Abstract
Objective
To investigate the neural correlates of verbal and non-verbal semantic processing in neurodegenerative disease.
Background
Semantic memory is often impaired in neurodegenerative disease. Neuropsychological and functional neuroimaging studies suggest that the semantic processing of verbal and non-verbal stimuli may depend on partially distinct brain networks.
Methods
We examined this possibility using voxel-based morphometry to correlate performance on verbal and non-verbal versions of a semantic association task with regional gray matter atrophy in 144 individuals with a variety of neurodegenerative diseases.
Results
Results showed that, regardless of stimulus type, semantic processing correlated with atrophy in both temporal lobes. In addition, material-specific correlations were found in left temporal regions for verbal stimuli and the right fusiform gyrus for non-verbal stimuli.
Conclusions
These results provide evidence for a differential role of the left and right hemispheres in the extraction of semantic information from verbal and pictorial representations. Areas in the right inferior temporal lobe may be necessary to access structural descriptions of visually presented objects.
Introduction
Semantic memory enables us to understand and categorize elements of the world. It comprises, inter alia, our knowledge of objects, animals, people and words. Semantic memory is often impaired in neurodegenerative diseases, most severely in patients with semantic dementia (SD) who have bilateral atrophy of the anterior and inferolateral temporal cortices 1-3. Such patients usually present with word-finding and comprehension difficulties and demonstrate loss of conceptual knowledge, despite preserved phonological and syntactic abilities 4-8.
Conceptual knowledge can be assessed with a variety of tasks including picture naming; word, picture, sound or color matching; object/non-object decision; feature generation and semantic association. Such tasks assess different components of semantic memory (e.g. knowledge of perceptual features or semantic associations), across a range of modalities (e.g. visual, auditory or tactile) and material types (e.g. verbal or non-verbal). In SD, the semantic deficit is usually highly consistent across trials, tasks, modality and material type 9-11 (but see Lauro-Grotto et al. 12 for alternative findings). This has led many authorities to propose that this syndrome reflects gradual degradation of a central, unitary semantic system dependent on the bilateral anterior temporal lobes 2, 13, 14.
In contrast, patients with non-neurodegenerative, focal brain lesions occasionally show dissociations in semantic memory with impairment apparently isolated to either verbal 15, 16 or non-verbal 17-19 material. Such cases suggest that some aspects of semantic processing are subserved by distinct brain regions according to the type of stimulus material.
Functional imaging studies of semantic memory, using a variety of stimulus modalities and categories, have consistently revealed activation in a predominantly left-sided network extending from the left inferior occipital cortex, through the middle and inferior temporal gyri to the inferior frontal gyrus 20-23. Additionally, a few studies have demonstrated right temporal activation specific to semantic processing 24, 25. Functional imaging studies that address the question of material-specificity in semantic processing using both visual 26, 27 and auditory 28 stimuli have identified hemispheric dissociations, with verbal material preferentially activating left-sided structures and non-verbal stimuli preferentially activating right-sided structures.
While functional imaging can help identify the networks active in semantic processing, only complementary data from lesion studies can determine which parts of those networks are necessarily involved. Furthermore, the proximity of air-filled sinuses to temporal regions involved in language processing, renders functional magnetic resonance imaging studies at risk of false-negative results due to susceptibility artefact and signal drop-out 29. Voxel-based morphometry (VBM) is a technique that has been used with structural MR brain images of patients with neurodegenerative disease to correlate behavioral 30 and cognitive data 2, 31, 32 with gray matter volumes in each voxel. In this study, we used VBM to address the question of which brain regions are necessary for conceptual processing and whether any areas show material-specificity. In patients with neurodegenerative disease we looked for correlations between gray matter atrophy and performance on a semantic association task administered with both pictorial and verbal stimuli. The ability to detect semantic associations between pairs of objects or words requires an understanding of the items that goes beyond their purely perceptual features. Nevertheless, it is clear that other, non-semantic, processes including perception and decision-making are also critical in successfully performing these tasks. Based on evidence from the case reports and functional imaging studies mentioned above, we hypothesized that areas in the left-temporal lobe would correlate most closely with semantic processing of both verbal and non-verbal stimuli, but that regions specifically correlating to verbal processing would be identified in the left and non-verbal processing in right temporal lobes.
Methods
Subjects
One hundred and forty four participants (79 males) were recruited at the Memory and Aging Center, University of California, San Francisco (UCSF). Evaluation consisted of neurological history and examination, nursing and neuropsychological assessment and magnetic resonance brain imaging (MRI). A team of clinicians arrived at a consensus diagnosis using published diagnostic criteria 33-37. The distribution of diagnoses was: Alzheimer’s disease (AD, n = 26), frontotemporal dementia (FTD, n = 24), semantic dementia (SD, n = 27), mild cognitive impairment (MCI, n = 12), progressive supranuclear palsy (PSP, n = 10), corticobasal degeneration (CBD, n = 10), progressive non-fluent aphasia (PNFA, n = 7) and dementia with Lewy bodies (DLB n = 2). For the purposes of analysis of demographic and neuropsychological data, patients with a predominantly motor presentation were grouped together (PSP/CBD/DLB). A further 26 normal control subjects were included in the study group. The inclusion of participants from diverse diagnostic groups with varying patterns of brain atrophy and a wide range of scores on the neuropsychological tests was intended to increase variance within the group and, thus improve, the power of the correlational VBM analyses. The study was approved by the UCSF Committee on Human Research. All subjects or their caregivers provided written, informed consent before participating.
Differences in performance on neuropsychological measures were assessed for each diagnostic group in comparison with the remaining participants using independent sample t tests or the Mann-Whitney U test where appropriate. Relations between neuropsychological measures were investigated by calculating Pearson’s correlation coefficient. Statistical analyses were performed in SPSS for Windows version 15 (SPSS, Chicago, IL).
Neuropsychological assessment
General intellectual function was tested with the Mini Mental Status Examination (MMSE) 38 and functional status using the sum of box scores from the Clinical Dementia Rating scale (CDR) 39. Memory, language, visuospatial ability and executive function were evaluated using a previously published protocol 40. Semantic associative knowledge was assessed using the word (PPTW) and picture (PPTP) versions of the Pyramids and Palm Trees test 41. This test is widely used for the clinical assessment of semantic memory in neurodegenerative disease. Subject are shown a triad of stimuli (either words or pictures) – a reference (eg. PYRAMID) and two choices (eg. palm tree OR pine tree). The subject is then asked to identify which of the two choices is most closely associated with the reference stimulus. The word and picture versions of the test use the same objects and consist of 52 trials each. A chance-level score is thus 26/52. The word and picture versions were administered in separate testing sessions.
Table 1 shows the demographic and neuropsychological data for the whole group and by diagnosis. The mean age of the group was 63.3 years (standard deviation = 10.3, range = 35 to 95) and mean duration of full-time education was 16.1 years. When compared with the rest of the group, patients with: i) AD were significantly impaired on the MMSE (p<0.001), Rey figure recall (p=0.002) and digits backwards (p<0.001); ii) FTD were significantly younger (p<0.001) and obtained higher CDR scores (p<0.001); iii) SD were significantly impaired on naming (BNT (p<0.001)) and semantic association tasks (PPTW (p<0.001) and PPTP (p<0.001)); and iv) PSP/CBD/DLB were impaired on the MMSE (p=0.015), Rey figure copy (p=0.001) and digits backwards (p<0.001).
Table 1.
Demographic and neuropsychological characteristics of study participants
| Whole group n=144 Mean (StD) | NC§ n=26 Mean (StD) | MCI n=12 Mean (StD) | AD n=26 Mean (StD) | FTD n=24 Mean (StD) | SD n=27 Mean (StD) | PNFA n=7 Mean (StD) | PSP/CBD/DLB n=22 Mean (StD) | |
|---|---|---|---|---|---|---|---|---|
| Age | 63.4 (10.2) | 58.8 (12.8)* | 75.4 (9.7)† | 62.3 (8.6) | 58.9 (6.1)* | 63.5 (7.4) | 60.7 (12.1) | 69.3 (7.6)† |
| Education (yrs) | 16.1 (2.7) | 16.6 (2.3) | 16.5 (2.6) | 16.0 (2.6) | 15.8 (2.2) | 16.6 (3.1) | 14.3 (2.4) | 15.8 (2.7) |
| M/F | 79/65 | 10/16 | 6/6 | 14/12 | 21/3 | 13/14 | 1/6 | 14/8 |
| CDR (sum of boxes) | 3.3 (2.9) | 0.8 (1.8) | 0.6 (0.6)* | 3.8 (2.4) | 5.8 (2.7)† | 3.7 (2.6) | 2.6 (2.4) | 4.4 (2.5) |
| MMSE | 24.9 (5.3) | 29.4 (1.0) | 29.3 (1.0)† | 21.5 (5.5)* | 25.8 (3.0) | 23.3 (5.7) | 24.4 (3.7) | 22.4 (6.4)* |
| Rey figure copy | 14.1 (3.6) | 15.8 (1.4) | 14.8 (1.4) | 12.5 (5.5) | 14.8 (2.1) | 15.4 (1.5) | 14.7 (1.6) | 11.0 (4.5)* |
| Rey figure recall (30 min) | 8.1 (4.9) | 13.3 (1.5) | 8.8 (4.4) | 5.5 (5.1)* | 7.4 (4.5) | 7.2 (4.8) | 7.3 (5.3) | 7.1 (4.0) |
| Digits backwards | 4.0 (1.4) | 4.7 (0.9) | 4.6 (0.8) | 3.1 (1.1)* | 4.5 (1.4) | 4.7 (1.1) | 3.1 (0.9) | 2.8 (1.5)* |
| BNT | 11.1 (4.5) | 14.6 (0.8) | 13.3 (2.5) | 11.6 (3.3) | 12.3 (3.2) | 4.1 (3.7)* | 10.7 (2.9) | 12.8 (2.5) |
| PPTW | 46.8 (6.7) | 51.4 (1.0) | 51.3 (0.8)* | 48.2 (3.9) | 47.3 (6.1) | 37.8 (8.1)* | 49.1 (2.5) | 47.1 (4.1) |
| PPTP | 45.9 (6.9) | 50.6 (1.7) | 50.8 (1.2)† | 46.4 (6.7) | 46.1 (6.8) | 38.1 (7.4)* | 47.3 (4.3) | 45.7 (5.0) |
significantly different on all neuropsychological measures from other diagnostic groups combined (p<0.05)
significantly lower than other diagnostic groups combined (p<0.05)
significantly higher than other diagnostic groups combined (p<0.05)
NC = normal control subjects; MCI = mild cognitive impairment; AD = Alzheimer’s Disease; FTD = fronto-temporal dementia; SD = semantic dementia; PNFA = progressive non-fluent aphasia; PSP = progressive supranuclear palsy; CBD = corticobasal degeneration; DLB = diffuse Lewy body dementia
Across all 144 participants, scores on the words (PPTW) and pictures (PPTP) subsets of the Pyramids and Palm Trees test correlated highly with each other (Pearson’s r = 0.789, p < 0.001) and with scores on the MMSE (PPTW: r = 0.514, p<0.001; PPTP: r = 0.536, p<0.001) and the BNT (PPTW: r = 0.693, p < 0.001; PPTP: r = 0.656, p < 0.001). PPTP scores also correlated weakly but significantly with the Rey copy (r = 0.188, p = 0.024).
Magnetic Resonance Imaging
MRI scans were obtained on a 1.5T Magnetom VISION system (Siemens, Iselin, NJ). A volumetric magnetization prepared rapid gradient-echo MRI (MPRAGE, TR/TE/TI = 10/4/300 milliseconds) was used to obtain T1-weighted images of the entire brain, 15-degree flip angle, coronal orientation perpendicular to the double spin-echo sequence, 1.0 × 1.0 mm 2 in-plane resolution and 1.5 mm slab thickness. Scanning was carried out within 365 days of behavioral assessment.
Voxel-based morphometry (VBM)
VBM is a technique for voxel-wise analysis of local changes in brain tissue content which has been used to study several varieties of brain disorder including neurodegenerative disease. The technique utilizes a spatial pre-processing stage followed by statistical analysis. Both stages were performed using the SPM2 software package (Wellcome Department of Cognitive Neurology, London; http://www.fil.ion.ucl.ac.uk/spm) running on Matlab 6.5.1 (MathWorks, Natick, MA).
MRI images were pre-processed following standard procedures of the optimized method 42. Customized template and a priori images were created by averaging 30 age-matched normal control subjects. A two-step segmentation procedure was applied to each scan included in the study. First, raw T1-weighted images were segmented in native space. Each gray matter image was then spatially normalized to the custom gray matter template. The parameters obtained from the gray matter normalization were subsequently applied to the original T1-weighted images. Finally, these normalized images were again segmented into gray and white matter and CSF compartments. In order to preserve the volume of tissue within each voxel a further modulation procedure was carried out in which voxel values of the normalized images were multiplied by the Jacobian determinates derived from the spatial normalization step. Spatially normalized, segmented and modulated images were then smoothed using a 12mm full-width at half-maximum isotropic Gaussian kernel.
A covariates-only statistical analysis was used to investigate correlations between gray matter volume and performance on verbal (PPTW) and pictorial (PPTP) versions of the Pyramids and Palm Trees test. Subjects from all diagnostic categories were entered as a single group. Age and sex were entered as nuisance covariates, together with score on the copy of the Rey complex figure. This test is highly sensitive, although not necessarily specific, to deficits in visuospatial perception. Variation in global brain atrophy was accounted for by scaling each image by its total gray matter volume. The significance of each effect of interest was determined by using the theory of Gaussian fields.
We first examined the effect of gray matter atrophy on PPTW and PPTP scores independently by constructing separate a statistical model for each. Then, in order to determine material-type specific effects, we entered both PPTW and PPTP scores into the design matrix and used [1, 0] and [0, 1] t-contrasts respectively as shown in figure 1. We accepted a statistical threshold of p<0.05, family-wise error (FWE) corrected for multiple comparisons, for the main effect analyses. Since previous studies indicate a central role for the temporal lobes in semantic processing 43, material-type specific effects were investigated within a region of interest comprising the bilateral temporal lobes at a significance threshold of p<0.001 uncorrected.
Figure 1.
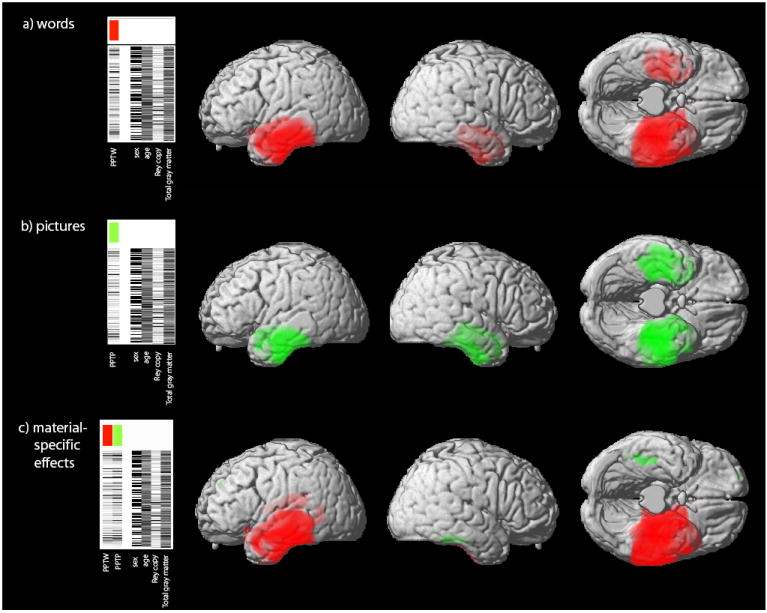
Brain areas that positively correlate with a) the verbal semantic task (PPTW); b) the non-verbal semantic task (PPTP) and c) the independent effects of words (red) and pictures (green). Design matrices and contrasts are displayed for each analysis. The threshold for display in a) and b) is p<0.05 (FWE corrected for multiple comparisons) and in c) is p<0.001 uncorrected. Maps of significant correlation are superimposed n a 3D rendering of the MNI standard brain.
Results
Main effects of material type
The regions of gray matter that correlated with scores on the word and picture versions of the PPT are shown at a significance level of p<0.05 FWE corrected for multiple comparisons in table 2 and figure 1 (a) and (b).
Table 2.
Main effects of material type. Results of individual VBM correlation analyses for the Pyramids and Palm Trees test (peaks significant at p<0.05 FWE corrected)
| Brain region | x | y | z | Z score |
|---|---|---|---|---|
| Pyramids and Palm Trees (Words) | ||||
| Left hemisphere | ||||
| hippocampus | -30 | -24 | -17 | >8 |
| fusiform gyrus | -32 | -9 | -39 | 7.8 |
| temporal pole | -44 | 13 | -24 | 7.62 |
| superior temporal gyrus | -47 | 2 | -12 | 7.42 |
| middle temporal gyrus | -62 | -11 | -20 | 7.08 |
| inferior temporal gyrus | -39 | -13 | -37 | >8 |
| caudate nucleus | -5 | 8 | 1 | 4.86 |
| cerebellum | -36 | -37 | -46 | 5.46 |
| Right hemisphere | ||||
| hippocampus | 32 | -17 | -21 | 5.4 |
| fusiform gyrus | 35 | -9 | -34 | 6.11 |
| parahippocampal gyrus | 37 | -20 | -26 | 5.77 |
| temporal pole | 44 | 12 | -22 | 4.95 |
| superior temporal gyrus | 49 | -2 | -14 | 5.4 |
| middle temporal gyrus | 52 | -29 | -11 | 5.01 |
| inferior temporal gyrus | 52 | -16 | -29 | 5.06 |
| Pyramids and Palm Trees (Pictures) | ||||
| Left hemisphere | ||||
| fusiform gyrus | -38 | -26 | -19 | 7 |
| hippocampus | -29 | -8 | -25 | 6.17 |
| temporal pole | -42 | 12 | -23 | 5.83 |
| superior temporal gyrus | -46 | 3 | -12 | 5.68 |
| middle temporal gyrus | -58 | -17 | -19 | 5.49 |
| inferior temporal gyrus | -44 | -19 | -30 | 6.45 |
| Right hemisphere | ||||
| fusiform gyrus | 40 | -24 | -24 | 6.3 |
| hippocampus | 24 | 2 | -28 | 5.11 |
| temporal pole | 31 | 13 | -32 | 5.95 |
| superior temporal gyrus | 47 | -1 | -15 | 5.03 |
| middle temporal gyrus | 49 | -13 | -12 | 5.42 |
| inferior temporal gyrus | 54 | -18 | -28 | 5.69 |
Words: When PPTW scores were considered as the single covariate in the analysis, two large clusters of significant voxels were found in the left and right temporal lobes. Peaks were identified in the bilateral hippocampus, fusiform gyrus, temporal pole and superior, middle and inferior temporal gyri, and in the left caudate nucleus and cerebellum.
Pictures: Similarly, large bitemporal voxel clusters were seen when PPTP scores were entered as the single covariate in the analysis. Peaks were identified in the bilateral hippocampus, fusiform gyrus, temporal pole and superior, middle and inferior temporal gyri.
To assess the relative volume of left and right temporal lobe gray matter that correlated with PPTW and PPTP scores, a laterality index (LI) was calculated by dividing the number of suprathreshold voxels (p<0.05 FWE corrected) in the left hemisphere by the number in the right. For the PPTW, the LI was 3.74 and for the PPTP the LI was 1.30, indicating that cortical regions supporting semantic association with pictorial stimuli are more symmetrical than those implicated when using verbal stimuli, which are predominantly left-sided.
In order to ensure that these results were not due to a general effect of disease severity, we reran the analyses using MMSE score as a nuisance covariate, and again using CDR box-scores as a nuisance covariate. The overall pattern and significance of gray matter correlations was unchanged for both verbal and pictorial versions of the Pyramids and Palm Trees test, and the laterality indices remained approximately the same.
The temporal lobes are atrophied bilaterally in semantic dementia raising the possibility that the observed correlations are a diagnosis-related effect. Therefore, we repeated the analyses entering diagnosis (‘SD’ or ‘not-SD’) into the design matrices as a nuisance covariate. Scores on the Pyramids and Palm Trees test were still associated with bilateral temporal lobe voxels, albeit at a predictably lower significance threshold. PPTW scores correlated with a left temporal peak at p<0.001 (uncorrected) and a right temporal peak at p=0.004 (uncorrected). PPTP scores correlated with a right temporal peak at p<0.001 (uncorrected) and a left temporal peak at p=0.003 (uncorrected).
Specific effects of Pictures and Words
Material-type specific effects were investigated within the temporal lobes bilaterally by entering both PPTW and PPTP scores into the design matrix as covariates of interest. Results are shown at a significance level of p<0.001 uncorrected for multiple comparisons in table 3 and figure 1(c).
Table 3.
Specific effect of material type. Coordinates of material-specific maxima (at p<0.001 uncorrected) with corresponding Z scores for the same region under the reverse comparison.
| Words>Pictures | Pictures>Words | |||||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | Z score | x | y | z | Z score | |
| Left hemisphere | ||||||||
| hippocampus | -26 | -23 | -16 | 6.5* | -37 | -27 | -15 | 1.2 |
| fusiform gyrus | -26 | 2 | -42 | 5.2* | n/a | |||
| temporal pole | -24 | 8 | -40 | 5.4* | -15 | 4 | -35 | 0.9 |
| superior temporal gyrus (ant) | -49 | -1 | -9 | 5.0* | -59 | -6 | -19 | 1.1 |
| superior temporal gyrus (post) | -43 | -37 | 18 | 4.5 | n/a | |||
| middle temporal gyrus | -63 | -25 | -18 | 4.7 | -51 | -25 | -18 | 1.3 |
| inferior temporal gyrus (ant) | -31 | -5 | -44 | 5.4 | -34 | -15 | -39 | 0.9 |
| inferior temporal gyrus (post) | -48 | -35 | -23 | 4.5 | -44 | -26 | -18 | 1.6 |
| Right hemisphere | ||||||||
| fusiform gyrus | 48 | -44 | -33 | 1.6 | 44 | -42 | -22 | 3.5† |
| middle temporal gyrus | 63 | -29 | -10 | 0.8 | 74 | -25 | -8 | 3.2† |
| inferior temporal gyrus | 45 | -16 | -31 | 1.7 | 55 | -20 | -26 | 3.1† |
| temporal pole | 26 | 6 | -37 | 2.1 | 32 | 15 | -32 | 3.1† |
= p<0.05 FWE corrected
= p<0.001 uncorrected
n/a = no voxels significant in this region at p<0.25
Specific effect of words: There were significant correlations between gray matter volume and PPTW score, independent of PPTP score, in left-sided structures only: the hippocampus/amygdala, temporal pole, and superior/middle and inferior temporal gyri. Correlations remained solely within the left hemisphere even at an uncorrected significance threshold of p<0.01.
Specific effect of pictures: There were significant correlations between gray matter volume and PPTP score, independent of PPTW score, in right-sided structures only: the fusiform gyrus, middle and inferior temporal gyri and temporal pole. Correlations remained solely within the right hemisphere even at an uncorrected significance threshold of p<0.01.
Post-hoc analysis: lobar volumes
We investigated this dissociation further, using an alternative volumetric method, amongst the group of patients with a diagnosis of SD, since they show the most marked semantic memory deficit and the greatest variance in temporal lobe volume. We divided the SD patients into those with predominantly ‘left’ (LTLV; n=17) and ‘right’ (RTLV; n=10) sided atrophy, as calculated using an automated Talairach-based method of regional classification 44 implemented in the BRAINS2 software package (Mental Health Clinical Research Center, University of Iowa http://www.psychiatry.uiowa.edu/mhcrc/). The method is described in detail elsewhere 45. Briefly, Z scores were calculated for patients’ left and right temporal lobe volumes, after correction for total intracranial volume, using the mean and standard deviation of the corrected left and right temporal lobe volumes respectively of control subjects. Patients were then classified as LTLV or RTLV according to which temporal lobe had the more negative Z score. The neuropsychological and volumetric data are presented in table 4. The RTLV group scored significantly higher on the CDR scale, reflecting the early behavioral changes in this presentation. The only other measure on which the groups were significantly different was PPTP score (LTL = 40.2; RTL = 34.5; p=0.05).
Table 4.
Regional volumes and neuropsychological data from semantic dementia patients with predominantly left temporal (LTL group) or right temporal (RTL group) atrophy.
| LTL group n=17 mean (StD) | RTL group n=10 mean (StD) | p | |
|---|---|---|---|
| Age | 63.3 (7.8) | 63.8 (7.1) | 0.868 |
| LTL volume Z score | -1.91 (1.37) | -0.92 (0.92) | 0.053 |
| RTL volume Z score | 0.28 (1.10) | -2.38 (0.89) | <0.001 |
| Total tissue volume (cc) | 858.4 (92.4) | 789.5 (84.4) | 0.065 |
| CDR (sum of boxes) | 2.7 (2.3) | 5.4 (2.2) | 0.006 |
| MMSE (30) | 22.8 (5.5) | 24.0 (6.1) | 0.612 |
| Rey copy (17) | 15.7 (1.6) | 14.9 (1.2) | 0.217 |
| Rey figure recall (17) | 7.7 (4.9) | 6.3 (4.7) | 0.488 |
| Digit span backwards | 4.8 (1.1) | 4.5 (1.3) | 0.572 |
| PPTW (52) | 36.7 (8.3) | 39.7 (7.8) | 0.354 |
| PPTP (52) | 40.2 (6.3) | 34.5 (8.1) | 0.050 |
| BNT (15) | 3.6 (3.0) | 5.1 (4.7) | 0.377 |
LTL = left temporal lobe; RTL = right temporal lobe
CDR = Clinical Dementia Rating; MMSE = mini-mental state examination; PPTW = Pyramids and Palm Trees (words version); PPTP = Pyramids and Palm Trees (pictures version); BNT = Boston Naming Test
In order to investigate the possibility of a differential performance on word and picture versions of the PPT test between the LTL and RTL groups, we conducted a repeated-measures ANOVA with Rey figure copy score as a covariate. This revealed no significant differences between groups (LTL or RTL: F(24,1)=0.23) or test material-type (PPTW or PPTP: F(24,1)=1.3) but showed a significant group by material-type interaction (F(24,1)=8.2, p=0.009). Two sample t tests showed that the RTL group scored lower than the LTL group on the PPTP (LTL = 40.2; RTL = 34.5), a difference that approached statistical significance (p=0.051), but that there was no significant difference in PPTW score (LTL = 36.6; RTL = 39.7; p=0.354).
Discussion
In this study we correlated scores on a pictorial and a verbal semantic association task with regional gray matter volume, using VBM in 144 patients with neurodegenerative disease. The degree of impairment on both tasks correlated with atrophy in the temporal lobes bilaterally, although the region of significant correlation was more symmetrical in the pictorial than in the verbal task. In addition, we have shown material-type specific effects for words in the left temporal lobe and for pictures in the right temporal lobe. The possible confound of impairment in lower-level visuospatial processing was taken into account by inclusion of performance on the Rey figure copy in the design matrix. Within the SD group, there was an interaction between the laterality of temporal lobe atrophy and relative performance on the verbal or pictorial semantic association task. We discuss the implications of these results for the understanding of semantic processing in the brain.
Our finding that scores on a test of semantic association correlated with gray matter volumes in the temporal lobe bilaterally, regardless of stimulus material-type, is in keeping with previous work in patients with semantic dementia 2, 6, 31 and other forms of neurodegenerative disease 46. The most highly correlated regions found in this study – the temporal pole, the lateral temporal gyri, the fusiform gyrus and the hippocampus – are consistent with those identified by functional neuroimaging studies of associative semantic knowledge 21, 22. It is important to recognize that the finding of correlations in these regions between atrophy and task performance does not imply that they are all necessarily involved in semantic processing. In neurodegenerative diseases, atrophy in distinct brain regions, such as the left and right temporal lobes, may be closely correlated. In the present study, it is not possible to say to what degree the bilateral findings in the main effects analyses are attributable to symmetrical atrophy rather than independent effects in each hemisphere. Notably, Lambon-Ralph et al. have recently demonstrated that transcranial magnetic stimulation of either the left or right temporal pole leads to disruption of performance on a synonym judgement task 47, supporting the hypothesis that both are independently necessary for semantic processing. Our failure to identify regions of correlation in the left prefrontal cortex, even at a reduced significance threshold (p<0.001 uncorrected), may reflect the fact that the PPT test is relatively easy compared with the tasks generally used in functional imaging paradigms with normal subjects. In addition, performance on the PPT has been shown to be comparatively spared in patients with frontal lobe atrophy (e.g. amyotrophic lateral sclerosis with frontotemporal dementia), although performance on a similar task involving action-based stimuli is impaired 48, 49.
Our observation of differential performance on the word and pictures versions of the PPT test according to the relative laterality of temporal lobe atrophy in SD is novel. Patients with predominant right temporal atrophy scored significantly worse than those with left-sided atrophy on the PPTP. There was no difference between the groups in performance on the PPTW. This latter result, which is at variance with the findings of the whole group VBM analysis, may be partially explained by the observation that all SD patients had significant left temporal lobe atrophy (see Table 4). Previous studies of associative semantics have found that patients with semantic dementia perform significantly better on the picture than word version of the PPT test 10, a modality-preference that, in some cases, extends across many semantic tasks 12. Thompson et al. 50 studied performance on a range of semantic tasks including picture naming and the word and picture versions of the PPT test, in a group of 47 SD patients, classified as L>R (more left than right temporal atrophy) or R>L (more right than left temporal atrophy) according to visual inspection of MRI or CT imaging by a neuroradiologist. They found that the L>R group was significantly worse than the R>L group on picture naming, category fluency and word-picture matching. There were no tasks on which the R>L group was significantly more impaired than the L>R group. They did not observe differential impairment on the PPT test according to modality of stimulus presentation. A number of factors may explain the discrepancy with the present study. Importantly, the classification of patients by an automated volumetric method has less potential for bias than visual inspection. In addition, the SD patients in the present study performed more poorly overall on the PPTW and PPTP, so it is possible that material-specific differences arise later in the disease. Furthermore, the repeated-measures ANOVA, as used in this study to analyze PPTW and PPTP scores, takes into account the correlation between the two.
The word-specific correlation that we observed with gray matter volume in the left temporal lobe is in accordance with several functional imaging studies that have compared activations on verbal and non-verbal semantic tasks in the visual 21, 22, 27 or auditory 27, 28 modalities. Vandenberghe et al. 21 used PET to study a semantic association task derived from the PPT test and found that the verbal task preferentially activated the left superior temporal sulcus, anterior middle temporal gyrus and inferior frontal sulcus. Deficits specific to semantic processing of verbal stimuli may be accounted for by their lack of perceptually salient features in comparison with pictorial representations, where meaning may be partially contained in the visual structure of the object (e.g. the ‘glove’ picture has the same shape as the ‘hand’ picture) that therefore enjoy ‘privileged access’51 to the semantic system. In addition, left temporal damage may lead to deficits in processes such as word-form analysis or lexical access that are difficult to separate from the semantic processes of interest.
Our finding that right temporal lobe regions correlated specifically with semantic association of pictorial stimuli also parallels functional imaging findings. Bright et al. 26, in an analysis of four PET studies using differing tasks (semantic categorization and lexical decision) and stimulus types (words or pictures), found overall greater activation for pictures than words in the bilateral fusiform gyri, left lingual gyrus and right inferior occipital gyrus and cerebellum. Thierry and Price 27, comparing conceptual processing of verbal and non-verbal stimuli in both visual and auditory modalities, found greater activation in the non-verbal trials in the right fusiform gyrus and right superior, middle and inferior temporal gyri. Of particular relevance is a report by Vandenbulcke et al. 52 of patient with a circumscribed ischemic lesion in the right fusiform gyrus, very close to the region identified in the present study, and an associated, isolated deficit in knowledge of visual attributes of concrete entities. The lesion overlapped with an area that, in normal controls, activated preferentially with pictorial stimuli in an fMRI-based semantic association paradigm derived from the PPT test.
With these findings in mind, we interpret our data as implying that semantically-driven, high-level visuoperceptual processes are dependent on the integrity of structures in the right inferomedial temporal lobe, perhaps associated with the storage of long-term visual memory in the form of structural descriptions 53-55. However, whilst the PPT test is widely used in clinical settings, it is not designed to assess in detail the nature of semantic memory impairment. Each trial can be solved using a variety of strategies – not necessarily limited to conceptual knowledge. Our study has highlighted the potential benefits of studying semantic memory in large groups of patients using VBM. More precise tests that probe finer-grained features of semantic impairment, such as loss of knowledge of visual or other sensory attributes, will be necessary to delineate more clearly the neural basis of conceptual knowledge.
Acknowledgments
This work was supported by the National Institute of Neurological Diseases and Stroke (R01 NS50915), the National Institute on Aging (P01 AG019724 and P50 AG-03-006), and the California Department of Health Services (DHS 04-35516).
References
- 1.Galton CJ, Patterson K, Graham K, et al. Differing patterns of temporal atrophy in Alzheimer’s disease and semantic dementia. Neurology. 2001;57:216–225. doi: 10.1212/wnl.57.2.216. [DOI] [PubMed] [Google Scholar]
- 2.Mummery CJ, Patterson K, Price CJ, et al. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- 3.Rosen HJ, Gorno-Tempini ML, Goldman WP, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- 4.Snowden J, Goulding P, Neary D. Semantic dementia: A form of circumscribed cerebral atrophy. Behavioural Neurology. 1989;2:167–182. [Google Scholar]
- 5.Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(Pt 6):1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- 6.Adlam AL, Patterson K, Rogers TT, et al. Semantic dementia and fluent primary progressive aphasia: two sides of the same coin? Brain. 2006;129:3066–3080. doi: 10.1093/brain/awl285. [DOI] [PubMed] [Google Scholar]
- 7.Hodges JR, Patterson K. Nonfluent progressive aphasia and semantic dementia: a comparative neuropsychological study. J Int Neuropsychol Soc. 1996;2:511–524. doi: 10.1017/s1355617700001685. [DOI] [PubMed] [Google Scholar]
- 8.Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garrard P, Carroll E. Lost in semantic space: a multi-modal, non-verbal assessment of feature knowledge in semantic dementia. Brain. 2006;129:1152–1163. doi: 10.1093/brain/awl069. [DOI] [PubMed] [Google Scholar]
- 10.Bozeat S, Lambon Ralph MA, Patterson K, et al. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38:1207–1215. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- 11.Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain. 2006;129:2132–2147. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- 12.Lauro-Grotto R, Piccini C, Shallice T. Modality-specific operations in semantic dementia. Cortex. 1997;33:593–622. doi: 10.1016/s0010-9452(08)70720-2. [DOI] [PubMed] [Google Scholar]
- 13.Patterson K, Hodges J. Semantic dementia: one window on the structure and organisation of semantic memory. In: Boller F, Grafman J, editors. Handbook of Neuropsychology: Memory and its disorders. 2. Vol. 2. Amsterdam: Elsevier Science; 2000. pp. 313–333. [Google Scholar]
- 14.Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- 15.Coltheart M, Marshall JC, Patterson K. Deep Dyslexia: Routledge and Kegan Paul. 1980. [Google Scholar]
- 16.Seliger GM, Lefever F, Lukas R, et al. Word deafness in head injury: implications for coma assessment and rehabilitation. Brain Inj. 1991;5:53–56. doi: 10.3109/02699059108998511. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy RA, Warrington EK. Evidence for modality-specific meaning systems in the brain. Nature. 1988;334:428–430. doi: 10.1038/334428a0. [DOI] [PubMed] [Google Scholar]
- 18.Shallice T. Multiple semantics: whose confusions? Cognitive neuropsychology(Print) 1993;10:251–261. [Google Scholar]
- 19.Fujii T, Fukatsu R, Watabe S, et al. Auditory sound agnosia without aphasia following a right temporal lobe lesion. Cortex. 1990;26:263–268. doi: 10.1016/s0010-9452(13)80355-3. [DOI] [PubMed] [Google Scholar]
- 20.Demonet JF, Chollet F, Ramsay S, et al. The anatomy of phonological and semantic processing in normal subjects. Brain. 1992;115(Pt 6):1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- 21.Vandenberghe R, Price C, Wise R, et al. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383:254–256. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- 22.Mummery CJ, Patterson K, Hodges JR, Price CJ. Functional neuroanatomy of the semantic system: divisible by what? J Cogn Neurosci. 1998;10:766–777. doi: 10.1162/089892998563059. [DOI] [PubMed] [Google Scholar]
- 23.Mummery CJ, Patterson K, Wise RJ, et al. Disrupted temporal lobe connections in semantic dementia. Brain. 1999;122(Pt 1):61–73. doi: 10.1093/brain/122.1.61. [DOI] [PubMed] [Google Scholar]
- 24.Devlin JT, Russell RP, Davis MH, et al. Is there an anatomical basis for category-specificity? Semantic memory studies in PET and fMRI. Neuropsychologia. 2002;40:54–75. doi: 10.1016/s0028-3932(01)00066-5. [DOI] [PubMed] [Google Scholar]
- 25.Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature. 1996;379:649–652. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- 26.Bright P, Moss H, Tyler LK. Unitary vs multiple semantics: PET studies of word and picture processing. Brain Lang. 2004;89:417–432. doi: 10.1016/j.bandl.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Thierry G, Price CJ. Dissociating Verbal and Nonverbal Conceptual Processing in the Human Brain. Journal of Cognitive Neuroscience. 2006;18:1018. doi: 10.1162/jocn.2006.18.6.1018. [DOI] [PubMed] [Google Scholar]
- 28.Thierry G, Giraud AL, Price C. Hemispheric Dissociation in Access to the Human Semantic System. Neuron. 2003;38:499–506. doi: 10.1016/s0896-6273(03)00199-5. [DOI] [PubMed] [Google Scholar]
- 29.Devlin JT, Russell RP, Davis MH, et al. Susceptibility-Induced Loss of Signal: Comparing PET and fMRI on a Semantic Task. NeuroImage. 2000;11:589–600. doi: 10.1006/nimg.2000.0595. [DOI] [PubMed] [Google Scholar]
- 30.Rosen HJ, Wilson MR, Schauer GF, et al. Neuroanatomical correlates of impaired recognition of emotion in dementia. Neuropsychologia. 2006;44:365–373. doi: 10.1016/j.neuropsychologia.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Brambati SM, Myers D, Wilson A, et al. The anatomy of category-specific object naming in neurodegenerative diseases. J Cogn Neurosci. 2006;18:1644–1653. doi: 10.1162/jocn.2006.18.10.1644. [DOI] [PubMed] [Google Scholar]
- 32.Williams GB, Nestor PJ, Hodges JR. Neural correlates of semantic and behavioural deficits in frontotemporal dementia. Neuroimage. 2005;24:1042–1051. doi: 10.1016/j.neuroimage.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 33.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol. 2003;54(Suppl 5):S15–19. doi: 10.1002/ana.10570. [DOI] [PubMed] [Google Scholar]
- 35.Peterson RC, Doody R, Kurz A. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 36.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 37.McKahn G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 38.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 39.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 40.Kramer JH, Jurik J, Sha SJ, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16:211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Howard D, Patterson K. Pyramids and palm trees: A test of semantic access from pictures and words. Bury St. Edmunds, UK: Thames Valley Test Company; 1992. [Google Scholar]
- 42.Good CD, Ashburner J, Frackowiak RS. Computational neuroanatomy: new perspectives for neuroradiology. Reviews in Neurology (Paris) 2001;157:797–806. [PubMed] [Google Scholar]
- 43.Rogers TT, Lambon Ralph MA, Garrard P, et al. Structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychol Rev. 2004;111:205–235. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- 44.Magnotta VA, Harris G, Andreasen NC, et al. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 45.Brambati SM, Rankin KP, Narvid J, et al. Atrophy progression in semantic dementia with asymmetric temporal involvement: A tensor-based morphometry study. Neurobiology of Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.05.014. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grossman M, McMillan C, Moore P, et al. What’s in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer’s disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127:628–649. doi: 10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- 47.Lambon Ralph MA, Pobric G, Jefferies E. Conceptual Knowledge Is Underpinned by the Temporal Pole Bilaterally: Convergent Evidence from rTMS. Cereb Cortex. 2008:bhn131. doi: 10.1093/cercor/bhn131. [DOI] [PubMed] [Google Scholar]
- 48.Bak TH, Hodges JR. The effects of motor neurone disease on language: Further evidence. Brain and Language. 2004;89:354–361. doi: 10.1016/S0093-934X(03)00357-2. [DOI] [PubMed] [Google Scholar]
- 49.Grossman M, Anderson C, Khan A, et al. Impaired action knowledge in amyotrophic lateral sclerosis. Neurology. 2008;71:1396–1401. doi: 10.1212/01.wnl.0000319701.50168.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology. 2003;61:1196–1203. doi: 10.1212/01.wnl.0000091868.28557.b8. [DOI] [PubMed] [Google Scholar]
- 51.Caramazza A, Hillis AE, Rapp BC, Romani C. The multiple semantics hypothesis: Multiple confusions. Cognitive Neuropsychology. 1990;7:161–189. [Google Scholar]
- 52.Vandenbulcke M, Peeters R, Fannes K, Vandenberghe R. Knowledge of visual attributes in the right hemisphere. Nat Neurosci. 2006;9:964–970. doi: 10.1038/nn1721. [DOI] [PubMed] [Google Scholar]
- 53.Kellenbach ML, Hovius M, Patterson K. A pet study of visual and semantic knowledge about objects. Cortex. 2005;41:121–132. doi: 10.1016/s0010-9452(08)70887-6. [DOI] [PubMed] [Google Scholar]
- 54.Humphreys GW, Forde EM. Hierarchies, similarity, and interactivity in object recognition: “category-specific” neuropsychological deficits. Behav Brain Sci. 2001;24:453–476. discussion 476-509. [PubMed] [Google Scholar]
- 55.Schacter DL, Reiman E, Uecker A, et al. Brain regions associated with retrieval of structurally coherent visual information. Nature. 1995;376:587–590. doi: 10.1038/376587a0. [DOI] [PubMed] [Google Scholar]


