Abstract
Cancer is a multistep process involving cooperation between oncogenic or tumor suppressor mutations and interactions between the tumor and surrounding normal tissue. Here we present the first description of cooperative tumorigenesis in Drosophila, by using a system that mimics the development of tumors in mammals. We have used the MARCM system to generate mutant clones of the apical–basal cell polarity tumor suppressor gene, scribble, in the context of normal tissue. We show that scribble mutant clones in the eye disc exhibit ectopic expression of cyclin E and ectopic cell cycles, but do not overgrow due to increased cell death mediated by the JNK pathway and the surrounding wild-type tissue. In contrast, when oncogenic Ras or Notch is expressed within the scribble mutant clones, cell death is prevented and neoplastic tumors develop. This demonstrates, for the first time in Drosophila, that activated alleles of Ras and Notch can act as cooperating oncogenes in the development of epithelial tumors, and highlights the importance of epithelial polarity regulators in restraining oncogenes and preventing tumor formation.
Keywords: cell cycle/cell death/cell polarity/cooperative tumorigenesis/Drosophila
Introduction
Drosophila has long been recognized as a valuable tool for understanding many aspects of cancer biology, mainly because of the high degree of conservation in signaling pathways between Drosophila and mammals, and the ease with which genetic analyses can be carried out in the fly. Studies on a group of three Drosophila genes, discs large (dlg), lethal giant larvae (lgl) and scribble (scrib), are now beginning to highlight the critical relationship that exists between loss of epithelial cell polarity and tumor development. Homozygous mutants of dlg, lgl or scrib all exhibit very similar phenotypes. Larvae develop normally; however, as maternal supplies of these proteins are exhausted, cells within the normally monolayered epithelial imaginal discs lose structure and polarity, fail to differentiate and overproliferate to become multilayered amorphous masses of cells that fuse with adjacent tissues (reviewed by Wodarz, 2000). The larvae are unable to initiate pupal development and eventually die as giant overgrown larvae. The three-dimensional and invasive overgrowth exhibited by the mutant tissue, which fails to differentiate properly and lacks morphology, has led to the classification of these three genes as Drosophila neoplastic tumor suppressors (Gateff, 1994). Detailed analysis has revealed that dlg, lgl and scrib participate in a common genetic pathway involved in the regulation of both cell polarity and proliferation (Bilder et al., 2000). Scrib is a LAP4 protein (16 leucine-rich repeats with four PDZ domains; Bilder and Perrimon, 2000) and Dlg a MAGUK (membrane-associated guanylate kinase) containing three PDZ domains (Woods and Bryant, 1991). In epithelia, both proteins normally localize to a lateral domain of the cell membrane, just basal to the adherens junction, known as the septate junction, a solute barrier thought to be analogous to the tight junction of mammalian cells. Lgl is a cytoplasmic and cortically localized protein with WD40 repeats. In epithelia lacking Scrib, apically localized proteins become redistributed over the baso-lateral surface, and spot adherens junctions remain scattered rather than coalescing into the defined lateral band of the zonula adherens (Bilder and Perrimon, 2000). It is thought that this disruption to cellular architecture perturbs signaling pathways, resulting in a loss of proliferation control.
At present, it is not known if human homologs of Scrib (hScrib or Vartul), Dlg (a family of four proteins; hDlg/SAP97, PSD-93, NE-Dlg/SAP102 and PSD-95/SAP90) and Lgl (Hugl) are tumor suppressors in mammalian cells; however, both hScrib and hDlg are targeted for degradation by the E6 oncoprotein of high-risk human papillomaviruses (Gardiol et al., 1999; Nakagawa and Huibregtse, 2000) and, at least in MDCK cells, E6 expression is accompanied by a loss of tight junction integrity (Nakagawa and Huibregtse, 2000). This suggests that the loss of these proteins may have important functional consequences for cell polarity, and hence may contribute to neoplasia progression by the E6 oncoprotein.
In this study, we use an in vivo Drosophila model that more closely resembles the clonal nature of mammalian cancer to investigate the role of cell polarity, and Scrib in particular, in tumor development. In mammals, tumorigenesis involves cooperative interactions between tumor suppressor genes and oncogenes (reviewed by Hanahan and Weinberg, 2000), as well as complex interactions between the overproliferating tumor itself and the surrounding stroma (Bissell and Radisky, 2001; Liotta and Kohn, 2001). Models of tumor development in flies must therefore attempt to mimic these added levels of complexity. Using an FLP/FRT-mediated clonal analysis, we show that clones of scrib– tissue within an otherwise wild-type animal lose polarity and overproliferate; however, the surrounding wild-type tissue ensures that this proliferative advantage is compensated for by Jun N-terminal kinase (JNK) pathway-mediated apoptosis of the mutant tissue. If secondary mutations of activated oncogenes, in particular Ras and Notch, are introduced into the scrib– clones, this fate is avoided, resulting in unrestrained tissue overgrowth. The cooperative effect of activated Ras or Notch on scrib– tissue cannot be explained solely by the ability of these oncogenes to promote cell survival or cell cycle progression, suggesting that other downstream targets are required for their tumorigenic effects. This study therefore provides the first demonstration of cooperative tumorigenesis in Drosophila, and shows how defects in cell polarity could cooperate with oncogenes in the development of cancer.
Results
Somatic clones of scrib mutant tissue in the eye disc lose polarity and overproliferate
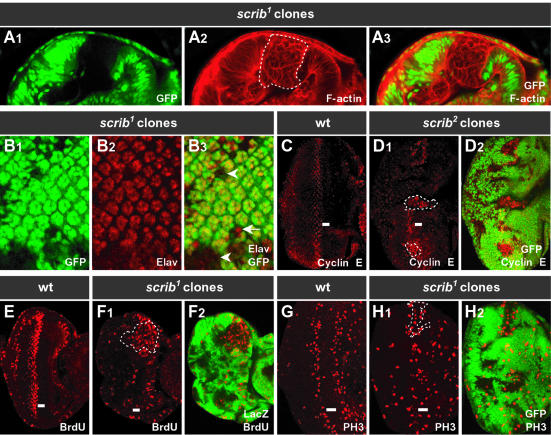
The absence of Scrib in homozygous scrib– larvae causes a loss of cell polarity and excessive cell proliferation of larval wing imaginal discs and brain lobes, resulting in the formation of neoplastic tumors (Bilder et al., 2000). We wished to examine the effects of removing Scrib in somatic clones of tissue within a wild-type tissue context, a situation more akin to the clonal development of mammalian tumors. For this analysis, we used the larval eye imaginal disc, a columnar, monolayered neuroepithelium. During late larval and early pupal stages of development, the morphogenetic furrow (MF) moves across the eye disc (posterior to anterior), inducing cells to differentiate into the photoreceptors and accessory cells that make up the mature ommatidia of the adult eye. Using FRT-mediated recombination and FLPase expressed from the eyeless (ey) promoter (Newsome et al., 2000), mitotic clones of scrib– tissue were induced early in eye development and examined at the late larval third instar stage. In clones of scrib– tissue [scrib1 and scrib2; both null alleles marked by the absence of green fluorescent protein (GFP) in Figure 1, but positively marked by GFP expression in subsequent figures], cells lost their monolayered and columnar shape to become rounded and multilayered (Figure 1A). A marker of photoreceptor differentiation, Elav, showed that many scrib– cells, but not all, were defective in their ability to initiate differentiation (Figure 1B). These effects were not strictly cell autonomous, as the regular spacing of ommatidia in the wild-type tissue immediately surrounding scrib– clones was also often disrupted.
Fig. 1. Somatic eye clones of scrib mutant tissue become multilayered and overproliferate. (A) Cross-section and (B–H) planar-sections, anterior to the right, through the monolayered epithelium of third instar larval eye discs. ey-FLP scrib1 or scrib2 mutant clones (sometimes outlined by dashed lines) are marked by the absence of GFP, except for BrdU detection (E and F) in which mutant clones are marked by the absence of LacZ staining. Similar results were obtained with both scrib1 and scrib2 alleles. In the third instar larval eye disc, the morphogenetic furrow (MF; indicated by a bar), having initiated from the posterior edge of the disc, has progressed half way across, inducing cells to differentiate behind it (posterior). (A) Phalloidin staining for F-actin (A2 and 3) shows the columnar epithelium of the wild-type eye disc tissue (GFP-positive), and the multilayered, rounded cells of scrib– tissue (GFP-negative). (B) Developing photoreceptor cells, marked by Elav staining (B2 and 3), are still able to differentiate in scrib– clones (arrow); however, some scrib– cells remain undifferentiated (arrowhead). There is also disruption to the regular spacing of ommatidial clusters, which extends into the wild-type tissue. (C–H) In a wild-type eye disc, cyclin E is expressed in a band of cells just posterior to the MF (C), which undergo a synchronous S phase (E; BrdU incorporation), followed by mitosis (G; phospho-histone H3 staining). In scrib– clones, and in immediately adjacent wild-type cells, cyclin E levels are elevated, particularly within and anterior to the MF (D), resulting in ectopic DNA replication (F) and mitoses (H).
We next determined if cell proliferation control was defective in scrib– clones. Normally, cell proliferation is tightly regulated during third instar larval eye development. Cells in the anterior of the eye disc, which are undifferentiated, cycle asynchronously, until arresting in G1 within the MF. Subsets of cells initiate differentiation within the MF, and the remainder express a pulse of cyclin E (Figure 1C) and undergo a synchronous S phase (Figure 1E) just posterior to the furrow. Most of these G2 cells will then also progress into mitosis (Figure 1G), and the resulting pool of cells is recruited into the developing ommatidia. Levels of cyclin E were elevated within scrib– clones (marked by the absence of GFP), as well as in wild-type cells immediately surrounding the clones, and this was most apparent within the zone where cyclin E is usually expressed just posterior to the MF and extended further anteriorly through the MF where cells normally arrest in G1 (Figure 1D). This was accompanied by ectopic S phases as revealed by bromodeoxyuridine (BrdU) staining (Figure 1F), as well as ectopic mitoses (Figure 1H).
We conclude that during eye development, clones of scrib– tissue lose their regular monolayered columnar shape, become multilayered and rounded, and undergo ectopic cell proliferation. Non-cell-autonomous defects in both photoreceptor differentiation and ectopic cyclin E expression are also observed within wild-type tissue immediately surrounding scrib– clones.
Clones of scrib mutant tissue are eliminated by JNK pathway-mediated apoptosis
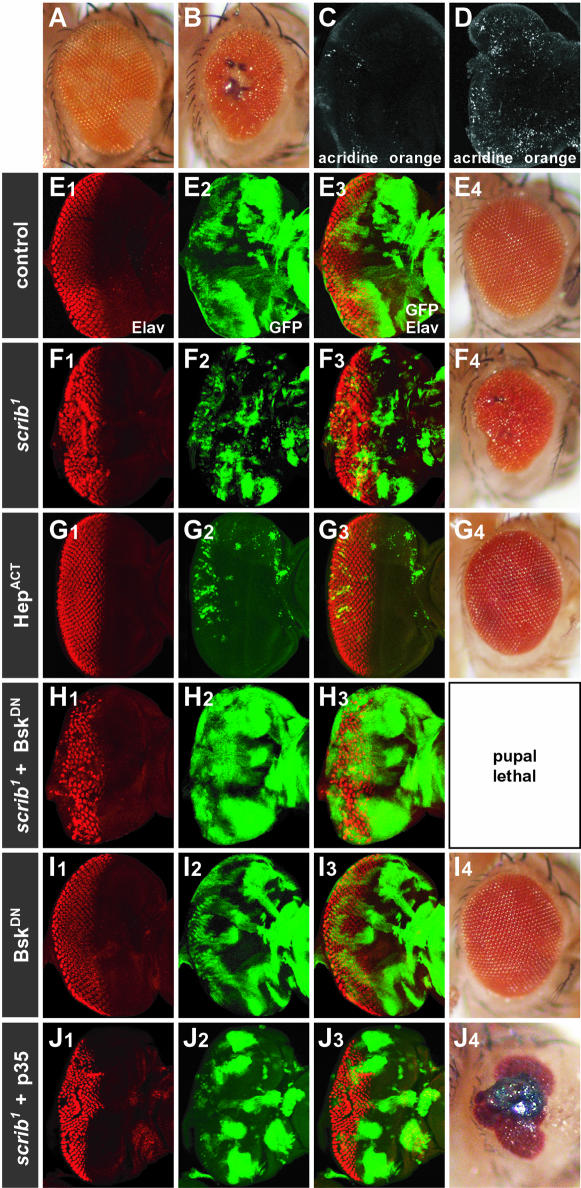
Despite increased proliferation of scrib– tissue during third instar larval development, this tissue must eventually be removed by cell death, since by the time the larvae eclose after pupation, little scrib– tissue remains in the adult eye (marked as w– tissue lacking red pigment), and there are signs of necrosis, especially in the middle of the eye (Figure 2B, compared with wild-type eye mosaic in Figure 2A). Indeed, even during third instar larval eye disc development, there is considerable apoptosis (Figure 2D compared with a wild-type disc in Figure 2C), and scrib– tissue (GFP positive), which should account for 50% of the eye antennal disc, is much less represented than wild-type tissue, particularly posterior to the MF where differentiation is occurring (Figure 2F compared with a control mosaic eye disc, Figure 2E).
Fig. 2. Clones of scrib mutant eye tissue are eliminated by JNK pathway-mediated apoptosis. (A and B) By using a w+ gene to mark wild-type tissue in the adult eye, an ey-FLP control eye incorporates an equal amount of w+ and w– tissue (A). An ey-FLP scrib1 mosaic eye incorporates little scrib– tissue (w–) in the adult, the eyes are reduced in size and necrotic spots are often observed in the centre of the eye (B). (C and D) Acridine orange staining of third instar larval eye discs reveals few apoptotic cells in a wild-type eye/antennal disc (C), but in an ey-FLP scrib1 mosaic eye/antennal disc (D) many apoptotic cells are detected. (E–J) The MARCM system was used to generate ey-FLP clones expressing different transgenes, with GFP as a positive clonal marker, in either wild-type control clones (E, G and I) or scrib1 clones (F, H and J). Developing photoreceptor cells in third instar larval eye discs are shown by Elav staining. In wild-type clones, only expressing GFP as a clonal marker, clonal tissue makes up ∼50% of the tissue (E2 and 3) and the adult eyes are normal (E4). In scrib1 clones, mutant tissue is significantly less represented than wild-type tissue (F2 and 3), and the adult eyes are often reduced in size and disorganized (F4). Ectopic activation of the JNK pathway by expressing an activated allele of JNKK (HepACT) in control wild-type clones (G1–3) nearly eliminates all clonal tissue, resulting in slightly roughened adult eyes (G4). Blocking the JNK pathway, by expressing a dominant-negative form of JNK (BskDN), in scrib1 clones (H1–3) causes a pronounced expansion of the scrib– tissue in the eye disc (H2; compared with scrib1 clones, F2), and complete pupal lethality. Expression of BskDN in control clones (I1–3) results in adult flies with only slight eye disorganization (I4). The expression of the pan-caspase inhibitor, p35, in scrib1 mutant clones (J1–3) results in an increase in the size of scrib– clonal tissue compared with clones of scrib1 alone (F2), and the eyes of the resulting adult flies are more disorganized and necrotic (J4) compared with scrib1 clones alone (F4).
In both mammals and Drosophila, JNK can induce a stress response leading to apoptosis (reviewed by Davis, 2000). We therefore wished to test whether ectopic activation of the JNK pathway in scrib– eye clones could account for the removal of the scrib– tissue. To do this, we used the MARCM system, a method for ectopically and constitutively expressing any GAL4-dependent (UAS-) transgene, just within mitotic clones of tissue (marked by UAS–GFP expression), and not in the surrounding wild-type tissue (Lee and Lou, 1999; see Materials and methods for a more detailed description of the system). Using this technique, with ey-FLP to generate the clones, we observed that the ectopic expression of an activated form of JNKK (Hemipterous), within GFP-expressing clones of otherwise wild-type tissue, resulted in very small clones, consistent with a pro-apoptotic role for the JNK pathway in the eye disc (Figure 2G; HepACT). To test if activation of the JNK pathway was responsible for the removal of scrib– tissue, we blocked JNK pathway activity by expressing a dominant-negative version of the Drosophila JNK homolog, Basket (BskDN), specifically within scrib– eye clones. This resulted in a significant increase in the size of the scrib– clonal tissue (GFP-positive cells, Figure 2H), compared with scrib– control clones (Figure 2F), consistent with the notion that BskDN rescues cell death of scrib– tissue. Furthermore, while the expression of BskDN in control eye clones resulted in adult flies with only mild eye disorganization (Figure 2I), expression of BskDN in scrib– cells resulted in pupal lethality. In contrast, using the MARCM system to downregulate other signaling pathways in scrib– clones, including blocking epidermal growth factor receptor (EGFR)/Ras signaling with a dominant-negative EGFR, Wingless (Wg) signaling with a dominant-negative form of TCF, or Decapentaplegic (Dpp) signaling with ectopic expression of Dad, had the opposite effect, dramatically reducing the viability of the mutant tissue (data not shown). Thus blocking the JNK pathway, specifically, led to increased viability of scrib– tissue. In fact, the effects of downregulating the JNK pathway were even more potent than those achieved by blocking apoptosis with the baculovirus pan-caspase inhibitor, p35. Although expressing p35 in scrib– eye clones also increased the size of the mutant tissue, some flies were capable of eclosing, with eyes showing signs of considerable necrosis (Figure 2J).
We therefore conclude that although the loss of Scrib is associated with ectopic cell proliferation, a JNK-mediated apoptotic response ensures that the mutant tissue does not overgrow.
The surrounding wild-type tissue is important in limiting the overgrowth of scrib mutant clones
The removal of scrib– tissue by JNK-mediated apoptosis could be a purely cell-intrinsic response to a general loss of cell polarity and detachment from the basement membrane, or, alternatively, it could be regulated by the surrounding wild-type tissue context acting to limit the overgrowth of aberrant cells. To distinguish between these two possibilities, we made use of two different genetic techniques to remove the wild-type eye disc tissue surrounding the scrib– clones. First, we expressed the cell death inducer, Hid, from an eye disc driver (GMR), specifically in the differentiating wild-type tissue posterior to the MF. Strikingly, removing the wild-type tissue dramatically increased the viability of scrib– clones (GFP-positive cells) posterior to the MF (Figure 3A compared with Figure 2F). This resulted in tissue overgrowth and lethality. In a second, and more extreme experimental option, we removed not just the wild-type tissue posterior to the MF, but nearly all of the wild-type tissue in the eye/antennal disc by using a homozygous cell lethal mutation [l(3)cl-R31; Newsome et al., 2000]. Similarly, in this context, the scrib– tissue (GFP-positive) was not eliminated by apoptosis but, instead, overproliferated, losing recognizable structure (Figure 3B, and data not shown), resulting in lethality.
Fig. 3. The surrounding wild-type tissue limits the overgrowth of the scrib mutant tissue. (A) scrib1 mosaic eye discs were generated, and the remaining wild-type tissue posterior to the MF was eliminated by expressing the cell death inducer, Hid, from the GMR promoter. The scrib1 clonal tissue, marked by the expression of GFP, is not eliminated posterior to the MF. Elav staining marks developing photoreceptor cells. (B) scrib1 mosaic eye discs were generated, and the surrounding wild-type tissue eliminated by the presence of a homozygous cell-lethal mutation. The scrib1 clonal tissue, marked by the expression of GFP, overgrows in three dimensions, with limited differentiation as judged by Elav staining of developing photoreceptor cells.
Taken together, these data indicate that the surrounding wild-type tissue is responsible for preventing overgrowth of the scrib– tissue through the induction of JNK-mediated cell death.
Oncogenic forms of Ras and Notch cooperate with scrib mutant clones to result in dramatic overgrowth
Clones of scrib– tissue share many characteristics of mammalian tumors, including loss of cell polarity, defective differentiation capacity and ectopic cell proliferation; however, scrib– tissue overgrowth is kept in check by a propensity to undergo JNK pathway-mediated apoptosis. We therefore wondered what the consequences would be of using the MARCM system to express secondary oncogenic mutations in the scrib– background.
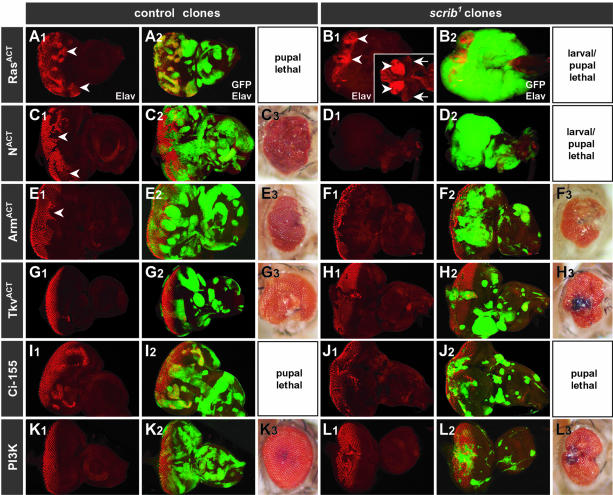
Most striking were the effects of ectopically expressing activated alleles of Ras (RasACT) or Notch (NACT) in scrib– clones (Figure 4B and D). Both of these oncogenes were capable of dramatically inducing the overgrowth of the scrib– tissue (GFP-positive); the wild-type tissue was outcompeted, and the mutant tissue failed to differentiate and continued to grow in three dimensions to many times the normal size of the eye/antennal disc, fusing with the brain lobes and other imaginal tissues. Many larvae failed to pupate, and grew to resemble the giant larvae of homozygous scrib– larvae. Importantly, although the ectopic expression of either RasACT or NACT in otherwise wild-type clones of tissue exerted hyperproliferative effects and resulted in pupal lethality, neither gene caused the massive synergistic tissue overgrowth observed in the absence of Scrib (Figure 4A and C).
Fig. 4. Ectopic activation of Ras and Notch signaling pathways, but not Wg, Dpp, Hh or PI3 kinase pathways, in scrib mutant eye clones induces massive cooperative overgrowth. (A–L) The MARCM system was used ectopically to activate the EGFR/Ras pathway (RasACT; A and B); Notch pathway (NACT; C and D); Wg pathway (ArmACT; E and F); Dpp pathway (TkvACT; G and H); Hh pathway (Ci-155; I and J); or PI3 kinase pathway (PI3K; K and L) in either wild-type control eye clones (A, C, E, G, I and K) or scrib1 clones (B, D, F, H, J and L). All clones are marked by the expression of GFP. Developing photoreceptors are shown by Elav staining (A1–L1; merged with GFP in A2–L2). The resulting adult eyes (A3–L3), or pharate adults dissected from the pupal case (C3), are shown where applicable. Expression of RasACT in control clones (A) induces precocious photoreceptor differentiation anterior to the MF (arrowhead). In scrib1 clones (B), RasACT fails to initiate differentiation, and instead, massive three-dimensional tissue overgrowth is induced, resulting in fusion of the eye/antennal discs to each other as well as the brain lobes (arrowheads). The insert (B1) shows wild-type brain lobes (arrowheads) and eye antennal discs (arrows) at the same magnification (magnification is half that of the other panels in this figure) for comparison. Expression of NACT in control clones (C) interferes with photoreceptor differentiation (arrowheads), and adult flies die before eclosure with overgrown eyes. In scrib– clones (D), NACT induces a similar degree of three-dimensional overgrowth, and lack of differentiation, as RasACT (a planar section is shown, but overgrowth is in three dimensions). Ectopic activation of Wg pathway signaling effectively blocks photoreceptor differentiation in control clones (E; arrowhead), and this remains the case in scrib1 clones (F). Ectopic activation of Dpp or Hh signaling induces patterning defects in both control clones (G and I), as well as scrib1 clones (H and J). Ectopic PI3 kinase signaling in scrib1 clones (L) has little effect.
The consequences of ectopically activating the major growth and patterning pathways of Hedgehog (Hh), Dpp and Wg in scrib– clones (GFP-positive) were very different from the effects of RasACT and NACT. An oncogenic, activated form of β-catenin (ArmACT) which mimics ectopic Wg pathway signaling effectively blocked photoreceptor differentiation in control clones and maintained the tissue in a proliferative state (Figure 4E), but in scrib– clones, although photoreceptor differentiation remained blocked by the expression of ArmACT, the scrib– tissue did not exhibit unrestrained overgrowth, and occasionally adult flies eclosed, albeit with severely disorganized eyes (Figure 4F). Activation of either Dpp (TkvACT; Figure 4G and H) or Hh (Ci-155; Figure 4I and J) signaling also caused patterning defects, but even ectopic Hh signaling in scrib– clones, which resulted in pupal lethality, did not induce the strong cooperative overgrowth effect with scrib– observed with either RasACT or NACT. Furthermore, while the ectopic activation of Wg, Dpp or Hh signaling pathways in scrib– clones generally increased the size of the clonal tissue, this was not with the same consistency, nor extent, as that induced by blocking the JNK pathway.
In summary, these data demonstrate that extensive tumor overgrowth can be induced in Drosophila by cooperative interactions between the loss of the tumor suppressor, scrib, and, specifically, the oncogenic forms of Ras or Notch.
The effects of activated Ras on scrib mutant tissue overgrowth are not solely a consequence of blocking apoptosis and enhancing cell cycle progression
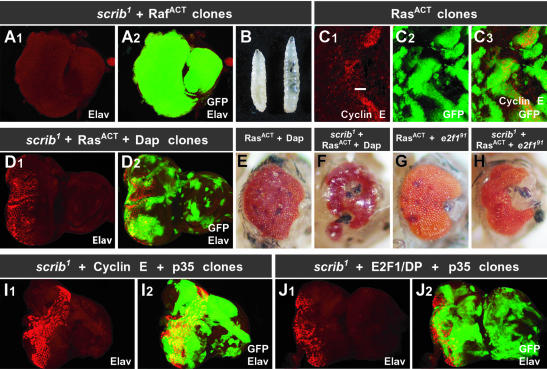
We chose to focus on the effects of RasACT on scrib– tissue in more detail to determine which effectors of Ras might be responsible for the synergistic overgrowth observed in combination with the absence of Scrib. In mammals, oncogenic Ras is thought to exert its effect through a number of different effectors including Raf and the canonical mitogen-activated protein kinase (MAPK) pathway, the growth regulator phosphatidylinositol 3-kinase (PI3 kinase) and cell architecture regulators such as Ral and Rho family members (reviewed by Shields et al., 2000). However, in the Drosophila eye disc, ectopic expression of PI3 kinase in scrib– clones did not mimic the effects of activated Ras (Figure 4L), nor could activated alleles of the cytoskeletal regulators, Ral, Rho, Rac and Cdc42 (data not shown). Thus it seemed likely that downstream targets of the MAPK pathway would be responsible for the effects of RasACT. Indeed, expression of a gain-of-function allele of Raf (RafACT) in scrib– clones (GFP-positive) completely phenocopied the overgrowth effects of RasACT in scrib– tissue (Figure 5A and B).
Fig. 5. The effects of RasACT in scrib mutant eye clones are mediated through the MAPK cascade, but cannot be mimicked by enhancing cell cycle progression and preventing apoptosis. (A and B) Expression of RafACT in scrib1 eye clones mimics the effects of RasACT. Elav staining shows a block to photoreceptor differentiation in third instar larval eye discs (A1), and the scrib– tissue, marked by the expression of GFP, greatly overproliferates (A2). As a consequence, many larvae fail to pupate and overgrow (B, wild-type larvae on the left) like homozygous scrib mutants. (C) Expressing RasACT in control wild-type eye clones, marked by GFP expression (C2 and 3), induces cyclin E (C1 and 3), and is pupal lethal. The position of the MF is indicated by a bar. (D–F) Blocking cyclin E activity by expressing Dacapo (Dap) in RasACT-expressing control clones can rescue the pupal lethality associated with the constitutive expression of RasACT, although the resulting adult eyes are severely disorganized (E). Expressing Dap in RasACT-expressing scrib1 clones can also rescue the overproliferation phenotype, although Elav staining (D) shows that RasACT is still not effective at inducing photoreceptor differentiation of scrib– tissue, and the developing adult flies fail to eclose and have severely disorganized eyes (F). (G and H) Mutating E2F1 (e2f191) in RasACT-expressing control eye clones rescues the pupal lethality associated with the constitutive expression of RasACT in wild-type clones (G), as does mutating E2F1 (e2f191) in RasACT-expressing scrib1 mutant clones (H). (I and J) Co-expression of cyclin E with the apoptosis inhibitor, p35, in scrib1 eye clones (I) results in overgrowth but fails to reproduce the effects of RasACT in scrib1 clones. Co-expression of E2F1, DP and p35 in scrib1 eye clones (J) results in overgrowth; however, the overgrowth is still not as aggressive as that produced by RasACT or RafACT in scrib1 clones.
Ras and the MAPK pathway have been implicated in a number of different developmental processes in the Drosophila eye disc, including differentiation (Halfar et al., 2001; Yang and Baker, 2001), protection from cell death (Bergmann et al., 1998, 2002; Kurada and White, 1998; Sawamoto et al., 1998), and increased cell proliferation (Karim and Rubin, 1998). We had already demonstrated that blocking apoptosis in scrib– clones enhanced overgrowth of the mutant tissue (Figure 2) but, as this was not to the same extent as RasACT, we considered it likely that downstream effectors of Ras involved in cell proliferation would also be important. Indeed, one of the downstream effectors of RasACT is cyclin E, since the ectopic expression of RasACT in otherwise wild-type eye clones (GFP-positive) increases cyclin E protein levels (Figure 5C). Although scrib– clones also ectopically express cyclin E, RasACT may increase the levels of cyclin E further and thereby enhance the overgrowth of scrib– tissue.
To test the importance of cyclin E in the RasACT-induced overgrowth of scrib– tissue, we blocked cyclin E activity by overexpressing the Drosophila p21 cyclin E–Cdk inhibitor homolog, Dacapo (Dap), in RasACT-expressing scrib– eye clones, and this effectively rescued the tumorigenic overgrowth phenotype (Figure 5D and F). Similarly, we also tested the involvement of another important cell cycle regulator, E2F1, in RasACT-induced overgrowth. Attenuating E2F1 activity in RasACT-expressing scrib– clones, by using an e2f1 null allele (e2f191; Duronio et al., 1995; Royzman et al., 1997), similarly rescued the overgrowth effects (Figure 5H).
Since increased proliferation mediated through cyclin E and E2F1 was therefore clearly important in mediating the tumorigenic effects of RasACT in scrib– clones, we next tested to see whether we could recapitulate the synergistic overgrowth effects of RasACT in scrib– clones by promoting cell proliferation with the ectopic expression of cyclin E or E2F1 while also blocking apoptosis. However, neither the co-expression of cyclin E with the apoptosis inhibitor p35, nor that of E2F1/DP with p35 could mimic the effects of RasACT on scrib– (GFP-positive) tissue (Figure 5I and J). Although pupal lethality resulted in both instances, analysis of larval eye discs revealed that the aggressive overgrowth and lack of differentiation induced by RasACT in scrib– clones did not occur (Figure 5I and J). As blocking JNK pathway activity had more pronounced effects on the viability of the scrib– mutant tissue than the use of p35, we also expressed cyclin E, or E2F1/DP, in BskDN-expressing scrib– clones, but this also failed to mimic the dramatic overgrowth effects of RasACT (data not shown).
These results suggest that although protection from apoptosis and enhanced cell cycle progression are important factors in mediating RasACT-induced overgrowth of scrib– tissue, other effectors of RasACT signaling are also likely to be important in mediating the cooperative overgrowth. The general tissue disruption induced by the ectopic expression of RasACT in wild-type clones, when the proliferative effects were minimized by either the expression of Dacapo (Figure 5E) or the absence of E2F1 (Figure 5G), is also suggestive of additional effectors other than those involved in cell proliferation and protection from apoptosis.
Discussion
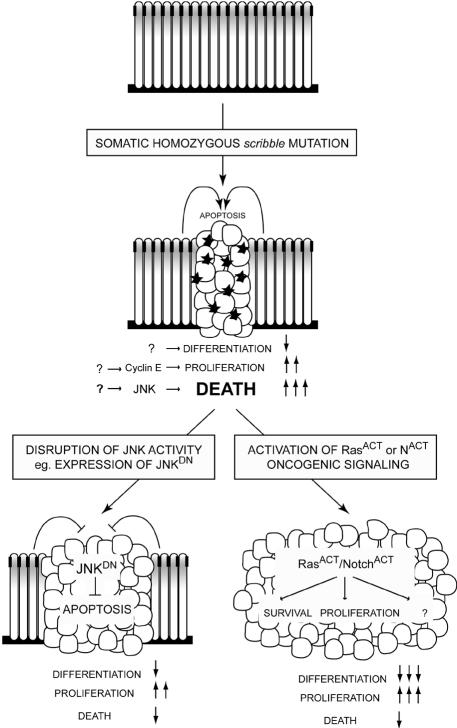
In this study on the Drosophila tumor suppressor, scribble, we have adopted a clonal approach, more closely resembling the clonal nature of mammalian cancer, to analyze the effects of removing Scrib function on tumor formation. Our analysis indicates that Drosophila scrib– tumors: (i) lose tissue architecture, including apical–basal cell polarity; (ii) fail to differentiate properly; (iii) exert non-cell-autonomous effects upon the surrounding wild-type tissue; (iv) upregulate cyclin E and undergo excessive cell proliferation; (v) are restrained from overgrowing by the surrounding wild-type tissue via a JNK-dependent apoptotic response; and (vi) show strong cooperation with oncogenic alleles of Ras and Notch to produce large amorphous tumors. These conclusions are summarized in a model for tumor development in Drosophila in Figure 6. We suggest that the role of epithelial cell polarity regulators in restraining oncogenes is likely to be of general significance in mammalian tumorigenesis.
Fig. 6. A model for cooperative tumorigenesis in Drosophila. A wild-type larval eye disc is a monolayered columnar epithelium, in which cell proliferation is tightly regulated. Cell architecture is maintained by the formation of adherens junctions (black boxes), the apical localization of Scribble (gray shading) and adhesion to the basement membrane. Mutation of scrib results in loss of apical–basal polarity, leading to multilayering and rounding up of cells. scrib– tissue also shows impaired differentiation, and ectopic cyclin E expression (by an unknown mechanism) leads to ectopic cell proliferation. Unrestrained overgrowth and tumor formation of scrib– cells is held in check by compensatory JNK-mediated apoptosis (black stars), dependent upon the presence of surrounding wild-type cells. Secondary mutations are required to avoid this apoptotic fate. If JNK activity is blocked within scrib– cells, by expressing a dominant-negative form of JNK, apoptosis is prevented, resulting in tissue overgrowth and lethality. Even more aggressive overgrowth results from the addition of activating oncogenic alleles of Ras or Notch. In addition to promoting cell survival, these oncogenes must also promote tumor cell proliferation; however, we propose that other downstream effectors of these oncogenes are likely also to be important, since we could not mimic the cooperative overgrowth effects of RasACT or NACT on scrib– tissue by simply blocking apoptosis and enhancing cell proliferation.
Upregulation of cyclin E and overproliferation of scrib mutant clones
scrib– clones ectopically express cyclin E and undergo ectopic S phases and mitoses. Since cyclin E is rate limiting for cell cycle progression in the developing eye (Richardson et al., 1995), it is likely that upregulation of cyclin E in scrib– clones is critical for the ectopic cell proliferation. Indeed we originally isolated alleles of scrib and lgl as dominant suppressors of a hypomorphic cyclin E allele, DmcycEJP (A.M.Brumby, J.Secombe, J.Horsfield, M.Coombe, N.Amin, D.Coates, R.Saint and H.E.Richardson, in preparation), suggesting that these cell polarity genes normally play a critical role in limiting cyclin E expression. We currently are investigating which signaling pathways are altered in scrib, dlg or lgl mutants that could be responsible for cyclin E upregulation. A recent study in human lung epithelial cells showed that disrupting cell polarity allowed mixing of the heregulin-α ligand and the erbB2-4 receptor, which are normally physically separated, resulting in activation of the pathway and cell proliferation (Vermeer et al., 2003). Further studies are required to determine whether the ectopic expression of cyclin E observed in the absence of Scrib is simply a consequence of the tissue disorganization induced by disrupting cell polarity, or if Scrib has a direct role in limiting cell proliferation independent of cell polarity. Interestingly, the rounding up of cells in the absence of Scrib appeared to be predominantly a cell-autonomous effect, yet clearly non-cell-autonomous defects were also apparent, including the upregulation of cyclin E. This would suggest that altered cell–cell interactions between wild-type and mutant cells can also alter signaling pathways within wild-type cells, and that the loss of apical–basal polarity and collapse of the columnar epithelium is not intrinsically responsible for the deregulated expression of cyclin E. A deeper understanding of the relationship between epithelial cell polarization and cell proliferation is clearly important for understanding the development of cancer, since a loss of cell polarity often accompanies tumor progression and metastasis.
Compensatory JNK-mediated elimination of scrib mutant tissue
Overproliferation of scrib– clones in the eye disc is compensated for by JNK-mediated apoptosis. Blocking JNK pathway activity in scrib– eye clones greatly increases the proportion of clonal tissue, and results in lethality to the host. As downregulating JNK pathway activity in otherwise wild-type clones of tissue did not induce increased cell proliferation (A.M.Brumby and H.E.Richardson, unpublished data), we suggest that JNK pathway activity in scrib– clones induces apoptosis. This is consistent with previous reports on the pro-apoptotic effects of the JNK pathway in the Drosophila eye (Takatsu et al., 2000) and our own observations (Figure 2G). Recent studies in mammals would also suggest that activation of the JNK pathway can limit the growth of tumors in situ, possibly by increasing apoptosis (Kennedy et al., 2003).
How JNK-mediated apoptosis is induced in scrib– clones is not known. While Scrib could play a direct role in repressing JNK pathway activity, it is also possible that the JNK pathway is activated indirectly, in response to other cellular defects. In the wing disc, removal of cells by JNK-mediated apoptosis is linked to discontinuities in a cell’s response to morphogen gradients, most notably the antero-posterior patterning regulator, Dpp, in a process probably related to cell competition, with the purpose of eliminating aberrant or slow growing cells (Adachi-Yamada et al., 1999; Adachi-Yamada and O’Connor, 2002; Moreno et al., 2002a). Although this form of compensatory JNK-mediated apoptosis has not yet been demonstrated within the eye disc, the observation that the surrounding wild-type tissue context plays an important role in limiting the overgrowth of scrib– tissue argues against a simple cell-intrinsic apoptotic response of scrib– cells to a loss of cell polarity, and is more consistent with an integrative response mediated by both the tumor cells and the surrounding wild-type cells, as exemplified by cell competition. Whether this is dependent on a failure of scrib– cells to transduce Dpp signaling is not known; however, other interesting possibilities also warrant further investigation. Notable is the recent identification of a tumor necrosis factor-induced apoptotic signaling pathway involving the JNK pathway (Igaki et al., 2002; Moreno et al., 2002b). We are also mindful of the involvement of the JNK pathway in orchestrating cell shape changes during the morphogenetic movements of dorsal and thorax closure (reviewed by Kockel et al., 2001) and wound healing (Ramet et al., 2002). We note that clones of scrib– tissue expressing BskDN (JNKDN) appeared morphologically different from those expressing the apoptosis inhibitor p35; most notably, the clones were generally larger and less rounded than those expressing p35 (Figure 2H, and data not shown). This would imply either that p35 is not as effective as BskDN in preventing cell death, or that there are other effectors of the JNK pathway that are important in the inhibition of scrib– tumor overgrowth. We currently are investigating the possibility that JNK activation could play a role in eliminating scrib– tissue from the epithelium in a process reminiscent of wound healing.
It has been reported previously that dlg and lgl mutant clones also show poor viability (Woods and Bryant, 1991; Agrawal et al., 1995), suggesting that JNK-mediated apoptosis could be a common response to the loss of cell polarity and overproliferation induced by the absence of these tumor suppressors. Indeed, while other regulators of epithelial cell polarity, such as Crumbs and E-cadherin, apparently do not act as tumor suppressors in Drosophila, the effects of these mutations on cell proliferation when cell death is blocked warrant further examination. Interestingly, in mammalian systems, the polarized nature of epithelia is also important in protecting cells from an apoptotic response, and this acts as a brake on tumor development when polarity is disrupted (reviewed by Jacks and Weinberg, 2002).
Cooperative tumorigenesis induced by oncogenic Ras and Notch
In Drosophila, activated Ras exerts its oncogenic effects through Raf and the MAPK pathway (Figure 5A and B). Downstream targets of MAPK in the eye disc previously have been shown to promote differentiation, cell survival and cell proliferation. Our work also demonstrates that Ras can increase cyclin E protein levels in the eye disc. In combination with scrib–, the differentiation output of RasACT signaling appears to be attenuated, and the proliferative and anti-apoptotic responses prevail.
Activated Notch also cooperates with scrib–, resulting in neoplastic overgrowth, and although no anti-apoptotic role for Notch signaling in the eye has been described previously, NACT exerts hyperproliferative effects in flies (Go et al., 1998; Baonza and Garcia-Bellido, 2000), and Notch signaling is required for proliferation of eye disc cells (Cho and Choi, 1998; Dominguez and de Celis, 1998). Although we do not know if NACT induces the same critical downstream targets as RasACT to cause overgrowth of scrib– tissue, removing ras function in scrib– cells overexpressing NACT rescues the overgrowth phenotype, suggesting that the effects of NACT are at least partially dependent on Ras (A.M.Brumby and H.E.Richardson, unpublished data).
Initially it seemed likely that the cooperative effects of RasACT or NACT on scrib– tissue could be explained by the ability of these oncogenes to promote cell proliferation while blocking apoptosis. However, the expression of neither cyclin E nor E2F1/DP, in combination with the apoptosis inhibitor p35 (or with the inhibitor of JNK pathway activity, BskDN), was capable of phenocopying the effect of RasACT or NACT in scrib– clones. We therefore suggest that other downstream effectors, apart from anti-apoptotic and cell cycle regulators, must be important in mediating the oncogenic effects of RasACT or NACT. In fact, in Drosophila, Ras has also been shown to be a potent inducer of cellular growth (Prober and Edgar, 2000), while cyclin E and E2F1 mainly promote cell cycle progression (Neufeld et al., 1998). Whether NACT also promotes cell growth in Drosophila has not been examined in detail. If growth promotion targets downstream of RasACT or NACT are critical in promoting the overgrowth of scrib– tumors, these are likely to be independent of the PI3 kinase pathway since ectopic PI3 kinase signaling in scrib– clones did not induce synergistic overgrowth, and RafACT was able to induce overgrowth as equally extensive as RasACT.
Finally, we note that in mammalian systems, evidence exists for a role for Ras signaling in modulating cell junction complexes and enhancing epithelial to mesenchymal transitions (e.g. Chen et al., 2000; Janda et al., 2002), and in Drosophila also, constitutive RasACT signaling in clones alters cell affinities and changes the levels of E-cadherin and β-catenin (Prober and Edgar, 2002). Whether RasACT or NACT signaling destabilizes adherens junctions in Drosophila and this potentiates scrib– neoplastic overgrowth or whether alterations in the structure of the adherens junction resulting from the absence of Scrib alters a cells response to constitutive activation of these oncogenes are important future questions.
Concluding remarks
In this study, we have described a novel multi-hit model of tumorigenesis in Drosophila (Figure 6). Furthermore, although it has been suspected that disruptions to cell polarity could potentiate tumor progression and metastasis, our work in Drosophila demonstrates for the first time how the oncogenic effects of activated Ras and Notch are unleashed in the absence of epithelial polarity regulators. We predict that in mammals also, defects in apical–basal polarity could cooperate with oncogenes during neoplastic development. Our approach in Drosophila can now be used to screen for novel oncogenes that, when specifically overexpressed in scrib– clones, are capable of inducing cooperative tumorigenesis, and can also be extended to identify cooperative interactions between other tumor suppressors and oncogenes within a whole animal context.
Materials and methods
Fly stocks
Mutants used were scrib1, scrib2 and e2f191. For the generation of eye mosaics, we used w ey-FLP1, FRT82B, FRT82B P[Ubi-nlsGFP], FRT82B P[mini-w] P[arm-lacZ], FRT82B scrib1, FRT82B scrib2, FRT82B P[Ubi-nlsGFP] scrib1, FRT82B e2f191 and FRT82B e2f191 scrib1.
For the generation of eye mosaics with the wild-type tissue removed, the stocks employed were ey-GAL4, UAS-FLP1;;FRT82B GMR-hid and ey-FLP2;;FRT82B l(3)cl-R31, and for the generation of MARCM eye mosaics, w ey-FLP1,UAS-mCD8-GFP;;tub-GAL4 FRT82B tub-GAL80.
Transgenes used for expression of: RasACT, UAS-Ras1V12; RafACT, UAS-DrafGOF; EGFRDN, UAS-EgfrDN; NACT, UAS-Nintra; ArmACT, UAS-armS10; dTCFDN, UAS-dTCFΔN3; TkvACT, UAS-tkvQ253D; Dad, UAS-dad; Ci-155, UAS-ci; PI3K, UAS-Dp110; PI3KDN, UAS-Dp110D945A; HepACT, UAS-hepACT; BskDN, UAS-bskDN; cyclin E, UAS-DmcycEII; p35, UAS-p35; E2F1/DP, UAS-dE2F1,UAS-dDP; Dap, UAS-dap; RalACT, UAS-RalV20; RacACT, UAS-Drac1V12; RhoACT, UAS-Rho1V12; Cdc42ACT, UAS-Dcdc42V12.
Generation of mitotic eye clones
Mitotic eye clones, marked by the absence of GFP or LacZ, were generated by crossing ey-FLP1;;FRT82B P[Ubi-nlsGFP] flies or ey-FLP1;;FRT82B P[mini-w] P[arm-lacZ] flies to FRT82B scrib1 or FRT82B scrib2 stocks.
To generate scrib1 eye clones with the surrounding wild-type tissue removed, ey-GAL4, UAS-FLP1;;FRT82B GMR-hid or ey-FLP2;;FRT82B l(3)cl-R31 flies were crossed to FRT82B P[Ubi-nlsGFP] scrib1 flies. scrib1 mutant tissue was marked by the expression of GFP.
To express different transgenes within mutant clones, the MARCM system was used (Lee and Lou, 1999). With the MARCM system, both the transcriptional activator GAL4 and the repressor GAL80 are ubiquitously expressed from tubulin promoters, but upon FLP-mediated recombination, a FRT site flanking tub-GAL80 ensures the loss of GAL80 in clones, and subsequent derepression of GAL4-dependent transcription, including a UAS-mCD8-GFP transgene as a positive clonal marker, specifically within the mutant clonal tissue. ey-FLP1,UAS-mCD8-GFP;;tub-GAL4 FRT82B tub-GAL80 flies were crossed to flies carrying the UAS-transgene with either FRT82B as a control, or FRT82B scrib1 to express the transgene in scrib1 clones. To express RasACT in e2f191 mutant clones, FRT82B e2f191 or FRT82B e2f191 scrib1 stocks were used. If the UAS-transgene was on the third chromosome, recombinants were generated carrying the UAS transgene with either FRT82B or FRT82B scrib1.
Immunohistochemistry
For the analysis of eye/antennal discs, larvae were picked at the third instar stage and tissues were fixed in 4% formaldehyde for 20 min. Antibodies used were mouse anti-Elav (Developmental Studies Hybridoma Bank, 1:5), rat anti-cyclin E (1:1000), rabbit anti-phosphohistone H3 (Santa Cruz, 1:400), mouse anti-BrdU (Becton-Dickinson, 1:50) and rabbit anti-β-galactosidase (Rockland, 1:400). F-actin was detected with phalloidin–tetramethylrhodamine isothiocyanate (TRITC; Sigma, 0.3 µM). For detection of apoptotic cells, discs were dissected in phosphate-buffered saline (PBS) and mounted in 10 µg/ml acridine orange (Sigma). For the detection of S phase cells, a 1 h BrdU pulse was followed by fixation, immunodetection of LacZ, further fixation, acid treatment and detection of the BrdU epitope. All fluorescent labelled samples were analyzed by confocal microscopy (Bio-Rad MRC1000).
Acknowledgments
Acknowledgements
We thank Spyros Artavanis-Tsakonas, David Bilder, Bruce Edgar, Mathew Freeman, Iswar Hariharan, Gary Hime, Michael Hoffman, Phil Ingham, Laura Johnston, Elisabeth Knust, Sally Leevers, Marek Mlodzik, Hideyuki Okano, Norbert Perrimon, Tetsuya Tabata, Jessica Treisman and the Bloomington Drosophila Stock Centre for supplying Drosophila stocks, Karen Goulding for assistance with the preparation of figures, and Patrick Humbert, Sarah Russell and Joe Sambrook for comments on the manuscript. This work was supported by the National Health and Medical Research Foundation, Australia, and a Wellcome Senior Research Fellowship to H.E.R.
References
- Adachi-Yamada T. and O’Connor,M.B. (2002) Morphogenetic apoptosis: a mechanism for correcting discontinuities in morphogen gradients. Dev. Biol., 251, 74–90. [DOI] [PubMed] [Google Scholar]
- Adachi-Yamada T., Fujimura-Kamada,K., Nishida,Y. and Matsumoto,K. (1999) Distortion of proximodistal information causes JNK-dependent apoptosis in Drosophila wing. Nature, 400, 166–169. [DOI] [PubMed] [Google Scholar]
- Agrawal N., Kango,M., Mishra,A. and Sinha,P. (1995) Neoplastic transformation and aberrant cell–cell interactions in genetic mosaics of lethal(2)giant larvae (lgl), a tumor suppressor gene of Drosophila. Dev. Biol., 172, 218–229. [DOI] [PubMed] [Google Scholar]
- Baonza A. and Garcia-Bellido,A. (2000) Notch signaling directly controls cell proliferation in the Drosophila wing disc. Proc. Natl Acad. Sci. USA, 97, 2609–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann A., Agapite,J., McCall,K.A. and Steller,H. (1998) The Drosophila gene hid is a direct molecular target of Ras-dependent survival signaling. Cell, 95, 331–341. [DOI] [PubMed] [Google Scholar]
- Bergmann A., Tugentman.,M., Shilo,B.-Z. and Steller,H. (2002) Regulation of cell number by MAPK-dependent control of apoptosis: a mechanism for trophic survival signaling. Dev. Cell, 2, 159–170. [DOI] [PubMed] [Google Scholar]
- Bilder D. and Perrimon,N. (2000) Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature, 403, 676–680. [DOI] [PubMed] [Google Scholar]
- Bilder D., Li,M. and Perrimon,N. (2000) Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science, 289, 113–116. [DOI] [PubMed] [Google Scholar]
- Bissell M.L. and Radisky,D. (2001) Putting tumors in context. Nature Rev. Cancer, 1, 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Lu,Q., Schneeberger,E.E. and Goodenough,D.A. (2000) Restoration of tight junction structure and barrier function by down-regulation of the mitogen-activated protein kinase pathway in Ras-transformed Madin–Darby canine kidney cells. Mol. Biol. Cell, 11, 849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K.-O. and Choi,K.-W. (1998) Fringe is essential for mirror symmetry and morphogenesis in the Drosophila eye. Nature, 396, 272–276. [DOI] [PubMed] [Google Scholar]
- Davis R.J. (2000) Signal transduction by the JNK group of MAP kinases. Cell, 103, 239–252. [DOI] [PubMed] [Google Scholar]
- Dominguez M. and de Celis,J. (1998) A dorsal/ventral boundary established by Notch controls growth and polarity in the Drosophila eye. Nature, 396, 276–278. [DOI] [PubMed] [Google Scholar]
- Duronio R.J., O’Farrell,P.H., Xie,J.-E., Brook,A. and Dyson,N. (1995) The transcription factor E2F is required for S phase during Drosophila embryogenesis. Genes Dev., 9, 1456–1468. [DOI] [PubMed] [Google Scholar]
- Gardiol D., Kuhne,C., Glaunsinger,B., Lee,S.S., Javier,R. and Banks,L. (1999) Oncogenic human papillomavirus E6 proteins target the discs large tumor suppressor for proteasome-mediated degradation. Oncogene, 18, 5487–5496. [DOI] [PubMed] [Google Scholar]
- Gateff E. (1994) Tumor suppressor and overgrowth suppressor genes of Drosophila melanogaster: developmental aspects. Int. J. Dev. Biol., 38, 565–590. [PubMed] [Google Scholar]
- Go M.J., Eastman,D.S. and Artavanis-Tsakonas,S. (1998) Cell proliferation control by Notch signaling in Drosophila development. Development, 125, 2031–2040. [DOI] [PubMed] [Google Scholar]
- Halfar K., Rommel,C., Stocker,H. and Hafen,E. (2001) Ras controls growth, survival and differentiation in the Drosophila eye by different thresholds of MAP kinase activity. Development, 128, 1687–1696. [DOI] [PubMed] [Google Scholar]
- Hanahan D. and Weinberg,R.A. (2000) The hallmarks of cancer. Cell, 100, 57–70. [DOI] [PubMed] [Google Scholar]
- Igaki T., Kanda,H., Yamamoto-Goto,Y., Kanuka,H., Kuranaga,E., Aigaki,T. and Miura,M. (2002) Eiger, a TNF superfamily ligand that triggers the Drosophila JNK pathway. EMBO J., 21, 3009–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T. and Weinberg,R.A. (2002) Taking the study of cancer cell survival to a new dimension. Cell, 111, 923–925. [DOI] [PubMed] [Google Scholar]
- Janda E., Lehmann,K., Killisch,I., Jechlinger,M., Herzig,M., Downward,J., Beug,H. and Grunert,S. (2002) Ras and TGFβ cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J. Cell Biol., 156, 299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim F.D. and Rubin,G.M. (1998) Ectopic expression of activated Ras1 induces hyperplastic growth and increased cell death in Drosophila imaginal tissues. Development, 125, 1–9. [DOI] [PubMed] [Google Scholar]
- Kennedy N.J., Sluss,H.K., Jones,S.N., Bar-Sagi,D., Flavell,R.A. and Davis,R.J. (2003) Suppression of Ras-stimulated transformation by the JNK signal transduction pathway. Genes Dev., 17, 629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kockel L., Homsy,J.G. and Bohmann,D. (2001) Drosophila AP-1: lessons from an invertebrate. Oncogene, 20, 2347–2364. [DOI] [PubMed] [Google Scholar]
- Kurada P. and White,K. (1998) Ras promotes cell survival in Drosophila by down-regulating hid expression. Cell, 95, 319–329. [DOI] [PubMed] [Google Scholar]
- Lee T. and Luo,L. (1999) Mosaic analysis with a repressible neurotechnique cell marker for studies of gene function in neural morphogenesis. Neuron, 22, 451–461. [DOI] [PubMed] [Google Scholar]
- Liotta L.A. and Kohn,E.C. (2001) The microenvironemnt of the tumor–host interface. Nature, 411, 375–379. [DOI] [PubMed] [Google Scholar]
- Moreno E., Basler,K. and Morata,G. (2002a) Cells compete for Decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature, 416, 755–759. [DOI] [PubMed] [Google Scholar]
- Moreno E., Yan,M. and Basler,K. (2002b) Evolution of TNF signaling mechanisms: JNK-dependent apoptosis triggered by Eiger, the Drosophila homolog of the TNF superfamily. Curr. Biol., 12, 1263–1268. [DOI] [PubMed] [Google Scholar]
- Nakagawa S. and Huibregtse,J.M. (2000) Human Scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol. Cell Biol., 20, 8244–8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld T.P., de la Cruz,A.F.A., Johnston,L.A. and Edgar,B.A. (1998) Coordination of growth and cell division in the Drosophila wing. Cell, 93, 1183–1193. [DOI] [PubMed] [Google Scholar]
- Newsome T.P., Asling,B. and Dickson,B.J. (2000) Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development, 127, 851–860. [DOI] [PubMed] [Google Scholar]
- Prober D.A. and Edgar,B.A. (2000) Ras1 promotes cellular growth in the Drosophila wing. Cell, 100, 435–446. [DOI] [PubMed] [Google Scholar]
- Prober D.A. and Edgar,B.A. (2002) Interactions between Ras1, dMyc and dPI3K signaling in the developing Drosophila wing. Genes Dev., 16, 2286–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramet M., Lanot,R., Zachary,D. and Manfruelli,P. (2002) JNK signaling pathway is required for efficient wound healing in Drosophila. Dev. Biol., 241, 145–156. [DOI] [PubMed] [Google Scholar]
- Richardson H., O’Keefe,L.V., Marty,T. and Saint,R. (1995) Ectopic cyclin E expression induces premature entry into S phase and disrupts pattern formation in the Drosophila eye imaginal disc. Development, 121, 3371–3379. [DOI] [PubMed] [Google Scholar]
- Royzman I., Whittaker,A.J. and Orr-Weaver,T.L. (1997) Mutations in Drosophila DP and E2F distinguish G1–S progression from an associated transcriptional program. Genes Dev., 11, 1999–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamoto K., Taguchi,A., Yamada,C., Jin,M. and Okano,H. (1998) Argos induces cell death in the developing Drosophila eye by inhibition of the Ras pathway. Cell Death Differ., 5, 262–270. [DOI] [PubMed] [Google Scholar]
- Shields J.M., Pruitt,K., McFall,A., Shaub,A. and Der,C.J. (2000) Understanding Ras: ‘it ain’t over ‘til it’s over’. Trends Cell Biol., 10, 147–154. [DOI] [PubMed] [Google Scholar]
- Takatsu Y., Nakamura,M., Stapleton,M., Danos,M.C., Matsumoto,K., O’Connor,M.B., Shibuya,H. and Ueno,N. (2000) TAK1 participates in c-Jun N-terminal kinase signaling during Drosophila development. Mol. Cell. Biol., 20, 3015–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer P.D., Einwalter,L.A., Moninger,T.O., Rokhlina,T., Kern,J.A., Zabner,J. and Welsh,M.J. (2003) Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature, 422, 322–326. [DOI] [PubMed] [Google Scholar]
- Wodarz A. (2000) Tumor suppressors: linking cell polarity and growth control. Curr. Biol., 10, R624–R626. [DOI] [PubMed] [Google Scholar]
- Woods D.F. and Bryant,P.J. (1991) The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell, 66, 451–464. [DOI] [PubMed] [Google Scholar]
- Yang L. and Baker,N.E. (2001) Role of the EGFR/Ras/Raf pathway in specification of photoreceptor cells in the Drosophila retina. Development, 128, 1183–1191. [DOI] [PubMed] [Google Scholar]