Abstract
Cyclic mechanical strain produced by pulsatile blood flow regulates the orientation of endothelial cells lining blood vessels, and influences critical processes such as angiogenesis. Mechanical stimulation of stretch-activated calcium channels is known to mediate this reorientation response, however, the molecular basis remains unknown. Here we show that cyclically stretching capillary endothelial cells adherent to flexible extracellular matrix substrates activates mechanosensitive TRPV4 ion channels that, in turn, stimulate phosphatidyl inositol-3-kinase-dependent activation and binding of additional ·1 integrin receptors, which promotes cytoskeletal remodeling and cell reorientation. Inhibition of integrin activation using blocking antibodies and knockdown of TRPV4 channels using specific siRNA suppress strain-induced capillary cell reorientation. Thus, mechanical forces that physically deform extracellular matrix may guide capillary cell reorientation through a strain-dependent ‘integrin to integrin’ signaling mechanism mediated by force-induced activation of mechanically-gated TRPV4 ion channels on the cell surface.
Keywords: mechanical strain, integrin, TRPV4, endothelial cell, reorientation, cytoskeleton
Mechanical forces regulate vascular growth and development by influencing endothelial cell growth, survival, differentiation and migration1, 2. Local mechanical cues conveyed by extracellular matrix (ECM) due to cyclic deformation of blood vessels, hemodynamic forces or cell-generated traction forces are also potent inducers of directional capillary blood vessel growth and vascular remodeling in vitro and in vivo3-10. For example, the initial step in neovascularization involves reorientation of a subset of capillary endothelial (CE) cells that spread and migrate perpendicular to the main axis of the pre-existing vessel towards the angiogenic stimulus11; however, the molecular mechanism responsible for this CE cell reorientation response is unknown. Many cell types, including large vessel endothelial cells, realign perpendicular to the direction of the applied force when they experience cyclic stretching (mechanical strain)12-15. In the case of macrovascular endothelium, this reorientation response can be prevented by treatment with chemical inhibitors of stretch-activated (SA) ion channels15. But neither the identity of these channels nor the mechanism by which they elicit cell reorientation is known.
Endothelial cells express most members of the Transient Receptor Potential (TRP) family of ion channels16-18 and TRPV4 has been reported to mediate flow-induced vasodilation in large vessel endothelium19-22. Here we show that calcium influx through TRPV4 channels stimulated by mechanically stretching CE cells through their integrin-ECM adhesions promotes cell reorientation by activating phosphatidyl inositol-3-kinase (PI3K), and thereby stimulating activation of additional ·1 integrin receptors. This mechanism is distinct from the one used by macrovascular endothelium to sense fluid shear stresses, which is mediated by a mechanosensory complex containing PECAM1, VEGFR and VE-cadherin23.
Materials and Methods
Cell Culture
CE cells were isolated from bovine adrenal cortex, cloned and passaged as decribed previously24. Frozen aliquots of these cells (passage <15), which we have confirmed retain their functionality and differentiation potential3, were maintained at 37°C in 10% CO2 on gelatin-coated tissue culture dishes in low glucose Dulbecco's Modified Eagle's Medium (DMEM; Invitrogen) supplemented with 10% fetal calf serum (FCS) (Hyclone), 10 mM HEPES (JRH-Biosciences) and L-glutamine (292 ·g/ml), penicillin (100 U/ml), streptomycin (100 ·g/ml) (GPS), as described 25. Human microvascular endothelial cells from dermis (HMVECs) (Cambrex, Walkersville, MD) were cultured in EBM-2 (Cambrex), supplemented with 5% FBS and growth factors (bFGF, IGF, VEGF) according to the manufacturer's instructions.
Mechanical Strain Application
CE cells cultured on fibronectin-coated 6 well Uniflex (Flex Cell International) plates for 24 hours were subjected to uniaxial cyclic stretch (10% elongation; 1 Hz frequency) for 1-2 h using a Flexercell® Tension Plus™ System (Flex Cell International)26. In some experiments, CE cells were plated on fibronectin-coated 6 well Bioflex (Flex Cell International) for 1h and subjected to static stretch (15% elongation) for 1-15 min. Control cells were maintained under identical conditions in the absence of strain application.
Measurement of Cell Orientation
To measure the orientation of cells in cyclic strain experiments, fluorescent images of cells were traced to measure angle with the direction of cyclic strain using ImageJ software (NIH) and reported as percent cells aligned at 90° ± 30°. For each condition, 5-6 fields were evaluated, with ∼ 15-30 cells per field. Statistical differences between experimental groups were determined using the student's t-test. All data were obtained from at least three separate experiments and are expressed as mean + SEM.
siRNA knock down of TRPV channels
smart pool siRNAs (10 nM) of TRPV2, TRPV4 (both from Dharmacon), TRPC1 (Ambion) or control (Qiagen) siRNAs was transfected into CE cells using silentfect reagent (BioRad) as described 27. Three days later cells were used for calcium imaging or reorientation experiments. The knock down of TRPV channel expression was assessed using RT-PCR with species-specific primers and Western blotting.
Results
Capillary cell reorientation induced by cyclic strain
Directional CE cell motility and angiogenesis have been shown to be stimulated by mechanical strain (distortion) in ECM gels and living tissues7-10. To begin to analyze the molecular mechanism by which mechanical strain influences CE cell orientation, we cultured bovine CE cells on flexible fibronectin-coated substrates and subjected them to 10% uniaxial cyclical strain (1 Hz) using a FlexerCell Tension Plus system. Fluorescence microscopic analysis of cells labeled with Alexa 488-phalloidin combined with computerized morphometry revealed that stress fibers thickened in these cells, and most (∼80%) realigned perpendicular to the main axis of the applied strain within 2 hr after force application (Fig. 1A,B). Stress fiber realignment was accompanied by redistribution and reorientation of focal adhesions containing vinculin (Fig. 1C), focal adhesion kinase (FAK) and talin (not shown), which appeared in close association with the ends of newly aligned stress fibers (Fig.1C).
Fig. 1. Capillary endothelial (CE) cells reorient in response to uniaxial cyclic strain.
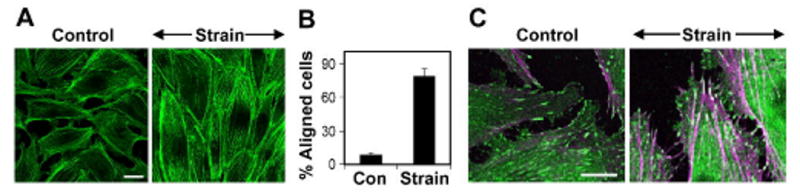
A) Fluorescence micrographs of CE cells cultured on fibronectin-coated flexible silicone membranes subjected to 0 or 10% uniaxial cyclic strain (2 h, 1 Hz) and stained with Alexa488-phalloidin to visualize actin stress fibers; arrow indicates the direction of applied strain. Scale bar: 25 ·m. B) Percentage of cells oriented 90 ± 30 ° degrees (aligned) relative to the direction of applied strain in control and strain exposed cells (p < 0.0006); error bars indicated S.E.M. C) Immunofluorescence micrographs of CE cells subjected to 0 or 10% uniaxial cyclic strain and stained for vinculin (green) and actin stress fibers (magenta) showing that application of strain causes enhanced recruitment of vinculin to large focal adhesions that colocalize with the ends of reinforced stress fibers (shown in white). Scale bar: 25 ·m.
Strain-induced capillary cell reorientation requires ·1 integrin activation
The effects of fluid shear on large vessel endothelium28 and mechanical strain on fibroblasts29 are mediated by stress-dependent activation of integrin receptors within minutes after force application. When CE cells cultured on flexible fibronectin-coated substrates were exposed to static stretch (15% elongation), ·1 integrin activation increased within 1 min after force application, as indicated by increased phosphorylation of the T788/789 site of the ·1 integrin cytoplasmic tail in Western blots (Fig. 2A), which has been shown to correlate with integrin activation30-32. Immunofluorescence staining using 12G10 antibodies that only recognize the activated conformation of ·1 integrins33, 34 also showed increased clustering of activated ·1 integrins within large streak-like focal adhesions at the cell periphery within 15 min after static strain application (Fig. 2B). The ability of the 12G10 antibody to detect activated ·1 integrins in our CE cells was confirmed using flow cytometry, which demonstrated a significantly increased 12G10 signal after globally activating integrins by treatment with manganese (see Supplementary Fig. S1). Static stretch-induced activation of integrin signaling was confirmed independently by demonstrating increased phosphorylation of MAP kinase (ERK1/2) (Fig. 2C) and FAK (Supplementary Fig. S2) within 5 to 15 min after exposure to mechanical strain. Application of uniaxial cyclic strain (10%; 1 Hz) also induced ·1 integrin activation within minutes, as measured by enhanced binding to the fibronectin fragment, GST-FNIII8-11 (Fig. 2D and Supplementary Information Fig. S3B) and to the 12G10 antibody that only ligate the activated form of the ·1 integrin receptor35 (Fig. 2E), as well as by increased T788/789 phosphorylation of ·1 integrin (Supplementary Fig. S3C). Cyclic strain also increased ·1 integrin activation in human CE cells as measured by enhanced binding of GST-FNIII8-11 (Supplementary Fig. S3B), and thus, this appears to be a generalized response in CE cells.
Fig. 2. ·1 integrin activation is required for cyclic strain-induced reorientation of CE cells.
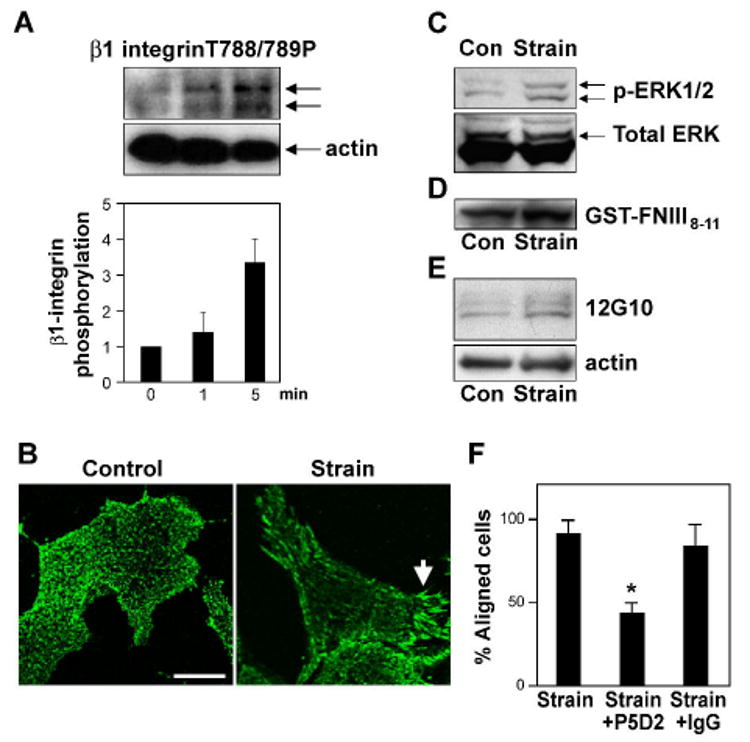
A) Western blot analysis of CE cell lysates showing time-dependent phosphorylation of ·1 integrin cytoplasmic tail at threonine T788/789 in response to static stretch. Histogram shows the corresponding densitometric quantification of ·1 integrin phosphorylation. B) Immunofluorescence micrographs of control and strain-exposed CE cells stained for activated β1 integrin using 12G10 antibody. Arrow indicates increased clustering of activated ·1 integrins within large streak-like focal adhesions at the cell periphery. Scale bar: 25 ·m. c-e) Western blots showing MAP kinase (ERK1/2) phosphorylation (C) and binding of GST-FNIII8-11 (D) and 12G10 (E) in CE cells in the absence and presence of static (C) or cyclic strain (D, E). F) Percentage of cells oriented 90 ± 30 ° degrees (aligned) relative to the direction of applied cyclic strain in the absence or presence of the ·1 integrin blocking antibody P5D2 (p < 0.001) or isotype-matched IgG.
To explore if this mechanical strain-induced wave of ·1 integrin activation is required for CE cell reorientation, cells were pre-incubated with function-blocking anti-·1 integrin (P5D2) antibody for 30 min, and then the cells were subjected to uniaxial cyclical strain (10%) for 2 hr. Treatment with this inhibitory antibody, but not isotype-matched control IgG, inhibited strain-induced cell realignment by almost 70% (p < 0.001) (Fig. 2F), and it prevented reorientation of stress fibers and focal adhesions (Supplementary Fig. S4). Prior to stretching, we did not find any changes in cell morphology or actin staining in antibody-treated cells confirming that binding of these antibodies did not affect existing adhesions. These results indicate that application of mechanical strain to CE cells through existing integrins that are bound to substrate-immobilized ECM molecules (and hence activated) induces focal adhesion remodeling, stress fiber realignment, and cell reorientation through a mechanism that requires activation of additional ·1 integrin receptors.
PI3K is upstream of ·1 integrin activation in this mechanical signaling cascade
PI3K has been implicated in the activation of ·3 integrins by fluid shear stress in large vessel endothelium23; however, it also can act downstream of integrin activation36. To explore whether PI3K is involved in early mechanical signaling in microvascular endothelium, CE cells were transfected with GFP-fused to an AKT-PH domain that translocates to the plasma membrane when it binds to the PI3K product, phosphatidyl inositol-3-phosphate37. Bright linear AKT-PH-GFP staining was detected at the peripheral membrane within 1 min after application of static stretch (15%), whereas it remained diffusely distributed throughout the cytoplasm in control (unstrained) CE cells (Fig. 3A). Quantification of AKT-PH-GFP translocation by two independent parameters (fraction of AKT-PH-GFP in total perimeter of the membrane, or GFP-fluorescence intensity ratio between membrane and cytosol) revealed a significant increase in response to mechanical strain that was inhibited by treatment with the PI3K inhibitor, LY294002 (Fig. 3B). Static stretch also activated PI3K as determined by enhanced phosphorylation of its downstream target AKT at ser-473 within minutes after force application, as detected in Western blots (Fig. 3C). Moreover, stretch-induced translocation of AKT-PH-GFP to the membrane and AKT phosphorylation were both abolished by inhibiting PI3K with LY294002 (Fig. 3A,D). LY294002 treatment also prevented ·1 integrin activation (Fig. 3D,E) and suppressed FAK activation (see Supplementary Fig. S2). Thus, force application through ECM-integrin adhesions activates additional cell surface ·1 integrin receptors by stimulating PI3K.
Fig. 3. Mechanical strain-induced ·1 integrin activation requires the PI3K/AKT pathway.
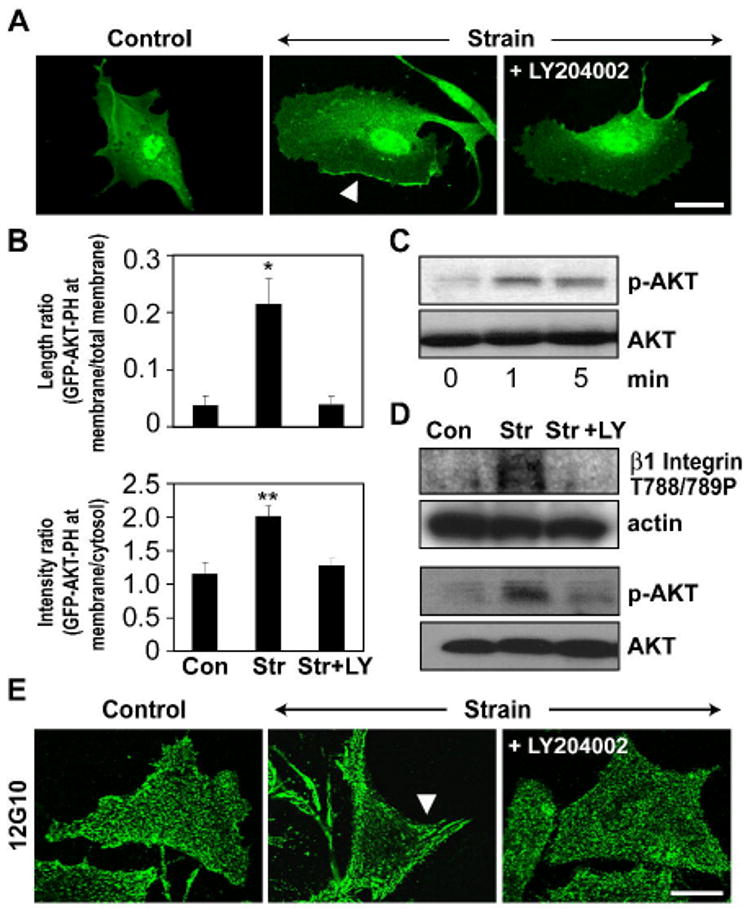
A) Fluorescence micrographs of CE cells transfected with GFP-AKT-PH and subjected to 0 or 15% static stretch in the absence or presence of the PI3-Kinase inhibitor, LY 294002 (LY, 40 ·M). Note that LY 294002 inhibits strain induced translocation of GFP-AKT-PH domain to the plasma membrane (arrow). Scale bar: 25 ·m. B) Quantification of mechanical strain-induced GFP-AKT-PH domain translocation to the membrane in the absence or presence of the PI3K inhibitor LY294002, measured as a fraction of total cell membrane perimeter that is enhanced with GFP-AKT-PH in randomly selected cells and the ratio of GFP fluorescence intensity in the membrane versus cytosol (*, p < 0.05). C-D) Representative Western blots showing time dependent activation of AKT (C) and phosphorylation of ·1 integrin cytoplasmic tail at T788/789 and AKT at ser-473 in response to static stretch in the presence and absence of the PI3K inhibitor, LY294002 (D). E) Fluorescence micrographs of CE cells subjected to 0 or 15% mechanical strain in the absence or presence of the PI3-Kinase inhibitor, LY 294002 and stained for activated ·1 integrin using the12G10 antibody. Arrow indicates increased clustering of activated ·1 integrins within large streak-like focal adhesions at the cell periphery. Scale bar: 25 ·m.
Strain-induced cell reorientation is mediated by stress-activated ion channels
SA ion channels have been implicated in force-dependent alignment of large vessel endothelial cells15. Direct force application to cell surface ·1 integrins using magnetic tweezers also results in rapid (within 2-5 sec) calcium influx in our bovine CE cells, and this response can be blocked using the general SA channel inhibitor, gadolinium chloride25, 38. To confirm that mechanical strain activates SA channels in these CE cells, cells adherent to flexible ECM substrates were loaded with the calcium reporter dye Fluo-4, subjected to static stretch (15% elongation) and calcium influx was measured using microfluorimetry25. Stretching CE cells for as little as 3 sec induced rapid calcium influx, and this response could be almost completely abolished by treatment with gadolinium chloride (Supplemental Fig. S3A). Pre-treatment of bovine and human CE cells for 30 min with gadolinium chloride also significantly inhibited ·1 integrin activation in response to static stretch, as measured by decreased binding of GST-FNIII8-11 and reduced ·1 integrin phosphorylation (Supplemental Fig. S3B,C). In addition, blocking SA channels with gadolinium chloride inhibited PI3K activity, as measured by membrane translocation of GFP-AKT-PH (Supplemental Fig. S3D). Finally, the cell and cytoskeletal reorientation normally induced by cyclic strain were greatly suppressed in the presence of this SA ion channel blocker (Supplemental Fig. S3E). Thus, mechanical stretch-dependent activation of mechanosensitive calcium channels appears to be required for activation of both PI3K and ·1 integrins, as well as subsequent cytoskeletal reorientation in CE cells.
TRPV4 channels mediate strain-induced capillary cell reorientation
We then set out to identify the specific type of mechanosensitive ion channel that mediates the effects of mechanical strain on CE cell orientation. TRPV4 is an interesting potential candidate because it mediates cell sensitivity to osmotic stresses39 and shear stress-induced vasodilation19. To determine whether TRPV4 is the candidate mechanosensitive channel, first we measured its expression in CE cells. Western blot analysis showed a strong band around 85 kDa (and a fainter band at ∼100 kDa) in both bovine and human CE cells (Fig. 4A). RT-PCR analysis also confirmed the presence of TRPV4 mRNA in both bovine and human CE cells (Fig. 4B and Supplementary Fig. S5). We then found that a specific activator of TRPV4 channels, 4-·-PDD40, induced a robust calcium signal in bovine and human CE cells, thus suggesting that both cell types express functional TRPV4 channels (Supplementary Fig. S6). Next, we measured TRPV4 channel activation directly by whole-cell clamp using BCE cells transiently transfected with TRPV4-EGFP that gave robust TRPV4 currents in response to 4-·-PDD, and we found that substitution of NMDG (N-methyl-D-glucamine) for cations in the bathing solution, inhibited activation of inward, but not outward, currents by 4-·-PDD in these cells (Supplementary Fig.S7). We used this approach because TRPV4-like currents in primary endothelial cells are small, transient and difficult to characterize, as previously described22, and as we observed as well. Thus, taken together these findings strongly suggest that capillary endothelial cells express functional TRPV4 channels, although at a low level.
Fig. 4. TRPV4 channels mediate mechanical strain induced calcium signaling in CE cells.
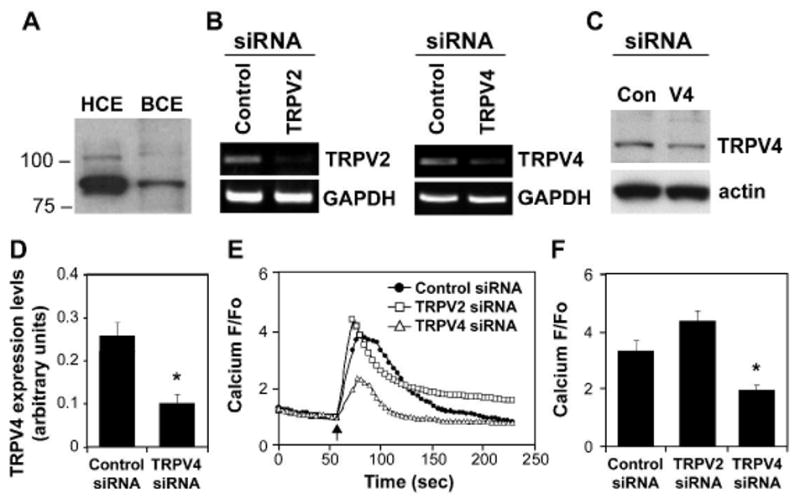
A) Western blotting analysis showing the expression of TRPV4 in human and bovine CE cells B) Representative RT-PCR results confirming knockdown of TRPV2 and TRPV4 mRNA levels in bovine CE cells using specific siRNAs and that the same TRPV4 siRNA produced comparable suppression of protein expression (C,D; (*, p < 0.05). E) Relative change in cytosolic calcium in response to static stretch (15%, 4 sec, arrow) in Fluo-4 loaded CE cells treated with indicated siRNA. F) Average relative increases in cytosolic calcium induced by mechanical strain in CE cells treated with the indicated siRNAs (*, p < 0.02).
To confirm that calcium influx through TRPV4 channels mediates the effects of cyclic strain on CE cell orientation, we knocked down the expression of TRPV4 in bovine and human CE cells using specific siRNA; sham siRNA and siRNA directed against the closely related channel, TRPV2, were used as controls. Sequence analysis of smart pool siRNAs confirmed that both siRNA sequences exhibit 80-100% homology with bovine and human TRPV4. RT-PCR analysis revealed that TRPV2 and TRPV4 mRNA levels were knocked down by 70% and 90% in bovine and human CE cells, respectively, using this approach, whereas use of a sham control siRNA had no effect (Fig. 4B and Supplementary Fig. S5). We found that TRPV4 protein expression was also knocked down by ∼60% and 80% in bovine and human CE cells, respectively (Fig. 4C,D and Supplementary Fig.S5).
Importantly, microfluorimetric analysis revealed that application of static stretch (15%) for 4 sec induced a large wave of calcium influx in bovine CE cells transfected with control siRNAs, whereas this response was significantly inhibited (p < 0.02) in cells treated with TRPV4 siRNA (Fig. 4E,F). In contrast, use of siRNA directed against the closely related SA channel TRPV2 had no effect (Fig. 4E,F). siRNA knock down of TRPV4 also inhibited cyclic strain-induced activation of ·1 integrins, AKT and ERK1/2 further confirming that TRPV4 activation is upstream of integrin activation (Fig. 5). Pretreatment of CE cells with the general TRPV inhibitor, ruthenium red41, or with TRPV4 siRNA also significantly suppressed calcium signaling and cell reorientation induced by application of cyclic strain in CE cells, whereas addition of siRNA against two different related SA channels, TRPV2 or TRPC1(see Supplementary Fig.S5), were ineffective (Fig. 6A-D). This inhibition was specific for reorientation as transfection of cells with TRPV4 siRNA did not alter the number of viable adherent CE cells when they were cultured on standard tissue culture substrates (see Supplementary Fig. S8). Moreover, we found that application of similar cyclic strain, in the presence or absence of ruthenium red, did not effect CE cell proliferation or apoptosis as measured by Ki 67 staining and PARP cleavage (Supplementary Fig. S9). Taken together, these results indicate that TRPV4 channels are mechanosensitive calcium channels in CE cells that are activated by mechanical strain applied through the integrin-mediated cell-ECM adhesions, and that calcium influx through these channels is required for downstream signaling events that drive the cell and cytoskeletal reorientation response triggered by cell stretching.
Fig. 5. TRPV4 channel knockdown inhibits cyclic strain-induced activation of · 1 integrins, AKT and ERK in CE cells.
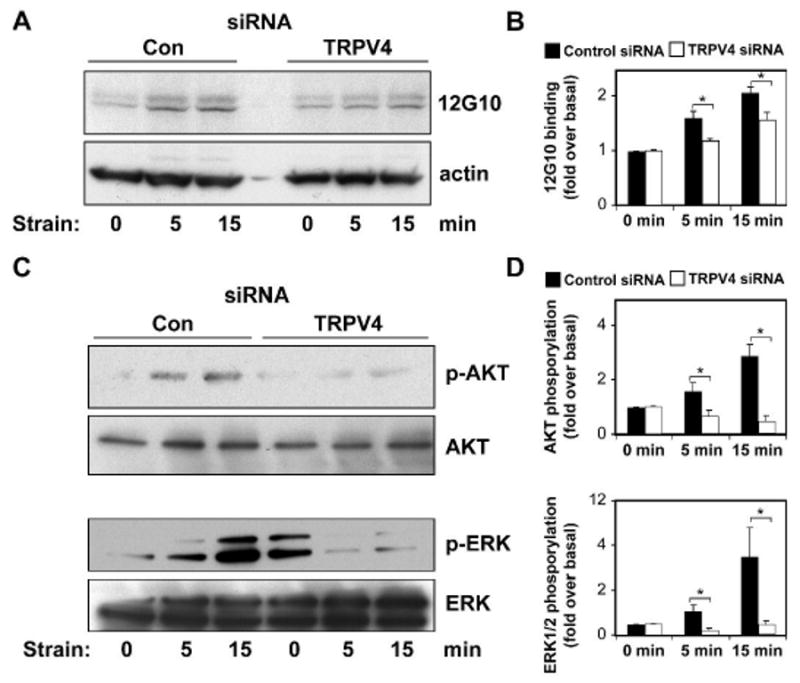
Representative Western blots showing activation of ·1 integrins as measured by binding to 12G10 antibody (A,B) and phosphorylation of AKT at ser-473 and ERK1/2 (C,D) in response to cyclic strain in the control and TRPV4 siRNA transfected CE cells at indicated times. Phosphorylation/activation of signaling protein levels were measured as a percentage of total protein/actin levels and normalized to basal levels (*, p < 0.05 for comparison between control siRNA versus TRPV4 siRNA treated cells).
Fig. 6. TRPV4 channel mediates cyclic strain-induced CE cell reorientation.
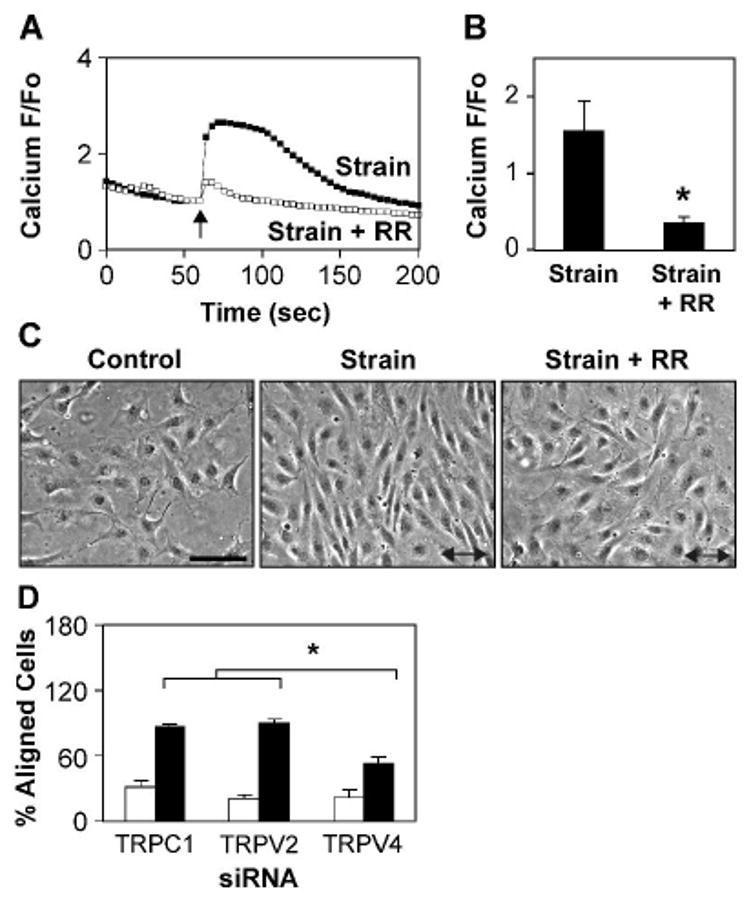
A-B) Relative changes in cytosolic calcium in Fluo-4 loaded CE cells in response to static stretch (15%, 4 sec, arrow) in the absence (■) and presence (□) of the TRPV inhibitor ruthenium red (RR) (*, p < 0.02). C) Phase contrast photomicrographs of CE cells showing the effects of cyclic strain on cell reorientation in the absence and presence of ruthenium red. Arrow indicates the direction of applied strain. Note that ruthenium red inhibits cyclic strain-induced cell reorientation. Scale bar: 50 ·m. D) Percentage of cells oriented 90 ± 30 ° degrees (aligned) relative to the direction of applied strain in control (white bars) and strain exposed (black bars) human CE cells treated with the indicated siRNA. Note that TRPV4 siRNA treated cells failed to reorient fully compared to TRPV2 or TRPC1 treated cells (*, p < 0.0025).
Discussion
In this study, we showed that application of mechanical strain to bound integrins on the CE cell surface stimulates calcium influx through mechanosensitive TRPV4 ion channels, which activates additional ·1 integrins and subsequent downstream cytoskeletal reorientation responses. Although cyclic strain induces reorientation of large vessel endothelial cells and this process has been shown to be mediated by activation of SA channels15, the present study is the first to analyze this process in microvascular CE cells and to determine the specific molecular identity of these channels. Our work shows the TRPV4 is at least one of the SA channels that is required for activation of ·1 integrins and subsequent reorientation of CE cells in response to mechanical strain.
Cell stretching and strain application to integrins have both been implicated as critical regulators of endothelial cell proliferation, migration and angiogenesis in the past3, 5, 6, 9, 42-44, but how these mechanical signals control vascular development is not known. The present findings provide direct evidence to show that mechanical strain activates ·1 integrins in bovine and human CE cells, and that this is required for downstream cell and cytoskeleletal remodeling events that mediate cell reorientation critical for directional cell motility. Given that we exposed cells to both static and cyclic stretch and similar results were obtained using multiple different assays and probes to assess ·1 integrin activation, we believe that these findings unequivocally confirm that mechanical strain activates ·1 integrins in CE cells.
The most important finding of this study is the identification of TRPV4 as the SA channel responsible for ·1 integrin activation in response to mechanical strain application to microvascular cells. We make this conclusion based on the following observations: 1) bovine and human CE cells functionally express TRPV4 channels that are activated by the selective TRPV activator, 4·-PDD, 2) the TRPV4 blocker, ruthenium red, inhibits calcium influx and cell reorientation in response to mechanical strain, and 3) siRNA knockdown of TRPV4, but not TRPV2 or TRPC1, inhibits strain-induced calcium influx and capillary cell reorientation. Among all known TRP channels, only TRPV4 has been reported to be mechanosensitive in that it transduces osmotic signals39 and plays a role in shear stress-induced vasodilation19, 20. TRPV4 is also important for the mechanical behavior of C. elegans45, and mice lacking TRPV4 are insensitive to normal levels of noxious mechanical stimuli46. Here, we show that activation of TRPV4 by mechanical distortion of cell-ECM adhesions plays a critical role in control of downstream signaling pathways that mediate cell reorientation and vascular development in response to mechanical strain of integrin-mediated cell-ECM adhesions.
Although, TRPV2 and TRPC1 channels were shown to mediate stretch-induced calcium signaling when overexpressed in CHO cells and oocytes47, 48, we found that knocking down of either TRPV2 and TRPC1 expression using siRNAs did not influence calcium influx or cytoskeletal reorientation in response to mechanical strain, suggesting that these candidate SA channels do not appear to contribute to stretch-activated calcium entry in CE cells49. TRPV4 channel activation by mechanical strain could be mediated through its interaction with integrins. Other types of TRP channels, such as polycystins and ENaC channels, co-immunoprecipitate with ·1 integrins50, 51, and TRPV4 has been found to co-immunoprecipitate with ·2 integrins52, suggesting that it resides in a common mechanosignaling complex with these ECM receptors.
Regardless of the precise mechanism by which TRPV4 channels sense changes in the forces that are balanced across integrins, our findings show that strain-induced calcium influx through these channels activates PI3K53. PI3K, in turn, activates additional integrins and related downstream signaling molecules that result in activation of Rho and its target Rho-associated kinase (ROCK)13, 26, which promote focal adhesion and stress fiber remodeling. The fact that this structural remodeling occurs in a highly oriented manner that is perpendicular to the applied tension field in non-confluent cells, provides additional evidence to suggest that these events occur locally at the cell surface-ECM interface where forces are exerted, rather than homogenously throughout the cytoplasm or within lateral membrane junctional complexes that form between cells in a confluent endothelial cell monolayer, as is required for shear stress sensation23.
These findings are important because CE cell reorientation plays a crucial role in the directional migration and oriented sprouting that drive angiogenesis. Ion signaling through SA channels has been previously shown to be important for both cell migration44 and reorientation15 in response to stress. However, these SA channels were never identified, and the importance of this type of mechanotransduction response for angiogenesis has not been explored previously. The possibility that TRP channels might be involved in vascular development has been raised in the past54, 55; however, there has been no evidence to suggest that they play a direct role in endothelial cell reorientation. Importantly, our data show that TRPV4 channels are the SA channels that mediate CE cell responses to mechanical forces and cell-ECM interactions, which are critical for control of cell migration and tube formation during capillary development. Mechanically-gated TRPV4 channels therefore appear to mediate a novel stretch-sensitive “integrin-to-integrin” mechanical signaling that is required for CE cell reorientation during angiogenesis, and thus, these channels may represent new targets for future therapeutic intervention in angiogenesis-dependent diseases, such as cancer, arthritis and macular degeneration.
Supplementary Material
Acknowledgments
We would like to thank Scott Ramsey and David Clapham (Children's Hospital, Boston) for performing patch-clamp experiments and their helpful comments in preparing manuscript; Richard Clark and X.-D. Ren (Stony Brook University, New York, USA) for providing the GST-FN III 8-11 domain; and Dr. Martin Schwartz (University of Virginia, Charlottesville, USA) for the GFP-AKT-PH construct.
Sources of Funding This work was supported by grants from NIH (CA55833 & CA45548) and American Heart Association (0635095N).
Footnotes
Disclosures: None
References
- 1.Chien S, Li S, Shyy YJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31(1 Pt 2):162–169. doi: 10.1161/01.hyp.31.1.162. [DOI] [PubMed] [Google Scholar]
- 2.Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res. 2002;91(10):877–887. doi: 10.1161/01.res.0000039537.73816.e5. [DOI] [PubMed] [Google Scholar]
- 3.Ingber DE, Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J Cell Biol. 1989;109(1):317–330. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies PF, Mundel T, Barbee KA. A mechanism for heterogeneous endothelial responses to flow in vivo and in vitro. J Biomech. 1995;28(12):1553–1560. doi: 10.1016/0021-9290(95)00102-6. [DOI] [PubMed] [Google Scholar]
- 5.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276(5317):1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 6.Dike LE, Chen CS, Mrksich M, Tien J, Whitesides GM, Ingber DE. Geometric control of switching between growth, apoptosis, and differentiation during angiogenesis using micropatterned substrates. In Vitro Cell Dev Biol Anim. 1999;35(8):441–448. doi: 10.1007/s11626-999-0050-4. [DOI] [PubMed] [Google Scholar]
- 7.Korff T, Augustin HG. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J Cell Sci. 1999;112(Pt 19):3249–3258. doi: 10.1242/jcs.112.19.3249. [DOI] [PubMed] [Google Scholar]
- 8.Joung IS, Iwamoto MN, Shiu YT, Quam CT. Cyclic strain modulates tubulogenesis of endothelial cells in a 3D tissue culture model. Microvasc Res. 2005 doi: 10.1016/j.mvr.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto T, Yung YC, Fischbach C, Kong HJ, Nakaoka R, Mooney DJ. Mechanical strain regulates endothelial cell patterning in vitro. Tissue Eng. 2007;13(1):207–217. doi: 10.1089/ten.2006.0058. [DOI] [PubMed] [Google Scholar]
- 10.Pietramaggiori G, Liu P, Scherer SS, Kaipainen A, Prsa MJ, Mayer H, Newalder J, Alperovich M, Mentzer SJ, Konerding MA, Huang S, Ingber DE, Orgill DP. Tensile forces stimulate vascular remodeling and epidermal cell proliferation in living skin. Ann Surg. 2007;246(5):896–902. doi: 10.1097/SLA.0b013e3180caa47f. [DOI] [PubMed] [Google Scholar]
- 11.Ausprunk DH, Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977;14(1):53–65. doi: 10.1016/0026-2862(77)90141-8. [DOI] [PubMed] [Google Scholar]
- 12.Kato T, Ishiguro N, Iwata H, Kojima T, Ito T, Naruse K. Up-regulation of COX2 expression by uni-axial cyclic stretch in human lung fibroblast cells. Biochem Biophys Res Commun. 1998;244(3):615–619. doi: 10.1006/bbrc.1998.8335. [DOI] [PubMed] [Google Scholar]
- 13.Kaunas R, Nguyen P, Usami S, Chien S. Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc Natl Acad Sci U S A. 2005;102(44):15895–15900. doi: 10.1073/pnas.0506041102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qu MJ, Liu B, Wang HQ, Yan ZQ, Shen BR, Jiang ZL. Frequency-dependent phenotype modulation of vascular smooth muscle cells under cyclic mechanical strain. J Vasc Res. 2007;44(5):345–353. doi: 10.1159/000102278. [DOI] [PubMed] [Google Scholar]
- 15.Naruse K, Yamada T, Sokabe M. Involvement of SA channels in orienting response of cultured endothelial cells to cyclic stretch. Am J Physiol. 1998;274(5 Pt 2):H1532–1538. doi: 10.1152/ajpheart.1998.274.5.H1532. [DOI] [PubMed] [Google Scholar]
- 16.Kwan HY, Huang Y, Yao X. TRP channels in endothelial function and dysfunction. Biochim Biophys Acta. 2007;1772(8):907–914. doi: 10.1016/j.bbadis.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Vennekens R, Owsianik G, Nilius B. Vanilloid transient receptor potential cation channels: an overview. Curr Pharm Des. 2008;14(1):18–31. doi: 10.2174/138161208783330763. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe H, Murakami M, Ohba T, Takahashi Y, Ito H. TRP channel and cardiovascular disease. Pharmacol Ther. 2008;118(3):337–351. doi: 10.1016/j.pharmthera.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Kohler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol. 2006;26(7):1495–1502. doi: 10.1161/01.ATV.0000225698.36212.6a. [DOI] [PubMed] [Google Scholar]
- 20.Hartmannsgruber V, Heyken WT, Kacik M, Kaistha A, Grgic I, Harteneck C, Liedtke W, Hoyer J, Kohler R. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS ONE. 2007;2(9):e827. doi: 10.1371/journal.pone.0000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loot AE, Popp R, Fisslthaler B, Vriens J, Nilius B, Fleming I. Role of cytochrome P450-dependent transient receptor potential V4 activation in flow-induced vasodilatation. Cardiovasc Res. 2008 doi: 10.1093/cvr/cvn207. [DOI] [PubMed] [Google Scholar]
- 22.Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, Nilius B. Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res. 2005;97(9):908–915. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- 23.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437(7057):426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 24.Folkman J, Haudenschild CC, Zetter BR. Long-term culture of capillary endothelial cells. Proc Natl Acad Sci U S A. 1979;76(10):5217–5221. doi: 10.1073/pnas.76.10.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthews BD, Overby DR, Mannix R, Ingber DE. Cellular adaptation to mechanical stress: role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J Cell Sci. 2006;119(Pt 3):508–518. doi: 10.1242/jcs.02760. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh K, Thodeti CK, Dudley AC, Mammoto A, Klagsbrun M, Ingber DE. Tumor-derived endothelial cells exhibit aberrant Rho-mediated mechanosensing and abnormal angiogenesis in vitro. Proc Natl Acad Sci U S A. 2008;105(32):11305–11310. doi: 10.1073/pnas.0800835105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mammoto A, Huang S, Ingber DE. Filamin links cell shape and cytoskeletal structure to Rho regulation by controlling accumulation of p190RhoGAP in lipid rafts. J Cell Sci. 2007;120(Pt 3):456–467. doi: 10.1242/jcs.03353. [DOI] [PubMed] [Google Scholar]
- 28.Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. Embo J. 2001;20(17):4639–4647. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katsumi A, Naoe T, Matsushita T, Kaibuchi K, Schwartz MA. Integrin activation and matrix binding mediate cellular responses to mechanical stretch. J Biol Chem. 2005;280(17):16546–16549. doi: 10.1074/jbc.C400455200. [DOI] [PubMed] [Google Scholar]
- 30.Nilsson S, Kaniowska D, Brakebusch C, Fassler R, Johansson S. Threonine 788 in integrin subunit beta1 regulates integrin activation. Exp Cell Res. 2006;312(6):844–853. doi: 10.1016/j.yexcr.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Stawowy P, Margeta C, Blaschke F, Lindschau C, Spencer-Hansch C, Leitges M, Biagini G, Fleck E, Graf K. Protein kinase C epsilon mediates angiotensin II-induced activation of beta1-integrins in cardiac fibroblasts. Cardiovasc Res. 2005;67(1):50–59. doi: 10.1016/j.cardiores.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Wennerberg K, Fassler R, Warmegard B, Johansson S. Mutational analysis of the potential phosphorylation sites in the cytoplasmic domain of integrin beta1A. Requirement for threonines 788-789 in receptor activation. J Cell Sci. 1998;111(Pt 8):1117–1126. doi: 10.1242/jcs.111.8.1117. [DOI] [PubMed] [Google Scholar]
- 33.Humphries MJ. Monoclonal antibodies as probes of integrin priming and activation. Biochem Soc Trans. 2004;32(Pt3):407–411. doi: 10.1042/BST0320407. [DOI] [PubMed] [Google Scholar]
- 34.Thodeti CK, Albrechtsen R, Grauslund M, Asmar M, Larsson C, Takada Y, Mercurio AM, Couchman JR, Wewer UM. ADAM12/syndecan-4 signaling promotes beta 1 integrin-dependent cell spreading through protein kinase Calpha and RhoA. J Biol Chem. 2003;278(11):9576–9584. doi: 10.1074/jbc.M208937200. [DOI] [PubMed] [Google Scholar]
- 35.Orr AW, Ginsberg MH, Shattil SJ, Deckmyn H, Schwartz MA. Matrix-specific suppression of integrin activation in shear stress signaling. Mol Biol Cell. 2006;17(11):4686–4697. doi: 10.1091/mbc.E06-04-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berrier AL, Mastrangelo AM, Downward J, Ginsberg M, LaFlamme SE. Activated R-ras, Rac1, PI 3-kinase and PKCepsilon can each restore cell spreading inhibited by isolated integrin beta1 cytoplasmic domains. J Cell Biol. 2000;151(7):1549–1560. doi: 10.1083/jcb.151.7.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watton SJ, Downward J. Akt/PKB localisation and 3′ phosphoinositide generation at sites of epithelial cell-matrix and cell-cell interaction. Curr Biol. 1999;9(8):433–436. doi: 10.1016/s0960-9822(99)80192-4. [DOI] [PubMed] [Google Scholar]
- 38.Yang XC, Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989;243(4894 Pt 1):1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]
- 39.Liedtke W. TRPV4 plays an evolutionary conserved role in the transduction of osmotic and mechanical stimuli in live animals. J Physiol. 2005;567(Pt 1):53–58. doi: 10.1113/jphysiol.2005.088963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002;277(16):13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- 41.Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22(15):6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ingber DE. Extracellular matrix as a solid-state regulator in angiogenesis: identification of new targets for anti-cancer therapy. Semin Cancer Biol. 1992;3(2):57–63. [PubMed] [Google Scholar]
- 43.Meredith JE, Jr, Winitz S, Lewis JM, Hess S, Ren XD, Renshaw MW, Schwartz MA. The regulation of growth and intracellular signaling by integrins. Endocr Rev. 1996;17(3):207–220. doi: 10.1210/edrv-17-3-207. [DOI] [PubMed] [Google Scholar]
- 44.Munevar S, Wang YL, Dembo M. Regulation of mechanical interactions between fibroblasts and the substratum by stretch-activated Ca2+ entry. J Cell Sci. 2004;117(Pt 1):85–92. doi: 10.1242/jcs.00795. [DOI] [PubMed] [Google Scholar]
- 45.Liedtke W, Tobin DM, Bargmann CI, Friedman JM. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2003;100 2:14531–14536. doi: 10.1073/pnas.2235619100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alessandri-Haber N, Dina OA, Yeh JJ, Parada CA, Reichling DB, Levine JD. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J Neurosci. 2004;24(18):4444–4452. doi: 10.1523/JNEUROSCI.0242-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res. 2003;93(9):829–838. doi: 10.1161/01.RES.0000097263.10220.0C. [DOI] [PubMed] [Google Scholar]
- 48.Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005;7(2):179–185. doi: 10.1038/ncb1218. [DOI] [PubMed] [Google Scholar]
- 49.Gottlieb P, Folgering J, Maroto R, Raso A, Wood TG, Kurosky A, Bowman C, Bichet D, Patel A, Sachs F, Martinac B, Hamill OP, Honoré E. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflügers Arch - Eur J Physiol. 2007 doi: 10.1007/s00424-007-0359-3. [DOI] [PubMed] [Google Scholar]
- 50.Shakibaei M, Mobasheri A. Beta1-integrins co-localize with Na, K-ATPase, epithelial sodium channels (ENaC) and voltage activated calcium channels (VACC) in mechanoreceptor complexes of mouse limb-bud chondrocytes. Histol Histopathol. 2003;18(2):343–351. doi: 10.14670/HH-18.343. [DOI] [PubMed] [Google Scholar]
- 51.Wilson PD. Polycystin: new aspects of structure, function, and regulation. J Am Soc Nephrol. 2001;12(4):834–845. doi: 10.1681/ASN.V124834. [DOI] [PubMed] [Google Scholar]
- 52.Alessandri-Haber N, Dina OA, Joseph EK, Reichling DB, Levine JD. Interaction of transient receptor potential vanilloid 4, integrin, and SRC tyrosine kinase in mechanical hyperalgesia. J Neurosci. 2008;28(5):1046–1057. doi: 10.1523/JNEUROSCI.4497-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Danciu TE, Adam RM, Naruse K, Freeman MR, Hauschka PV. Calcium regulates the PI3K-Akt pathway in stretched osteoblasts. FEBS Lett. 2003;536(13):193–197. doi: 10.1016/s0014-5793(03)00055-3. [DOI] [PubMed] [Google Scholar]
- 54.Nilius B, Droogmans G, Wondergem R. Transient receptor potential channels in endothelium: solving the calcium entry puzzle? Endothelium. 2003;10(1):5–15. doi: 10.1080/10623320303356. [DOI] [PubMed] [Google Scholar]
- 55.Yao X, Garland CJ. Recent developments in vascular endothelial cell transient receptor potential channels. Circ Res. 2005;97(9):853–863. doi: 10.1161/01.RES.0000187473.85419.3e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


