Abstract
In her initial research, Elizabeth D. Hay studied amphibian limb regeneration, but later switched her focus, and for the remainder of her career addressed the role of the extracellular matrix (ECM) in regulating embryonic morphogenesis. Much of that work used the embryonic chick corneal epithelial model. This review highlights many of the discoveries that she made using this model. Hay was the first to show that embryonic corneal epithelial cells produce fibrillar collagen. Her lab was among the first to demonstrate that corneal epithelial cells respond to a collagenous substrate by increasing ECM production, and that purified ECM molecules, added to cultures of epithelial sheets, induce a reorganization of the actin cytoskeleton. These data led to the first theories of cell-matrix interactions, illustrated in a ‘hands across the membrane’ sketch drawn by Hay. Recent work with the epithelial sheet model system has elucidated many of the signal transduction pathways required for actin reorganization in response to the ECM. In all, this body of work has amply supported Hay's belief that the embryonic corneal epithelium is a powerful model system for exploring the role of the ECM in regulating the cytoskeleton, in directing cell migration, and in profoundly influencing cell growth and differentiation during development.
Keywords: embryonic corneal epithelium, cell-matrix interactions, cytoskeleton, actin, extracellular matrix, signal transduction
Fibrillar Collagen Production by Epithelial Cells
Elizabeth D. Hay, called Betty, by all who knew her, was a pioneer in cell and developmental biology. She began her career investigating limb regeneration in salamanders, and when electron microscopy was being integrated with autoradiography, she was among the first to fruitfully apply them to study regeneration. In several early papers, Hay reported that both the salamander epithelial and fibroblast cells of regenerating limbs appeared to be producing, organizing, and assembling collagen matrices (Hay and Revel, 1963). At that time, the importance of the extracellular matrix (ECM) was just starting to be appreciated, and the word “collagen” referred to the only known collagen: fibrillar type I collagen. Hay's and Revel's conclusions about the synthesis of salamander epithelial type I collagen were based on morphological, “pulse chase” experiments. Systemic injection of tritiated proline demonstrated that, shortly after injection, 3H was localized within intracellular organelles, mainly the ER and the Golgi apparatus. However, at later time points, the label was found in the ECM underlying the epithelium, then referred to as the basement lamella (Hay and Revel, 1963). Publication of this work elicited substantial controversy, because it went against the current dogma of that time, which was that collagen (type I) was produced only by fibroblasts (Kallman and Grobstein, 1965; Bernfield, 1970).
Hay took the criticism as a personal challenge to prove that epithelial cells could synthesize ECM molecules, including type I collagen. To accomplish this she needed an epithelium that could be dissected free of other tissue types, and would survive sufficient lengths of time in culture to demonstrate collagen synthesis and assembly. The salamander limb epithelium was not a good candidate for in vitro studies because the tissue was not sterile and its biosynthetic levels were insufficient to address the core questions. Hay read with interest the paper published by the Coulombres, which contained exquisite electron micrographs of collagen in the developing chick cornea. The micrographs showed that the fibrils were secreted and assembled in orthogonal arrays, that is, the fibrils were arranged in mat-like layers, where within a layer the fibrils were unidirectional. Any given layer would have fibrils running at nearly a 90-degree angle with respect to the fibrils in the layer above it or below it (Coulombre and Coulmbre, 1958).
Embryonic Chick Corneal Epithelial Model System
Hay turned to the embryonic chick corneal model system because the organization of the matrix was similar to the pattern observed in the basement lamella of the salamander limb. The collagen fibrils first laid down by the corneal epithelium were assembled into an acellular “primary” stroma in the absence of fibroblasts. This occurred 2 days before the stroma was invaded by neural crest-derived cells, which synthesized the “secondary,” mature stroma. These features made the embryonic chick cornea a perfect model for her subsequent experiments.
By the mid 1960s, Jean Paul Revel and Hay had completed a comprehensive study of the fine structure of the developing chick cornea. Their results were published in a monograph that is referenced to this day (Hay and Revel, 1969). The early development of the cornea was described, carefully documenting the attributes of the epithelial-derived primary stroma before fibroblast invasion. The fibrils made by the epithelium had a periodicity of approximately 600Å, with an interband striation pattern identical to fibrils assembled in the secondary corneal stroma, synthesized by the fibroblasts. At the time, this research provided the most compelling evidence that epithelial cells produced the collagenous primary stroma. The discovery of a second collagen, type II (Miller and Matukas, 1969), was still 2 years away. If Hay's embryonic epithelial fibrillar collagen papers had been published after the discovery of type IV collagen, she would still have been likely to face skepticism, because type IV would have been considered “the” epithelial collagen. Because her observations were novel, they were bound to create discord. No matter. Hay's persistence in her research, along with subsequent work by others, has supported her discovery that embryonic chick corneal epithelia synthesize fibrillar collagens. It is now known that this epithelium can secrete a minimum of three fibrillar collagens: type I, type II (Hendrix et al., 1982), and type V (Gordon et al., 1994).
Influence of Matrix on the Embryonic Chick Corneal Epithelium
The next series of experiments using cultured corneal epithelia launched a body of work that has spanned 40 years and remains an active field to this day. James W. Dodson joined Hay's laboratory as a postdoctoral fellow in the late 1960s. He had grown chick skin in organ culture on a variety of “cell-free” substrata (Dodson, 1967). These substrata were made by dissecting an organ known to have an abundant matrix and killing its cells with low temperature, leaving the matrix intact. Dodson was quick to apply this approach to the corneal epithelium. By culturing sheets of corneal epithelia on “cell-free” lens capsules (Fig. 1A), he demonstrated that the epithelium produced a proline rich ECM, that was absent when the same cells were cultured on plastic. This was additional evidence for collagen synthesis by epithelia (Dodson and Hay, 1971). It also suggested to Hay that the components of the ECM might regulate embryonic growth and differentiation. Her group exploited a combination of morphological and autoradiographic approaches to examine the response of corneal epithelia to various substrata (Hay and Dodson, 1973; Dodson and Hay, 1974). Stephen Meier, a graduate of Michael Solursh's laboratory, joined the Hay lab in 1972. His background in chondrogenesis included cell–matrix interactions, and he brought biochemical analytical skills to the project. His work provided the initial quantitation of collagen synthesis (Meier and Hay, 1974b) and glycosaminoglycan (GAGs) synthesis (Meier and Hay, 1973) induced in corneal epithelia in response to specific substrata. From this work, it became evident that an appropriate substratum was required for the embryonic epithelium to synthesize and assemble a primary stroma in vitro.
Fig. 1.
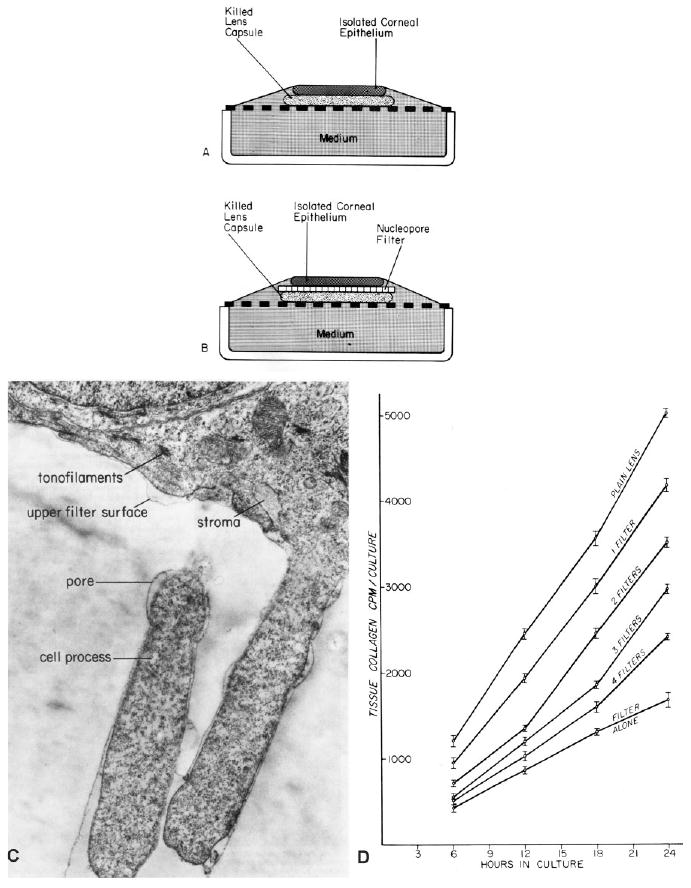
The culture system developed by Dodson, Meier, and Hay placing epithelium on killed lens capsule. A: Epithelium cultured directly on lens capsule. B: Epithelium separated from lens capsule by a Nucleopore filter barrier. Reprinted from Meier and Hay, 1975. C: Transmission electron micrograph of a 24-hr culture showing epithelial cell processes extending into the filter. Reprinted from Hay and Meier, 1976. D: Corneal epithelia grown directly on one Nucleopore filter, 0.8 μm pore size (bottom curve), on stacks of 0.8μm pore size filters with lens capsule beneath the filter, or directly on lens capsule (top curve). Cultures were grown in the presence of 5μCi/ml H3 proline and harvested at various times up to 24 hr. The bottom curve demonstrates the base line level of collagen synthesis, while the top curve corresponds to the “induced state.” Stimulation of synthetic activity decreased with the increasing filter thickness, consistent with the observation that the cell processes traversed the filter contacting the lens capsule. Vertical bars = standard deviation for four assays. Reprinted from Meier and Hay (1975).
Meier next designed a set of experiments to demonstrate that physical contact with the substratum enhanced the epithelial response. He observed that the basal surface of corneal epithelial sheets, enzymatically released from the 5-day chick embryo, contained protrusions termed “blebs.” Meier sandwiched Nucleopore filters between the epithelial sheet and killed lens capsule (Fig. 1B,C). When a single filter was used in the culture system (Meier and Hay, 1975), the basal blebs traversed the filter's pores and made contact with the lens capsule substratum (Fig. 1C). However, when more than one filter was interposed, the response to the lens capsule diminished in proportion to the number of filters (Fig. 1D). In addition, there was a direct relationship between the size of the pore and the response to the substrate (Meier and Hay, 1975). This was strong evidence that cell contact with the substratum was required for stimulating the epithelial cells to synthesize collagen. Not only did these experiments reinforce the concept that the developing corneal epithelium needed attachment to ECM to induce synthesis of the primary stroma, but in their 1975 papers Meier and Hay proposed that epithelial cell processes might contain specific surface sites for collagen-ECM interaction (Meier and Hay, 1975), foreshadowing the discovery of integrins.
Matrix Induced Actin Reorganization
During the same time period, Richard Hynes, while exploring the differences between normal and tumor cells, identified and isolated a large molecular weight protein that bound to normal cells (Hynes, 1973). Hynes termed the protein LETS (large, external transformation-sensitive) protein. It was later proven to be a ubiquitous extracellular protein, and renamed fibronectin (Wartiovaara et al., 1974; Mautner et al., 1977). In the latter part of the 1970s, several laboratories demonstrated that extracellular bundles of fibronectin, attached to the basal surface of epithelial cells, were aligned with the submembranous actin filaments in the cytoplasm (Hynes et al., 1978; Singer, 1979; Heggeness et al., 1978). Additional work showed that fibronectin was able to induce the rearrangement of actin filaments in fibroblasts and myoblasts (Ali et al., 1978). The term “fibronexus” was coined to describe this conjoined extra- and intracellular structural complex (Singer, 1979).
Stephen Sugrue joined Hay's lab in the late 1970s to determine whether purified ECM proteins, such as specific collagen types (I, II, and IV), laminin and fibronectin, would promote the synthesis of differentiation markers by corneal epithelium. Sugrue's data demonstrated that epithelia grown on Millipore filters, with no soluble matrix component added to the culture, continued to bleb for the duration of the experiments (12–14 hr; Fig. 2A). No fibrillar collagen was synthesized. However, if soluble collagen (COLL), laminin (LAM), or fibronectin (FN) were added to the medium, blebs were withdrawn within 2–6 hr and the basal cell surfaces flattened (Table 1; Fig. 2B). Sugrue and Hay demonstrated that soluble ECM molecules added to the medium bound to the basal epithelial surface, and these induced and regulated collagen synthesis. In addition, it was shown that the binding sites for the soluble matrix components were proteinaceous (Sugrue and Hay, 1986). In contrast, epithelia grown on filters in the presence of added albumin, IgG, or glycosaminoglycans continued to form basal blebs, and did not attain the flattened basal phenotype (Table 1; Fig. 2A). Also, epithelia cultured on solid matrices, such as glass, continued to bleb in the absence of ECM components (Sugrue and Hay, 1981). It was clear that cell–ECM interactions were central to actin reorganization and expression of a differentiated phenotype. Ultimately, myosin S-1 decoration definitively established that the basal submembranous filament bundles were F-actin (Fig. 2C). Of interest, new protein synthesis was not required for the corneal epithelial cells to respond to collagen or laminin, but was needed for the cell's response to fibronectin (Sugrue and Hay, 1982). These data suggested that actin reorganization in response to the ECM was responsible for bleb retraction (Fig. 2B). The intracellular basal sheet occupied by actin was termed the “actin cortical mat” (ACM). Hay, who was very artistic, drew her interpretation of the interactions between the basal epithelia and the extracellular matrix in an illustration referred to by lab members as “hands across the membrane” (Fig. 2D). This drawing was used by the group for conceptual discussions, but was never published. It was given to Sugrue when he left Harvard because it represented a summary of his work. As a tribute to Hay, he framed the original drawing and presented it back to her at a dinner following the Matrix and Morphogenesis conference, held in Boston to honor her 75th birthday and her contributions to the field.
Fig. 2.
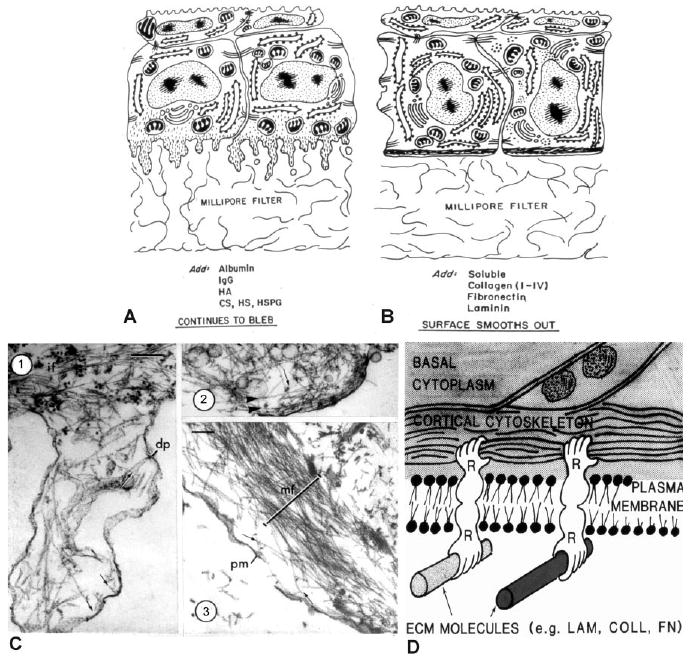
Diagrams and electron micrographs illustrating the effects of different experimental conditions on the organization of the basal cell surface. A: The basal surface blebbed when the basal lamina was removed by EDTA or enzyme treatment, and the blebbing persisted on Millipore filters in the presence of nonmatrix proteins or glycosaminoglycans. B: Soluble collagens, fibronectin and laminin added to the medium stimulated the bleb retraction and reformation of the basal actin cytoskeleton. Reprinted from Sugure and Hay (1982). C: Corneal epithelial tissues were detergent extracted and treated with S-1 fragments of heavy meromyosin before EM preparation. C-1 is a typical bleb demonstrating a core of actin filaments decorated with myosin S-1 fragments, which aligns on the actin filaments indicating the polarity of the filament that is pointing toward the plasma membrane (indicated by small arrows) and some apparently inserting into a dense plaque (dp). Intermediate filaments (IF) were in the basal cell area. C-2 is a smaller bleb with some actin filaments parallel to the cell surface (arrowheads) and others perpendicular to the surface. C-3 presents a cell 6 hr after immersion in soluble type IV collagen (100 μg/ml). The actin bundle (MF) was in the basal compartment of this slightly tangential section containing a damaged plasma membrane (PM). The actin filaments were organized in opposite directions (small arrows). Scale bars = 0.2μm. Reprinted from Sugrue and Hay (1982). D. Hay's concept drawing illustrating the influence of the ECM on the actin cytoskeleton. It was used in lab meetings and titled “Hands across the membrane.”
Table 1. Summary of Stimulators and Inhibitors of Corneal Epithelial Actin Reorganizationa.
| No Effect on Actin Reorganization | Stimulate Organization of the Actin Cortical Mat | Blocked Actin Cortical Mat Organization |
|---|---|---|
| Albumin | Collagen (I–IV) #α2β1,α3β1, α6β1, αvβ5, α6β4 | Actin inhibitors (Cytochalasin D) |
| Hyaluronic Acid | Fibronectin - α3β1, α5β1, α6β1, α9β1, αvβ1, αvβ6, α6β4 | Phospho-tyrosine inhibitors |
| Antibodies IgG | Laminin (type I) α2β1, α3β1, α6β1, αvβ1, αvβ5, α6β4 | Decreasing Rho protein or activity |
| Proteoglycans and glycosaminoglycans including CS, HS, HSPG | Vitronectin - αvβ3, αvβ6 | ROCK inhibitors |
| Serum | LPA* | PI-3 Kinase inhibitors |
| Bombesin* | MAP Kinase inhibitors |
Molecules that had no effect on organizing the corneal epithelial actin cortical mat included proteoglycans, glycosaminoglycans, serum, albumin and antibodies (Sugrue and Hay, 1982). Most of the extracellular matrix (ECM) molecules that bound to integrin receptor subunits (Stepp, 2006) reorganized the actin cytoskeleton: these included collagens, fibronectin, laminin (Sugrue and Hay, 1982) and vitronectin (Svoboda, unpublished). Molecules that directly stimulated the Rho pathway, including lysophosphatidic acid (LPA) and bombesin, reorganized the actin cortical mat faster (15 min) than integrin stimulation (2–6 hr; Svoboda et al., 1999b). Inhibitors of actin reorganization or the signaling pathways (broad phospho-tyrosine blockers, MAP-kinase, PI-3 kinase, ROCK inhibitors, or interference with Rho or its activity) blocked reorganization of the actin cortical mat (Svoboda and Hay, 1987; Svoboda, et al., 1999b, 2004, Chu et al., 2000; Reenstra et al., 2002).
Integrin subunits identified in corneal epithelia (Stepp, 2006).
G-protein receptor- direct Rho stimulator with an Actin response in 15 min.
Cell-Matrix Interactions
Because ECM binding to the cell surface clearly involves specific molecular interactions, a search for ways to block these interactions was begun in several labs. Once techniques were refined to purify ECM molecules, monoclonal and polyclonal antibodies were generated against whole molecules and their proteolytic fragments. The goal was to identify blocking antibodies that would inhibit cell adhesion to specific substrates. In the early 1980s, review papers highlighted the experimental data demonstrating evidence for integral membrane linkers (Fig. 3). Hay was an author on several of these reviews (Hay, 1977, 1981b, 1984; Hynes et al., 1982; Singer, 1979). Yet the identity of the transmembrane linker proteins proved elusive. Finally, in the mid 1980s the first integrin was identified and cloned (Tamkun et al., 1986); for review see (Hynes, 2004). As with many protein families, once the first integrin was cloned, the flood gates opened and many others were quickly identified. Hynes organized a Gordon Conference in early 1987, inviting everyone he knew who was working on cell adhesion proteins. By the end of that week, the integrin family was pieced together and a common terminology accepted (Hynes, 1987). In the late 1980s and early 1990s many groups were using the newly characterized integrin antibodies to identify tissue-specific subunits. The Trinkaus-Randall and Gipson labs were the first to describe integrin subunits in the corneal epithelial cells (Stepp et al., 1990a,b; Trinkaus-Randall et al., 1990; Grushkin-Lerner and Trinkaus-Randall, 1991; Stepp, 2006).
Fig. 3.
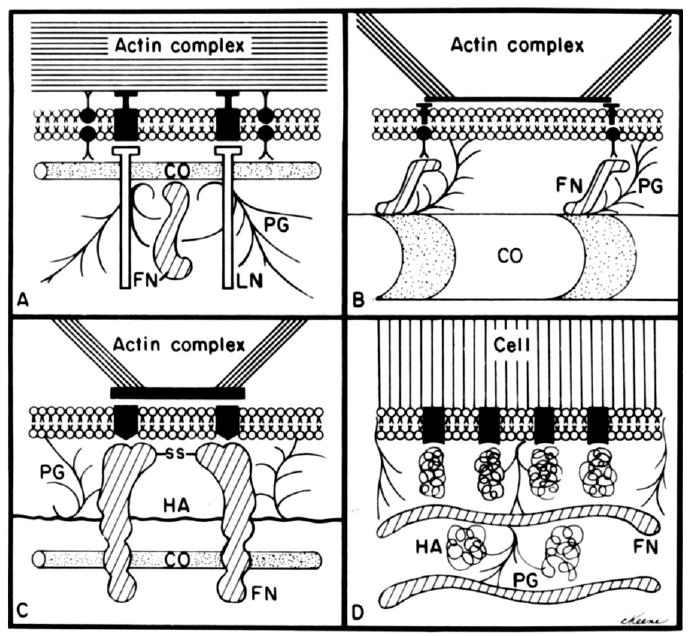
Diagrams of several models depicting the possible relation of extracellular matrix (ECM) molecules to the cell surface. All models envision plasma membrane receptors for one or more molecules. CO, collagen; FN fibronectin; PG, proteoglycans; HA, hyaluronic acid. A: Model based on the data from Sugrue and Hay (1982). B: Model based on data from Singer (1979) and Kleinman et al. (1981). C: Model of FN interaction with fibroblasts by Hynes et al. (1982). D: Model of glycosaminoglycans and the mesenchymal cell surface from Toole (1981). Reprinted from Hay, (1981a,b).
In 1982, Kathy Svoboda joined the Hay lab as a post doctoral fellow, being interested in the role of cytoskeletal reorganization during morphogenesis. Her goal was to determine whether actin reorganization in response to the ECM is a necessary prerequisite for increased collagen production within cultured corneal epithelia. Her data indicated that increased collagen synthesis was, indeed, actin dependent, and if the actin mat was disrupted, the rough endoplasmic reticulum became displaced from the basal cytoplasm (Svoboda and Hay, 1987).
Impact of Hay's Corneal Epithelial Research on Epithelial Cell Biology
For the most part in the late 1980s, Hay's work with the corneal epithelial sheet model was superseded by research on epithelial mesenchymal transitions. However, her impact on corneal epithelial cell biology continues to this day. For example, after leaving the Hay laboratory, Svoboda has continued to use the corneal epithelial sheets in her own laboratory, further characterizing binding of the cell sheets to soluble matrix, identifying the signal transduction pathways, and actin associated proteins involved in the reorganization of the actin cortical mat (Svoboda, 1990, 1992; Khoory et al., 1993; Hirsch et al., 1994; Svoboda et al., 1999a). To visualize the changes that Hay's group had been showing biochemically, confocal microscopy was refined as a tool to evaluate actin reorganization (Fig. 4). En face images with fluorescently labeled phalloidin showed bright fluorescent spots when the cells were optically sectioned transverse to the epithelial blebs (Fig. 4C), and large actin bundles formed in parallel on the base of the cells as the ACM was reorganized (Fig. 4D). This morphological method unraveled the three-dimensional organization of actin and its associated proteins, intracellular organelles and mRNAs for collagen I and actin. (Svoboda, 1991, 1992; Khoory et al., 1993; Yeh and Svoboda, 1993, 1994; Svoboda et al., 1999a). The time-course of binding was measured using fluorescein isothiocyante (FITC) -labeled fibronectin and collagen I (Fig. 5A,B). The corneal epithelial sheets needed 15 min in the presence of soluble matrix before the ACM began reforming, and the mat was complete by 2 hr (Fig. 5C). Binding and competition experiments also showed that binding was saturable (Fig. 5D). These experiments complemented both Meier and Sugrue's experiments on the corneal epithelial response to collagen (Meier and Hay, 1974a; Sugrue and Hay, 1981, 1982).
Fig. 4.
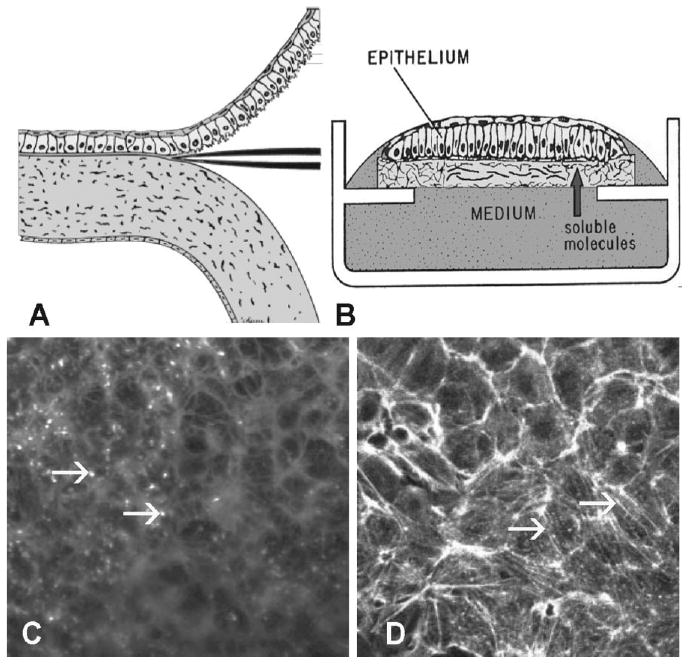
Confocal microscopy of actin in corneal epithelial sheets. Schematic drawing of embryonic corneal epithelia isolation and culture. A: Epithelia were isolated as a sheet of tissue and placed on a polycarbonate filter, basal side down, then cultured at the air–media interface, B: Epithelia isolated without the basal lamina (−BL) extended basal cellular processes termed “blebs.” C: Sheets of corneal epithelia were stained with fluorescent phalloidin to label all filamentous actin (F-actin). In single confocal optical sections, the blebs are punctate spots in the plane of the basal cells. The cortical actin near cell membranes is also in the micrograph. D: If the tissue is cultured with soluble extracellular matrix (ECM) molecules, the basal actin reorganizes into an actin cortical mat (ACM) that phalloidin labels as bundles of F-actin that align from cell to cell after COL treatment for 2 hr. Scale bar = 10 μm. Reprinted from Svoboda et al. (1999b).
Fig. 5.
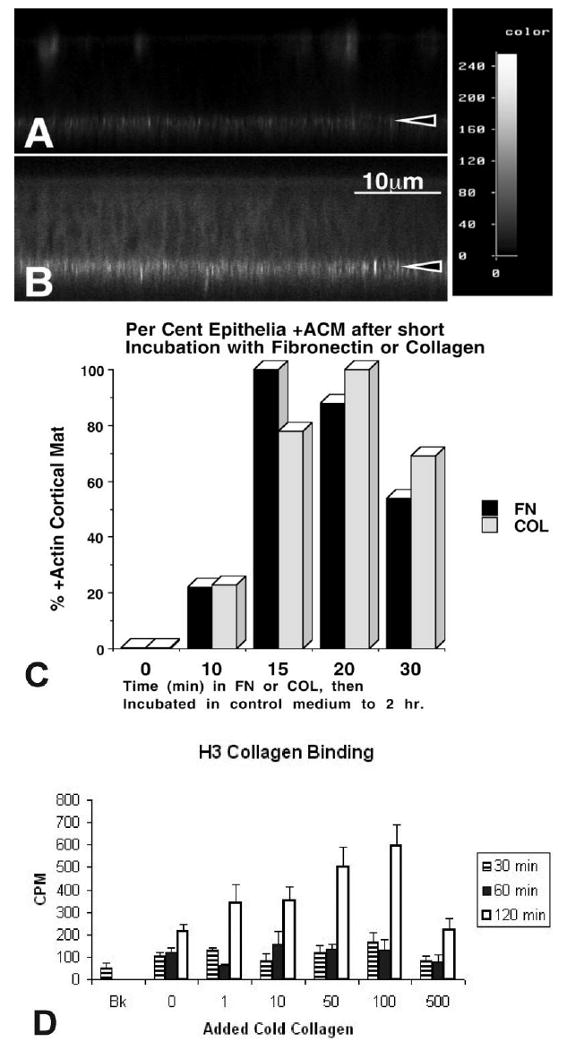
Confocal images of epithelia grown on filters with fluorescently labeled matrix components. A,B: Corneal epithelia were isolated without the basal lamina (−BL), incubated with media containing fluorescein isothiocyanate–fibronectin (FITC-FN) for short time intervals (15 or 30 min.). The epithelia were rinsed, fixed and viewed on the confocal microscope in the xz optical plane. The intensity of bound FITC-labeled FN was recorded at the basal cell surface in a nonuniform pattern. The intensity level of FITC-FN increased from 0 to 30 min as analyzed with NIH Image. C: Corneal epithelia −BL were isolated, incubated with media containing FN or COL for a short time interval (0, 10, 15, 20, or 30 min). The cultures were rinsed and immersed in control non-ECM media, and then incubated for a total time of 2 hr. Samples were fixed and stained with fluorescent phalloidin, and then groups coded (numbered) and scored by at least two people as +, +/−, or −actin cortical mat (ACM). The experiments were repeated three times, with an n = 5–7 epithelia/group. Folded and damaged tissue was not scored. Greater than 80% of the epithelia responded to the ECM molecules within 20-30 min, 22% with 10 min. D: Tritium labeled collagen was added to cultured epithelium in the presence of unlabeled collagen (1, 10, 50, 100 or 500 μg/ml) for 30, 60 or 120 min. Increased binding of 3H-collagen was observed up to 100 μg/ml but binding decreased at 500 μg/ml, suggesting competition at high levels of cold collagen. Reprinted from Svoboda et al. (1999b).
Tyrosine phosphorylation of many signaling proteins was observed in corneal epithelia as they bound exogenous fibronectin or collagen, beginning within 15 min of exposure to the soluble matrix (Svoboda et al., 1999b). Focal adhesion kinase (FAK) and p190RhoGAP were tyrosine phosphorylated within 5 min. Other MAP kinase signaling pathways (Erk-1, 2) and PI-3 kinase were activated later (30–60 min). These phosphorylations were required for actin reorganization, as shown with a tyrosine and Src kinase inhibitor, Herbimycin A (Table 1; Svoboda et al., 1999b). Furthermore, blocking the MAP kinase and PI-3 kinase pathways with specific inhibitors also prevented actin reorganization and decreased ECM binding (Table 1) (Chu et al., 2000). Monolayer cultures of corneal epithelial cells grown on laminin, fibronectin and collagen I showed that ACM reorganization was accompanied by an increase in intracellular pH (Wu et al., 1995). Reorganization of the actin cortical mat in the epithelial sheets was Rho-dependent (Reenstra et al., 2002) and ROCK-dependent (Svoboda et al., 2004). All of this work has been derived from the elegantly simple conclusion of Elizabeth Hay that epithelial cells in the embryonic chick cornea can synthesize and assemble fibrillar collagen and do so in response to its unique ECM.
Some of Hay's other trainees have continued their research on the cornea. May Griffith, a former postdoctoral fellow, has developed corneal constructs, resulting in many publications, including one in Science (Griffith et al., 1999). Others, including Stephen Sugrue, expanded their interest to other epithelia. In Sugrue's case, this led to the discovery of the molecule pinin (Ouyang et al., 1997; Shi et al., 2000; Zimowska et al., 2003; Joo et al., 2005), a multifunctional molecule involved in cell–cell interactions and much more. Additionally, colleagues saw the potential of Hay's corneal model system: Ilene Gipson adapted the model to rabbit cornea (Geggel and Gipson, 1985; Gipson et al., 1985; Spurr and Gipson, 1985). This began a body of work unraveling the components of the adhesion complex between the epithelium and the stroma in the cornea.
Perspectives
This embryonic corneal epithelial organ culture model provides investigators with a more physiological system than monolayer cell cultures. Epithelial–stromal interactions, actin-associated protein changes, the influence of soluble matrix, and downstream signaling molecules, have all been studied using it. This model preserves many characteristics of in vivo epithelium, including apical-basal polarity and cell–cell junctions. Corneal epithelial cells have a rich integrin subset that also responds to ECM molecules (Table 1, Stepp, 2006). Powerful new techniques are emerging using fluorescently tagged proteins and real-time cell imaging that will enable a much better understanding of the intracellular events that take place when the epithelium is challenged by trauma, toxins or transforming agents.
Because signaling triggers alterations in cell metabolism, in gene expression and in cell behavior, continued use of the corneal epithelial model could link signal transduction cell biology with embryonic morphogenesis.
Acknowledgments
This review is dedicated to the memory of Elizabeth D. Hay. We thank Steve Sugrue for helpful insights and for providing the “hands across the membrane” illustration. We thank Developmental Biology and Journal of Cell Biology for permission to reprint figures. Dedication: K.K.S. and M.K.G. were funded by NIH/NEI.
Grant sponsor: NIH/NEI; Grant number: EY08886; Grant number: EY014389; Grant number: EY09056; Grant sponsor: UMDNJ/Rutgers Center; Grant number: AR055073.
References
- Ali IU, Hynes RO, Ali IU, Hynes RO. Effects of LETS glycoprotein on cell motility. Cell. 1978;14:439–446. doi: 10.1016/0092-8674(78)90129-0. [DOI] [PubMed] [Google Scholar]
- Bernfield MR. Collagen synthesis during epitheliomesenchymal interactions. Dev Biol. 1970;22:213–231. doi: 10.1016/0012-1606(70)90151-x. [DOI] [PubMed] [Google Scholar]
- Chu CL, Reenstra RW, Orlow DL, Svoboda KKH. Erk and PI3 kinase are necessary for collagen binding and actin reorganization in avian corneal epithelia. Invest Ophthalmol Vis Sci. 2000;41:3374–3382. [PMC free article] [PubMed] [Google Scholar]
- Coulombre AJ, Coulmbre JL. Corneal development. II. Transparency changes during rapid hydration. Am J Ophthalmol. 1958;46:276–280. discussion 281. [PubMed] [Google Scholar]
- Dodson JW. The differentiation of epidermis. I. The interrelationship of epidermis and dermis on embryonic chicken skin. J Embryol Exp Morphol. 1967;17:83–105. [PubMed] [Google Scholar]
- Dodson JW, Hay ED. Secretion of collagenous stroma by isolated epithelia grown in vitro. Exp Cell Res. 1971;65:215–220. doi: 10.1016/s0014-4827(71)80069-1. [DOI] [PubMed] [Google Scholar]
- Dodson JW, Hay ED. Secretion of collagen by corneal epithelium. II. Effect of the underlying substratum on secretion and polymerization of epithelial products. J Exp Zool. 1974;189:51–72. doi: 10.1002/jez.1401890106. [DOI] [PubMed] [Google Scholar]
- Geggel HS, Gipson IK. Removal of viable sheets of conjunctival epithelium with dispase II. Invest Ophthalmol Vis Sci. 1985;26:15–22. [PubMed] [Google Scholar]
- Gipson IK, Friend J, Spurr SJ. Transplant of corneal epithelium to rabbit corneal wounds in vivo. Invest Ophthalmol Vis Sci. 1985;26:425–433. [PubMed] [Google Scholar]
- Gordon MK, Foley JW, Birk DE, Fitch JM, Linsenmayer TF. Type V collagen and Bowman's membrane. Quantitation of mRNA in corneal epithelium and stroma. J Biol Chem. 1994;269:24959–24966. [PubMed] [Google Scholar]
- Griffith M, Osborne R, Munger R, Xiong X, Doillon CJ, Laycock NL, Hakim M, Song Y, Watsky MA. Functional human corneal equivalents constructed from cell lines. Science. 1999;286:2169–2172. doi: 10.1126/science.286.5447.2169. [DOI] [PubMed] [Google Scholar]
- Grushkin-Lerner L, Trinkaus-Randall V. Localization of integrin and syndecan in vivo in a corneal epithelial abrasion and keratectomy. Curr Eye Res. 1991;10:75–85. doi: 10.3109/02713689109007612. [DOI] [PubMed] [Google Scholar]
- Hay ED. Interaction between the cell surface and extracellular matrix in corneal development. In: Lash JW, Burger MM, editors. Cell and tissue interactions. New York: Raven Press; 1977. pp. 115–137. [PubMed] [Google Scholar]
- Hay ED. Cell biology of the extracellular matrix. 1st. New York: Plenum Press; 1981a. p. 468. [Google Scholar]
- Hay ED. Extracellular matrix. J Cell Biol. 1981b;91:205s–223s. doi: 10.1083/jcb.91.3.205s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay ED. Cell-matrix interaction in the embryo: cell shape, cell surface, cell skeletons, and their role in differentiation. In: Hay ED, editor. Role of extracellular matrix in development. New York: Alan R. Liss, Inc.; 1984. pp. 379–409. [Google Scholar]
- Hay ED, Dodson JW. Secretion of collagen by corneal epithelium: I. Morphology of the collagenous products produced by isolated epithelia grown on frozen-killed lens. J Cell Biol. 1973;57:190–213. doi: 10.1083/jcb.57.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay ED, Revel JP. Autoradiographic studies of the origin of the basement lamella in Ambystoma. Dev Biol. 1963;7:152–168. doi: 10.1016/0012-1606(63)90114-3. [DOI] [PubMed] [Google Scholar]
- Hay ED, Meier S. Stimulation of corneal differentiation by interaction between cell surface and extracellular matrix. II. Further studies on the nature and site of transfilter “induction”. Dev Biol. 1976;52:141–157. doi: 10.1016/0012-1606(76)90014-2. [DOI] [PubMed] [Google Scholar]
- Hay ED, Revel JP. Fine structure of the developing avian cornea. Basel, Switzerland: Karger; 1969. [PubMed] [Google Scholar]
- Heggeness MH, Ash JF, Singer SJ. Transmembrane linkage of fibronectin to intracellular actin-containing filaments in cultured human fibroblasts. Ann N Y Acad Sci. 1978;312:414–417. doi: 10.1111/j.1749-6632.1978.tb16822.x. [DOI] [PubMed] [Google Scholar]
- Hendrix MJ, Hay ED, von der Mark K, Linsenmayer TF. Immunohistochemical localization of collagen types I and II in the developing chick cornea and tibia by electron microscopy. Invest Ophthalmol Vis Sci. 1982;22:359–375. [PubMed] [Google Scholar]
- Hirsch MS, Law LY, Trinkaus-Randall V, Svoboda KKH. The intracellular distribution of vinculin and alpha 2 integrin in epithelial cells and chondrocytes. Scanning. 1994;16:275–284. [PubMed] [Google Scholar]
- Hynes RO. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973;70:3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: a family of cell surface receptors. Cell. 1987;48:549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes RO. The emergence of integrins: a personal and historical perspective. Matrix Biol. 2004;23:333–340. doi: 10.1016/j.matbio.2004.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO, Ali IU, Mautner VM, Destree A. LETS glycoprotein: arrangement and function at the cell surface. Birth Defects Orig Artic Ser. 1978;14:139–153. [PubMed] [Google Scholar]
- Hynes RO, Destree AT, Wagner DD. Relationships between microfilaments, cell-substratum adhesion, and fibronectin. Cold Spring Harb Symp Quant Biol. 1982;46(pt 2):659–670. doi: 10.1101/sqb.1982.046.01.062. [DOI] [PubMed] [Google Scholar]
- Joo JH, Alpatov R, Munguba GC, Jackson MR, Hunt ME, Sugrue SP. Reduction of Pnn by RNAi induces loss of cell-cell adhesion between human corneal epithelial cells. Mol Vis. 2005;11:133–142. [PubMed] [Google Scholar]
- Kallman F, Grobstein C. Source of collagen at epitheliomesenchymal interfaces during inductive interaction. Dev Biol. 1965;11:169–183. doi: 10.1016/0012-1606(65)90055-2. [DOI] [PubMed] [Google Scholar]
- Khoory W, Wu E, Svoboda KKH. Intracellular relationship between actin and alpha-actinin in a whole corneal epithelial tissue. J Cell Sci. 1993;106:703–717. doi: 10.1242/jcs.106.3.703. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, Klebe RJ, Martin GR, Kleinman HK, Klebe RJ, Martin GR. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981;88:473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mautner V, Hynes RO, Mautner V, Hynes RO. Surface distribution of LETS protein in relation to the cytoskeleton of normal and transformed cells. J Cell Biol. 1977;75:743–768. doi: 10.1083/jcb.75.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier S, Hay ED. Synthesis of sulfated glycosaminoglycans by embryonic corneal epithelium. Dev Biol. 1973;35:318–331. doi: 10.1016/0012-1606(73)90027-4. [DOI] [PubMed] [Google Scholar]
- Meier S, Hay ED. Control of corneal differentiation by extracellular materials. Collagen as a promoter and stabilizer of epithelial stroma production. Dev Biol. 1974a;38:249–270. doi: 10.1016/0012-1606(74)90005-0. [DOI] [PubMed] [Google Scholar]
- Meier S, Hay ED. Stimulation of extracellular matrix synthesis in the developing cornea by glycosaminoglycans. Proc Natl Acad Sci U S A. 1974b;71:2310–2313. doi: 10.1073/pnas.71.6.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier S, Hay ED. Stimulation of corneal differentiation by interaction between cell surface and extracellular matrix. I. Morphometric analysis of transfilter “induction”. J Cell Biol. 1975;66:275–291. doi: 10.1083/jcb.66.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EJ, Matukas VJ. Chick cartilage collagen: a new type of alpha 1 chain not present in bone or skin of the species. Proc Natl Acad Sci U S A. 1969;64:1264–1268. doi: 10.1073/pnas.64.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang P, Zhen YY, Sugrue SP. Cloning and analysis of cDNA encoding murine pinin. Gene. 1997;197:115–120. doi: 10.1016/s0378-1119(97)00249-7. [DOI] [PubMed] [Google Scholar]
- Reenstra WR, Orlow DL, Svoboda KKH. ECM-stimulated signaling and actin reorganization in embryonic corneal epithelia are Rho dependent. Invest Ophthalmol Vis Sci. 2002;43:3181–3189. [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Tabesh M, Sugrue SP. Role of cell adhesion-associated protein, pinin (DRS/memA), in corneal epithelial migration. Invest Ophthalmol Vis Sci. 2000;41:1337–1345. [PubMed] [Google Scholar]
- Singer II. The fibronexus: a transmembrane association of fibronectin-containing fibers and bundles of 5 nm microfilaments in hamster and human fibroblasts. Cell. 1979;16:675–685. doi: 10.1016/0092-8674(79)90040-0. [DOI] [PubMed] [Google Scholar]
- Spurr SJ, Gipson IK. Isolation of corneal epithelium with Dispase II or EDTA. Effects on the basement membrane zone. Invest Ophthalmol Vis Sci. 1985;26:818–827. [PubMed] [Google Scholar]
- Stepp MA. Corneal integrins and their functions. Exp Eye Res. 2006;83:3–15. doi: 10.1016/j.exer.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Stepp MA, Park CC, Spurr-Michaud S, Gipson I. Identification of integrins in stationary and migrating corneal epithelia. Invest Ophthalmol Vis Sci. 1990a;31:538a. [Google Scholar]
- Stepp MA, Spurr-Michaud S, Tisdale A, Elwell J, Gipson IK. Alpha 6 beta 4 integrin heterodimer is a component of hemidesmosomes. Proc Natl Acad Sci U S A. 1990b;87:8970–8974. doi: 10.1073/pnas.87.22.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue SP, Hay ED. Response of basal epithelial cell surface and cytoskeleton to solubilized extracellular matrix molecules. J Cell Biol. 1981;91:45–54. doi: 10.1083/jcb.91.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue SP, Hay ED. Interaction of embryonic corneal epithelium with exogenous collagen, laminin, and fibronectin: role of endogenous protein synthesis. Dev Biol. 1982;92:97–106. doi: 10.1016/0012-1606(82)90154-3. [DOI] [PubMed] [Google Scholar]
- Sugrue SP, Hay ED. The identification of extracellular matrix (ECM) binding sites on the basal surface of embryonic corneal epithelium and the effect of ECM binding on epithelial collagen production. J Cell Biol. 1986;102:1907–1916. doi: 10.1083/jcb.102.5.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda KKH. Spatial localization of collagen mRNA and cytoskeletal proteins in embryonic corneal epithelium with confocal image analysis. Scanning. 1990;90:112–113. [Google Scholar]
- Svoboda KKH. Intracellular localization of types I and II collagen mRNA and endoplasmic reticulum in embryonic corneal epithelia. J Cell Sci. 1991;100:23–33. doi: 10.1242/jcs.100.1.23. [DOI] [PubMed] [Google Scholar]
- Svoboda KKH. Embryonic corneal epithelial actin alters distribution in response to laminin. Invest Ophthalmol Vis Sci. 1992;33:324–333. [PMC free article] [PubMed] [Google Scholar]
- Svoboda KKH, Hay ED. Embryonic corneal epithelial interaction with exogenous laminin and basal lamina is F-actin dependent. Dev Biol. 1987;123:455–469. doi: 10.1016/0012-1606(87)90403-9. [DOI] [PubMed] [Google Scholar]
- Svoboda KKH, Orlow DL, Ashrafzadeh A, Jirawuthiworavong G. Zyxin and vinculin distribution at the cell-extracellular matrix attachment complex (CMAX) in corneal epithelial tissue are actin dependent. Anat Rec. 1999a;254:336–347. doi: 10.1002/(sici)1097-0185(19990301)254:3<336::aid-ar4>3.0.co;2-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda KKH, Orlow DL, Chu CL, Reenstra WR. ECM-stimulated actin bundle formation in embryonic corneal epithelia is tyrosine phosphorylation dependent. Anat Rec. 1999b;254:348–359. doi: 10.1002/(sici)1097-0185(19990301)254:3<348::aid-ar5>3.0.co;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda KKH, Moessner P, Field T, Acevedo J. ROCK inhibitor (Y27632) increases apoptosis and disrupts the actin cortical mat in embryonic avian corneal epithelium. Dev Dyn. 2004;229:579–590. doi: 10.1002/dvdy.20008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamkun JW, DeSimone DW, Fonda D, Patel RS, Buck C, Horwitz AF, Hynes RO. Structure of integrin, a glycoprotein involved in the transmembrane linkage between fibronectin and actin. Cell. 1986;46:271–282. doi: 10.1016/0092-8674(86)90744-0. [DOI] [PubMed] [Google Scholar]
- Toole BP. Glycosamionglycans in morphogenesis. In: Hay ED, editor. Cell biology of the extracellular matrix. New York: Plenum Press; 1981. pp. 259–294. [Google Scholar]
- Trinkaus-Randall V, Newton AW, Franzblau C. The synthesis and role of integrin in corneal epithelial cells in culture. Invest Ophthalmol Vis Sci. 1990;31:440–447. [PubMed] [Google Scholar]
- Wartiovaara J, Linder E, Ruoslahti E, Vaheri A. Distribution of fibroblast surface antigen: association with fibrillar structures of normal cells and loss upon viral transformation. J Exp Med. 1974;140:1522–1533. doi: 10.1084/jem.140.6.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XY, Svoboda KKH, Trinkaus-Randall V. Intracellular distribution of cytoskeletal and adhesion proteins in response to corneal substrata. Exp Eye Res. 1995;60:445–458. doi: 10.1016/s0014-4835(05)80101-0. [DOI] [PubMed] [Google Scholar]
- Yeh B, Svoboda KKH. Intracellular labeling of β-actin mRNA using reverse transcriptase incorporated biotin dUTP into actin cortical mat of corneal epithelial cells. Micron. 1993;24:595–602. [Google Scholar]
- Yeh B, Svoboda KKH. Intracellular distribution of beta-actin mRNA is polarized in embryonic corneal epithelia. J Cell Sci. 1994;107:105–115. doi: 10.1242/jcs.107.1.105. [DOI] [PubMed] [Google Scholar]
- Zimowska G, Shi J, Munguba G, Jackson MR, Alpatov R, Simmons MN, Shi Y, Sugrue SP. Pinin/DRS/memA interacts with SRp75, SRm300 and SRrp130 in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:4715–4723. doi: 10.1167/iovs.03-0240. [DOI] [PubMed] [Google Scholar]


