Abstract
ESRD can affect the pharmacokinetic disposition of drugs subject to nonrenal clearance. Cytochrome P450 (CYP) enzymes, including CYP3A, and multiple intestinal and hepatic drug transporters are thought to mediate this process, but the extent to which kidney disease alters the function of these proteins in humans is unknown. We used midazolam and fexofenadine to assess CYP3A (intestinal and hepatic) and drug transport, respectively, in patients with ESRD and healthy control subjects. We evaluated the effect of uremia on CYP3A and transporter expression in vitro by incubating normal rat hepatocytes and enterocytes with serum drawn from study participants. ESRD dramatically reduced nonrenal transporter function, evidenced by a 63% decrease in clearance (P < 0.001) and a 2.8-fold increase in area under the plasma concentration–time curve for fexofenadine (P = 0.002), compared with control subjects. We did not observe significant differences in midazolam or 1′-hydroxymidazolam clearance or area under the curve after oral administration, suggesting that CYP3A function is not changed by ESRD. Changes in hepatocyte and enterocyte protein expression in the presence of uremic serum were consistent with in vivo results. These findings demonstrate a mechanism for altered drug disposition in kidney disease, which may partially account for the high rates of drug toxicity in this population.
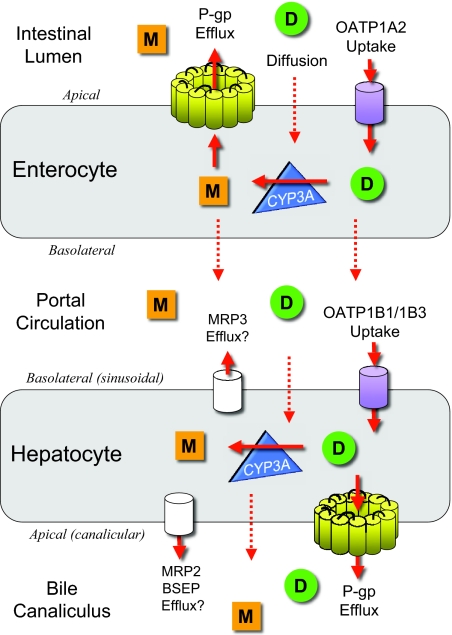
The prevalence of chronic kidney disease (CKD) in the adult US population was recently estimated to be >13%, and the number of patients with ESRD continues to rise rapidly.1 Patients with CKD and particularly ESRD require numerous medications, are at high risk for drug accumulation and toxicity, and experience a high rate of adverse drug events.2–4 Mild, moderate, and advanced CKD (i.e., stages 2 through 5) have been shown to affect the disposition of drugs subject to nonrenal clearance (CLNR) in both humans and animal models.5 As depicted in Figure 1, CLNR is mediated in large part by the concerted actions of cytochrome P450 (CYP) metabolic enzymes such as CYP3A4 and CYP3A5 (collectively CYP3A), multiple drug transporters including the cellular efflux transporter P-glycoprotein (P-gp), and uptake transporters known as organic anion-transporting polypeptides (OATPs)6; however, the manner and extent to which individual pathways of nonrenal clearance are affected in humans with CKD is largely unknown.
Figure 1.
The concerted actions of OATP, P-gp, and CYP3A determine the disposition of many drugs. Briefly, after oral administration, a drug (D, green circle) comes into contact with enterocytes lining the lumen of the gut wall, where it may diffuse or undergo active OATP-mediated transport (uptake) across the apical membrane into the enterocyte, frequently followed by CYP3A-mediated metabolism. Subsequently, the parent drug and/or metabolite (M, orange square) may be either (1) actively countertransported (effluxed) via P-gp back across the apical membrane into the gut lumen to be fecally excreted or endure repeated uptake-metabolism-efflux cycling or (2) translocated across the basolateral membrane into the portal circulation. Once in the portal circulation, drugs and metabolites may either diffuse or undergo active OATP-mediated uptake across the basolateral (sinusoidal) membrane of the hepatocyte, again followed by CYP3A-mediated metabolism, then diffusion or P-gp–mediated efflux across the apical (canalicular) membrane into the bile for excretion. Multidrug resistance–associated protein 2 (MRP2) and bile salt export pump (BSEP) may also be involved in the biliary excretion and MRP3 in the basolateral efflux of fexofenadine. Enterohepatic recirculation of drugs from the bile back into the gut is possible.
Determination of which drug-metabolizing enzymes or transporters are affected in humans with kidney disease is inherently complicated. There is a complex interplay between enzymes and transporters in the liver, intestine, and other extrarenal organs, making it difficult to interpret clinical pharmacokinetic data mechanistically. Published studies to date have not simultaneously and separately evaluated the function of individual pathways of drug metabolism and transport in multiple organs in patients with ESRD. We hypothesized that uremia differentially affects the functional expression of drug-metabolizing enzymes and transporters, which may explain the seeming inconsistent effects of uremia on the clinical pharmacokinetics of various intravenously and orally administered CYP and transporter substrates.5
To elucidate the physiologic mechanism by which kidney disease affects the nonrenal clearance of drugs in humans, we phenotypically evaluated hepatic and intestinal CYP3A-mediated metabolism and drug transporter activity in patients with ESRD. We investigated the clinical pharmacokinetics of midazolam, a pharmacologic probe of CYP3A metabolism, and fexofenadine, a nonspecific probe of drug transport, in vivo, and we assessed the effect of uremic serum on the expression of Cyp3a, Oatp, and P-gp in rat hepatocyte and enterocyte cultures.
Results
Study Participants
A total of 10 patients with ESRD (all white, six male) and 10 healthy control subjects without kidney disease (all white, six male) participated in this study. The patients with ESRD were 51.5 ± 15.6 yr of age; weighed 78.1 ± 20.0 kg; and had a body mass index (BMI) of 27.1 ± 4.9 kg/m2, a Kt/V urea value of 1.8 ± 0.3, a blood urea nitrogen (BUN) concentration of 47.8 ± 8.7 mg/dl, and a serum creatinine (SCR) concentration of 7.5 ± 1.6 mg/dl. Control subjects were 45.9 ± 13.2 yr of age; weighed 87.8 ± 33.0 kg; and had a BMI of 26.4 ± 3.0 kg/m2, a BUN concentration of 14.4 ± 2.2 mg/dl, a SCR concentration of 0.9 ± 0.1 mg/dl, and an estimated GFR7 of 83.9 ± 13.4 ml/min per 1.73 m2. The ESRD and control groups were well matched, with no significant differences in demographic variables except kidney function, BUN, and SCR.
Effect of ESRD on the Pharmacokinetics of Fexofenadine
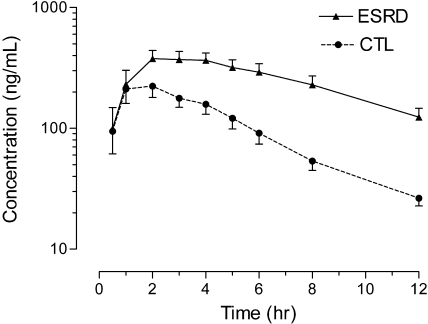
Mean fexofenadine plasma concentration–time curves observed in patients with ESRD and control subjects are depicted in Figure 2, and the corresponding pharmacokinetic parameters are presented in Table 1. The findings in control subjects are comparable to the results of previous studies of healthy subjects.8,9 Fexofenadine clearance after oral administration (CL/F), where F is the bioavailability, was 63% slower, 102.8 ± 37.9 L/h in control subjects to 37.9 ± 19.5 L/h (P < 0.001) in patients with ESRD, leading to a 2.8-fold higher fexofenadine area under the plasma concentration–time curve from 0 to infinity (AUC0 to ∞) in patients with ESRD (P < 0.001; Figure 2). A corresponding 35% longer fexofenadine half-life was also observed (3.4 ± 0.9 h in control subjects to 4.6 ± 1.3 h in patients with ESRD; P = 0.019); maximum plasma concentration (Cmax) was 74% higher (P = 0.023), and the time Cmax occurred (tmax) was not significantly different.
Figure 2.
Mean ± SEM fexofenadine serum concentration–time curves observed in patients with ESRD (solid line) and healthy control subjects (dashed line) after administration of a single oral dose of 120 mg.
Table 1.
Pharmacokinetic parameter estimates of fexofenadine after oral administration of 120mg
| Parameter | Control Subjects | Patients with ESRD | P |
|---|---|---|---|
| Cmax (ng/ml; mean ± SD) | 267.2 ± 130.3 | 464.2 ± 194.7 | 0.023 |
| tmax (h; median [range]) | 2 (1 to 4) | 2 (1 to 6) | NS |
| t1/2 (h; mean ± SD) | 3.4 ± 0.9 | 4.6 ± 1.3 | 0.019 |
| CL/F (L/h; mean ± SD) | 102.8 ± 37.9 | 37.9 ± 19.5 | <0.001 |
| AUC0 to ∞ (h/ng per ml; mean ± SD) | 1380.2 ± 674.1 | 3926.2 ± 1842.8 | <0.001 |
Effect of ESRD on the Pharmacokinetics of Midazolam and 1′-Hydroxymidazolam
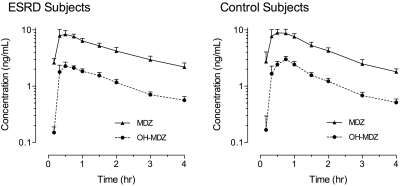
No significant differences were observed in the midazolam and 1′-hydroxymidazolam pharmacokinetic parameters after oral administration in patients with ESRD compared with healthy control subjects (Table 2), which resulted in nearly superimposable plasma concentration–time curves in the two groups (Figure 3). Midazolam CL/F values were 88.9 ± 24.6 and 96.9 ± 46.8 L/h in control subjects and patients with ESRD, respectively (NS), similar to what has been reported previously in healthy subjects.9–11
Table 2.
Pharmacokinetic parameter estimates of midazolam and 1′-hydroxymidazolam after oral administration of midazolam 2 mg
| Parameter | Control Subjects | Patients with ESRD | P |
|---|---|---|---|
| Midazolam | |||
| Cmax (ng/ml; mean ± SD) | 12.3 ± 3.9 | 11.0 ± 5.3 | NS |
| tmax (h; median [range]) | 0.50 (0.33 to 0.75) | 0.50 (0.33 to 1.50) | NS |
| t1/2 (h; mean ± SD) | 1.8 ± 0.7 | 2.6 ± 2.4 | NS |
| CL/F (L/h; mean ± SD) | 88.9 ± 24.6 | 96.9 ± 46.8 | NS |
| AUC0 to ∞ (h/ng per ml; mean ± SD) | 23.8 ± 5.7 | 26.1 ± 13.9 | NS |
| 1′-Hydroxymidazolam | |||
| Cmax (ng/ml; mean ± SD) | 3.6 ± 1.5 | 3.0 ± 1.3 | NS |
| tmax (h; median [range]) | 0.750 (0.330 to 0.750) | 0.625 (0.330 to 1.500) | NS |
| AUC0 to ∞ (h/ng per ml; mean ± SD) | 6.4 ± 2.5 | 6.5 ± 2.3 | NS |
Figure 3.
Mean ± SEM midazolam (solid line) and 1′-hydroxymidazolam (dashed line) serum concentration–time curves observed in patients with ESRD (left) and healthy control subjects (right) after administration of a single oral dose of midazolam 2 mg.
Expression of Oatp, P-gp, and Cyp3a in Rat Hepatocytes and Enterocytes Incubated with Serum from Patients with ESRD
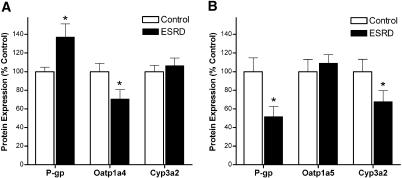
There was a 29% decrease (P < 0.05) in Oatp1a4 and a 37% increase (P < 0.05) in P-gp protein expression in normal hepatocytes incubated with uremic serum compared with control serum, whereas Cyp3a2 protein expression was not affected (Figure 4A). Uremic serum also significantly decreased P-gp (48%; P < 0.05) and Cyp3a2 (32%; P < 0.05) protein expression in enterocytes compared with control serum and did not affect Oatp1a5 protein expression (Figure 4B).
Figure 4.
(A and B) Protein expression of P-gp, Oatp1a4, and Cyp3a2 (A) in normal rat hepatocytes, and P-gp, Oatp1a5, and Cyp3a2 (B) in normal rat enterocytes incubated for 48 h with serum from healthy control subjects or patients with ESRD. The standardized densitometry units of control samples were defined as 100%. Data are means ± SEM of three experiments. *P < 0.05 compared with control subjects.
Discussion
We present a phenotypic assessment of hepatic and intestinal CYP3A and transporter function in patients who had ESRD and were receiving conventional hemodialysis and demonstrate that whereas fexofenadine clearance after oral administration is significantly reduced, midazolam clearance after oral administration is not significantly altered in patients with ESRD. These results suggest that nonrenal transporter function (likely intestinal P-gp and/or hepatic OATP) is dramatically reduced, whereas intestinal and hepatic CYP3A function is similar in patients with ESRD compared with healthy control subjects. Changes in protein expression observed in hepatocytes and enterocytes incubated with serum from study participants are consistent with these in vivo findings and suggest that kidney disease differentially affects these clearance pathways in the liver and gastrointestinal tract. These data establish a specific uremic phenotype at a molecular and cellular level in vivo, which has proved difficult in patients with kidney disease.12
The short-acting benzodiazepine midazolam was used as a selective phenotypic probe of CYP3A13 in this study, because it is metabolized predominantly to 1′-hydroxymidazolam by both CYP3A4 and CYP3A5,14 and, unlike many CYP3A substrates, it is neither a P-gp nor an OATP substrate.15 No significant differences in the oral midazolam and 1′-hydroxymidazolam pharmacokinetic parameters based on total drug concentrations (protein bound plus unbound) were observed in patients with ESRD compared with healthy control subjects (Table 2). These findings contrast with those of Vinik et al.,16 who reported higher clearance of intravenously administered midazolam (based on total drug concentrations) in hemodialysis patients than in matched healthy control subjects; however, after correction for protein binding changes, midazolam pharmacokinetic parameters were nearly identical in the two groups.16 Overall, these data suggest that neither hepatic nor intestinal CYP3A function is substantially altered in patients who have ESRD and receive conventional hemodialysis therapy.
Several studies using experimental models have suggested that kidney disease leads to decreased activity of hepatic and intestinal CYP3A secondary to reduced gene and protein expression,17,18 findings that differ from this in vivo human study. These discrepancies may be due to the more sustained, severe degree of uremia often observed in experimental models, in which, for example, plasma urea nitrogen concentrations have been reported to range from >100 to >500 mg/dl.19,20 These concentrations are considerably higher than are observed in well-dialyzed patients, and our study participants had mean BUN concentrations of 47.8 ± 8.7 mg/dl. It is also possible that dialysis depletes serum of uremic toxins, which may directly affect CYP3A, thereby normalizing CYP3A function.
Previous phenotypic assessments of CLNR in patients with ESRD using the 14C-erythromycin breath test (ErBT) have been reported,21 and we recently demonstrated that hemodialysis acutely improves ErBT results, suggesting that erythromycin clearance is increased after dialysis.22 The ErBT is based on the principle that radiolabeled 14C-erythromycin undergoes N-demethylation by CYP3A4, and the demethylated carbon (14C) rapidly appears in breath as 14CO2.23 According to the standard interpretation, a decrease in ErBT results (i.e., decreased excretion of 14CO2) translates into a reduction in CYP3A4 activity, and vice versa. Although the ErBT is a widely used phenotypic trait measure long considered a specific index of hepatic CYP3A4 activity, recent data indicated that erythromycin undergoes OATP uptake and P-gp efflux in addition to CYP3A4 metabolism.24,25 It is now recognized that considerable overlapping substrate specificity and complex interplay exist between CYP3A and transporters,26 such that alterations in transporters but not CYP3A, per se, may result in significant changes in the clinical pharmacokinetics of many CYP3A substrates.25,27 Reduced OATP uptake lowers the amount of intracellular 14C-erythromycin available for CYP3A4 metabolism and ultimately manifests as decreased excretion of 14CO225; therefore, the previously reported decrease in ErBT results in patients with ESRD may indicate reduced OATP-mediated hepatic uptake of erythromycin rather than reduced CYP3A4 activity. A recent clinical pharmacokinetic study of orally and intravenously administered erythromycin to patients with ESRD demonstrated that hepatic clearance but not oral absorption was significantly reduced,28 further supporting the concept that hepatic OATP uptake is decreased in patients with ESRD. Conversely, an increase in ErBT results may indicate that OATP-mediated hepatic uptake of erythromycin is increased, which then increases the amount of intracellular substrate available for CYP3A4 metabolism, thereby leading to increased 14CO2 excretion. The latter explanation supports previously reported findings of improved ErBT results after dialysis,22 and our findings of significantly reduced fexofenadine clearance and unaltered hepatic and intestinal CYP3A activity in patients with ESRD mechanistically corroborate this reasoning.
There are several possible physiologic explanations for the observed reduction in fexofenadine clearance and corresponding increase in AUC: (1) Reduced intestinal P-gp efflux and increased bioavailability, (2) increased OATP1A2 uptake in the intestine and increased bioavailability, (3) reduced hepatic OATP1B1 or OATP1B3 uptake, and (4) decreased hepatic P-gp efflux. Taking the previously described ErBT data into consideration, however, the most likely explanation for decreased fexofenadine clearance is reduced hepatic OATP (likely OATP1B1 or OATP1B329) uptake and/or reduced enteric P-gp efflux with increased bioavailability (Figure 1). Previous evidence that P-gp plays a vital role in intestinal absorption and only a limited role in biliary excretion of fexofenadine,30 along with recent clinical data demonstrating that hepatic clearance but not oral absorption of the OATP, P-gp, and CYP3A substrate erythromycin is significantly reduced in patients with ESRD, further supports this explanation.28 A significant decrease in Oatp1a4 protein expression was observed whereas P-gp expression was increased in hepatocytes incubated with uremic sera (Figure 4A). Conversely, Oatp1a5 expression was unchanged and P-gp expression was significantly decreased in enterocytes incubated with uremic sera (Figure 4B). A reduction in enterocyte P-gp protein expression would likely translate into an increase in the bioavailability of P-gp substrates such as fexofenadine. Furthermore, with a decrease in the expression of liver OATP, a marked increase in serum concentrations, decreased clearance, and increased plasma half-life would be expected, as we observed in vivo (Table 1; Figure 2).
There are several limitations to this study. Polymorphisms in the genes encoding for P-gp31 and OATP32 have been associated with altered activity of the transporters and may lead to increased plasma concentrations of fexofenadine. Because study participants were not genotyped, the possibility of polymorphically expressed P-gp or OATP in some individuals contributing to the observed reduction in fexofenadine clearance cannot be ruled out; however, given the magnitude of the difference in fexofenadine clearance between patients with ESRD and healthy subjects and the absence of outliers (i.e., individuals who exhibited serum concentrations and pharmacokinetic parameters that were profoundly higher or lower than most), it is unlikely that genotypic differences would explain the observed findings. Moreover, although CYP3A4 and CYP3A5 are polymorphically expressed, allelic variants of each do not seem to have functional phenotypic implications for midazolam.33–35
Another potential limitation of this work is that midazolam and fexofenadine protein binding (or unbound fraction) and pharmacokinetics of the unbound moieties were not determined. Midazolam is normally 96% protein bound, so it is possible that the midazolam unbound fraction was increased in patients with ESRD, resulting in increased hepatic clearance; however, in a previous study, unbound midazolam concentrations and corresponding pharmacokinetic parameters were nearly identical in patients with ESRD and healthy control subjects.16 Because the majority of patients with ESRD are functionally anuric, urine could not be collected for determination of 1′-hydroxymidazolam formation clearance to confirm in vivo findings of unaltered midazolam systemic clearance.
Although this study was well powered to detect a difference in fexofenadine clearance between patients with ESRD and control subjects, the power to detect a difference in midazolam clearance (the primary surrogate of CYP3A activity) was sufficient only to detect a comparatively large difference (50%); however, this magnitude of difference in midazolam clearance is consistent with moderate inhibitors of CYP3A activity (e.g., diltiazem, fluconazole) and is more likely to be clinically relevant than differences of smaller magnitude. Thus, we cannot entirely exclude the possibility that a true difference exists in midazolam clearance between well dialyzed patients with ESRD and healthy control subjects; however, given that we observed nearly superimposable plasma concentration–time curves in the two groups for midazolam and 1′-hydroxymidazolam (Figure 3), the magnitude of any true difference in midazolam clearance is likely to be small.
Fexofenadine is a widely used probe drug for phenotypically assessing P-gp and OATP in humans36,37; however, recent animal studies suggested that it is also a substrate of multidrug resistance–associated proteins and the bile salt export pump.38–40 Consequently, it is possible that alterations in multidrug resistance–associated protein or bile salt export pump transport contributed to the reduction in fexofenadine clearance observed in this study; however, our results clearly show that transporter function is altered in patients with ESRD and that further study is warranted.
In conclusion, we present a phenotypic assessment of hepatic and intestinal CYP3A and transporter function in patients who have ESRD and receive conventional hemodialysis and demonstrate that transporter function (likely hepatic OATP and/or intestinal P-gp) is significantly reduced whereas hepatic and intestinal CYP3A function is not affected in patients with ESRD compared with healthy control subjects. These data establish a specific in vivo phenotype of uremia at the molecular and cellular levels. Moreover, these results provide important mechanistic insight for understanding the physiologic effects of uremia on nonrenal drug metabolism and transport pathways that are critical determinants of drug disposition, response, and outcome and have direct clinical implications because they may ultimately lead to a better understanding of optimal drug choices and dosages to maximize efficacy while minimizing toxicity.
Concise Methods
Study Participants
After providing written informed consent, all participants underwent a screening evaluation based on complete medical history, physical examination, medication history, and laboratory testing. Eligibility criteria included age 18 to 70 yr, normal hepatic function, BMI ≤35 kg/m2, dialysis Kt/V ≥1.20, nonsmoking status, and a negative pregnancy test for women of child-bearing potential. Kidney function in control subjects was estimated according to the method of Levey et al.7 (Modification of Diet in Renal Disease [MDRD] prediction equation). Individuals who had known hepatic or gastrointestinal disease; were taking drugs or dietary products that are known to inhibit or induce CYP3A, P-gp, or OATP; or had a known sensitivity or previous adverse reaction to midazolam or fexofenadine were excluded. Participants were instructed to abstain from consuming any grapefruit products, alcoholic beverages, herbal supplements/teas, and over-the-counter remedies for at least 72 h before and during the study day, and caffeine-containing products were prohibited on the study day.
Study Design
This was an open-labeled, prospective, fixed-order, parallel-group study, which adhered to the Declaration of Helsinki and was approved by the Maine Medical Center institutional review board. Patients with ESRD were studied the day after regularly scheduled hemodialysis. Each patient received 120 mg of fexofenadine (Teva Pharmaceuticals, Sellersville, PA) orally at time 0, followed by a 2-mg oral dose of midazolam (1 ml, 2 mg/ml oral syrup; Ranbaxy Pharmaceuticals, Jacksonville, FL) at time +2 h. Participants fasted overnight before the study day until time +4 h, when they received a small snack. Standardized meals were given at time +5 and +10 h. Venous blood samples (3 ml) were collected through an indwelling catheter immediately before and serially after administration of each probe drug for pharmacokinetic determination as follows: at baseline and at 30, 60, 120, 180, 240, 300, 360, 480, and 720 min after oral fexofenadine administration and immediately before and at 10, 20, 30, 45, 60, 90, 120, 180, and 240 min after oral midazolam administration. Blood samples were drawn into prechilled EDTA tubes, kept on ice, and centrifuged within 30 min of collection. Plasma was stored at −80°C until analysis.
Analytical Assays
The total (protein bound plus unbound) plasma concentrations of midazolam, 1′-hydroxymidazolam, and fexofenadine were determined by liquid chromatography–tandem mass spectrometry using positive electrospray ionization in the selected reaction monitoring mode. The liquid chromatography–tandem mass spectrometry system consists of a Surveyor HPLC autosampler, a Surveyor MS quaternary pump, and a TSQ Quantum Discovery triple quadruple mass spectrometer (ThermoFinnigan, San Jose, CA).
Midazolam and 1′-Hydroxymidazolam
Briefly, plasma (250 μl) was combined with 2 N sodium hydroxide (100 μl) and internal standards (30 μl) and vortex-mixed briefly before the addition of methyl tert-butyl ether (1 ml). Tubes were capped, vortex-mixed, and centrifuged at 20,817 × g for 5 min. The ether layer was transferred, evaporated under nitrogen, reconstituted with 150 μl of methanol:water (1:1), and then injected into the system. The mobile phases used for the analysis were 0.1% formic acid in deionized water (mobile phase A) and 0.1% formic acid in methanol (mobile phase B), delivered in a gradient at a flow rate of 0.2 ml/min. The analytical column was a Phenomenex Synergi Polar-RP, 75.0 × 2.0 mm, 4-μ column. The MS acquisition parameters included a spray voltage of 4.0 kV and heated capillary temperature at 300°C. Nitrogen was used as the sheath and auxiliary gas and set to 30 and 15 arbitrary units, respectively. The argon collision gas pressure was set to 1.5 mTorr, and the collision energy was 47 eV for 1′-hydroxymidazolam, 37 eV for midazolam, 38 eV for alprazolam (1′-hydroxymidazolam internal standard), and 42 eV for diazepam (midazolam internal standard). The selected reaction monitoring scheme followed transitions of the [M+H]+ precursor to selected product ions with the following values: m/z 326.12 → 291.09 for midazolam, m/z 342.10 → 167.90 for 1′-hydroxymidazolam, m/z 309.10 → 281.00 for alprazolam, and m/z 285.10 → 193.08 for diazepam. The lower limit of quantification for midazolam and 1′-hydroxymidazolam was 0.2 ng/ml using 0.25 ml of plasma. The within-run and between-run precision (percentage coefficients of variation) was <7%.
Fexofenadine
Briefly, plasma (100 μl) was combined with acetonitrile (300 μl) containing internal standard in a Captiva filter plate and vortex-mixed briefly before allowing the inverted plate to stand at room temperature for 5 min. The filter plate was fitted with a collection plate before the application of the vacuum; the filtrate was then injected into the system. The mobile phase was 0.1% formic acid, 5 mM ammonium acetate in deionized water, and methanol (35:65, vol/vol) delivered at a flow rate of 0.20 ml/min; the column used was a Phenomenex Gemini, 150.0 × 2.0 mm, 5-μ analytical column. The MS acquisition parameters included a spray voltage of 4.6 kV and heated capillary temperature of 375°C. Nitrogen was used as the sheath and auxiliary gas and set to 35 and 10 arbitrary units, respectively. The argon collision gas pressure was set to 1.5 mTorr, and the collision energy was 41 eV for both fexofenadine and fexofenadine-D6 (internal standard). The selected reaction monitoring scheme followed the transitions of m/z 502.3 → 171.0 for fexofenadine and 508.3 → 177.0 for fexofenadine-D6. The limit of quantification for fexofenadine was 0.1 ng/ml using 0.1 ml of plasma, and the within-run and between-run precision was <5%.
Pharmacokinetic Analysis
Fexofenadine pharmacokinetic parameters were estimated from plasma concentration data using standard noncompartmental methods (WinNonlin Professional 4.1; PharSight Corp., Mountain View, CA). The Cmax, the tmax, and the last plasma concentration measured (i.e., the 12-h concentration in plasma [C12]) were obtained directly from the individual plasma concentration–time profiles. The terminal elimination rate constant (λz) was estimated by linear regression of the terminal phase of the logarithmic plasma concentration–time curve. Terminal elimination half-life (t½) was calculated by dividing 0.693 by λz. Area under the plasma concentration–time curve from 0 to 12 h (AUC0 to 12) was calculated with the log-linear trapezoidal rule. The AUC0 to ∞ was calculated as AUC0 to 12 + C12/λz. The CL/F of fexofenadine was calculated as the oral dosage/AUC0 to ∞. Midazolam and 1′-hydroxymidazolam pharmacokinetic parameters were also calculated using noncompartmental methods. The AUC0 to 4 was calculated with the log-linear trapezoidal rule. AUC0 to ∞, Cmax, tmax, λz, t½, and CL/F were calculated as described already.
In Vitro Studies
For investigation of the effects of kidney disease on the expression of P-gp, Oatp, and Cyp3a in liver and intestine, primary cultures of normal rat hepatocytes and enterocytes were incubated with serum drawn from each patient as described previously.20,41 Hepatocytes were isolated from normal male Sprague-Dawley rats (Charles River, Saint-Charles, Quebec, Canada) using the two-step liver perfusion method with minor modification using collagenase type 4 (Worthington, Lakewood, NJ) for digestion of hepatic tissue, then incubated for 48 h with 2 ml of William's E medium containing 10% serum obtained at baseline from patients with ESRD or control subjects.19,20 Intestinal membrane proteins were prepared from rat enterocytes as described previously41 using two thirds of the small intestine, then incubated for 48 h in culture medium containing 10% serum from patients with ESRD or control subjects. All experiments were conducted according to the Canadian Council on Animal Care guidelines for care and use of laboratory animals and under the supervision of our local animal care committee.
Western Blot Analysis
Protein expression of intestinal and hepatic Cyp3a2,18 intestinal P-gp and Oatp1a5, and hepatic P-gp and Oatp1a4 was assessed via Western blotting as described previously.41,42 β-Actin and villin were used as loading controls with hepatocytes and enterocytes, respectively. Immune reaction intensity was determined by computer-assisted densitometry on a Fujifilm LAS-3000 camera system coupled to MultiGauge software (Fujifilm Life Science USA, Stamford, CT). Every blot was repeated three times, and results were pooled to obtain the final reported values.
Statistical Analysis
For the in vivo study, determination of the target sample size was based on the reported between-subject coefficient of variation for fexofenadine CL/F of approximately 26%.43 The sample size of n = 10 subjects per group was estimated a priori to have 84% power (two-sided type I error of 0.05) to detect a 37% difference in fexofenadine clearance. Moreover, on the basis of the reported between-subject coefficient of variation for midazolam total clearance after oral administration of approximately 36%,33 a sample size of n = 10 subjects per group was estimated a priori to have 82% power (two-sided type I error of 0.05) to detect a 50% difference in midazolam clearance. For avoiding the assumption of normally distributed data, group-wise (patients with ESRD versus control subjects) comparisons of fexofenadine and midazolam pharmacokinetic parameters were made by the Mann-Whitney U test. Power calculations were carried out with G*Power 3.0.10,44 and all other statistical calculations were performed with Prism 4.02 (GraphPad Software, San Diego, CA). Data are presented as means ± SD unless otherwise noted. For in vitro studies, differences between groups were assessed by the unpaired two-sided t test or an ANOVA test. Significant ANOVA was followed by a post hoc Scheffe analysis. P < 0.05 was considered significant for all comparisons.
Disclosures
None.
Acknowledgments
This work was supported in part by grants from the Maine Medical Center Research Institute (T.D.N.), the Satellite Healthcare Research Foundation (T.D.N.), the Canadian Institute of Health Research (V.P.), and the Fonds de la Recherche en Santé du Québec (V.P. and J.N.).
This study was presented in part at the annual meeting of the American Society for Clinical Pharmacology and Therapeutics; April 2 through 5, 2008; Orlando, FL; and has appeared in abstract form (Clin Pharmacol Ther 83[Suppl 1]: S58, 2008).
During this research, Dr. Nolin was a clinical pharmacologist, Dr. Sadr was a renal fellow, and Dr. Himmelfarb was Director, Division of Nephrology and Transplantation, Department of Medicine, Maine Medical Center (Portland, ME).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Manley HJ, Cannella CA, Bailie GR, St. Peter WL: Medication-related problems in ambulatory hemodialysis patients: A pooled analysis. Am J Kidney Dis 46: 669–680, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Bates DW, Miller EB, Cullen DJ, Burdick L, Williams L, Laird N, Petersen LA, Small SD, Sweitzer BJ, Vander Vliet M, Leape LL: Patient risk factors for adverse drug events in hospitalized patients. ADE Prevention Study Group. Arch Intern Med 159: 2553–2560, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Evans RS, Lloyd JF, Stoddard GJ, Nebeker JR, Samore MH: Risk factors for adverse drug events: A 10-year analysis. Ann Pharmacother 39: 1161–1168, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Nolin TD, Naud J, Leblond FA, Pichette V: Emerging evidence of the impact of kidney disease on drug metabolism and transport. Clin Pharmacol Ther 83: 898–903, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Sun H, Frassetto L, Benet LZ: Effects of renal failure on drug transport and metabolism. Pharmacol Ther 109: 1–11, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Lemma GL, Wang Z, Hamman MA, Zaheer NA, Gorski JC, Hall SD: The effect of short- and long-term administration of verapamil on the disposition of cytochrome P450 3A and P-glycoprotein substrates. Clin Pharmacol Ther 79: 218–230, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Robertson SM, Davey RT, Voell J, Formentini E, Alfaro RM, Penzak SR: Effect of Ginkgo biloba extract on lopinavir, midazolam and fexofenadine pharmacokinetics in healthy subjects. Curr Med Res Opin 24: 591–599, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Thummel KE, O'Shea D, Paine MF, Shen DD, Kunze KL, Perkins JD, Wilkinson GR: Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin Pharmacol Ther 59: 491–502, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Masica AL, Mayo G, Wilkinson GR: In vivo comparisons of constitutive cytochrome P450 3A activity assessed by alprazolam, triazolam, and midazolam. Clin Pharmacol Ther 76: 341–349, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Meyer TW, Hostetter TH: Uremia. N Engl J Med 357: 1316–1325, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Lee JI, Chaves-Gnecco D, Amico JA, Kroboth PD, Wilson JW, Frye RF: Application of semisimultaneous midazolam administration for hepatic and intestinal cytochrome P450 3A phenotyping. Clin Pharmacol Ther 72: 718–728, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Lin YS, Dowling AL, Quigley SD, Farin FM, Zhang J, Lamba J, Schuetz EG, Thummel KE: Co-regulation of CYP3A4 and CYP3A5 and contribution to hepatic and intestinal midazolam metabolism. Mol Pharmacol 62: 162–172, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Kim RB, Wandel C, Leake B, Cvetkovic M, Fromm MF, Dempsey PJ, Roden MM, Belas F, Chaudhary AK, Roden DM, Wood AJ, Wilkinson GR: Interrelationship between substrates and inhibitors of human CYP3A and P-glycoprotein. Pharm Res 16: 408–414, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Vinik HR, Reves JG, Greenblatt DJ, Abernethy DR, Smith LR: The pharmacokinetics of midazolam in chronic renal failure patients. Anesthesiology 59: 390–394, 1983 [DOI] [PubMed] [Google Scholar]
- 17.Leblond FA, Guevin C, Demers C, Pellerin I, Gascon-Barre M, Pichette V: Downregulation of hepatic cytochrome P450 in chronic renal failure. J Am Soc Nephrol 12: 326–332, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Leblond FA, Petrucci M, Dube P, Bernier G, Bonnardeaux A, Pichette V: Downregulation of intestinal cytochrome P450 in chronic renal failure. J Am Soc Nephrol 13: 1579–1585, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Guevin C, Michaud J, Naud J, Leblond FA, Pichette V: Down-regulation of hepatic cytochrome P450 in chronic renal failure: role of uremic mediators. Br J Pharmacol 137: 1039–1046, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michaud J, Dube P, Naud J, Leblond FA, Desbiens K, Bonnardeaux A, Pichette V: Effects of serum from patients with chronic renal failure on rat hepatic cytochrome P450. Br J Pharmacol 144: 1067–1077, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dowling TC, Briglia AE, Fink JC, Hanes DS, Light PD, Stackiewicz L, Karyekar CS, Eddington ND, Weir MR, Henrich WL: Characterization of hepatic cytochrome P4503A activity in patients with end-stage renal disease. Clin Pharmacol Ther 73: 427–434, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Nolin TD, Appiah K, Kendrick SA, Le P, McMonagle E, Himmelfarb J: Hemodialysis acutely improves hepatic CYP3A4 metabolic activity. J Am Soc Nephrol 17: 2363–2367, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Watkins PB: Noninvasive tests of CYP3A enzymes. Pharmacogenetics 4: 171–184, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Kurnik D, Wood AJ, Wilkinson GR: The erythromycin breath test reflects P-glycoprotein function independently of cytochrome P450 3A activity. Clin Pharmacol Ther 80: 228–234, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Frassetto LA, Poon S, Tsourounis C, Valera C, Benet LZ: Effects of uptake and efflux transporter inhibition on erythromycin breath test results. Clin Pharmacol Ther 81: 828–832, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Lam JL, Okochi H, Huang Y, Benet LZ: In vitro and in vivo correlation of hepatic transporter effects on erythromycin metabolism: Characterizing the importance of transporter-enzyme interplay. Drug Metab Dispos 34: 1336–1344, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Lau YY, Huang Y, Frassetto L, Benet LZ: Effect of OATP1B transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clin Pharmacol Ther 81: 194–204, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Frassetto LA, Sun H, Huang Y, Benet LZ: Uremic toxins decrease hepatic clearance but not oral bioavailability of erythromycin in patients with end stage renal disease [Abstract]. J Am Soc Nephrol 19: 929A, 2008 [Google Scholar]
- 29.Matsushima S, Maeda K, Ishiguro N, Igarashi T, Sugiyama Y: Investigation of the inhibitory effects of various drugs on the hepatic uptake of fexofenadine in humans. Drug Metab Dispos 36: 663–669, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Tahara H, Kusuhara H, Fuse E, Sugiyama Y: P-glycoprotein plays a major role in the efflux of fexofenadine in the small intestine and blood-brain barrier, but only a limited role in its biliary excretion. Drug Metab Dispos 33: 963–968, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Yi SY, Hong KS, Lim HS, Chung JY, Oh DS, Kim JR, Jung HR, Cho JY, Yu KS, Jang IJ, Shin SG: A variant 2677A allele of the MDR1 gene affects fexofenadine disposition. Clin Pharmacol Ther 76: 418–427, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Niemi M, Kivisto KT, Hofmann U, Schwab M, Eichelbaum M, Fromm MF: Fexofenadine pharmacokinetics are associated with a polymorphism of the SLCO1B1 gene (encoding OATP1B1). Br J Clin Pharmacol 59: 602–604, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wandel C, Witte JS, Hall JM, Stein CM, Wood AJ, Wilkinson GR: CYP3A activity in African American and European American men: Population differences and functional effect of the CYP3A4*1B5′-promoter region polymorphism. Clin Pharmacol Ther 68: 82–91, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Floyd MD, Gervasini G, Masica AL, Mayo G, George AL, Jr, Bhat K, Kim RB, Wilkinson GR: Genotype-phenotype associations for common CYP3A4 and CYP3A5 variants in the basal and induced metabolism of midazolam in European- and African-American men and women. Pharmacogenetics 13: 595–606, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Kharasch ED, Walker A, Isoherranen N, Hoffer C, Sheffels P, Thummel K, Whittington D, Ensign D: Influence of CYP3A5 genotype on the pharmacokinetics and pharmacodynamics of the cytochrome P4503A probes alfentanil and midazolam. Clin Pharmacol Ther 82: 410–426, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Drescher S, Schaeffeler E, Hitzl M, Hofmann U, Schwab M, Brinkmann U, Eichelbaum M, Fromm MF: MDR1 gene polymorphisms and disposition of the P-glycoprotein substrate fexofenadine. Br J Clin Pharmacol 53: 526–534, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glaeser H, Bailey DG, Dresser GK, Gregor JC, Schwarz UI, McGrath JS, Jolicoeur E, Lee W, Leake BF, Tirona RG, Kim RB: Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther 81: 362–370, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Tian X, Zamek-Gliszczynski MJ, Li J, Bridges AS, Nezasa K, Patel NJ, Raub TJ, Brouwer KL: Multidrug resistance-associated protein 2 is primarily responsible for the biliary excretion of fexofenadine in mice. Drug Metab Dispos 36: 61–64, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian X, Swift B, Zamek-Gliszczynski MJ, Belinsky MG, Kruh GD, Brouwer KL: Impact of basolateral multidrug resistance-associated protein (Mrp) 3 and Mrp4 on the hepatobiliary disposition of fexofenadine in perfused mouse livers. Drug Metab Dispos 36: 911–915, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsushima S, Maeda K, Hayashi H, Debori Y, Schinkel AH, Schuetz JD, Kusuhara H, Sugiyama Y: Involvement of multiple efflux transporters in hepatic disposition of fexofenadine. Mol Pharmacol 73: 1474–1483, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Naud J, Michaud J, Boisvert C, Desbiens K, Leblond FA, Mitchell A, Jones C, Bonnardeaux A, Pichette V: Down-regulation of intestinal drug transporters in chronic renal failure in rats. J Pharmacol Exp Ther 320: 978–985, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Naud J, Michaud J, Lebond FA, Lefrancois S, Bonnardeaux A, Pichette V: Effects of chronic renal failure on liver drug transporters. Drug Metab Dispos 36: 124–128, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Russell T, Stoltz M, Weir S: Pharmacokinetics, pharmacodynamics, and tolerance of single- and multiple-dose fexofenadine hydrochloride in healthy male volunteers. Clin Pharmacol Ther 64: 612–621, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Faul F, Erdfelder E, Lang AG, Buchner A: G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175–191, 2007 [DOI] [PubMed] [Google Scholar]