Abstract
Purpose
To compare the effects of single-sitting vs. four-sitting panretinal photocoagulation (PRP) on macular edema in subjects with severe non-proliferative or early proliferative diabetic retinopathy with relatively good visual acuity and no or mild center involved macular edema.
Method
Subjects were treated with one sitting or 4 sittings of PRP in a non-randomized, prospective, multi-centered clinical trial.
Main Outcome Measures
Central subfield thickness on optical coherence tomography (OCT).
Results
Central subfield thickness was slightly greater in the 1 sitting group (n=84) than in the 4 sitting group (n=71) at the 3-day (P=0.01) and 4-week visits (P=0.003). At the 34-week primary outcome visit, the slight differences had reversed, with the thickness being slightly greater in the 4-sitting group than in the 1-sitting group (P=0.06). Visual acuity differences paralleled OCT differences.
Conclusions
Our results suggest that clinically meaningful differences are unlikely in OCT thickness or visual acuity following application of PRP in 1 sitting compared with 4 sittings in subjects in this cohort. More definitive results would require a large randomized trial.
Application to Clinical Practice
These results suggest PRP costs to some patients, in terms of travel and lost productivity, as well as to eye care providers, could be reduced.
Introduction
The Diabetic Retinopathy Study (DRS) demonstrated that panretinal (scatter) photocoagulation (PRP) reduced the risk of severe vision loss (<5/200 at 2 consecutive 4-month visits) due to complications of proliferative diabetic retinopathy (PDR) from 25% to 14% over 2 years.1 The Early Treatment Diabetic Retinopathy Study (ETDRS) subsequently demonstrated, for patients with severe non-proliferative or proliferative diabetic retinopathy, PRP and vitrectomy when necessary reduced the risk of severe vision loss to 4% over 5 years.2 Consequently, the use of PRP has been accepted as the standard care for patients with PDR. 3
PRP has been associated with numerous complications that can result in decreased visual acuity, including macular edema.3, 4 In the ETDRS, which was performed prior to the advent of OCT, 18% of eyes that underwent full PRP (1200 to 1600 spots) were noted to have macular edema on stereoscopic fundus photographs by 4 months (Ferris FL, unpublished data). There are multiple theories as to why the edema may occur, including oncotic fluid accumulation related to the tissue destruction or PRP induced inflammation leading to cytokine release and increased permeability of the retinal capillaries.5
In the ETDRS, PRP generally was given over 2 or more sittings, usually within 4 weeks.6 Some clinicians, however, have recommended doing the PRP in a single sitting.7 In a 2004 survey of investigators participating in the Diabetic Retinopathy Clinical Research Network (DRCR.net), about a quarter of the responding investigators indicated that they routinely performed PRP in a single sitting while three-quarters used multiple sittings. While completion of PRP in one sitting might be more convenient with respect to number of office visits and compliance with completion of treatment plan, there is at least a theoretical concern that this may increase the development of vision disabling macular edema, although there are few data published to support this hypothesis. Other potential side effects include pain sometimes requiring retrobulbar or peribulbar anesthesia, choroidal effusion or detachment, accommodative paresis, and acute elevation of intraocular pressure. 8–11, 13–15 Conversely, limiting the total number of spots at any one sitting by completing the scatter over multiple sittings may allow the edema to subside before the next sitting of PRP. As possible support for this hypothesis, Shimura et al12 found that macular edema was more likely when the PRP regimen was given in four sittings at weekly intervals compared with four sittings at bi-weekly intervals in a group of eyes with OCT evidence of pre-existing macular edema.
There are more than 63,000 new cases of PDR each year in the United States.16 PRP is indicated in the vast majority of these cases and in some of the 700,000 persons in the US with PDR at any given time. Thus, determining if the morbidity from macular edema and accompanying visual acuity loss from PRP differs when the treatment is completed in a single sitting versus multiple sittings would be of interest to the many people receiving PRP each year. If there are no relevant clinical differences, then patient and physician convenience as well as possible economic consequences can be major factors in determining the number of PRP sittings. Potentially better compliance with the recommended treatment could also be realized.
The DRCR.net conducted a study to compare PRP in one sitting with PRP distributed over four sittings. Although a randomized trial was proposed, a majority of investigators had a strong bias for one vs more than one sitting, precluding a randomized trial design. Therefore, as a potential prelude to a randomized trial protocol, a prospective nonrandomized study was conducted. In order to minimize selection bias, each investigator was required, prior to study initiation, to indicate whether he/she would perform PRP in one or four sittings for all study subjects they enrolled.
Methods
The study was conducted by the Diabetic Retinopathy Clinical Research Network (DRCR.net) at 27 clinical sites in the United States. The protocol and HIPAA-compliant informed consent forms were approved by multiple institutional review boards. Each subject gave written informed consent to participate in the study. Study oversight was provided by an independent data and safety monitoring committee. The study is listed on www.clinicaltrials.gov, under identifier NCT00687154 and the protocol is available on the DRCR.net website (www.drcr.net) and summarized below.
Study Population
Eligible patients had to be at least 18 years old with type 1 or type 2 diabetes. Patients were excluded if they had a history of chronic renal failure requiring dialysis, kidney transplant, pancreatic transplant, or intensive insulin therapy initiated within 4 months of randomization. The major eligibility criteria for a study eye included (1) early proliferative or severe nonproliferative diabetic retinopathy for which investigator intended to perform full scatter photocoagulation, (2) retinal thickness measured on Optical Coherence Tomography (OCT) < 300 microns in the central subfield (for the first 5 months of the enrollment period the eligibility criteria was center point thickness ≤200 microns and then the protocol was amended to increase the upper limit to 300 microns in the central subfield), and (3) visual acuity letter score ≥73 (20/32 or better) measured with the electronic ETDRS method.17 An eye was not eligible if it had received prior scatter photocoagulation, had high risk (severe proliferative) retinopathy, had macular edema that had been treated in the prior 6 months or for which treatment was planned, had a history of major ocular surgery (including cataract extraction, vitrectomy, scleral buckle, any intraocular surgery) within the prior 6 months or anticipated within the next 8 months, or had a history of YAG capsulotomy performed within the prior 2 months. A subject could have only one study eye.
Each investigator was required to declare, prior to enrollment, whether he or she was going to perform PRP in one or four sittings for all patients participating in this protocol under his or her care. The one-sitting regimen consisted of the application of 1200 to 1600 burns. The four-sitting regimen was spread over 12 weeks, with each sitting separated by four weeks (±4 days) and consisted of approximately 300 burns in each of the first two sittings and investigator judgment for number of burns for the third and fourth sittings as long as the total for the four sittings was between 1200 and 1600 burns. For both groups, treatment burns were to be spaced one burn width apart and were to extend from the vascular arcades to beyond the equator
The burn characteristics were as follows: (1) size: argon laser using 200 micron spot size with Rodenstock lens (or equivalent) or 500 micron spot size with three mirror contact lens, (2) exposure: 0.1 seconds recommended, 0.05 to 0.2 seconds allowed, (3) intensity: standard mild white retinal burns, i.e., 2+ to 3+ burns, no 4+ burns permitted,18, 19 (4) distribution: edges at least 1 burn width apart, no closer than one row within the arcades, no closer than two disc diameters temporal to the fovea, (5) extent: arcades (~3000 microns from the macular center) to at least the equator, (6) wavelength: green or yellow (red could be used if vitreous hemorrhage is present precluding use of green or yellow). A retrobulbar injection, peribulbar or sub-Tenon’s injection could be used at investigator discretion. An indirect laser delivery system could not be used. All treatments were performed with a laser which produced manually aimed burns, although a repeat mode could be used at investigator discretion.
Follow-up visits were performed in both groups after 3 days, 4 weeks, 17 weeks, and 34 weeks within pre-specified time windows. At baseline and at each of these follow-up visits, best-corrected visual acuity was measured at 3 meters. A certified visual acuity examiner completed a refraction following DRCR.net specific protocol and tested visual acuity using the ETDRS electronic visual acuity system.17 Optical coherence tomography (OCT) images were obtained through a dilated pupil by a certified operator using the Zeiss Stratus OCT (OCT3). Seven-field fundus photographs were obtained at baseline and 3-field photographs at 34 weeks. Both sets were sent to the DRCR.net Reading Center at the University of Wisconsin-Madison for grading.
If macular edema developed and resulted in a decrease of more than 10 letters in either treatment group, and this acuity decrease from macular edema still was present on a second examination at least two weeks later, focal/grid photocoagulation could be given at the discretion of the investigator and completion of PRP in the 4-sitting group could be deferred. If a vitreous hemorrhage occurred, additional PRP could be given.
OCT scans were 6 mm length and included the 6 radial line pattern (fast macular scan option with OCT3) for quantitative measures and the cross hair pattern (6–12 to 9-3 o’clock) for qualitative assessment of retinal morphology. The OCT scans were sent to the DRCR.net Reading Center at the University of Wisconsin-Madison for grading. Based on review of these scans by an expert grader at the Reading Center, a total of 11% of the 155 baseline scans and 11% of the 567 follow-up scans were judged by the Reading Center to have inaccurate automated thickness measurements because the automated placement of lines by the OCT software were judged morphologically not to be at the inner aspect of the retina or outer aspect of the retina or both. In these cases, center point thickness was measured manually and the resultant value used to impute a value for the central subfield (based on a correlation of the two measures of 0.98) as previously published. 20
Statistical Methods
A sample size was planned to be approximately 150 eyes (only one eye per subject was entered into the study), with approximately equal numbers receiving each of the two treatment regimens. If both eyes were eligible, the investigator at his/her discretion selected one of the eyes to be the study eye. As an observational study, this protocol aimed to determine if any trends exist and if the trends are strong enough to warrant a phase 3 trial.
The primary study outcome was OCT-measured central subfield thickening at 34 weeks; the main secondary outcome was visual acuity at 34 weeks. Another secondary outcome was the presence of retinal thickness in follow-up of at least 250 microns in the central subfield combined with an increase in OCT measured retinal thickness from baseline of at least 25 microns (representing the half-width of the 95% confidence interval on the difference between replicate OCT measurements in eyes with levels of macular edema similar to this cohort21). Outcomes related to PRP safety and efficacy included the need for additional PRP or the development of vitreous hemorrhage.
Burn number and average power were compared between 1-sitting and 4-sitting groups using Wilcoxon rank sum tests. Signed rank tests were performed on changes in OCT central subfield thickness from baseline at each follow up visit for 1-sitting and 4-sitting groups respectively. Statistical comparisons of 1-sitting group vs. 4-sitting group for the continuous outcomes were performed using non-parametric analysis based on ranks (van der Waerden scores) adjusted for baseline values. The models were also adjusted for race and baseline retinopathy severity to account for potential confounding due to the slight baseline imbalances between treatment groups. Logistic regression models adjusted for baseline values, race and baseline retinopathy severity were used to assess dichotomous outcomes. Subgroup analyses were also performed stratified by baseline retinopathy severity (non-proliferative vs. proliferative retinopathy). Missed visits were excluded from the analysis. Analyses using the last observation carried forward imputation method gave similar results (data not shown).
All P values are 2-tailed. SAS version 9.1 (Cary, NC) was used for all analyses.
Results
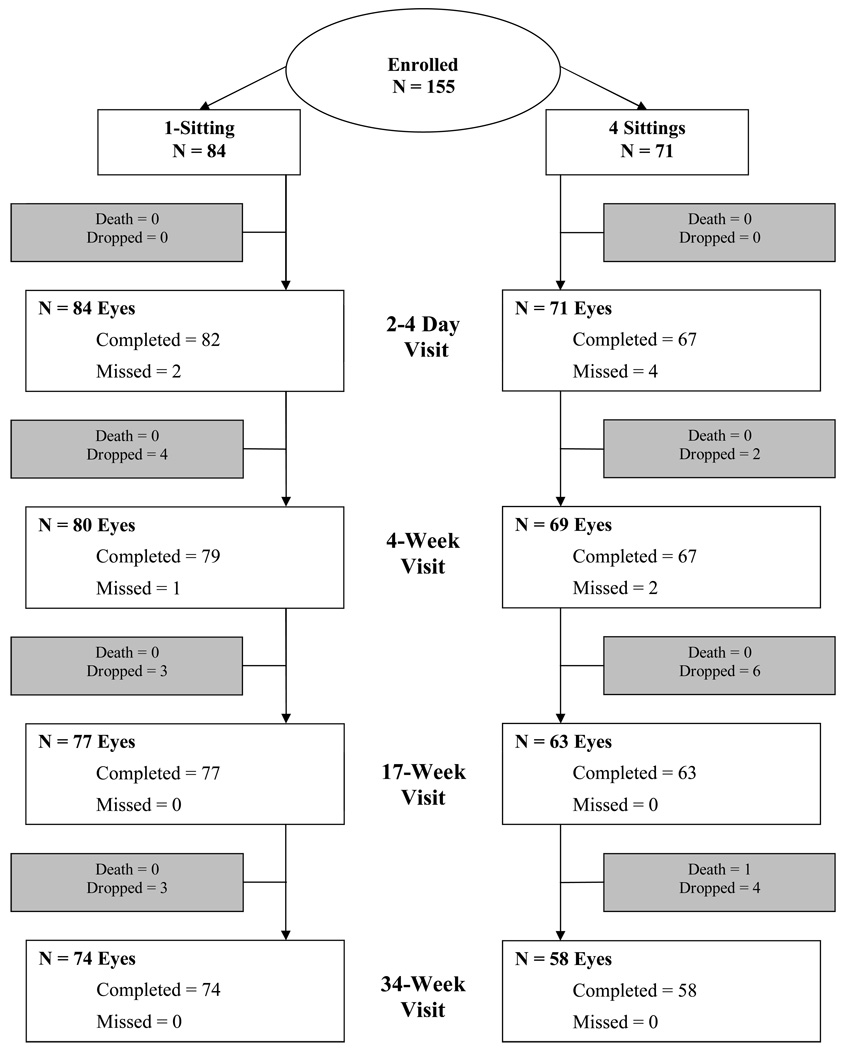
Between 2005 and 2007, 155 subjects (mean age 55±11 years; 46% women) were enrolled at 27 sites. Mean visual acuity was 20/25 (letter score 83±5) and mean OCT central subfield retinal thickness was 202±26 microns, with the central subfield being <250 microns in 151 (97%) eyes. Among the 155 subjects, 144 had fundus photographs which could be graded by the Reading Center; 73 eyes (51%) were classified as having nonproliferative retinopathy, 50 eyes (35%) had non-high risk PDR, and 21 (15%) eyes had high risk PDR (even though these latter 21 eyes were not considered to have high risk PDR by the enrolling ophthalmologist). Macular edema was considered to be present on baseline photographs in 27 eyes (18%). Eighty-four eyes were enrolled in the 1-sitting group and 71 in the 4-sitting group. The baseline characteristics of the two groups are presented in Table 1.
Table 1.
Baseline Characteristics by Treatment Group
| 1 Sitting N= 84 |
4 Sittings N= 71 |
|
|---|---|---|
| Gender: Women - n (%) | 40 (48%) | 31 (44%) |
| Age (yrs) - Median (25th, 75th percentile) | 56 (49, 64) |
54 (45, 61) |
| Race – n (%) | ||
| White | 54 (64%) | 33 (46%) |
| African-American | 20 (24%) | 20 (28%) |
| Hispanic or Latino | 9 (11%) | 13 (18%) |
| Asian | 0 | 4 (6%) |
| More than one race | 1 (1%) | 1 (1%) |
| Diabetes Type - n (%) | ||
| Type 1 | 16 (19%) | 18 (25%) |
| Type 2 | 68 (81%) | 53 (75%) |
| Duration of Diabetes (years)- | ||
| Median (25th, 75th percentile) | 18 (13, 24) |
20 (13, 27) |
| HbA1c (%) -Median (25th, 75th percentile) | 7.7 (6.8, 9.1) |
8.2 (7.2, 10.6) |
| Prior treatment for DME in study eye - n (%) | ||
| None | 72 (86%) | 63 (89%) |
| Focal laser photocoagulation | 12 (14%) | 8 (11%) |
| E-ETDRS Visual Acuity | ||
| Median (25th, 75th percentile) – letter score | 85 (79, 88) |
83 (77, 87) |
| Central Subfield Thickness | ||
| Median (25th, 75th percentile) - Microns | 207 (191, 222) |
198 (182, 222) |
| ≥ 250 – 299 Microns - n (%) | 3 (4%) | 1 (1%) |
| Retinal Volume | ||
| Median (25th, 75th percentile) - mm3 | 6.9 (6.6, 7.4) |
7.0 (6.5, 7.4) |
|
1 Sitting N=81 |
4 Sittings N=66 |
|
|
Retinopathy Severity - n (%) (ETDRS Severity Scale)* |
||
| Mild NPDR (35) | 5 (6%) | 0 |
| Moderate NPDR (43) | 7 (9%) | 2 (3%) |
| Moderately severe NPDR (47) | 24 (30%) | 25 (38%) |
| Severe NPDR (53) | 5 (6%) | 5 (8%) |
| Mild PDR (60, 61) | 19 (23%) | 10 (15%) |
| Moderate PDR (65) | 11 (14%) | 10 (15%) |
| High Risk PDR (71, 75) | 9 (11%) | 12 (18%) |
| Cannot Grade (90) | 1 (1%) | 2 (3%) |
HbA1c: Missing 7 in one-sitting group, 13 in four-sittings group
Early Treatment Diabetic Retinopathy Study Research Group. Fundus Photographic Risk Factors for Progression of Diabetic Retinopathy: ETDRS report no. 12. Ophthalmology 1991; 98:823–833
Photo missing 3 in one-sitting group and 5 in four-sittings group.
Panretinal (Scatter) Photocoagulation (PRP) Treatment
In the 1-sitting group, 84 eyes received a median of 1274 burns (interquartile range 1220 to 1406) compared with 1260 burns (interquartile range 1200 to 1456) in the 71 eyes in the 4-sitting group (P=0.73). Eleven eyes in the 4-sitting group had fewer than 4 sittings due to missed visits, with the median number of burns in these eyes being 618. Median average power was 280 mW and 250 mW in the two groups, respectively (P=0.89). Thirty-nine (46%) of the eyes in the 1-sitting group received a retrobulbar anesthetic injection prior to treatment while an injection was received for at least one sitting in 10 (14%) eyes in the 4-sitting group (P<0.001). Additional scatter photocoagulation after completion of the initial regimen, and before 34 weeks, was given to 6 eyes in the 1-stting group.
Follow Up
Visit completion was 98% at the 3-day visit, 94% at the 4-week visit, 92% at the 17-week visit, and 88% at the 34 week visit in the 1-sitting group and 94%, 94%, 89%, and 82%, respectively, in the 4-sitting group (Figure 1). One subject died (in the 4-sitting group). Among the 11 subjects in the 4-sitting group with an incomplete laser regimen, 2 completed both the 17-week and 34-week visit, 2 completed just the 17-week visit, and 7 dropped out prior to the 17-week visit.
Figure 1.
Retinal Thickness
Median OCT central subfield thickness at baseline was 207 microns in the 1-sitting group and 198 microns in the 4-sitting group. During follow up, thickness tended to increase slightly from baseline levels in both the 1-sitting and 4-sitting group (1-sitting group: P< 0.001 at each visit compared with baseline; 4-sitting group: P= 0.004 at 3 days and <0.001 at 4 weeks, 17 weeks, and 34 weeks; Table 2). However, in the 1-sitting group the median changes appear stable across all follow-up visits, while in the 4-sitting group the median changes appear to increase throughout the study.
Table 2.
OCT-measured Retinal Thickness at Follow-up Visits
| 1 Sitting N=84 |
4 Sittings N=71 |
P value | |
|---|---|---|---|
| Central Subfield | |||
| Baseline - Median (quartiles) microns | 207 (191, 222) | 198 (182, 222) | |
| ≥ 250 – 299 Microns - n (%) | 3 (4%) | 1 (1%) | |
| Change from Baseline - Median (quartiles) microns (p-values obtained based on van der Waerden scores) | |||
| 3 Day | +9 (+4, +14) | +5 (−2, +9) | 0.01 |
| 4 Weeks | +13 (+5, +21) | +5 (+1, +15) | 0.003 |
| 17 Weeks | +14 (+5, +20) | +15 (+6, +34) | 0.08 |
| 34 Weeks | +14 (+2, +25) | +22 (+5, +41) | 0.06 |
| Change from Baseline ≥ 25 Microns - n (%) (p-values obtained based on logistic regressions) | |||
| 3 Day | 7 (9%) | 3 (5%) | 0.32 |
| 4 Weeks | 14 (18%) | 5 (8%) | 0.04 |
| 17 Weeks | 13 (17%) | 26 (41%) | 0.001 |
| 34 Weeks | 18 (25%) | 25 (45%) | 0.005 |
| ≥250 Microns and Change from Baseline ≥ 25 Microns - n (%) (p-values obtained based on logistic regressions) | |||
| 3 Day | 1 (1%) | 2 (3%) | 0.40 |
| 4 Weeks | 8 (10%) | 3 (5%) | 0.14 |
| 17 Weeks | 5 (6%) | 11 (17%) | 0.003 |
| 34 Weeks | 9 (13%) | 13 (24%) | 0.02 |
| Retinal Volume | |||
| Baseline - median (quartiles) microns | 6.9 (6.6, 7.4) | 7.0 (6.5, 7.4) | |
| Change from Baseline - Median (quartiles) microns (p-values obtained based on van der Waerden scores) | |||
| 3 Day | +0.3 (+0.1, +0.4) | +0.1 (0, +0.2) | 0.001 |
| 4 Weeks | +0.4 (+0.3, +0.6) | +0.3 (+0.1, +0.4) | <.001 |
| 17 Weeks | +0.3 (+0.2, +0.5) | +0.6 (+0.3, +0.9) | 0.001 |
| 34 Weeks | +0.2 (0, +0.5) | +0.5 (0, +0.8) | 0.03 |
| N | |||
| 3 Day | 81 | 64 | |
| 4 Weeks | 78 | 65 | |
| 17 Weeks | 77 | 63 | |
| 34 Weeks | 72 | 55 | |
At the 3-day visit (after completion of the one sitting regimen and the first sitting of the four sitting regimen), the median change from baseline in central subfield thickness was +9 microns in the 1-sitting group compared with +5 microns in the 4-sitting group (P=0.01), with 9% and 5% of eyes in the two groups, respectively, having increased by 25 microns or more (P=0.32). At 4 weeks, the 1-sitting group had a greater median change in retinal thickness from baseline (+13 microns) than the 4-sitting group (+5 microns, P=0.003); at this time, there was also an increase from baseline retinal thickness of 25 microns or more in 18% of the 1 sitting group compared with 8% (P=0.04) in the 4 sitting group.
By 17 weeks (approximately 17 weeks after the 1-sitting group regimen had been completed and approximately 5 weeks after the 4-sitting group regimen had been completed), median central subfield thickness in the 4-sitting group had increased to a level similar to that in the 1-sitting group (median change from baseline +14 microns versus +15 microns, respectively, P=0.08). Seventeen percent of eyes in the 1-sitting group and 41% in the 4-sitting group had an increase of at least 25 microns (P=0.001), and 6% and 17%, respectively, had an increase of at least 25 microns from baseline with a thickness of 250 microns or greater (P=0.003).
At 34 weeks (approximately 34 weeks after the 1-sitting group regimen had been completed and approximately 22 weeks after the 4-sitting group regiment had been completed), the median change in the central subfield thickening in the 1-sitting group was stable at +14 microns but there was a further increase in the 4-sitting group to +22 microns (P=0.06). Twenty-five percent of the eyes in the one sitting group and 45% of eyes in the 4-sitting group had an increase in the central subfield thickening of at least 25 microns (P=0.005), including 13% and 24%, respectively having an increase of at least 25 microns to a thickness of 250 microns or greater (P=0.02). Results for OCT-measured retinal volume were similar to the central subfield results, with a greater increase seen in the 1-sitting group compared with the 4-sitting group at 3 days and 4 weeks, but a greater increase in the 4-sitting group compared with the 1-sitting group at 17 weeks and 34 weeks (Table 2).
Subgroup analysis of eyes with non-proliferative and proliferative retinopathy at baseline gave similar results to the pooled analyses for both central subfield thickening and retinal volume.
Prior to the 34-week visit, macular edema was treated with focal/grid photocoagulation in 1 eye in the 1-sitting group and 1 eye in the 4-sitting group. On the 34-week fundus photographs, macular edema was present in 17 eyes (23%) in the 1-sitting group and 15 eyes (29%) in the 4-sitting group.
Visual Acuity
The visual acuity results generally paralleled the retinal thickening results (Table 3). The visual acuity was slightly worse in the 1-sitting group compared with the 4-sitting group at the 3-day visit with a median change from baseline in letter score of −3 and −1, respectively (P=0.005). At weeks 4 and 17, the visual acuity change from baseline was similar with a median change from baseline in letter score of −1 in each group at both visits (P=0.37 and 0.66 respectively). By 34 weeks, the visual acuity was slightly worse in the 4-sitting group with a median change from baseline in letter score of 0 versus −2 (P=0.006). Although few eyes experienced a worsening of 10 or more letters from baseline, this change was greater at the 3-day visit in the 1-sitting group (13% versus 2%, P=0.004); thereafter, little difference for this outcome was observed between groups. At 34 weeks, 7% in the 1-sitting group and 9% in the 4-sitting group had visual acuity 10 or more letters worse than baseline (P=0.75). There was no obvious correlation of an increase in retinal thickening by 25 microns or more with visual acuity loss (5 or more letters, or 10 or more letters).
Table 3.
Visual Acuity at Follow-up Visits
| 1 Sitting N=84 |
4 Sittings N=71 |
P value* | |
|---|---|---|---|
| Baseline -median (quartiles) letter score | 85 (79, 88) | 83 (77, 87) | |
| Change from Baseline median (quartiles) letter score | |||
| 3 Day | −3 (−6, 0) | −1 (−3, +1) | 0.005 |
| 4 Weeks | −1 (−5, +1) | −1 (−3, +2) | 0.37 |
| 17 Weeks | −1 (−4, +2) | −1 (−4, +2) | 0.66 |
| 34 Weeks | 0 (−3, +3) | −2 (−6, −1) | 0.006 |
| Distribution of Change - n (%) | |||
| 3 Day | |||
| ≥ 10 letters improvement | 0 | 1 (2%) | |
| 5 – 9 letters improvement | 3 (4%) | 4 (6%) | |
| within ± 4 letters | 49 (60%) | 49 (74%) | |
| 5 – 9 letters worse | 19 (23%) | 11 (17%) | |
| ≥ 10 letters worse | 11 (13%) | 1 (2%) | |
| 4 Weeks | |||
| ≥ 10 letters improvement | 2 (3%) | 1 (1%) | |
| 5 – 9 letters improvement | 5 (6%) | 6 (9%) | |
| within ± 4 letters | 50 (64%) | 50 (75%) | |
| 5 – 9 letters worse | 20 (26%) | 8 (12%) | |
| ≥ 10 letters worse | 1 (1%) | 2 (3%) | |
| 17 Weeks | |||
| ≥ 10 letters improvement | 1 (1%) | 3 (5%) | |
| 5 – 9 letters improvement | 2 (3%) | 3 (5%) | |
| within ± 4 letters | 57 (74%) | 43 (68%) | |
| 5 – 9 letters worse | 14 (18%) | 9 (14%) | |
| ≥ 10 letters worse | 3 (4%) | 5 (8%) | |
| 34 Weeks | |||
| ≥ 10 letters improvement | 0 | 4 (7%) | |
| 5 – 9 letters improvement | 12 (16%) | 1 (2%) | |
| within ± 4 letters | 47 (64%) | 33 (57%) | |
| 5 – 9 letters worse | 10 (14%) | 15 (26%) | |
| ≥ 10 letters worse | 5 (7%) | 5 (9%) | |
| N | |||
| 2 – 4 Day | 82 | 66 | |
| 17 Weeks | 78 | 67 | |
| 17 Weeks | 77 | 63 | |
| 34 Weeks | 74 | 58 |
P values obtained based on van der Waerden scores.
Similar results were observed when analyzed separately for eyes with non-proliferative vs. proliferative retinopathy at baseline or with respect to the anesthesia used to apply PRP.
Vitreous Hemorrhage
Between the 17-week and 34-week visit, a vitreous hemorrhage reducing acuity by 10 or more letters from baseline occurred in 2 eyes in each group. These eyes had mild and moderate PDR (in the 1-sitting group) and one had high risk PDR in the 4-sitting group (the Reading Center was not able to grade the retinopathy level of the other eye) at baseline.
Discussion
This study evaluated 155 individuals undergoing panretinal (scatter) photocoagulation (PRP) for diabetic retinopathy with relatively good visual acuity and no or minimal macular edema within two nonrandomized groups, completion of PRP in 1 or 4 sittings. While some eyes had an increase in OCT-measured central retinal thickening following treatment in either treatment group, most eyes maintained a central retinal thickness within a normal range. Retinal thickening was greater in the first few days after completion of the entire treatment regimen of 1200 to 1600 burns in a single sitting compared with the initial 300 burns from the first of four sittings. However, this treatment group difference was transient, and the results at 34 weeks of follow up suggested that retinal thickening might be slightly greater with the 4-sitting regimen. It should be noted that at 34 week, the eyes in the 4-sitting group had a shorter time from the last laser sitting than in the 1-sitting group. The eyes within the 4-sitting group might have improved further on OCT and visual acuity measurements if more follow-up was obtained.
The number of burns administered in the two groups was similar. Not unexpectedly, retrobulbar/peribulbar anesthesia was used more commonly in the 1-sitting group, although without any apparent serious adverse events noted nor any differences in OCT or visual acuity outcomes. Although there was a slight increase in OCT-measured central subfield thickness in both groups overall, only 1 eye in the 1-sitting group and 1 eye in the 4-sitting group developed macular edema sufficient to be treated with focal/grid photocoagulation. The only difference judged clinically relevant between groups at any time point was the increased chance for visual acuity loss in the one-sitting group at the 3 day visit which no longer was apparent between 4 weeks and 17 weeks. By the 34 week visit, it is possible that the course of the disease, independent of the number of sittings used to complete the PRP, could have a greater influence on the OCT and visual acuity measurements than any differential impact of the number of sittings on these outcomes.
Fundus photographs were not obtained to evaluate level of retinopathy at the end of the 34 weeks of follow up; therefore, direct comparison of the efficacy of the scatter treatment in the two groups cannot be made. Few eyes required additional scatter treatment after completion of the initial regimen and few developed a vitreous hemorrhage.
In one study published in 1982, investigators reported a single-center randomized study evaluating the beneficial or adverse effects of argon laser photocoagulation for proliferative diabetic retinopathy depending on whether treatment was administered in a single sitting or multiple sittings. Results showed no major differences between groups in the effect of treatment on visual acuity, visual field scores, or retinopathy risk factors; OCT was not available at the time. According to Doft, exudative retinal detachment, choroidal detachment, and angle closure occurred more commonly in the single sitting regimen, but these side effects were transient, and no long-term difference between treatment groups was found. 22 These side effects also have been reported by others8, 9 10, 11 however, none of these side effects were identified in our study, although we did not perform ultrasonography to look for shallowing of the anterior chamber. We are not aware of any other prospective studies that have compared PRP administered in one sitting and multiple sittings. However, other prior studies have evaluated the development of macular edema following a multiple-sitting PRP regimen. Most of the literature consists of case reports and case series.4, 12, 23–25 Shimura et al 12 conducted a prospective study in which 36 patients with type 2 diabetes who had bilateral symmetric severe nonproliferative or early proliferative retinopathy without clinically significant macular edema received PRP in 4 sittings. Each subject received the treatment once a week for 4 weeks in one eye and once every other week over 8 weeks in the other eye. The authors reported that there was a greater increase in central retinal thickening in the eyes treated weekly than in the eyes treated biweekly and the resolution of the edema was slower in the eyes treated weekly. Their results suggest a somewhat greater increase in retinal thickening after scatter treatment than was reported in this study. However, since their results excluded 7 eyes that developed macular edema and 39% of eyes received additional scatter treatment after completion of the initial regimen (whereas only 4% of eyes in our cohort did), it is difficult to compare their results to the results presented in this study. In the Early Treatment Diabetic Retinopathy Study (ETDRS), which was performed prior to OCT availability, 18% of eyes that had been treated with full scatter photocoagulation in 2 or more sittings were noted to have macular edema graded on fundus photographs at 4 months. While this value is similar to what was reported in this study within the 4-sitting group based on OCT criteria for macular edema, the correlation between presence of edema on fundus photographs and OCT thickening is not strong.20
The strengths of this study were that it was prospective and followed a standardized protocol. However, the results of this study must be viewed in the context that treatment group assignment was not determined using randomization and the number of subjects enrolled was not large enough to derive definitive conclusions. The majority of DRCR.net investigators had a strong bias for one vs more than one sitting, precluding a randomized trial design. This was primarily due to concern of investigators who routinely performed PRP in multiple sittings who feared that a single sitting regimen would produce clinically relevant macular edema in their patients. It was thought that if results from a nonrandomized trial showed no large difference between one vs. four PRP sessions, randomization might be possible in a future trial. Some control of selection bias was obtained by requiring each participating investigator to indicate prior to the study whether they would perform scatter treatment in one sitting or four sittings for all of their subjects enrolled in the study. In an attempt to have treatment groups with similar levels of retinopathy severity, eyes with high-risk proliferative disease were excluded because scatter treatment generally would be given over a shorter period of time than the 12 week period mandated in the 4-sitting group. Baseline characteristics of the two treatment groups were fairly well balanced despite the lack of randomization, although, as expected from previous Network studies,26 the level of retinopathy as determined by the investigator did not always match the level as determined by the Reading Center. Nevertheless, selection bias cannot be excluded in this study since an investigator might not offer study participation to a patient for whom they did not believe that the study treatment regimen was appropriate. In addition, only 85% of subjects completed the 34-week follow-up period, a percentage lower than the goal of 90% or greater. Also, there were 11 eyes in the 4-sitting group in which the PRP was not completed due to missed visits, and in which the median number of laser spots was only 618. Though unlikely, this study does not allow one to determine if the outcomes in the 4-sitting group would have been different if the PRP was completed in these eyes and complete follow-up was obtained.
Results of the current study suggest that panretinal (scatter) photocoagulation (PRP) for diabetic retinopathy can be safely administered in a single sitting in patients with relatively good visual acuity and no or mild pre-existing center involved macular edema. Due to its nonrandomized design and small sample size, this study is not sufficient to determine unequivocally that applying PRP in one sitting is either non-inferior, or superior to applying PRP in more than one sitting. More definitive results would require a large randomized trial. The implications of this study, if true, have important public health implications, as the costs to the patient in terms of travel and lost productivity as well as to the eye care provider could be reduced.
Acknowledgments
Financial Support: Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases EY14231, EY14269, EY14229
Writing Committee: Writing Committee: Lead Authors: Alexander J. Brucker, Haijing Qin. Additional Writing Committee Members (Alphabetical): Andrew N Antoszyk, Roy W. Beck, Neil M. Bressler, David J. Browning, Michael J. Elman, Adam R. Glassman, Jeffrey G. Gross, Craig Kollman, , John A. Wells III.
Clinical Sites that Participated in this Protocol: Charlotte, NC Charlotte Eye, Ear, Nose and Throat Assoc., PA (24) David Browning(I); Andrew N. Antoszyk(I); Danielle R. Brooks (C,V); Melissa K. Cowen (C,V); Alison H. Stallings (C,V); Jennifer V. Helms (C,V); Angela K. Price (C,V); Heather L. Murphy(V); Rachel E. Pierce(V); Karen A. Ruiz(P); Richard J. George(P); Michael D. McOwen(P); Uma M. Balasubramaniam(P); Michele E. Powers(P); Donna McClain(P); Loraine M. Clark(P); Linda M Davis(P) Denver, CO Denver Health Medical Center (22) Jon M. Braverman(I); Antonio P. Ciardella(I); Leif S. Ryman(C); Brenner F. Dixon(C); Melissa A. Stillberger(C); Janelle Dane Zapata(V); Rosemary C. Rhodes(V); Debbie M. Brown(P) Baltimore, MD Elman Retina Group, P.A. (17) Michael J. Elman(I); Robert Z. Raden(I); Michelle D. Sloan(C); Tammy M. Butcher(C); JoAnn Starr (C,V); Dena Salfer-Firestone(V); Teresa Coffey(V); Pamela V. Singletary(V); Nancy Gore(V); Terri Cain(P); Peter Sotirakos(P); Giorya Shabi(P) New Albany, IN John-Kenyon American Eye Institute (15) Howard S. Lazarus(I); Debra Paige Bunch (C,V); Angela D. Ridge(C); Kelly Booth(V); Liana C. Davis(V); Margaret Trimble (P); Jay Moore(P) Dallas, TX Texas Retina Associates (13) Gary E. Fish(I); Robert C. Wang(I); Jean Arnwine(C); Sally Arceneaux(V); Brenda Sanchez(V); Keith Gray(P); Kimberly Cummings(P); Hank Aguado(P); Diana Jaramillo(P)
Columbia, SC Carolina Retina Center (8) Jeffrey G. Gross(I); Barron C. Fishburne(I); Michael A. Magee(I); Amy M. Flowers (C,V); Peggy W. Cummings(C); Regina A. Gabriel(V); Kristin K. Bland(V); Heidi K. Lovit(V); Chris N. Mallet(P); Randall L. Price(P); Rick Christoff(P)Statistician, DRCRnet Coordinating Center Detroit, MI Henry Ford Health System, Dept of Ophthalmology and Eye Care Services (6) Paul Andrew Edwards(I); Michael D. Ober(I); Sheila M Rock (C,V); Mary K. Monk (C,V); Janet Murphy (C,V); Dorena F. Wilson(V); Mark Croswell(P); Brian A. Rusinek(P); Lisa M. Schillace(P); Tracy A. Troszak(P); Bradley A. Stern(P) West Columbia, SC Palmetto Retina Center (6)
John A. Wells(I); W. Lloyd Clark(I); Marcia D. Gridine (C,V); Cassie P. Cahill(V); Amy B. Hickman(P); Robbin Spivey(P) Milwaukee, WI Medical College of Wisconsin (5) Judy E. Kim(I); Dennis P. Han(I); William J. Wirostko(I); Dawn Alvarez (C,V); Troy S. Drescher (C,V); Rowena J. Knapp(V); Vicki Barwick(V); Judy Flanders(V); Dennis B. Backes(P); Kathy J. Selchert(P); Joseph R. Beringer(P); Kristy L. Keller(P) Palm Springs, CA Southern California Desert Retina Consultants, MC (5) Clement K Chan(I); David M. Salib(I); Steven G Lin(I); Asha S.D. Nuthi(I); Eric D. Dickerson(C); Trina L. Keith(C); Kelly E. Sage(C); Teri A. Andresen(C); Isela Aldana(C); Tina B Wiskirchen(C); Sandra U. Castillo(V); Sara Warren(V); Kara Rollins(V); Kenneth M Huff(P); Donna J. Chesbrough(P); Sabrina E. Bretz(P) Boston, MA Joslin Diabetes Center (4) George S. Sharuk(I); Deborah K. Schlossman(I); Ann Kopple(C); Margaret E Stockman (C,V); Robert W. Cavicchi(P); Ellen L. Casazza(P) Loma Linda, CA Loma Linda University Health Care, Department of Ophthalmology (4) Joseph T. Fan(I); Michael E. Rauser(I); Kara E. Rollins (C,P,V); Carrousel J. Corliss (C,P,V); Sarina L. Osuna (C,P,V); William H. Kiernan(V); Johnathan D. Cloud(P); William Milam(P); Gene Saldana(P) Syracuse, NY Retina-Vitreous Surgeons of Central New York, PC (4) G. Robert Hampton(I); Samuel C. Spalding(I); Paul F. Torrisi(I); Bryan K. Rutledge (I); Cindy J. Grinnell(C); Fayth M. DiSano (C,V); Lynn M. Kwasniewski(V); Tanya C. Czajak(V); Lynn A. Capone(P); Bob Corey(P); Peter B. Hay(P) Austin, TX Austin Retina Associates (3) Jose A. Martinez(I); Chris A. Montesclaros(C); Carrie E. Odean (C,P,V); Richard A. Sabo(P)
Houston, TX Charles A. Garcia, P.A and Associates (3) Charles A. Garcia(I); John McCrary(I); Elizabeth Garibay(C); Emma M. Lessieur (C,P,V); Penelope Reyes Villeda(C); Hugo L. Paz(V); Juan P. Montoya(V); Cecilia Vi Nguyen(V); Edgardo Santisbon(V); Sindya M. Cerda(P); Rafael A. Lopez(P); Angela Ramirez(P) Lakeland, FL Central Florida Retina Institute (3) Scott M. Friedman(I); Kelly A. Blackmer(C); Jolleen S. Key (C,P,V); Steve Carlton (C,P,V); Damanda A. Fagan(V); Virginia Gregory(V)
Grand Rapids, MI Associated Retinal Consultants (2) Thomas M. Aaberg(I); Sarita Scott (C,V); Debra Markus (C,P,V); Sandy Kronlein (C,V); Sandra Lewis(P) Lubbock, TX Texas Retina Associates (2)
Michel Shami(I); Phyllis Pusser(C); Carrie L. Tarter (C,V); Linda Squires(V); Thom F. Wentlandt(P)
Chapel Hill, NC University of North Carolina, Dept of Ophthalmology (1) Mary Elizabeth R. Hartnett(I); Seema Garg(I); Cassandra J. Barnhart (C,V); Debra Cantrell(P); Rona Lyn Esquejo(P); Kelly D. Shields(P) Chicago, IL Northwestern Medical Faculty Foundation (1) Alice T. Lyon(I); Lori A. Kaminski (C,V); Lori E. Ackatz(C); Laima M. O'Donnell(V); Jonathan Shankle(P) Dublin, OH OSU Eye Physicians and Surgeons, LLC. (1) Frederick H. Davidorf(I); Alicia M. Green(C); Jerilyn G. Perry(V); Chhanda G. Chaudhuri(V); Scott J. Savage(P) Honolulu, HI Retina Associates of Hawaii, Inc. (1)
John H. Drouilhet(I); Susan Pelke(C); Deborah Nobler(P) Kansas city, MO Mid-America Retina Consultants, P.A. (1) Ivan R. Batlle(I); Karla A. Batlle(C); Gwendolyn M. Williams(V); Michelle Parks(P); R. Scott Varner(P) Knoxville, TN Southeastern Retina Associates, P.C. (1) Joseph Googe(I); Tod A. McMillan(I); Nicholas G. Anderson(I); Stephen L Perkins(I); Christina T. Higdon (C,V); Cecile Hunt(V); Jerry K. Whetstone(P); Michael Jacobus(P); Misty Moore(P); Paul A. Blais(P) New York, NY Manhattan Eye, Ear & Throat Hospital (1) Michael J. Cooney(I); John A. Sorenson(I); Leandro Maranan (C,V); Maria Scolaro(V); Eugene I. Agresta(P) Providence, RI Retina Consultants (1) Caldwell W. Smith(I); Collin L. DuCoty(C); Sylvia Varadian(C); Claudia Salinas(V); Erika Banalewicz(V); Mark Hamel(P); Alex L. Nagle(P) Sarasota, FL Sarasota Retina Institute (1) Melvin Chen(I); Keye L. Wong(I); Christine Holland(C); Karen Hagin(V); Mark Sneath(P); Rosa Miller(P)
DRCR.net Coordinating Center- Jaeb Center for Health Research, Tampa, FL (staff as of 5/1/08): Roy W. Beck (Director), Adam R. Glassman (Assistant Director), Joy Barros, Brian B. Dale, Sharon R. Constantine, Simone S. Dupre, Allison R. Edwards, Meagan Hutton, Paula A. Johnson, Craig Kollman, Lee Anne Lester, Brenda L. Loggins, Shannon L. McClellan, Patricia Murtaugh, Pamela S. Moke, Haijing Qin, Rosa Pritchard, Hiram Ramirez, Eureca Scott, Cynthia R. Stockdale
Fundus Photograph Reading Center – University of Wisconsin-Madison, Madison, WI (staff as of 5/1/08): Matthew D. Davis (Director Emeritus), Ronald P. Danis (Director), Larry Hubbard (Associate Director), James Reimers (Lead Color Photography Evaluator), Pamela Vargo (Lead Photographer), Ericka Moeller (Digital Imaging Specialist), Dawn Myers (Lead OCT Evaluator), Michael Daywalt (Project Manager)
DRCR.net Chair: Neil M. Bressler – Baltimore, MD (2006-current), Lloyd P. Aiello – Boston, MA (2002 – 2005)
National Eye Institute: Donald F. Everett, Päivi H. Miskala (2007)
Steering Committee: Alexander J. Brucker, M.D. (Protocol Chair 2005-Present), David Browning, M.D. (2005-Present), Emily Y. Chew, M.D. (2005- Present), Ronald P. Danis, M.D. (2003-Present), Julia A. Haller, M.D. (2005-Present), Lloyd P. Aiello, M.D., Ph.D. (2003-Present), Carl W. Baker, M.D. (2007 –2008), Neil M. Bressler, M.D.(2005-Present), Debra Paige Bunch (2007–2008), Donald F. Everett, M.A. (2006-Present), Frederick Ferris, M.D. (2005-Present), Adam R. Glassman, M.S. (2005-Present), Dennis M. Marcus, M.D. (2007 – 2008), Prior Members: Greg Anderson (2003–2004), Steve Carlton (2006 –2007), Tom Gardner (2003–2004) Jeffrey G. Gross, M.D. (2006 – 2007), Helen K. Li, M.D. (2006 –2007), Angela K. Price, M.P.H. (2005), Päivi Miskala, Ph.D. (2005–2007), Don S. Fong, M.D., M.P.H.(2003–2007),
Executive Committee: Neil M. Bressler (Chair 2006 - present), Lloyd P. Aiello (Chair 2002 – 2005), Roy W. Beck (2002-present), Abdhish Bhavsar (2007 –present), Ronald P. Danis (2004-present), Matthew D. Davis (2002-present), Donald F. Everett (2002-present), Frederick L. Ferris(2002-present), Joan Fish (2008 - present), Scott Friedman (2007 –2008), Adam R. Glassman (2002-present), Andreas Lauer (2007-present). Prior Members: David M. Brown (2006–2007), David J. Browning (2005–2006), Michael J. Elman (2006–2007), Kim McLead (2002–2006), Päivi H. Miskala(2005–2007), Cynthia J. Grinnell (2006– 2007).
DRCR.net Data and Safety Monitoring Committee: John Connett, (2003-Current), Deborah Barnbaum (2006 -Current), Harry W. Flynn, Jr. (2003-Current), Robert N. Frank (2003-Current), Saul Genuth (2003-Current), Lee Jampol (2003-Current), Stephen Wisniewski (2003-Current), Prior Member: Jeanette Resnick (2003-2000)
Footnotes
An address for reprints will not be provided.
Conflicts of interest statement: None
A complete list of all DRCR.net investigator financial disclosures can be found at www.drcr.net
References
- 1.The Diabetic Retinopathy Study Research Group. Preliminary report on effects of photocoagulation therapy. Am J Ophthalmol. 1976;81:383–396. doi: 10.1016/0002-9394(76)90292-0. [DOI] [PubMed] [Google Scholar]
- 2.Chew EY, Ferris FL, 3rd, Csaky KG, et al. The long-term effects of laser photocoagulation treatment in patients with diabetic retinopathy: the early treatment diabetic retinopathy follow-up study. Ophthalmology. 2003;110:1683–1689. doi: 10.1016/S0161-6420(03)00579-7. [DOI] [PubMed] [Google Scholar]
- 3.Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy ETDRS report number 9. Ophthalmology. 1991;98:766–785. [PubMed] [Google Scholar]
- 4.McDonald HR, Schatz H. Visual loss following panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology. 1985;92:388–393. doi: 10.1016/s0161-6420(85)34016-2. [DOI] [PubMed] [Google Scholar]
- 5.Dharma S, Bazan HE, Peyman GA, Atef MS. Production of platelet-activating factor in photocoagulated retinas. Curr Eye Res. 1991;10:1031–1035. doi: 10.3109/02713689109020341. [DOI] [PubMed] [Google Scholar]
- 6.ETDRS. Manual of Operations. In: US Department of Commerce NTIS.5285 Port Royal Rd, Springfield, VA 22161. PB85 223006/AS.
- 7.Blankenship GW. A clinical comparison of central and peripheral argon laser panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology. 1988;95:170–177. doi: 10.1016/s0161-6420(88)33212-4. [DOI] [PubMed] [Google Scholar]
- 8.Mensher JH. Anterior chamber depth alteration after retinal photocoagulation. Arch Ophthalmol. 1977;95:113–116. doi: 10.1001/archopht.1977.04450010113011. [DOI] [PubMed] [Google Scholar]
- 9.Liang JC, Huamonte FU. Reduction of immediate complications after panretinal photocoagulation. Retina. 1984;4:166–170. doi: 10.1097/00006982-198400430-00007. [DOI] [PubMed] [Google Scholar]
- 10.Yuki T, Kimura Y, Nanbu S, Kishi S, Shimizu K. Ciliary body and choroidal detachment after laser photocoagulation for diabetic retinopathy. A high-frequency ultrasound study. Ophthalmology. 1997;104:1259–1264. doi: 10.1016/s0161-6420(97)30149-3. [DOI] [PubMed] [Google Scholar]
- 11.Huamonte FU, Peyman GA, Goldberg MF, Locketz A. Immediate fundus complications 529 after retinal scatter photocoagulation. I. Clinical picture and pathogenesis. Ophthalmic Surg. 1976;7:88–99. [PubMed] [Google Scholar]
- 12.Shimura M, Yasuda K, Nakazawa T, Kano T, Ohta S, Tamai M. Quantifying alterations of macular thickness before and after panretinal photocoagulation in patients with severe diabetic retinopathy and good vision. Ophthalmology. 2003;110:2386–2394. doi: 10.1016/j.ophtha.2003.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Blondeau P, Pavan PR, Phelps CD. Acute pressure elevation following panretinal photocoagulation. Arch Ophthalmol. 1981;99:1239–1241. doi: 10.1001/archopht.1981.03930020113011. [DOI] [PubMed] [Google Scholar]
- 14.Zamir E, Anteby I, Merin S. Choroidal effusion causing transient myopia after panretinal photocoagulation. Arch Ophthalmol. 1996;114:1284–1285. doi: 10.1001/archopht.1996.01100140484028. [DOI] [PubMed] [Google Scholar]
- 15.Lerner BC, Lakhanpal V, Schocket SS. Transient myopia and accommodative paresis following retinal cryotherapy and panretinal photocoagulation. Am J Ophthalmol. 1984;97:704–708. doi: 10.1016/0002-9394(84)90501-4. [DOI] [PubMed] [Google Scholar]
- 16.Aiello LP. Angiogenic pathways in diabetic retinopathy. N Engl J Med. 2005;353:839–841. doi: 10.1056/NEJMe058142. [DOI] [PubMed] [Google Scholar]
- 17.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135:194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 18.The Diabetic Retinopathy Study Research Group. Four risk factors for severe visual loss in diabeticretinopathy The third report from the Diabetic Retinopathy Study. Arch Ophthalmol. 1979;97:654–655. doi: 10.1001/archopht.1979.01020010310003. [DOI] [PubMed] [Google Scholar]
- 19.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796–1806. [PubMed] [Google Scholar]
- 20.Diabetic Retinopathy Clinical Research Network. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114:525–536. doi: 10.1016/j.ophtha.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diabetic Retinopathy Clinical Research Network. Reproducibility of macular thickness and volume using Zeiss optical coherence tomography in patients with diabetic macular edema. Ophthalmology. 2007;114:1520–1525. doi: 10.1016/j.ophtha.2006.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doft BH, Blankenship GW. Single versus multiple treatment sessions of argon laser panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology. 1982;89:772–779. doi: 10.1016/s0161-6420(82)34734-x. [DOI] [PubMed] [Google Scholar]
- 23.Higgins KE, Meyers SM, Jaffe MJ, Roy MS, de Monasterio FM. Temporary loss of foveal contrast sensitivity associated with panretinal photocoagulation. Arch Ophthalmol. 1986 Jul;104:997–1003. doi: 10.1001/archopht.1986.01050190055039. [DOI] [PubMed] [Google Scholar]
- 24.Kleiner RC, Elman MJ, Murphy RP, Ferris FL., 3rd Transient severe visual loss after panretinal photocoagulation. Am J Ophthalmol. 1988;106:298–306. doi: 10.1016/0002-9394(88)90365-0. [DOI] [PubMed] [Google Scholar]
- 25.Myers SM. Macular edema after scatter laser photocoagulation for proliferative diabetic retinopathy. Am J Ophthalmol. 1980;90:210–216. doi: 10.1016/s0002-9394(14)74855-x. [DOI] [PubMed] [Google Scholar]
- 26.Scott IU, Bressler NM, Bressler SB, et al. Agreement between clinician and reading center gradings of diabetic retinopathy severity level at baseline in a phase 2 study of intravitreal bevacizumab for diabetic macular edema. Retina. 2008;28:36–40. doi: 10.1097/IAE.0b013e31815e9385. [DOI] [PMC free article] [PubMed] [Google Scholar]