46%) |
| Increased ≥10% and ≥25 microns–N(%) | 5 (8%) | 9 (33%) | 8 (26%) | 8 (16%) | 14 (24%) | 10 (19%) |
| Increased ≥20% and ≥50 microns–N(%) | 4 (7%) | 6 (22%) | 6 (19%) | 5 (10%) | 7 (12%) | 7 (13%) |
includes only eyes with OCT measurements at 2 years and 3 years.
Visits occurring between 609 and 852 days from randomization were used as completed 2-year visits.
Visits occurring between 1035 and 1156 days from randomization were used as completed 3-year visits.
When more than one visit occurred in either of these windows, data from the visit closest to the target date was used.
Glaucoma and Cataract
Four eyes in the 4 mg triamcinolone group had a procedure for glaucoma prior to the 2-year visit (1 had laser trabeculoplasty and 3 had glaucoma surgery), but there were no additional cases of glaucoma surgery in any treatment group during the third year of follow up. At 3 years, mean intraocular pressure was 16±3 mm Hg in the laser group, 17±3 mm Hg in the 1 mg triamcinolone group, and 16±4 mm Hg in the 4 mg triamcinolone group, with 6 (5%), 14 (15%), and 10 (10%) having an intraocular pressure ≥21 mm Hg. Intraocular pressure lowering medications were being used in 3 (3%), 2 (2%), and 12 (12%) eyes, respectively. Among completers of the 3-year visit, an intraocular pressure increase of ≥10 mm Hg occurred at any visit between baseline and 2 years in 4 (3%) eyes in the laser group, 16 (17%) in the 1 mg triamcinolone group, and 30 (31%) in the 4 mg triamcinolone group, and occurred at any visit between baseline and 3 years in 4%, 18%, and 33%, respectively.
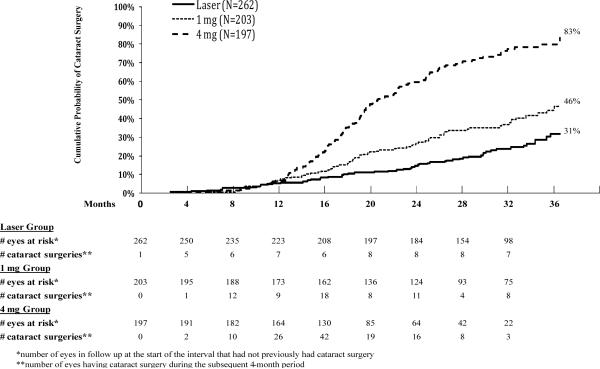
Among phakic eyes at baseline, the three-year cumulative probability of cataract surgery was 31% in the laser group, 46% in the 1 mg group, and 83% in the 4 mg group (P<0.001 for all pairwise comparisons). Excluding eyes in the laser group that received triamcinolone, the cumulative probability was 27%. The timing of the cataract surgery is depicted in Figure 2.
Figure 2.
Cumulative probability of cataract surgery for all eyes phakic at baseline. The “# eyes at risk” indicates the number of eyes still in follow up at the beginning of the interval.
Discussion
In the subset of the originally randomized cohort with DME who completed a third year of follow up, visual acuity improved more often than it worsened and residual macular edema tended to lessen. Treatment group differences seen at two years were in the same direction and of similar magnitude at three years, slightly favoring the laser group.
In the triamcinolone groups, intraocular pressure was generally in the normal range at three years although a greater proportion of eyes in the 4 mg triamcinolone group were being treated with intraocular pressure lowering medications. It is not known whether the intraocular pressure in these eyes would be abnormally high if treatment were discontinued. Similar to the reported findings with corticosteroid implants,9 most eyes treated with 4 mg triamcinolone developed lens changes requiring cataract surgery with the three-year cumulative probability estimated to be 83%.
An issue in the interpretation of the results is the completeness of follow up. The cohort with 3-year follow up was a subset (36%) of the total randomized cohort because a substantial number of subjects enrolled in the study less than 34 months (the open window for the 3 year visit) before the study closeout. Three-year follow up was complete for only 80% of the subjects who did have the potential for three years of follow up. However, the completion rate was similar among the three treatment groups. We evaluated the potential impact of incomplete follow up on the results. It appears that the three-year results likely slightly overestimate the amount of visual acuity improvement from baseline because visual acuity during follow up tended to be slightly worse in those not completing the 3-year visit who had the potential (based on date of randomization) to do so compared with those who completed the 3-year visit. However, there was no indication that the treatment group comparisons were affected by the missing data. Analyses with imputation for missing data gave similar results to analyses of the completed three-year examinations. In view of the smaller sample size than was present for the primary outcome analysis at 2 years, emphasis was placed on determining whether the 3-year treatment group comparison results appeared to be consistent with the 2-year results rather drawing conclusions based on statistical testing.
Our 3 year results analyzed from a subset of the randomized subjects are consistent with the previously published results after 2 years of follow up. There was no long-term benefit of intravitreal triamcinolone relative to focal/grid photocoagulation for patients with DME receiving treatment as performed in this clinical trial. Rather, visual acuity outcomes slightly favored the laser group compared with either of the two triamcinolone groups. It appears that most eyes receiving this 4 mg triamcinolone preparation will require cataract surgery although only a few will develop glaucoma requiring surgery.
Acknowledgments
The funding organization participated in oversight of the conduct of the study and review of the manuscript but not directly in the design of the study, the conduct of the study, data collection, data management, data analysis, interpretation of the data, or preparation of the manuscript.
Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Department of Health and Human Services EY14231, EY14269, EY14229
Footnotes
Allergan, Inc. provided the triamcinolone and topical antibiotics after successfully competing for a request for proposals issued by DRCR.net for a company to provide a preservative-free triamcinolone for the study. As per the DRCR.net Industry Collaboration Guidelines (available at www.drcr.net), the DRCR.net had complete control over the design of the protocol, ownership of the data, and all editorial content of presentations and publications related to the protocol. Allergan, Inc. has provided unrestricted funds to DRCR.net for its discretionary use.
A complete list of all DRCR.net investigator financial disclosures can be found at www.drcr.net
References
- 1.Klein R, Klein BE, Moss SE, et al. The Wisconsin epidemiologic study of diabetic retinopathy .IV. Diabetic macular edema. Ophthalmology. 1984;91(12):1464–74. doi: 10.1016/s0161-6420(84)34102-1. [DOI] [PubMed] [Google Scholar]
- 2.Moss SE, Klein R, Klein BE. Ten-year incidence of visual loss in a diabetic population. Ophthalmology. 1994;101(6):1061–70. doi: 10.1016/s0161-6420(94)31217-6. [DOI] [PubMed] [Google Scholar]
- 3.Moss SE, Klein R, Klein BE. The 14-year incidence of visual loss in a diabetic population. Ophthalmology. 1998;105(6):998–1003. doi: 10.1016/S0161-6420(98)96025-0. [DOI] [PubMed] [Google Scholar]
- 4.Diabetic Retinopathy Clinical Research Network A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115(9):1447–9. e1–10. doi: 10.1016/j.ophtha.2008.06.015. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ip MS, Bressler SB, Antoszyk AN, et al. A randomized trial comparing intravitreal triamcinolone and laser photocoagulation for diabetic macular edema: Baseline features. Retina. 2008;28(7):919–30. doi: 10.1097/IAE.0b013e31818144a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diabetic Retinopathy Clinical Research Network Comparison of the modified Early Treatment Diabetic Retinopathy Study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol. 2007;125(4):469–80. doi: 10.1001/archopht.125.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135(2):194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 8.Lee EW, Wei LJ, Amato DA. Cox-type regression analysis for large numbers of small groups of correlated failure time observations. Kluwer Academic; Dordrecht: 1992. pp. 237–47. [Google Scholar]
- 9.Callanan DG, Jaffe GJ, Martin DF, et al. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Arch Ophthalmol. 2008;126(9):1191–201. doi: 10.1001/archopht.126.9.1191. [DOI] [PubMed] [Google Scholar]