Abstract
This technical note describes a modification to a fundus camera that permits simultaneous recording of pattern electroretinograms (pERGs) and pattern visual evoked potentials (pVEPs). The modification consists of placing an organic light-emitting diode (OLED) in the split-viewer pathway of a fundus camera, in a plane conjugate to the subject’s pupil. In this way, a focused image of the OLED can be delivered to a precisely known location on the retina. The advantage of using an OLED is that it can achieve high luminance while maintaining high contrast, and with minimal degradation over time. This system is particularly useful for animal studies, especially when precise retinal positioning is required.
Keywords: Fundus camera, Organic light-emitting diode, Pattern electroretinogram, Pattern visual evoked potential
Introduction
Pattern-evoked potentials can be difficult to obtain in animal studies, because the stimulus must be focused on the retina for accurate interpretation. The problem is compounded when control of the locus of stimulation is important, such as, when the measurement of function at a specific area of the visual field is desired. One solution to the problem of focus has been to derive the animal’s manifest refraction and correct for optical errors using lenses of the appropriate strength, e.g., [1]; however, a manifest refraction may not yield the optimal refraction because the surface of reflection in a manifest refraction may not be co-planar with the photoreceptor layer, and there is still the problem of where exactly the animal is looking. Fortune and colleagues found that they could reliably estimate the animal’s gaze by recording a multifocal ERG and locating the highest response density; however, this technique takes time and assumes normal retinal function. Johnson et al. [2] developed a technique in which the image from an external CRT monitor was collimated and refocused to the conjugate pupil plane of the split-viewer pathway of a fundus camera. This technique ensured that when the fundus was in focus in the camera, the stimulus was in focus on the retina and, additionally, was centered in the area viewed. Unfortunately, variations in the animal’s gaze occurring after the experiment began necessitated moving the entire optical bench containing the CRT. Consequently, the animal had to be paralyzed under anesthesia for the duration of the experiment.
Recent technologic advances have dramatically simplified this technique. Herein, we describe minor modifications to a fundus camera that allow anyone with a vision electrophysiologic recording system having two input channels to record pattern electroretinograms (PERGs) and pattern visual-evoked potentials (VEPs) simultaneously in animals with a minimum dilated pupil size of 4 mm.
Methods
All animal studies abided by the guidelines of the ARVO Statement for the use of animals in ophthalmic and visual research, and were approved by our institutional IACUC.
A TRC-50IA Topcon fundus camera (TOPCON corporation, Tokyo, Japan) was altered by attaching a 0.61 inch diagonal (12.78 × 9.0 mm) organic light-emitting display (OLED; eMagin corp., Bellevue, WA, USA) to the split-viewer pathway. The display had a resolution of 600 × 800 pixels. Although any small display can be used for this purpose, an OLED has the advantages of maintaining a very high luminance, while preserving high contrast. This is because OLEDs are emissive devices, creating their own light when a current is applied. The high resolution monochrome OLED used in this study is comprised of over a million of individual diodes, each of which acts as an independent light source, emitting close to the entire visible spectrum of light. Color OLEDs are currently capable of delivering more than 24 bits of color. The individual pixels refresh in nanoseconds, depending on the display refresh rate, which can vary from 30 to 85 Hz.
The OLED used in this study had a maximum luminance of 2000 cd/m2, adjustable in 256 steps, and a maximum contrast ratio of >800:1. Both of these variables were adjusted by computer, using accompanying software and eMagin’s interface design reference kit. The reference kit connects to a personal computer via an RS232 cable. Contrast and luminance were calibrated using a Minolta chroma meter CS-100A (Minolta Corp, Ramsey, NJ USA).
OLEDs are very stable, being capable of hundreds of thousands of hours of continuous operation with little image degradation. The half-life of the OLED used for this application is 320,000 h, when run at maximum voltage.
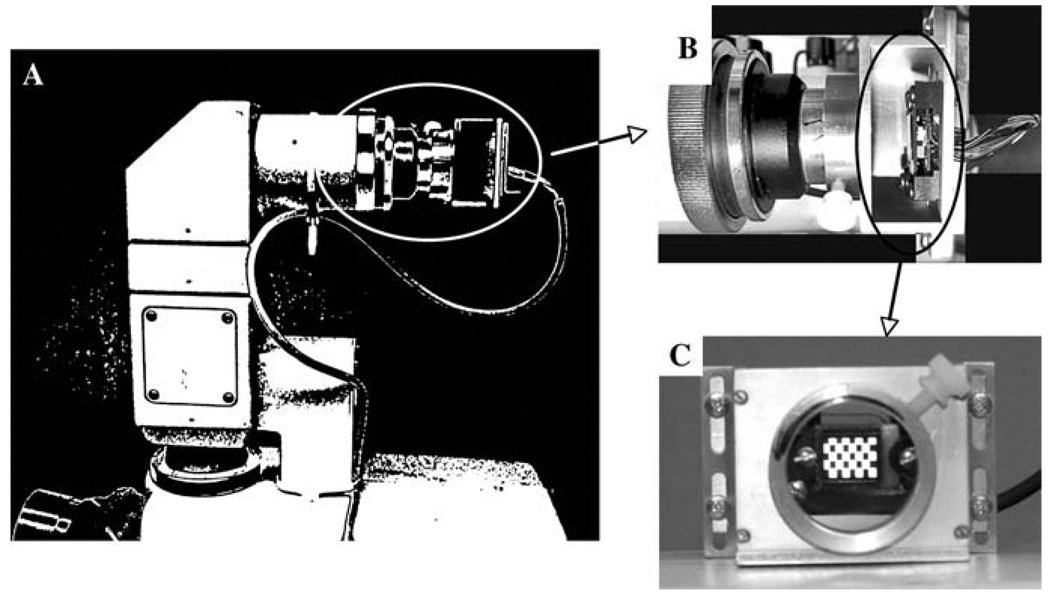
The OLED and its circuit board were attached by screws to an aluminum sleeve that was machined to fit over the entrance port of the split-viewer pathway (see Fig. 1c). The sleeve was slid along its z-axis until the OLED was focused in the plane conjugate to the animal’s pupil (Fig. 1b), and its position was maintained at this location using a set screw. Alignment was verified by dilating one of the author’s (MAJ) pupils; she then confirmed the location of the OLED after an image of her fundus had been focused.
Fig. 1.
a Fundus camera with OLED monitor and circuit board connected to the split-viewer pathway. b Exploded image of OLED mounted to fundus camera (circled area in a). c View of OLED, showing an 8 × 8 checkerboard
The input to the OLED was an alternating black-and-white checkerboard pattern generated by the VGA-compatible graphics board of a UTAS (LKC Technologies, Inc., Gaithersburg, MD, USA) electrodiagnostic recording system.
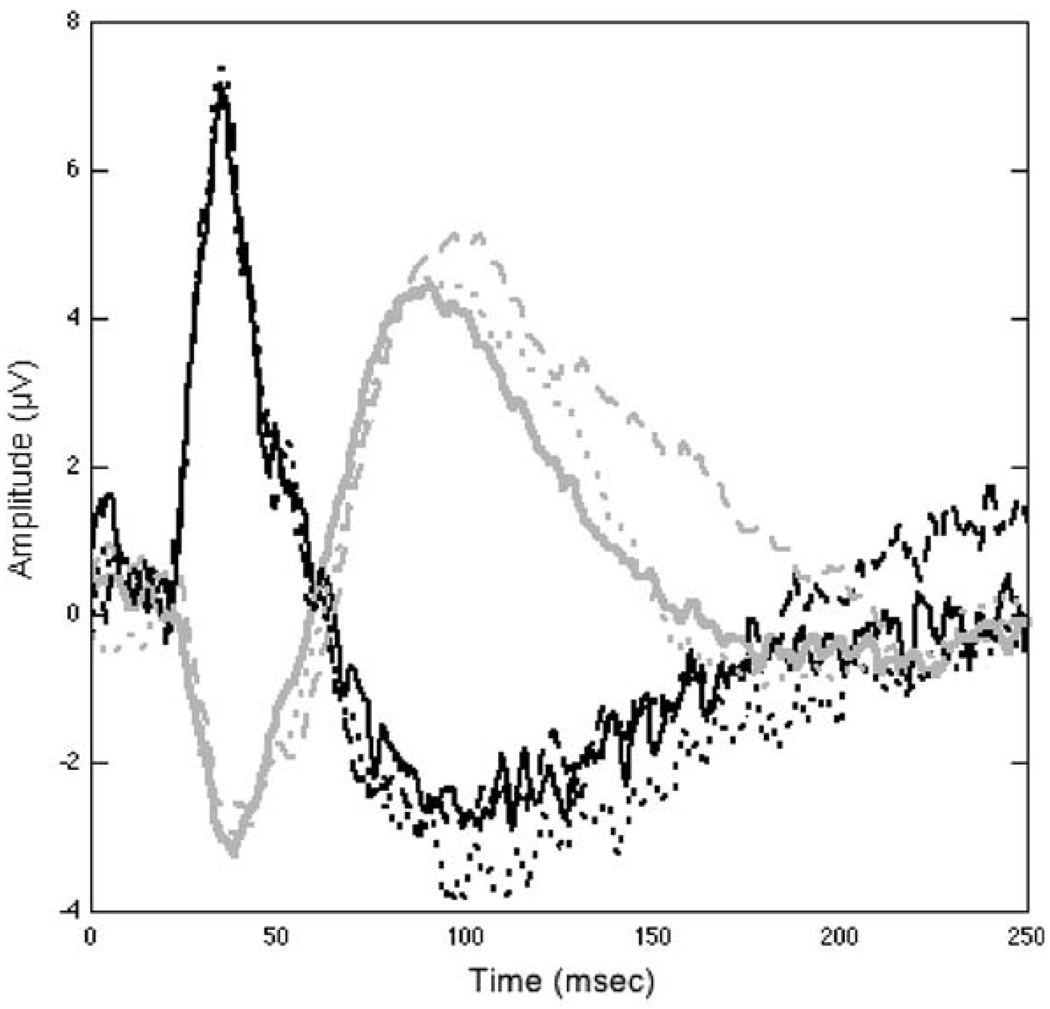
Figure 2 is an example of simultaneous PERGs and VEPs recorded from the normal eye of a rhesus monkey. A Burian–Allen bipolar contact lens, custom designed for the rhesus monkey eye by Hansen Labs (Coralville, IA, USA) was used to record the PERG, and subcutaneous needle electrodes (Advena Ltd., Herefordshire, UK) were inserted at Oz and Fz for recording of the pattern VEP. A subcutaneous electrode on the forearm served as ground for both signals, which were connected to input channels 1 and 2, respectively.
Fig. 2.
Three PERG (black lines) and VEP (gray lines) waveforms recorded from one eye of a rhesus macaque, using a 2 Hz alternating 8 × 8 (6°19′) checkerboard, which had a luminance of 150 cd/s2 and a contrast approaching 100%. The PERG, VEP pairs having the same line type were simultaneously recorded
Discussion
Even in the best experimental preparations, positional stability can be a problem. This system allows precise stimulus alignment and focus in anesthetized animals. However, eye movements can still occur during experimentation, depending on the idiosyncrasies of the particular animal and depth of anesthesia. We were alerted to a possible shift in eye position or loss in image clarity when a loss in amplitude of the pERG or VEP occurred during the session, as these two tests are very sensitive to focus and eccentricity. At that point in the experiment, the fundus was re-imaged and the animal dark-adapted again. However, insertion of an infrared source into the main optical pathway of the fundus camera will permit frequent monitoring of the preparation without the need to dark-adapt the animal. Kondo and colleagues [3] recently have described a method of monitoring eye position during focal retinal stimulation using an infrared light source and fundus camera.
We are currently using our system to record simultaneous PERGs and VEPs from rhesus monkeys in which experimental non-arteritic anterior ischemic optic neuropathy has been induced in one eye. We have recently reported early results using a similar system [4]. We hope that the ability to precisely and non-invasively determine the differential effects of therapies on ganglion cells and their axons will accelerate the identification of promising candidates for clinical trials.
Acknowledgments
We thank the Donegal Foundation whose contributions helped to make this study possible.
Glossary
Abbreviations
- CRT
Cathode ray tube
- OLED
Organic light-emitting diode
- PERG
Pattern electroretinogram
- VEP
Pattern visual evoked potential
Contributor Information
Mary A. Johnson, The Department of Ophthalmology and Visual Sciences, The University of Maryland School of Medicine, Baltimore, MD 21201, USA, mjohnson@umaryland.edu
Bernard J. Slater, The Department of Ophthalmology and Visual Sciences, The University of Maryland School of Medicine, Baltimore, MD 21201, USA
Neil R. Miller, The Wilmer Eye Institute, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA
Steven L. Bernstein, The Department of Ophthalmology and Visual Sciences, The University of Maryland School of Medicine, Baltimore, MD 21201, USA
Robert W. Flower, The Department of Ophthalmology and Visual Sciences, The University of Maryland School of Medicine, Baltimore, MD 21201, USA
References
- 1.Fortune B, Cull G, Wang L, Van Buskirk EM, Cioffi GA. Factors affecting the use of multifocal electroretinography to monitor function in a primate model of glaucoma. Doc Ophthalmol. 2002;105(2):151–178. doi: 10.1023/a:1020548919355. [DOI] [PubMed] [Google Scholar]
- 2.Johnson MA, Drum BA, Quigley HA, Sanchez RM, Dun-kelberger GR. Pattern-evoked potentials and optic nerve fiber loss in monocular laser-induced glaucoma. Invest Ophthalmol Vis Sci. 1989;30(5):897–907. [PubMed] [Google Scholar]
- 3.Kondo M, Kurimoto Y, Sakai T, Koyasu T, Miyata K, Ueno S, Terasaki H. Recording focal macular photopic negative response (PhNR) from monkeys. Invest Ophthalmol Vis Sci. 2008;49(8):3544–3550. doi: 10.1167/iovs.08-1798. [DOI] [PubMed] [Google Scholar]
- 4.Chen CS, Johnson MA, Flower RW, Slater BJ, Miller NR, Bernstein SL. A primate model of nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2008;49(7):2985–2992. doi: 10.1167/iovs.07-1651. Epub Mar 7, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]