Abstract
Long-chain omega-3 polyunsaturated fatty acid (PUFA) supplementation has been used for the secondary prevention of fatal and nonfatal myocardial infarction (MI). However, the benefit of this therapy is frequently confused with other established treatments in the therapeutic strategy among such patients. We review the data on omega-3 PUFA use in secondary care and consider indications for its use which include post-MI and raised triglycerides. We suggest that the available evidence supports the use of omega-3 supplementation as part of the comprehensive secondary care package for post-MI patients.
Keywords: omega-3 polyunsaturated fatty acids, myocardial infarction
Introduction
Long-chain omega-3 polyunsaturated fatty acids (PUFA) supplementation is used in the secondary prevention of fatal and nonfatal myocardial infarction (MI).1 The therapeutic actions of omega-3 PUFA are largely characterized by their ability to lower serum triglycerides, but additional less explicit cardio-protection mechanisms including reduced platelet aggregation, antithrombotic and fibrinolytic activities, antiarrhythmic effects, reduced blood viscosity, and anti-inflammatory properties have all been suggested.2,3
Several issues hinder the use of this treatment as part of a comprehensive cardiovascular disease management package that already includes antiplatelet agents, angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, anticoagulants, and lipid-lowering drugs. Specifically, the ‘additional’ benefits and mode of action of omega-3 PUFA supplementation for post-MI patients are not clear to clinicians, the arrhythmic benefits are poorly understood, and the indications for triglyceride lowering are commonly confused with the indication for post-MI secondary prevention.
This review addresses these issues, providing an overview of efficacy data on omega-3 PUFA in post-MI survivors.
Benefits of omega-3 in patients post-MI
Cardiovascular disease is a major health care focus as it accounts for nearly one half of all deaths in the developed and developing world,4–7 low-density lipoprotein (LDL) cholesterol is one of the major modifiable risk factors for cardiovascular disease8,9 and clinical trials using LDL cholesterol-lowering interventions have established its benefits following an ischemic event.10 However, even among patients at target levels of LDL and total cholesterol, cardiovascular events still occur,11–13 particularly among those patients who have survived a heart attack.14 Indeed, MI is often followed by an impairment of cardiac function which increases the risk of reinfarction and heart failure amongst patients with coronary artery disease (CAD). Paradoxically, levels of serum cholesterol are typically low in heart failure,15 and an inverse relationship exists between circulating concentrations and prognosis, particularly in advanced patients.16,17
The Diet And Reinfarction Trial (DART)
DART was the first randomized controlled trial of dietary fish intake in secondary cardiovascular prevention.18 It examined the effects of dietary changes related to increased fatty fish intake in 2033 men diagnosed with MI followed for 2 years. There was a reduction of 29% in 2-year all-cause mortality (P = 0.05) after MI among men that followed a high fish intake diet and 16% reduction in the risk of ischemic heart disease events. The benefit of the high fish intake group started to manifest early and persisted throughout study duration.
The GISSI-Prevenzione trial
In the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico Prevenzione (GISSI-Prevenzione) trial, in which omega-3 PUFAs were used, participants had a relative risk reduction of 15% in the composite primary end point (total mortality, nonfatal MI, stroke) despite a lack of change in cholesterol levels.1
This trial was the key clinical study investigating various aspects of omega-3 PUFA effects on post-MI patients (n = 11,324). Patients were randomized into 4 groups that included an omega-3 PUFA capsule containing 84% eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) 1 g/day, vitamin E (alpha-tocopherol 300 mg/day), a combination of omega-3 PUFA and vitamin E or no treatment. Male and female patients from across Italy with recent (≤3 months) MI were eligible and this included both ST segment elevation (STEMI) and non-ST segment elevation (NSTEMI) on electrocardiographic evidence. Age was not a restriction for entry into the trial and the mean age of the participants was 59.4 years. Importantly, patients were asked to adhere to cardiovascular treatments such as aspirin, beta-blockers and ACE inhibitors and statin therapy (some 5% at the start of the trial and 45% after 42 months, as clinical trial evidence of efficacy of these drugs was emerging). Mean levels of total cholesterol among patients at baseline were 6.67 mmol/L, HDL levels were 1.33 mmol/L, and serum triglycerides were 2.27 mmol/L. After 6 months, levels of HDL and total cholesterol were largely unchanged, while triglyceride levels were reduced by 3.4% in the omega-3 therapy group (P = 0.001).
The outcomes from the study showed that compared to the control group (no treatment), the patients taking omega-3 PUFA showed relative risk reductions of 20% for all deaths, 30% for cardiovascular deaths, and 45% for sudden deaths. Thus, there was a benefit of omega-3 PUFA therapy, which was over and above other secondary prevention drugs (beta-blockers, antiplatelet drugs, ACE inhibitors, statins), albeit in a patient group subscribing to a Mediterranean diet.
The GISSI-Prevenzione trial has also provided additional data from ancillary publications. For example, the time-course of the benefit associated with CHD patients surviving MI in the GISSI-Prevenzione trial was assessed by study investigators.19 On statistical survival analysis, Kaplan-Meier curves at 3 months following treatment with omega-3 PUFA showed a significant reduction in total mortality (compared to the control group), and a significant reduction in sudden death at 4 months. For sudden death, GISSI-Prevenzione investigators have also reported that omega-3 PUFAs are efficacious in the prevention of sudden death within high-risk groups such as those with systolic dysfunction.20 Further details on the heart failure data from GISSI-Prevenzione are provided later.
Japan EPA Lipid Intervention Study (JELIS)
JELIS was a randomized, open-label, blinded, endpoint trial of highly purified EPA with statin vs statin-alone therapy for prevention of major coronary events in hypercholesterolemic patients.21 A total of 18,645 Japanese patients were randomly assigned to receive 1800 mg of highly purified EPA in addition to statin therapy and followed up for 4.6 years. Out of these patients, 14,981 were subject to primary prevention and 3664 to secondary prevention.
The relative risk reduction of major coronary events in the EPA group was 19%, with a 24% reduction in the frequency in the unstable angina. The occurrence of coronary death and sudden cardiac death was not significantly reduced. However, the frequency of nonfatal coronary events (including nonfatal MI, unstable angina, angioplasty, stenting, or coronary artery bypass) was significantly lower in the EPA group compared to the controls. Of note, the study population was likely to have high levels of serum EPA because of the high fish consumption in the Japanese population. Both EPA with statin and statin-alone treatments lowered LDL cholesterol by the same amount of 25%. Hence, the increased reduction of major coronary events was likely to be unrelated to the LDL cholesterol-lowering effect per se.
Other evidence post-MI
Studer et al published a systematic review on 97 lipid-lowering trials carried out between 1965 and 2003.22 This included a total of 137,140 individuals subjected to intervention and 138,976 in the control group. The selection of the trials was oriented towards comparison of individual drugs to placebo or usual care. Based on pharmacological characteristics, the trials were divided into statin, fibrate, resin, niacin, omega-3 PUFA, and dietary intervention groups.
The omega-3 PUFA group included 14 trials with 9 trials on secondary cardiovascular disease prevention. The average relative reduction in total cholesterol was highest in the statin group and lowest in the omega-3 PUFA group. The risk ratios for overall mortality were not correlated with reductions in cholesterol level. Risk rates for cardiac mortality showed a significant reduction in the statin and omega-3 PUFA treatment groups, as did the group on resin treatment. Thus, omega-3 PUFAs reduced overall mortality and cardiac mortality in patients with CAD. The estimated number to treat for 1 year in this group was 140 patients for secondary prevention to prevent 1 death, whereas for the statin group, the number to treat was 248 patients for secondary prevention. In primary prevention (with a mortality rate of below 1% per year) the number to treat was 855 patients to prevent 1 death. Similar to JELIS, this review also concluded that the benefits of omega-3 PUFA were not related to reduction in the cholesterol level.
Heart failure
Heart failure is an increasing cause of cardiovascular morbidity and mortality within the Western world.23,24 Given that it is associated with marked impairment of quality of life, frequent hospital admissions, and poor prognosis25,26 (recognized to be worse than some cancers), heart failure is an important public health concern.27 MI is often followed by left ventricular systolic dysfunction (LVSD), and an increased risk of heart failure, and mortality.28–30 The symptoms and prognosis of CAD patients with LVSD are significantly improved by various cardiovascular therapies such as beta-blockers, ACE inhibitors, and spironolactone,31 which can delay or even prevent the progression of heart failure.
In the GISSI-Prevenzione trial the presence of overt congestive heart failure was an exclusion critera for the study. One could argue that this exclusion renders the GISSI-Prevenzione trial less representative of a post-MI population. Nonetheless, left ventricular systolic function was monitored by echocardiography on 9630 patients enrolled in the trial.32 These patients were stratified into 4 ordinal groups of ventricular systolic function (ejection fraction >50%, 46% to 50%, 45% to 41%, <40%). Omega-3 PUFA therapy across the groups reduced the total mortality within patients by 24% (P = 0.02) in those with LVSD and nonsignificantly by 19% in those without LVSD. Of note, patients with LVSD (ejection fraction <40%) had a 4-fold greater benefit from omega-3 PUFA treatment in the reduction of sudden death compared to those with normal systolic function (ejection fraction >50%). These benefits of omega-3 PUFA were independent of treatment with beta-blockers and ACE inhibitors.
This analysis of results from the GISSI-Prevenzione trial relating to LVSD were explorative and hypothesis-generating, and this formed the basis for a large clinical trial to specifically test the effects of omega-3 PUFA in the setting of heart failure – the GISSI-HF trial.33 The aim of this randomized, double-blind, placebo-controlled trial was to determine whether omega-3 PUFA would improve mortality and morbidity among patients with chronic heart failure (New York Heart Association class II–IV, of any cause and with any level of left ventricular systolic function). In total, 3494 were randomly assigned to omega-3 PUFA (1 g daily) and 3481 to placebo for a median time of 3.9 years where primary endpoints were time to death and time to death or admission to hospital for cardiovascular reasons. Compared to the placebo arm, the omega-3 PUFA group resulted in a 1.8% absolute reduction in all-cause mortality and 2.3% reduction in mortality or admission for cardiovascular reasons. This small, albeit significant, benefit from omega-3 PUFA (1 g/day) was not seen in the other arm of the GISSI-HF Trial, in which statin therapy did not reach the same clinical outcomes.34 These results consolidated the indication for omega-3 PUFA therapy in post-MI patients.
Antiarrhythmic and antithrombotic effects of omega-3 PUFAs
Arrhythmias are common complications and account for a large proportion of death post-MI. Results from the GISSI-Prevenzione trial show a significant reduction in sudden cardiac death of 45% over 3.5 years of follow-up.1 The reduction in risk of sudden cardiac death was reported to increase in significance with the duration of trial, becoming highly statistically significant by the end of study. The benefits were particularly attributed to the reduction of lethal arrhythmias, rather than cholesterol-lowering or anticoagulant effects per se.1
The mechanism of action of omega-3 PUFA is multifaceted. Low heart rate variability is associated with increased morbidity and mortality in post-MI patients.35–37 Indeed, Christensen et al reported an increase in heart rate variability with use of omega-3 PUFAs.38,39 However, Hamaad et al reported no changes in indices of heart rate variability (HRV) in patients post-MI after 3 months treatment of omega-3 PUFAs with the dose used in the GISSI-Prevenzione study.40 Direct correlations between the level of omega-3 PUFA in cell membranes and HRV indices have also been reported.41 Observations in vitro and in vivo imply that the antiarrythmogenic effect is related to the modulation of the Na+ and Ca2+ currents in the myocyte sarcolema, electrical stabilization of cardiomyocytes, enhanced myocardial efficiency, and increased resistance to reperfusion arrhythmias.42 Infusion of an emulsion of omega-3 PUFAs on an exercising dog model with surgically produced MI prevented ischemia-induced sudden cardiac death by preventing ventricular fibrillation.43
Recent studies suggest that omega-3 PUFAs have a protective effect against fatal ventricular arrythmias, particularly in post-MI patients,1 and atrial fibrillation (AF).44 AF is the most common complication after coronary artery bypass surgery (CABG).45 Calo et al conducted the first trial investigating the efficacy of perioperative treatment with n-3 PUFAs in preventing AF in patients undergoing CABG. The results of this open-label randomized controlled study show not only a significant reduction in the incidence of postoperative AF events but also a reduction in the hospital stay.46 There was a relative risk reduction of 54.4% and an absolute risk reduction of 18.1%, the effect being similar to that of the beta-blockers sotalol and amiodarone currently used for this purpose, and having a further benefit of being well tolerated and safe to use in the majority of patients.
There is evidence among patients with implantable cardioverter defibrillators that suggest that omega-3 PUFAs do not reduce the risk of ventricular tachycardia/ventricular fibrillation and may actually be pro-arrhythmic.47 These studies include patients with ventricular arrythmias without the ischemic background or recent MI, and hence different mechanisms of arrhythmia development.47 However, a recent study in patients with idiopathic dilated cardiomyopathy by Nodari et al found a beneficial effect in parameters related to arrhythmic risk independent from the anti-schemic effects, although again this was a small study.48 The trials published to date in nonischemic arrhythmia patients have either been inconclusive49 or underpowered.47,48,50
It is possible that omega-3 PUFAs can have antiarrhythmogenic or proarrhythmogenic effects depending on the origins of arrhythmias, and this question remains open for larger randomized controlled trials. In a study comparing the effects of different types of dietary fish consumption, baked and broiled fish intake (tuna) was associated with lower risk of incident AF, a situation which was reversed among people eating fried fish or fish sandwiches. However, this observed relationship may be related to other environmental factors associated with both fish intake and risk of AF.51
Sudden cardiac death events are not related only to the arrhythmogenic effects, and in many instances have a thrombotic basis. Some studies attribute the anti-inflammatory effect of the omega-3 PUFAs to the suppression of pro-inflammatory cytokines, such as interleukin 6, interleukin 1β and tumor necrosis factor α production.52,53 In addition, a reduction of platelet count and augmentation of fibrinolytic activity has been reported, and would be expected to reduce the potential for thrombus formation. Conversely, other studies have not found significant changes in prothrombosis markers with omega-3 PUFAs.54,55
Overall, there is no strong indication for the antithrombotic hypothesis of omega-3 PUFAs, and we have to conclude that on current evidence the major effect may perhaps be antiarrhythmic rather than antithrombotic.
Direct effects of omega-3 PUFAs on the heart
Despite clinical trial evidence of efficacy for post-MI and heart failure patients, it remains unclear as to whether omega-3 PUFAs have a direct effect on the heart. Little is known about the influence of omega-3 PUFA supplements on energy homeostasis in post-MI patients, where the competitive balance between glucose and nonesterified fatty acids (NEFA) may have a crucial impact on the vitality of the myocardium following ischemic damage.56
Metabolic disturbances following MI
Physiologically, for the myocardium to contract, the heart must successfully transform chemical energy into mechanical energy. Cytosolic mitochondrial oxidative phosphorylation of ADP to ATP is employed by the heart to fulfil this task, for which it requires oxygenated blood and a fuel source. The fuel requirements (determined by body size and composition, age, and gender) are met by glucose (4 kcal/g) or NEFA (9 kcal/g). The preferred fuels for myocardial oxidative metabolism are glucose (3:1) in the fed state, NEFA during fasting, and NEFA and lactate during exercise.56 Energy is derived from accessible stores of glycogen (for glucose) and triglyceride (for NEFA).
Acute myocardial ischemia generates a chain of compensatory metabolic reactions that are directed to preserve cardiac function in an environment of decreasing myocardial oxygen supply. Decreasing mitochondrial respiratory chain ATP production in the cardiac muscle is then met by an increase in glycolysis. Under oxygen-limiting conditions, glycolytic activity results in the formation of lactate and only a small fraction of the energy normally released from glucose aerobically is generated. Further electron transport activity associated with anaerobic glycolytic activity results in proton production (which would normally act as substrate for energy and water synthesis in the presence of oxygen). Excessive lactate and proton production promotes acidosis and damage (either direct action or through free radical formation) to the myocardium that further decrease cardiac efficiency at a crucial stage.57,58
Omega-3 PUFAs and fatty acid oxidation in the ischemic myocardium
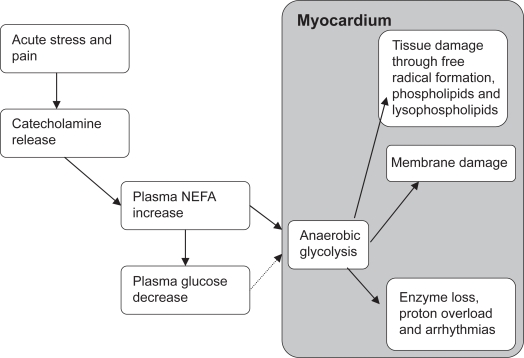
NEFA concentrations are raised and sustained throughout the process of severe ischemia and MI.59 Indeed, large infarcts have been associated with high plasma concentrations of fatty acids.10 Acute stress and pain at the time of MI is associated with an increase in circulating catecholamines, promoting the hydrolysis of NEFA from adipose stores.60 Effectively, high concentrations of circulating NEFA and low levels of insulin during (and after) the ischemic period ensure that metabolism within the myocardium is preferentially directed towards the oxidation of NEFA instead of glucose. Of note, the highly reduced structures of NEFA create an oxygen demand that is much higher than that required for the complete oxidation of glucose. Anaerobic metabolism of NEFA also results in the formation of glycolytic metabolites that are manifest as excessive lactate, proton production, free radical formation, and intracellular acidosis. In addition, acyl CoA and acylcarnitine, products of fatty acid catabolism, are associated with cardiac membrane damage, enzyme loss, Ca2+ overload, and arrhythmias (see Figure 1).
Figure 1.
Effects of glucose and fatty acids on myocardial ischemia and arrhythmias.
Abbreviation: NEFA, nonesterified fatty acids.
In a pilot randomized study we investigated the effects of omega-3 PUFAs in addition to usual care on levels of insulin, NEFA, triglycerides, glucose and other indices of markers of energy homeostasis.61 Short-term therapy with Omacor® (Solvay Healthcare, UK) (Figure 2) did not significantly change circulating concentrations of metabolically active hormones, lipids or glycemic control. However, such therapy was associated with raised insulin levels compared to usual care.
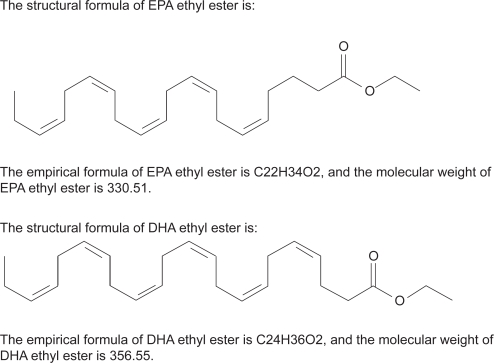
Figure 2.
Omacor is a highly purified omega-3 fatty acid formulation containing 900 mg of the ethyl esters of omega-3 fatty acids in each 1 g capsule (a combination of ethyl esters of eicosapentaenoic acid [EPA – approximately 465 mg] and docosahexaenoic acid [DHA – approximately 375 mg]).
There is also growing interest on the action of insulin on the myocardium to promote the extraction of glucose in the ischemic myocardium, kerbing the competitive edge of NEFA as a fuel source in this setting.62 Indeed, the antiarrhythmia action of omega-3 PUFAs could relate to an hyperinsulinemic effect among patients with MI. Perhaps omega-3 PUFAs could reduce the adverse effects of fatty acid toxicity on the myocardium (fatty acid phospholipid, lysophospholipid, and free radical formation). In support of such a theory, dietary intervention with omega-3 PUFAs has improved insulin sensitivity in overweight and obese European adults.63 Despite a major influence on metabolism, omega-3 PUFA supplements have little effect on glycemic control, at least among hypertriglyceridemic patients.64,65
Serum triglycerides as a risk factor in patients post-MI
Raised triglycerides are an independent risk factor for CAD66 and there is also evidence that raised triglycerides promote a synergistic risk through lipoproteins transporting triglycerides.67 In the Copenhagen Male Study, for example, which was an 8-year follow-up of 2906 men free of CAD, an increasing trend (P < 0.001) between triglyceride concentration and CAD events was shown to be independent of other risk factors including HDL cholesterol concentrations.68 Other data from the Atherosclerosis Risk in Communities Study (ARIC), which was a 10-year follow-up of 12,339 middle-aged North American subjects, showed that triglycerides were an independent risk factor in women, when associated with CAD events.69 In the Physician’ Health Study, a prospective study of 14,916 adult men, nonfasting triglyceride levels were a strong and independent predictor of future risk of MI, particularly in the presence of elevated total cholesterol.70 One meta-analysis of 17 population-based studies estimated that elevated triglycerides were associated with a 30% increase in cardiovascular disease in men and a 75% increase in women.71 Despite ongoing debate as to whether triglyceride concentrations should be measured in a fasted state,72 these data essentially establish triglycerides as a risk factor for primary prevention of CAD.
In the four secondary-prevention trials of triglyceride lowering using fibrate drugs, a significant decrease in CAD events occurred in only one of these trials.73–76 Hence the efficacy of omega-3 PUFA for all-cause mortality may not necessarily involve triglyceride lowering per se. Nonetheless, hypertriglyceridemia is often the most common hyperlipidemia found in MI survivors.77
Pathophysiological considerations
Triglyceride deposits within adipose tissue are the major store of energy in the body. Triglycerides accumulate by the esterification of NEFA with glycerol-3-phosphate (G3P).78 Hormone-sensitive-lipase (HSL) hydrolyzes adipocyte triglyceride to release NEFA into the circulation for uptake by skeletal muscle and liver during fasting.79 HSL is an intracellular enzyme under the control of both insulin and catecholamine release.80 NEFA levels at fasting are usually around 0.4 to 1.0 mmol/L.
During starvation, associated hormones stimulate hydrolysis, NEFA levels rise to 1.5 to 2.0 mmol/L after 2 to 5 days of fasting.78 Triglycerides are transported in the blood upon lipoproteins – those made in the intestine called chylomicrons, and smaller triglyceride-rich lipoproteins made in hepatocytes called very low-density lipoprotein (VLDL). The role of chylomicrons and VLDL is thought to relate to a fuel (triglyceride) delivery system. On metabolism, these particles release free fatty acids and glycerol from triglycerides, and these generate a source of cholesterol. As triglycerides are hydrolyzed, VLDL is converted to intermediate-density lipoprotein (IDL) and then to remnant particles, known as LDL.77 Triglyceride levels are nonparametrically distributed within populations and the upper limit of normal is considered to be 2.3 mmol/L77 although other studies suggest that triglyceride levels above 1.7 mmol/L are an indication of increased cardiovascular risk.81
Cardiovascular risk from raised triglycerides
The mechanism by which triglycerides promote atherogenicity may be difficult to separate from its synergistic effects on a host of lipid and lipoprotein variables, as well as other cardiovascular risk factors. For example, raised triglycerides are frequently manifest as part of a cluster of cardiovascular co-morbidities such as obesity, hypertension, insulin resistance, high blood pressure, and abnormal glucose and lipid metabolism.82–86 Moreover, the cardiovascular risk from raised serum cholesterol87 or low HDL cholesterol88 is much greater when triglycerides are raised.
The preponderance of LDL particles that are small and dense is another interrelated feature of the abnormal lipid distribution amongt individuals with raised triglycerides, providing an adverse consequence for cardiovascular risk.89,90 Patients with hypertriglyceridemia are also more likely to have a increased thrombotic risk91,92 and platelet activation.93 However, treatment with omega-3 PUFA in combination with statin therapy can reverse these effects.94
Lowering triglycides using omega-3 PUFAs in secondary care
The quantity of omega-3 PUFAs used in studies such as GISSI-Prevenzione trial was sufficient to significantly lower serum triglycerides. Also, Durrington et al investigated the benefits of omega-3 PUFAs in doses that lower serum triglycerides in CAD patients with persisting hypertriglyceridemia (>2.3 mmol/L) despite statin treatment.95 Patients were randomized to receive Omacor 2 g twice daily or corn oil in identical capsules, as placebo, for 24 weeks in a double-blind trial, after which both groups were invited to receive Omacor for a further 24 weeks. In patients receiving Omacor, there was a significant and sustained reduction of triglycerides (20% to 30%), with no adverse effects on HDL cholesterol, hematological and biochemical safety tests, or glycemic control. Interestingly, in another study of stable CAD patients (on statin therapy) with raised triglycerides, a reduction of approximately 20% was demonstrated with just DHA 1 g daily. However, it is unclear whether a preparation of ‘pure’ DHA has the same efficacy for incident CAD events as ‘mixtures’ such as Omacor.96 While both EPA and DHA have triglyceride-lowering effects, their impact on fatty acids metabolism differs.97
Conclusion
Given the public health focus on the epidemic of cardiovascular disease, which accounts for nearly one half of all deaths in the developed and developing world,98 there is certainly scope for the inclusion of omega-3 PUFA therapy as part of a comprehensive cardiovascular disease management package. The evidence provided in this review underlines the efficacy of this treatment alongside antiplatelet agents, ACE inhibitors, beta-blockers, anticoagulants, and statins. Importantly, the additional benefits of omega-3 PUFA over these other treatments does not include the lowering of total or LDL cholesterol in post-MI patients. There is no evidence on whether concentrated preparations of omega-3 PUFA such as Omacor are interchangeable with individual preparations of DHA or EPA. The utility of omega-3 PUFAs in this patient group is possibly derived through its arrhythmic benefits and triglyceride lowering.
Footnotes
Disclosures
The authors declare no conflicts of interest.
References
- 1.GISSI-Prevention Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico) Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-prevention trial. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 2.Bhatnagar D, Durrington PN. Omega-3 fatty acids: their role in the prevention and treatment of atherosclerosis related risk factors and complications. Int J Clin Pract. 2003;57:305–314. [PubMed] [Google Scholar]
- 3.Marchioli R, Levantesi G, Macchia A, et al. GISSI-Prevenzione Investigators. Antiarrhythmic mechanisms of n-3 PUFA and the results of the GISSI-Prevenzione trial. J Membr Biol. 2005;206:117–128. doi: 10.1007/s00232-005-0788-x. [DOI] [PubMed] [Google Scholar]
- 4.Mathers CD, Stein C, Ma Fat M, et al. The Global Burden of Disease 2000 Study (version 2): Methods and Results Discussion Paper 50 Geneva: Global Program on Evidence for Health Policy World Health Organization; 2002http://www.who.int/infobase IBRef: 19998. [Google Scholar]
- 5.Michaud CM, Murray CJ, Bloom BR. Burden of disease-implications for future research. JAMA. 2001;285(5):535–539. doi: 10.1001/jama.285.5.535. [DOI] [PubMed] [Google Scholar]
- 6.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases. Part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 7.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases. Part II: variations in cardiovascular disease by specific ethnic groups and geographic regions and prevention strategies. Circulation. 2001;104:2855–2864. doi: 10.1161/hc4701.099488. [DOI] [PubMed] [Google Scholar]
- 8.Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 9.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 10.Durrington P. Dyslipidaemia. Lancet. 2003;362:717–731. doi: 10.1016/S0140-6736(03)14234-1. [DOI] [PubMed] [Google Scholar]
- 11.Kita Chun A, McGee SR. Bedside diagnosis of coronary artery disease: a systematic review. Am J Med. 2004;117:334–343. doi: 10.1016/j.amjmed.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Patel JV, Lim HS, Gunarathne A, et al. Ethnic differences in myocardial infarction in patients with hypertension: effects of diabetes mellitus. QJM. 2008;101:231–236. doi: 10.1093/qjmed/hcm151. [DOI] [PubMed] [Google Scholar]
- 13.Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 1986;256:2835–2838. [PubMed] [Google Scholar]
- 14.Kannel WB, Sorlie P, McNamara PM. Prognosis after initial myocardial infarction: the Framingham study. Am J Cardiol. 1979;44:53–59. doi: 10.1016/0002-9149(79)90250-9. [DOI] [PubMed] [Google Scholar]
- 15.Kjekshus J, Dunselman P, Blideskog M, et al. A statin in the treatment of heart failure? Controlled rosuvastatin multinational study in heart failure (CORONA): study design and baseline characteristics. Eur J Heart Fail. 2005;7:1059–1069. doi: 10.1016/j.ejheart.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Rauchhaus M, Clark AL, Doehner W, et al. The relationship between cholesterol and survival in patients with chronic heart failure. J Am Coll Cardiol. 2003;42:1933–1940. doi: 10.1016/j.jacc.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Horwich TB, Hamilton MA, Maclellan WR, Fonarow GC. Low serum total cholesterol is associated with marked increase in mortality in advanced heart failure. J Card Fail. 2002;8:216–224. doi: 10.1054/jcaf.2002.0804216. [DOI] [PubMed] [Google Scholar]
- 18.Burr ML, Fehily AM, Gilbert JF, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;2:757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 19.Marchioli R, Barzi F, Bomba E, et al. GISSI-Prevenzione Investigators Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–1903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 20.Macchia A, Levantesi G, Franzosi MG, et al. GISSI Prevenzione Investigators Left ventricular systolic dysfunction, total mortality, and sudden death in patients with myocardial infarction treated with n-3 polyunsaturated fatty acids. Eur J Heart Fail. 2005;7:904–909. doi: 10.1016/j.ejheart.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Yokoyama M, Origasa H, Matsuzaki M, et al. Japan EPA lipid intervention study (JELIS) Investigators Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 22.Studer M, Briel M, Leimenstoll B, Glass TR, Bucher HC. Effect of different antilipidemic agents and diets on mortality: a systematic review. Arch Intern Med. 2005;165:725–730. doi: 10.1001/archinte.165.7.725. [DOI] [PubMed] [Google Scholar]
- 23.Hobbs FDR, Kenkre J, Roalfe AK, Davis RC, Hare R, Davies MK. Impact of heart failure and left ventricular systolic dysfunction on quality of life. Eur Heart J. 2002;23:1867–1876. doi: 10.1053/euhj.2002.3255. [DOI] [PubMed] [Google Scholar]
- 24.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 25.Sutton GC. Epidemiological aspects of heart failure. Am Heart J. 1990;120:1538–1540. doi: 10.1016/0002-8703(90)90055-3. [DOI] [PubMed] [Google Scholar]
- 26.Bonneaux L, Barendregt JJ, Meeter K, Bonsel GJ, Van der Maas PJ. Estimating clinical morbidity due to ischaemic heart disease and congestive heart failure: the future rise of heart failure. Am J Public Health. 1994;84:20–28. doi: 10.2105/ajph.84.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMurray JJ, Stewart S. Epidemiology, aetiology and prognosis of heart failure. Heart. 2000;83:596–602. doi: 10.1136/heart.83.5.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spencer F, Meyer T, Goldberg RJ, et al. Twenty year trends (1975–1995) in the incidence, in-hospital and long-term death rates associated with heart failure complicating acute myocardial infarction: a community-wide perspective. J Am Coll Cardiol. 1999;34:1378–1387. doi: 10.1016/s0735-1097(99)00390-3. [DOI] [PubMed] [Google Scholar]
- 29.Nicod P, Gilpin E, Dittrich H, et al. Influence on prognosis and morbidity of left ventricular ejection fraction with and without signs of left ventricular failure after acute myocardial infarction. Am J Cardiol. 1988;61:1165–1171. doi: 10.1016/0002-9149(88)91148-4. [DOI] [PubMed] [Google Scholar]
- 30.Emanuelsson H, Karlson W, Herlitz J. Characteristics and prognosis of patients with acute myocardial infarction in relation to occurrence of congestive heart failure. Eur Heart J. 1994;15:761–768. doi: 10.1093/oxfordjournals.eurheartj.a060583. [DOI] [PubMed] [Google Scholar]
- 31.National Institute for Clinical Excellence Chronic heart failure: management of chronic heart failure in adults in primary and secondary careClinical Guideline 5. London: National Institute for Clinical Excellence; July 2003. URL http://guidance.nice.org.uk/cg5 Accessed 08/08/2008
- 32.Macchia A, Levantesi G, Franzosi MG, et al. GISSI-Prevenzione Investigators Left ventricular systolic dysfunction, total mortality, and sudden death in patients with myocardial infarction treated with n-3 polyunsaturated fatty acids. Eur J Heart Fail. 2005:904–909. doi: 10.1016/j.ejheart.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Gissi-HF Investigators. Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 34.Gissi-HF Investigators. Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1231–1239. doi: 10.1016/S0140-6736(08)61240-4. [DOI] [PubMed] [Google Scholar]
- 35.Lombardi F, Mäkikallio TH, Myerburg RJ, Huikuri HV. Sudden cardiac death: role of heart rate variability to identify patients at risk. Cardiovasc Res. 2001;50:210–217. doi: 10.1016/s0008-6363(01)00221-8. [DOI] [PubMed] [Google Scholar]
- 36.Stein PK, Kleiger RE. Insights from the study of heart rate variability. Ann Rev Med. 1999;50:249–261. doi: 10.1146/annurev.med.50.1.249. [DOI] [PubMed] [Google Scholar]
- 37.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 38.Christensen JH, Skou HA, Fog L, et al. Marine n-3 fatty acids, wine intake, and heart rate variability in patients referred for coronary angiography. Circulation. 2001;103:651–657. doi: 10.1161/01.cir.103.5.651. [DOI] [PubMed] [Google Scholar]
- 39.Christensen JH, Gustenhoff P, Korup E, et al. n-3 polyunsaturated fatty acids, heart rate variability and ventricular arrhythmias in post-AMI-patients. A clinical controlled trial [Danish] Ugeskr Laeger. 1997;159:5525–5529. [PubMed] [Google Scholar]
- 40.Hamaad A, Kaeng Lee W, Lip GY, MacFadyen RJ. Oral omega n3-PUFA therapy (Omacor) has no impact on indices of heart rate variability in stable post myocardial infarction patients. Cardiovasc Drugs Ther. 2006;20:359–364. doi: 10.1007/s10557-006-0295-z. [DOI] [PubMed] [Google Scholar]
- 41.Christensen JH, Christensen MS, Dyerberg J, et al. Heart rate variability and fatty acid content of blood cell membranes: a dose-response study with n-3 fatty acids. Am J Clin Nutr. 1999;70:331–337. doi: 10.1093/ajcn/70.3.331. [DOI] [PubMed] [Google Scholar]
- 42.Pepe S, McLennan PL. Cardiac membrane fatty acid composition modulates myocardial oxygen consumption and postischemic recovery of contractile function. Circulation. 2002;105:2303–2308. doi: 10.1161/01.cir.0000015604.88808.74. [DOI] [PubMed] [Google Scholar]
- 43.Billman GE, Kang JX, Leaf A. Prevention of sudden cardiac death by dietary pure omega-3 polyunsaturated fatty acids in dogs. Circulation. 1999;99:2452–2457. doi: 10.1161/01.cir.99.18.2452. [DOI] [PubMed] [Google Scholar]
- 44.Mozaffarian D, Psaty BM, Rimm EB, et al. Fish intake and risk of incident atrial fibrillation. Circulation. 2004;110:368–373. doi: 10.1161/01.CIR.0000138154.00779.A5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ommen SR, Odell KA, Stanton MS. Atrial arrythmias after cardiothoracic surgery. N Engl J Med. 1997;336:1429–1434. doi: 10.1056/NEJM199705153362006. [DOI] [PubMed] [Google Scholar]
- 46.Calo L, Bianconi L, Colivicchi F, et al. N-3 fatty acids for the prevention of atrial fibrillation after coronary artery bypass sugery. J Am Coll Cardiol. 2005;45:1723–1728. doi: 10.1016/j.jacc.2005.02.079. [DOI] [PubMed] [Google Scholar]
- 47.Raitt MH, Connor WE, Morris C, et al. Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators: a randomized controlled trial. JAMA. 2005;293:2884–2891. doi: 10.1001/jama.293.23.2884. [DOI] [PubMed] [Google Scholar]
- 48.Nodari S, Metra M, Milesi G, et al. The role of n-3 PUFAs in preventing the arrhythmic risk in patients with idiopathic dilated cardiomyopathy. Cardiovasc Drugs Ther. 2009;23:5–15. doi: 10.1007/s10557-008-6142-7. [DOI] [PubMed] [Google Scholar]
- 49.Brouwer IA, Zock PL, Camm AJ, et al. for the SOFA Study Group Effect of Fish Oil on Ventricular Tachyarrhythmia and Death in Patients With Implantable Cardioverter Defibrillators: The Study on Omega-3 Fatty Acids and Ventricular Arrhythmia (SOFA) Randomized Trial. JAMA. 2006;295:2613–2619. doi: 10.1001/jama.295.22.2613. [DOI] [PubMed] [Google Scholar]
- 50.Leaf A, Albert CM, Josephson M, et al. Fatty Acid Antiarrhythmia Trial Investigators Prevention of fatal arrhythmias in high-risk subjects by fish oil n-3 fatty acid intake. Circulation. 2005;112:2762–2768. doi: 10.1161/CIRCULATIONAHA.105.549527. [DOI] [PubMed] [Google Scholar]
- 51.Mozaffarian D, Psaty BM, Rimm EB, et al. Fish intake and risk of incident atrial fibrillation. Circulation. 2004;110:368–337. doi: 10.1161/01.CIR.0000138154.00779.A5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meydani SN, Endres S, Woods MM, et al. Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older women. J Nutr. 1991;121:547–555. doi: 10.1093/jn/121.4.547. [DOI] [PubMed] [Google Scholar]
- 53.Endres S, Ghorbani R, Kelley VE, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989;320:265–271. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 54.Lee KW, Blann AD, Lip GY. Effects of omega-3 polyunsaturated fatty acids on plasma indices of thrombogenesis and inflammation in patients post-myocardial infarction. Thromb Res. 2006;118:305–312. doi: 10.1016/j.thromres.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 55.Hansen J, Grimsgaard S, Nordoy A, Bonaa KH. Dietary supplementation with highly purified eicosapentaenoic acid and docosahexaenoic acid does not influence PAI-1 activity. Thromb Res. 2000;98:123–132. doi: 10.1016/s0049-3848(99)00223-6. [DOI] [PubMed] [Google Scholar]
- 56.Oliver MF, Opie LH. Effects of glucose and fatty acids on myocardial ischemia and arrhythmias. Lancet. 1994;343:155–158. doi: 10.1016/s0140-6736(94)90939-3. [DOI] [PubMed] [Google Scholar]
- 57.Neely JR, Grotyohann LW. Role of glycolytically derived protons and lactate and damage to the myocardium. Dissociation of adenosine triphosphate levels and recovery function of reperfused ischaemic hearts. Circ Res. 1984;55:816–824. doi: 10.1161/01.res.55.6.816. [DOI] [PubMed] [Google Scholar]
- 58.Wolff AA, Rotmensch HH, Stanley WC, Ferrari R. Metabolic approaches to the treatment of ischaemic heart disease: the clinician’s perspective. Heart Fail Rev. 2002;7:187–203. doi: 10.1023/a:1015384710373. [DOI] [PubMed] [Google Scholar]
- 59.Oliver MF. Sudden cardiac death: the lost fatty acid hypothesis. QJM. 2006;99:701–709. doi: 10.1093/qjmed/hcl084. [DOI] [PubMed] [Google Scholar]
- 60.Shillingford J. Free noradrenaline and adrenaline excretion in relation to clinical syndromes following myocardial infarction. Am J Cardiol. 1967;20:605. doi: 10.1016/0002-9149(67)90001-x. [DOI] [PubMed] [Google Scholar]
- 61.Patel JV, Lee KW, Tomson J, Dubb K, Hughes EA, Lip GY. Effects of omega-3 polyunsaturated fatty acids on metabolically active hormones in patients post-myocardial infarction. Int J Cardiol. 2007;115:42–45. doi: 10.1016/j.ijcard.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 62.McNulty PH. Comparison of local and systemic effects of insulin on myocardial glucose extraction in ischemic heart disease. Am J Physiol Heart Circ Physiol. 2000;278:H741–H747. doi: 10.1152/ajpheart.2000.278.3.H741. [DOI] [PubMed] [Google Scholar]
- 63.Ramel A, Martinéz A, Kiely M, Morais G, Bandarra NM, Thorsdottir I. Beneficial effects of long-chain n-3 fatty acids included in an energy-restricted diet on insulin resistance in overweight and obese European young adults. Diabetologia. 2008;51:1261–1268. doi: 10.1007/s00125-008-1035-7. [DOI] [PubMed] [Google Scholar]
- 64.Sirtori CR, Paoletti R, Mancini M, et al. N-3 fatty acids do not lead to an increased diabetic risk in patients with hyperlipidemia and abnormal glucose tolerance. Italian Fish Oil Multicenter Study. Am J Clin Nutr. 1997;65:1874–1881. doi: 10.1093/ajcn/65.6.1874. [DOI] [PubMed] [Google Scholar]
- 65.Toft I, Bonaa KH, Ingebretsen OC, Nordoy A, Jenssen T. Effects of n-3 polyunsaturated fatty acids on glucose homeostasis and blood pressure in essential hypertension. A randomized, controlled trial. Ann Intern Med. 1995;123:911–918. doi: 10.7326/0003-4819-123-12-199512150-00003. [DOI] [PubMed] [Google Scholar]
- 66.Miller M. Is hypertriglyceridaemia an independent risk factor for coronary heart disease. Eur Heart J. 1998;19:H18–H22. [PubMed] [Google Scholar]
- 67.Manninen V, Tenkanen L, Koskinen P, et al. Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study. Implications for treatment. Circulation. 1992;85:37–45. doi: 10.1161/01.cir.85.1.37. [DOI] [PubMed] [Google Scholar]
- 68.Jeppesen J, Hein MO, Suadicani P, Gyntelberg F. Triglyceride concentration and ischaemic heart disease. An eight-year follow-up in the Copenhagen male study. Circulation. 1998;97:1029–1036. doi: 10.1161/01.cir.97.11.1029. [DOI] [PubMed] [Google Scholar]
- 69.Sharrett AR, Ballantyne CM, Coady SA, et al. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein (a), apolipoproteins A-I and B, and HDL density subfractions. The Atherosclerosis Risk In Communities (ARIC) study. Circulation. 2001;104:1108–1113. doi: 10.1161/hc3501.095214. [DOI] [PubMed] [Google Scholar]
- 70.Stampfer MJ, Krauss RM, Ma J, et al. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA. 1996;276:882–888. [PubMed] [Google Scholar]
- 71.Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3:213–299. [PubMed] [Google Scholar]
- 72.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 73.Rubins HB, Robins SJ, Collins D, et al. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 74.The BIP Study Group Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) Study. Circulation. 2000;102:21–27. doi: 10.1161/01.cir.102.1.21. [DOI] [PubMed] [Google Scholar]
- 75.Meade T, Zuhrie R, Cook C, Cooper J. MRC General Practice Research Framework, Bezafibrate in men with lower extremity arterial disease: randomised controlled trial. BMJ. 2002;325:1139–1141. doi: 10.1136/bmj.325.7373.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Keech A, Simes RJ, Barter P, et al. FIELD study investigators Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 77.Durrington PN. Hyperlipidaemia, Diagnosis and Management. 2nd edition. Wright Publishers; 1989. Lipoproteins and their metabolism. [Google Scholar]
- 78.Kruszynska YT. Normal metabolism: the physiology of fuel homeostasis. In: Pickup J, Williams G, editors. Textbook of Diabetes. Blackwell Science; 1997. [Google Scholar]
- 79.Frayn KN. Non esterified fatty acid metabolism and postprandial lipaemia. Atherosclerosis. 1998;141(Suppl):S41–S46. doi: 10.1016/s0021-9150(98)00216-0. [DOI] [PubMed] [Google Scholar]
- 80.Meijssen S, Castro Cabezas M, Ballieux CGM, Derksen RJ, Bilecen S, Erkelens DW. Insulin mediated Inhibition of hormone sensitive lipase activity in vivo in relation to endogenous catecholamines in healthy subjects. J Clin Endocrinol Metab. 2001;86:4193–4197. doi: 10.1210/jcem.86.9.7794. [DOI] [PubMed] [Google Scholar]
- 81.British Cardiac Society. British Hypertension Society. Diabetes UK. HEART UK. Primary Care Cardiovascular Society. Stroke Association JBS 2: Joint British Societies’ guidelines on prevention of cardiovascular disease in clinical practice. Heart. 2005;91(Suppl 5):v1–v5. doi: 10.1136/hrt.2005.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, National Heart, Lung, and Blood Institute. American Heart Association Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 83.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 84.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 85.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 86.Alberti KGMM, Zimmet P, Shaw J, for the IDF Epidemiology Task Force Consensus Group The metabolic syndrome – a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 87.Carlson LA, Böttiger LE. Ischaemic heart-disease in relation to fasting values of plasma triglycerides and cholesterol. Stockholm prospective study. Lancet. 1972;1:865–868. doi: 10.1016/s0140-6736(72)90738-6. [DOI] [PubMed] [Google Scholar]
- 88.Castelli WP. The triglyceride issue: a view from Framingham. Am Heart J. 1986;112:432–437. doi: 10.1016/0002-8703(86)90296-6. [DOI] [PubMed] [Google Scholar]
- 89.Abate N, Vega GL, Garg A, Grundy SM. Abnormal cholesterol distribution among lipoprotein fractions in normolipidemic patients with mild NIDDM. Atherosclerosis. 1995;118:111–122. doi: 10.1016/0021-9150(95)05597-p. [DOI] [PubMed] [Google Scholar]
- 90.Stampfer MJ, Krauss RM, Ma J, et al. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA. 1996;276:882–888. [PubMed] [Google Scholar]
- 91.Simpson HC, Mann JI, Meade TW, Chakrabarti R, Stirling Y, Woolf L. Hypertriglyceridaemia and hypercoagulability. Lancet. 1983;1:786–790. doi: 10.1016/s0140-6736(83)91849-4. [DOI] [PubMed] [Google Scholar]
- 92.Chadarevian R, Bruckert E, Dejager S, Presberg P, Turpin G. Relationship between triglycerides and factor VIIc and plasminogen activator inhibitor type-1: lack of threshold value. Thromb Res. 1999;96:175–182. doi: 10.1016/s0049-3848(99)00089-4. [DOI] [PubMed] [Google Scholar]
- 93.de Man FH, Nieuwland R, van der Laarse A, et al. Activated platelets in patients with severe hypertriglyceridemia: effects of triglyceride-lowering therapy. Atherosclerosis. 2000;152:407–414. doi: 10.1016/s0021-9150(99)00485-2. [DOI] [PubMed] [Google Scholar]
- 94.Nordøy A, Svensson B, Hansen JB. Atorvastatin and omega-3 fatty acids protect against activation of the coagulation system in patients with combined hyperlipemia. J Thromb Haemost. 2003;1:690–697. doi: 10.1046/j.1538-7836.2003.00140.x. [DOI] [PubMed] [Google Scholar]
- 95.Durrington PN, Bhatnagar D, Mackness MI, et al. An omega-3 poly-unsaturated fatty acid concentrate administered for one year decreased triglycerides in simvastatin treated patients with coronary heart disease and persisting hypertriglyceridaemia. Heart. 2001;85:544–548. doi: 10.1136/heart.85.5.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schwellenbach LJ, Olson KL, McConnell KJ, Stolcpart RS, Nash JD, Merenich JA, Clinical Pharmacy Cardiac Risk Service Study Group The triglyceride-lowering effects of a modest dose of docosahexaenoic acid alone versus in combination with low dose eicosapentaenoic acid in patients with coronary artery disease and elevated triglycerides. J Am Coll Nutr. 2006;25:480–485. doi: 10.1080/07315724.2006.10719562. [DOI] [PubMed] [Google Scholar]
- 97.Grimsgaard S, Bonaa KH, Hansen JB, Nordøy A. Highly purified eicosapentaenoic acid and docosahexaenoic acid in humans have similar triacylglycerol-lowering effects but divergent effects on serum fatty acids. Am J Clin Nutr. 1997;66:649–659. doi: 10.1093/ajcn/66.3.649. [DOI] [PubMed] [Google Scholar]
- 98.Mathers CD, Stein C, Ma Fat M, et al. The Global Burden of Disease 2000 Study (version 2): Methods and Results Discussion Paper 50 Geneva: Global Program on Evidence for Health Policy World Health Organization; 2002http://www.who.int/infobase IBRef: 19998. [Google Scholar]