Abstract
X-linked congenital nephrogenic diabetes insipidus (cNDI) results from inactivating mutations of the human arginine vasopressin (AVP) V2 receptor (hV2R). Most of these mutations lead to intracellular retention of the hV2R, preventing its interaction with AVP and thereby limiting water reabsorption and concentration of urine. Because the majority of cNDI-hV2Rs exhibit protein misfolding, molecular chaperones hold promise as therapeutic agents; therefore, we sought to identify pharmacochaperones for hV2R that also acted as agonists. Here, we describe high-affinity nonpeptide compounds that promoted maturation and membrane rescue of L44P, A294P, and R337X cNDI mutants and restored a functional AVP-dependent cAMP signal. Contrary to pharmacochaperone antagonists, these compounds directly activated a cAMP signal upon binding to several cNDI mutants. In addition, these molecules displayed original functionally selective properties (biased agonism) toward the hV2R, being unable to recruit arrestin, trigger receptor internalization, or stimulate mitogen-activated protein kinases. These characteristics make these hV2R agonist pharmacochaperones promising therapeutic candidates for cNDI.
The antidiuretic hormone arginine-vasopressin (AVP) is crucial for osmoregulation, cardiovascular control, and water homeostasis. The human AVP V2 receptor (hV2R), localized in the principal cells of the kidney collecting duct, mediates AVP antidiuretic effect and therefore helps in maintaining physiologic plasma osmolality, blood volume, and arterial pressure. Binding of AVP to hV2R first triggers a cAMP signal through activation of the G protein αs (Gs) subunit and adenylyl cyclase (AC). Then, the cAMP-activated protein kinase A phosphorylates aquaporin 2 water channels, resulting in their insertion into the luminal membrane of principal cells and finally to water reabsorption.1 AVP binding to hV2R also induces arrestin recruitment, receptor internalization,2 and mitogen-activated protein kinase (MAPK) activation.3
Mutations in the hV2R gene lead to the X-linked congenital nephrogenic diabetes insipidus (cNDI), a rare disease characterized by the kidney's inability to concentrate urine despite normal or elevated plasma concentrations of AVP.4 More than 200 different mutations have been described and are responsible for polyuria, a main consequence of the disease. Most of the mutant receptors (cNDI-hV2Rs), trapped in the endoplasmic reticulum (ER), cannot reach the cell surface and interact with AVP.5 cNDI is thus referred as a conformational or protein-misfolding disease.6
Various chaperones,7 either chemical (cellular osmolytes such as glycerol or DMSO) or pharmacologic (specific ligands),8,9 are promising therapeutic agents for future clinical treatment of protein-misfolding disorders. Because a majority of cNDI-hV2Rs are misfolded and many ligands are available for hV2R, the pharmacochaperone-based strategy is of particular interest for cNDI. Considering an efficient therapy for this disease, the ideal drug should combine pharmacochaperone properties together with hV2R agonist and noninternalizing activities, for stimulating AC and maintaining a long-lasting cAMP signal. This would classify such a molecule as a biased agonist or functionally selective compound.10
Small nonpeptide AVP antagonists (commonly named vaptans)—such as the hV2R-selective antagonists SR121463 (satavaptan), VPA985 (lixivaptan),11 OPC41061 (tolpavtan), and OPC31260 (mozavaptan)12; the V1a receptor (V1aR) antagonist SR49059 (relcovaptan); and the nonselective V1aR/V2R antagonist YM087 (conivaptan)13—were demonstrated to promote adequate maturation and cell surface rescue of cNDI-hV2Rs, with restoration of their capacity to initiate a cell response upon AVP binding. Although the vaptans display the expected pharmacochaperone beneficial effects, their antagonistic activity limits their use as a result of their inability to stimulate membrane-targeted cNDI-hV2Rs directly. Comparatively, agonist pharmacochaperones would combine crucial advantages for treating cNDI.
Here, we identified the first functionally selective hV2R agonist pharmacochaperones. The Wyeth-Ayerst WAY-VNA-932 and the Otsuka OPC23h nonpeptide hV2R antidiuretics,14,15 as well as a novel compound MCF57, were tested for their capacity to recruit intracellularly trapped cNDI-hV2Rs and to restore their functionality. In addition, we determined the capacity of the three ligands to act as hV2R biased agonists (i.e., their behavior in terms of Gs/AC agonism and arrestin antagonism).
Results
Nonpeptide Compounds Have a High Affinity for hV2R and Induce Receptor-Dependent cAMP Production
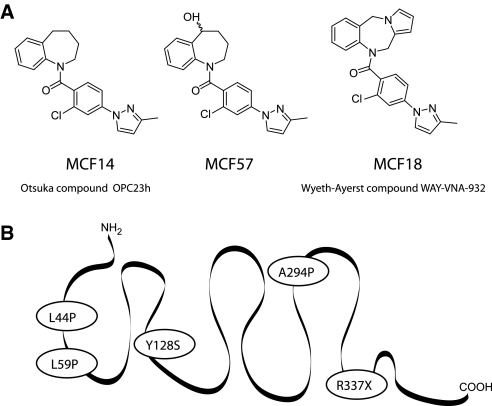
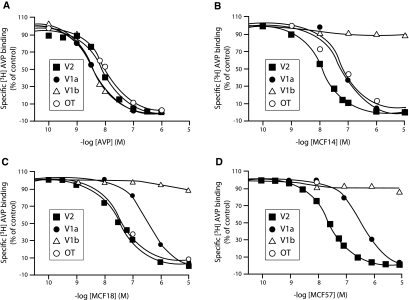
To develop ligands exhibiting both hV2R agonist properties and pharmacochaperone effects on cNDI-hV2Rs, we used the Otsuka OPC23h and the Wyeth-Ayerst WAY-VNA-932 nonpeptide hV2R agonist molecules as lead compounds. New molecules derived from these antidiuretics were synthesized for optimizing preferential affinity and activity toward hV2R. The pharmacologic properties of one of these hits, MCF57 (Figure 1A), were compared with those of OPC23h (termed MCF14 here) and WAY-VNA-932 (named MCF18) in terms of hV2R affinity and Gs/AC activation. The three ligands fully displaced [3H]AVP binding on tsA201 cells expressing the c-myc–tagged AVP hV2R and hV1aR receptors, and the human oxytocin (OT) receptor (hTOR) (Figure 2, A through D) but were unable to compete with [3H]AVP binding to the hV1bR. They all displayed a preferential affinity (6 to 20 nM) for the c-myc-hV2R versus other receptor subtypes (Table 1). MCF57 possessed the best hV2R selectivity index. [3H]AVP saturation binding experiments performed with various MCF57 concentrations on c-myc-hV2R–expressing cells revealed that the nonpeptide inhibited AVP binding competitively (data not shown).
Figure 1.
Structure of the three MCF nonpeptide compounds and snake-like plot of the hV2R. (A) Chemical structures of MCF14, MCF18, and MCF57. (B) L44P, L59P, Y128S, A294P, and R337X cNDI mutants of the hV2R used in the study.
Figure 2.
Binding profiles of AVP and MCF compounds for human AVP/OT receptor subtypes expressed in tsA201 cells. Competition experiments using [3H]AVP as radioligand on tsA201 transfected cells were done as described in the Concise Methods section and in the legend to Table 1. (A through D) [3H]AVP competition experiments to each human c-myc–tagged AVP/OT receptors (■, V2; ●, V1a; ▵, V1b; ○, OTR) were performed using increasing concentrations of unlabeled ligand: AVP (A), MCF14 (B), MCF18 (C), and MCF57 (D). Specific binding was expressed as percentage of the specific binding measured in the presence of vehicle only. Results illustrated correspond to an experiment representative of three independent experiments, each performed in duplicate.
Table 1.
Affinity of the MCF nonpeptide compounds for the human AVP/OT receptor subtypes
| Compound | Receptors |
|||||||
|---|---|---|---|---|---|---|---|---|
| c-myc-hV2R |
c-myc-hV1aR |
c-myc-hV1bR |
c-myc-hOTR |
|||||
| Ki nM | V2-SI | Ki nM | V2-SI | Ki nM | V2-SI | Ki nM | V2-SI | |
| AVP | 5.3 ± 0.5 | 1.0 | 1.1 ± 0.2 | 0.2 | 1.4 ± 0.3 | 0.3 | 6.3 ± 0.7 | 1.2 |
| MCF14 | 6.2 ± 0.8 | 1.0 | 19.0 ± 3.0 | 3.1 | >1000 | >100 | 37.0 ± 8.0 | 6.0 |
| MCF18 | 20.0 ± 3.0 | 1.0 | 106.0 ± 10.0 | 5.3 | >1000 | >100 | 31.0 ± 7.0 | 1.5 |
| MCF57 | 18.0 ± 3.0 | 1.0 | 122.0 ± 34.0 | 6.8 | >1000 | >100 | 214.0 ± 55.0 | 12.0 |
Binding assays were performed as described in the Concise Methods section and are illustrated in Figure 2. [3H]AVP binding was measured on whole tsA201 cells transfected with c-myc-hV2R, c-myc-hV1aR, c-myc-hV1bR, or c-myc-hOTR subtypes and displaced using increasing concentrations of AVP, MCF14, MCF18, or MCF57. Inhibition constants (Ki in nM) were determined from the competition experiments performed using [3H]AVP concentrations around Kd for the various receptors (i.e., 1.5 nM). Data are means ± SEM of three distinct experiments each done in duplicate. V2-SI (normalized selectivity index) = Ki MCF or AVP for VxR/Ki MCF or AVP for V2R. The Kd for [3H]AVP, calculated from saturation binding assays done on whole tsA201 cells were 2.20 ± 0.40, 0.76 ± 0.08, 0.85 ± 0.04, and 3.10 ± 0.30 nM for c-myc-hV2R, c-myc-hV1aR, c-myc-hV1bR, and c-myc-hOTR, respectively.
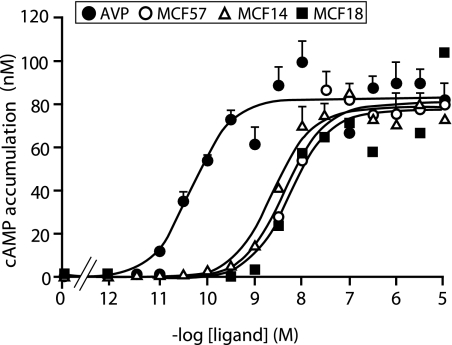
We characterized the agonist properties of the three MCFs to the c-myc-hV2R through their capacity to induce a cAMP accumulation (which correlates to Gs/AC stimulation). The ligands exhibited efficiencies similar to AVP (Emax values were close to that for AVP maximal response) but lower potencies (nanomolar EC50 values), as illustrated by a rightward shift of the dose-response curves (Figure 3; Table 2). The three MCFs, however, are full agonists of the hV2R regarding the Gs/AC pathway.
Figure 3.
Determination of agonist properties of the nonpeptide compounds for the c-myc–tagged hV2R using cAMP accumulation as a readout. The tsA201 cells transfected with the c-myc-hV2R were incubated for 30 min at 37°C in a cAMP buffer containing 0.1 mM RO 201724 with or without increasing amounts of AVP (●), MCF57 (○), MCF14 (▵), or MCF18 (■). As described in the Concise Methods section, cAMP accumulation was evaluated using the Cisbio International Dynamic 2 kit based on fluorescence resonance energy transfer measurements. Basal cAMP accumulation values were subtracted from values obtained with AVP or MCFs. Data are means ± SEM of cAMP concentration (nM). The figure illustrates an experiment representative of three distinct experiments, each performed in triplicate.
Table 2.
Agonist properties of the nonpeptide compounds on c-myc-hV2R–stimulated AC activity
| Compound | cAMP Accumulation |
|
|---|---|---|
| EC50 (nM) | Emax (%) | |
| AVP | 0.05 ± 0.01 | 100 |
| MCF14 | 4.00 ± 2.00 | 96 ± 5 |
| MCF18 | 7.00 ± 1.00 | 95 ± 3 |
| MCF57 | 6.00 ± 2.00 | 103 ± 7 |
Accumulation of cAMP was performed as described in the Concise Methods section and supplemental information, using an homogeneous time-resolved fluorescence-fluorescence resonance energy transfer technology developed by CisBio Int. TsA201 cells transfected with the myc-hV2R were stimulated for 30 min with increasing concentrations of AVP, MCF14, MCF18, or MCF57 in the presence of 0.1 mM of the phosphodiesterase inhibitor RO 201724. The cAMP accumulation was expressed as the mean ± SEM of cAMP concentration (nM) and reported as percentage of the maximal AVP response (Emax). EC50s in the table are the mean ± SEM from three distinct experiments performed in triplicate and described in the legend to Figure 3.
Nonpeptide Agonist Compounds Are Pharmacochaperones for Several cNDI-hV2Rs
We determined the capacity of MCFs to trigger plasma membrane expression of several cNDI-hV2Rs: L44P,16 L59P, Y128S, A294P, and R337X (Figure 1B).11 These mutants are known to be sequestered mainly either in the ER or in the ER-Golgi intermediate compartment (although Y128S was shown to be partially present at the cell surface11) and functionally rescued by the hV2R antagonist SR121463. We also tested the murine V2R (mV2R), which is predominantly retained within the ER as an immature protein17 but also membrane-targeted by the vaptans.18 Treatment of the cells with each MCF led to a significant increase in the L44P, A294P, and R337X membrane expression levels, compared with control cells treated with 0.1% DMSO only (Figure 4, A through C). The MCFs were very effective and their rescue properties equivalent to those of the reference pharmacochaperone SR121463. By contrast, whereas SR121463 was still efficient to rescue Y128S and L59P, MCFs were either less active or inactive, respectively (Figure 4, D through E). Interestingly, the agonists increased significantly membrane expression of the mV2R, such as SR121463 (Figure 4F). The three MCFs exhibited similar pharmacochaperone behavior, being active on several receptors (L44P, A294P, and R337X), less active on others (Y128S and mV2R), or inactive (L59P).
Figure 4.
Cell surface rescue of the different N-terminally c-myc–tagged cNDI-hV2Rs with the nonpeptide compounds. (A through F) Transiently transfected tsA201 cells with c-myc–tagged L44P (A), A294P (B), R337X (C), Y128S (D), L59P (E) or mV2 (F) receptors were incubated for 18 h at 37°C with nonpeptide compounds at 10 μM (SR121463, MCF14, MCF18, and MCF57) or with vehicle only (DMSO 0.1%, control). Each of these receptors (■) was compared with the wild-type hV2R (□). ELISA measurements with an anti–c-myc (9E10) antibody were performed on nonpermeabilized cells as described in the Concise Methods section. Results are expressed as percentage of the maximal hV2R expression under control conditions done in the same experiment and are the means ± SEM of at least three independent experiments, each performed in triplicate. For each nonpeptide compound, a statistical analysis between control and treated cells was done: *P < 0.05, **P < 0.01, and ***P < 0.001.
To avoid potential escape from the cell quality control system as a result of mutant overexpression, we defined conditions in which no cNDI-hV2Rs could be detected at the cell surface in control situation using varying plasmid quantities. Even with low cNDI-hV2R plasmid quantities, membrane targeting of the intracellularly retained cNDI-hV2Rs using MCF57 as a pharmacochaperone reference was evidenced (Supplemental Figure 1).
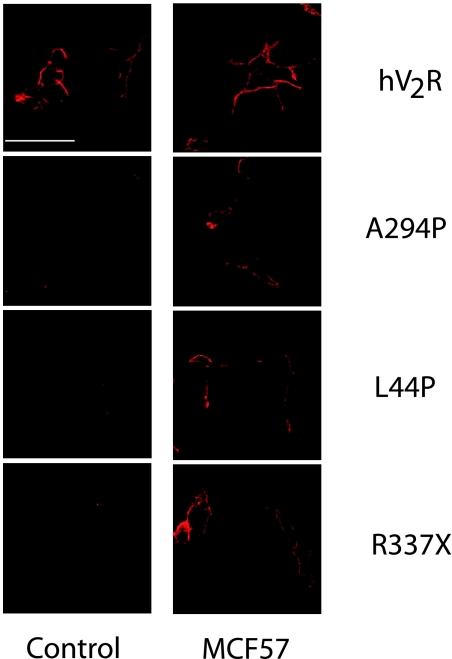
We also checked membrane rescue of cNDI-hV2Rs by directly visualizing the effects of MCF57 using confocal microscopy. We performed immunofluorescence experiments with nonpermeabilized cells to label only cell surface receptors. L44P, A294P, and R337X were shown to respond efficiently to the pharmacochaperone treatment. Incubation of cells with MCF57 allowed detection of a clear surface labeling that was not seen in control cells treated with DMSO (Figure 5).
Figure 5.
Confocal microscopy visualization of cell surface rescue of different cNDI-hV2Rs. The tsA201 cells were transfected with different N-terminally c-myc–tagged human V2 receptors, either wild-type or mutants (A294P, L44P, or R337X). Then, cells were treated for 18 h at 37°C with MCF57 at 10 μM or with vehicle only (DMSO 0.1%, control), fixed, and incubated with antibodies. Bar = 50 μm.
Agonist Pharmacochaperones Promote cNDI-hV2R Maturation
To evidence maturation of cNDI-hV2Rs under MCF treatment, we analyzed the glycosylation pattern of L44P, A294P, and R337X. In control conditions, the three cNDI-hV2Rs were detected only as immature proteins (apparent molecular weight approximately 40 kDa, or 36 kDa for R337X) compared with the complex-glycosylated hV2R (apparent molecular weight approximately 50 kDa; Figure 6). Treatment of the receptors with EndoH deglycosylated cNDI-hV2Rs immature forms without effect on the mature hV2R forms, indicating that the three cNDI-hV2Rs are probably high-mannose glycosylated proteins trapped in the ER. Interestingly, treatment of the cells with the pharmacochaperone MCF14 allowed efficient complex glycosylation of the cNDI-hV2Rs, an effect comparable to that of SR121463. This confirmed the ability of MCF compounds to promote maturation and plasma membrane translocation of L44P, A294P, and R337X.
Figure 6.
Effect of the MCF14 pharmacochaperone treatment on the maturation of the cNDI-hV2Rs. The tsA201 cells were transfected with different N-terminally c-myc–tagged human V2 receptors, either wild-type or mutants (A294P, L44P, or R337X). Then, cells were treated for 18 h at 37°C with MCF14 (10 μM), with SR121463 (10 μM), or with vehicle only (DMSO 0.1%, control). Cell lysates from various conditions were immunoprecipitated with c-myc antibodies. Immunoprecipitates were treated or not with EndoH and analyzed by Western blotting with c-myc antibodies as described in the Concise Methods section. Each result illustrated here corresponds to an experiment representative of three independent experiments. Molecular weight markers are indicated on the left of the panels.
Membrane-Rescued cNDI-hV2Rs Are Functional
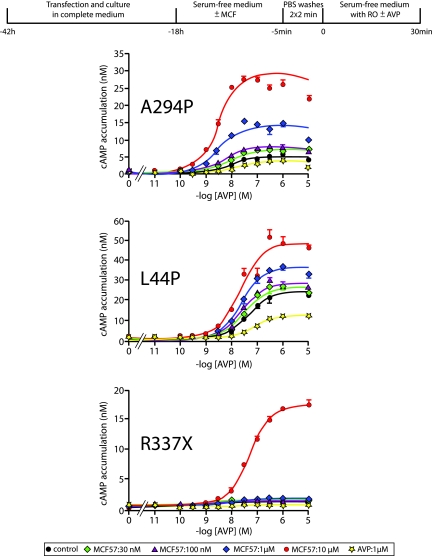
We checked the functionality of membrane-rescued cNDI-hV2Rs by measuring their ability to generate a cAMP response upon AVP stimulation. As in previous studies with antagonist pharmacochaperones,11,19,20 we determined cNDI-hV2R activity after washing the cells (elimination of the pharmacochaperone) and subsequently stimulating them with AVP. First, optimal plasmid quantities were determined to avoid cNDI-hV2R plasma membrane expression and a cAMP response in control conditions (Supplemental Figure 1). Then, using an 18-h preincubation with MCF57 concentrations from 30 nM to 10 μM, we were able to evidence further the cAMP responses to AVP with different efficiencies for L44P, A294P, and R337X (Figure 7). The L44P and A294P mutants were sensitive to AVP after pretreatment with 30 nM MCF57. R337X was sensitive to AVP only when preincubated with 10 μM MCF57. An 18-h AVP preincubation had no effect on the cAMP response whatever the cNDI-hV2R considered, as in control situation, confirming that AVP is not a pharmacochaperone (Figure 7). These data show that rescued L44P, A294P, and R337X are functional once targeted to the plasma membrane using low pharmacochaperone concentrations. The results obtained with MCF57 were reproducible with MCF14 and 18, although statistically less significant, these ligands used at 10 μM being more difficult to eliminate, even though extensive washes were performed (data not shown).
Figure 7.
Functional rescue of various cNDI-hV2Rs by the nonpeptide MCF57 compound. Transiently transfected tsA201 cells with N-terminally c-myc–tagged A294P (50 ng of PRK5-c-myc-A294P), L44P (25 ng of PRK5-c-myc-L44P), or R337X (50 ng of PRK5-c-myc-R337X) were incubated for 18 h at 37°C in serum-free medium supplemented with increasing concentrations of MCF57 (from 30 nM to 10 μM), with AVP (1 μM in DMSO 0.1%), or with vehicle only (DMSO 0.1%, control). After two PBS washes, 2 min each, cells were incubated again for 30 min at 37°C in serum-free medium containing 0.1 mM RO 201724 and BSA 0.5% with or without (basal) increasing concentrations of AVP (from 10 pM to 10 μM). The cAMP accumulation was measured as described in the Concise Methods section. Data are means ± SEM of cAMP concentration (nM) and illustrated from a representative experiment performed at least three times.
Nonpeptide Pharmacochaperones Directly Activate cNDI-hV2Rs
Because MCFs are able to membrane rescue cNDI-hV2Rs and trigger an hV2R-dependent cAMP accumulation, we measured their agonist activity on mutant receptors. Cells expressing cNDI-hV2Rs were treated with various pharmacochaperones or DMSO 0.1% (control condition) and then directly incubated for 30 min with RO 201724 for accumulating cAMP (Figure 8). Agonist properties of the MCFs were evaluated for L44P, A294P, and R337X. Compared with the strong cAMP response obtained for hV2R (Figure 8A), a lower but significant cAMP accumulation for A294P (Figure 8B) and L44P (Figure 8C) mutants was measured. The pharmacochaperone SR121463 was unable to stimulate a cAMP production, whatever the receptor considered. The competitive effect of SR121463 to MCF57-induced cAMP signal confirmed the specificity of interactions between cNDI-hV2Rs and the different pharmacochaperones; therefore, MCF ligands were unable to stimulate R337X (Figure 8D).
Figure 8.
Agonist properties of the nonpeptide compounds toward the cNDI-hV2Rs. (A through D) Transiently transfected tsA201 cells with N-terminally c-myc–tagged hV2R (A), A294P (B), L44P (C), or R337X (D) were incubated for 18 h at 37°C with 10 μM MCF14, MCF18, MCF57, SR121463, MCF57 and SR121463 mixed together (10 μM each), or vehicle only (DMSO 0.1%, control condition). Then, the medium was supplemented with 0.1 mM RO 201724, and cells were incubated for 30 min at 37°C. The cAMP accumulation was measured, and the results are expressed as in Figure 7. A statistical analysis between control and treated cells was done: *P < 0.05 and **P < 0.01.
Nonpeptide Pharmacochaperones Are Antagonists of Arrestin Recruitment
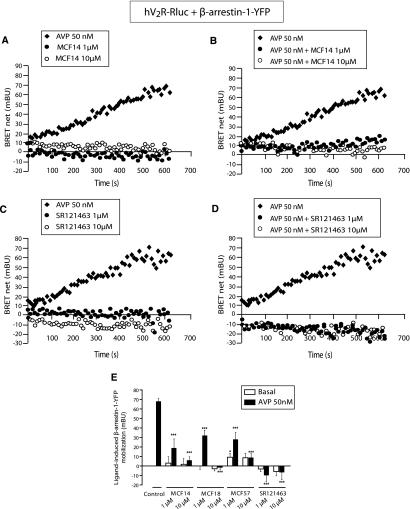
The capacity of the MCF agonists to elicit hV2R-arrestin binding was investigated by bioluminescence resonance energy transfer measurements (BRET), using the receptor fused with Luciferase as a donor and β-arrestin 1 or 2 fused with the yellow fluorescence protein as an acceptor. Control cells were treated with DMSO 0.1% and stimulated by AVP. MCF14, representative of the three agonists, could not induce β-arrestin 1 recruitment (Figure 9A) but inhibited AVP-stimulated hV2R-arrestin binding (Figure 9B). These effects were mimicked by the inverse agonist SR121463 (Figure 9, C and D), contrasting with what has been published previously.21 Statistical results of the β-arrestin 1 mobilization with each MCF alone or combined with AVP are shown in Figure 9E. MCF18 was unable to mobilize arrestin but inhibited the AVP effect. MCF57 induced a slight increase in arrestin recruitment (<10% of the AVP response) and inhibited AVP-dependent arrestin mobilization. The effects of MCF14 and SR121463 on arrestin recruitment were identical using β-arrestin 2 (Supplemental Figure 2). Contrary to AVP, the MCFs exhibited antagonistic effects on hV2R-dependent arrestin recruitment. They consequently display functional selectivity properties.
Figure 9.
Inhibitory effects of the nonpeptide compounds on the AVP-induced β-arrestin 1–YFP/hV2R-Rluc interactions. Real-time BRET experiments for arrestin recruitment were performed using tsA201 cells transiently co-transfected with hV2R-Rluc and β-arrestin 1–YFP and incubated at 37°C with coelenterazine-h either with the nonpeptide compounds alone or in combination with AVP. (A and B) Mobilization of β-arrestin 1–YFP was measured after treatment of the hV2R-expressing cells by 50 nM AVP (♦), 1 μM MCF14 (●) or 10 μM MCF14 (○) alone (A) or by 50 nM AVP (♦) in combination with either 1 μM (●) or 10 μM MCF14 (○; B). (C and D) Mobilization of β-arrestin 1–YFP was measured after treatment of the hV2R-expressing cells by 50 nM AVP (♦), 1 μM SR121463 (●), or 10 μM SR121463 (○) alone (C) or by 50 nM AVP (♦) in combination with either 1 μM (●) or 10 μM SR121463 (○; D). (E) Complete analysis of arrestin recruitment measured after a 600-s treatment in the absence (control) or presence of the nonpeptide compounds at 1 or 10 μM. In each condition, a 50 nM concentration of AVP was added (■) or not (basal; □). Data are means ± SEM obtained from at least three distinct experiments. A statistical analysis between control and treated cells was done: *P < 0.05 and ***P < 0.001.
Nonpeptide Pharmacochaperones Are Unable to Induce hV2R Internalization
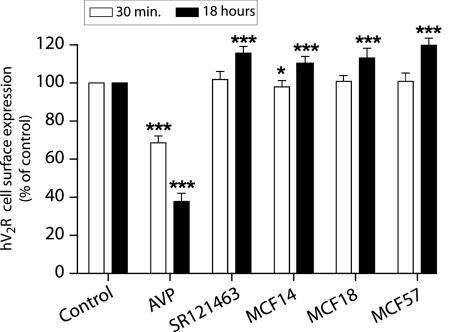
Because arrestins are involved in hV2R internalization, we evaluated the capacity of the MCFs potentially to induce this receptor-associated pathway. Indeed, hV2R internalization was investigated by ELISA using the N-terminal c-myc tag as a readout to measure disappearance of the receptor from the cell surface upon ligand binding. Neither a 30-min nor a long-lasting (18 h) application of the various compounds (MCF14, 18, or 57, 10 μM) provoked hV2R internalization (Figure 10). The antagonist SR121463 displayed the same behavior. As a positive control, AVP induced a significant hV2R internalization for a short or a long incubation period (31 ± 4 and 62 ± 5% of hV2R were internalized, respectively). The AVP-induced internalization could be inhibited by 10 μM of the various MCF compounds (data not shown). The MCFs are noninternalizing hV2R agonists.
Figure 10.
Inability of the various nonpeptide compounds to trigger hV2R internalization. Transiently transfected tsA201 cells with the c-myc–tagged hV2R were incubated for 30 min (□) or 18 h (■) at 37°C with vehicle (control), 100 nM AVP, 10 μM SR121463, MCF14, MCF18, or MCF57. Cell surface expression was measured by ELISA on nonpermeabilized cells. Results are expressed as percentage of the maximal hV2R expression under control conditions. Data are means ± SEM taken from at least three independent experiments, each performed in triplicate. A statistical analysis between control and treated cells was done for each incubation time, 30 min or 18 h: *P < 0.05 and ***P < 0.001.
Nonpeptide Pharmacochaperones Are Antagonists of the MAPK Signaling Pathway
AVP activation of the hV2R leads to arrestin recruitment, which in turn behaves as a scaffold protein for MAPK stimulation.22,23 MAPK activity can be measured through modification of the level of extracellular signal–regulated kinase 1 and 2 (ERK1/2) phosphorylation (p-ERK). We evaluated the effect of the MCFs used alone or in combination with AVP on the hV2R-dependent MAPK activity (Figure 11). Contrary to AVP, MCF14, 18, and 57 were not able to induce ERK phosphorylation, their effect being not significantly different from the control (cells treated with DMSO). The hV2R inverse agonist SR121463 was not able to induce MAPK activation on its own.21 The MCF compounds were able to inhibit significantly the AVP-induced p-ERK. These results confirmed that MCF14, 18, and 57 are biased agonists.
Figure 11.
Effects of the various nonpeptide compounds on the hV2R-mediated MAPK activation. Stably transfected HEK293 cells with the c-myc–tagged-hV2R were incubated for 3 min at 37°C in serum-free medium (control conditions) with either vehicle (□) or 100 nM AVP (■). Other cells were treated with 10 μM of MCF14, MCF18, MCF57, or SR121463 alone (basal; □) or in combination with 100 nM AVP (■). Then, p-ERK and total ERK from cell lysates were analyzed by Western blot. Molecular weight markers are indicated on the left of the top panel. Results are expressed as percentage of the AVP maximal p-ERK/total ERK ratio under control conditions and are the means ± SEM taken from at least three independent experiments, each performed in duplicate. A statistical analysis between control and treated cells was done: *P < 0.05.
Discussion
Chemical chaperones, pharmacochaperones, or cell-penetrating peptides have been developed to correct plasma membrane–targeting deficiency of misfolded G protein-coupled receptors (GPCRs).24,25 Pharmacochaperones are the most promising candidates because of their target specificity. For instance, the P23H rhodopsin mutant responsible for autosomal dominant retinitis pigmentosa and mutants of the gonadotropin-releasing hormone receptor responsible for hypogonadotropic hypogonadism were rescued by the inverse agonist 9-cis-retinal and an antagonist, respectively.26,27 The δ opioid receptor, displaying low intrinsic maturation efficiency, was membrane-targeted with selective opioid ligands.28 Considering AVP receptors, the nonpeptide antagonists SR121463 and VPA985 were first shown to rescue cell surface expression of several cNDI-hV2Rs.11 This effect was reproduced using the AVP antagonists SR49059,29 YM087,13 OPC41061, and OPC31260.12 Because most of the cNDI-hV2Rs are functional once targeted to the plasma membrane, a pharmacochaperone-based therapy represents a potential general treatment of the disease.
Using antagonists as pharmacochaperones, functional rescue is a subtle balance between the ability of the ligand to target cell surface expression of the cNDI-hV2Rs and its possibility to be displaced by AVP for receptor activation.12 In this regard, considering the antagonist affinity as an important feature for this challenge, either low concentrations of antagonists with high affinity (SR121463)11,12 or higher concentrations of nonspecific hV2R antagonists with low affinity (SR49059) may be used.13 Although SR49059 behaved as an efficient pharmacochaperone in patients with cNDI,13 it revealed hepatic toxicity during phase II of its clinical development. An important alternative to the antagonists would be the use of agonists able both to restore membrane trafficking of cNDI-hV2Rs and to promote their activation directly. These ligands would behave as more beneficial cNDI drugs.
We characterized here some hV2R nonpeptide agonists displaying such properties. OPC23h,15 WAY-VNA-932,14 and MCF57 have a nanomolar affinity for hV2R and behave as full agonists for AC activation. They possess a lower affinity for hV1aR and hOTR. These molecules do not bind to hV1bR, in agreement with available data for OPC23h and WAY-VNA-932.14,15 They membrane-rescued the L44P, Y128S, A294P, and R337X mutants and the mV2R but were inactive toward L59P. This suggests that these agonists preferentially rescue some given misfolded cNDI-hV2Rs, whereas the antagonist pharmacochaperones (SR121463, VPA985, YM087, and SR49059) may have a larger spectrum of efficiency.11,30 Efficiency of cNDI-hV2R membrane translocation between the various pharmacochaperones could be directly related to the affinity of each ligand for each mutant12; however, the nonpeptide agonists having an hV2R relatively high affinity (6 to 20 nM) are unable to rescue L59P, contrary to the low-affinity SR49059 antagonist.13,29 Even though pharmacochaperones need to bind to mutants, affinity seems not to be the only critical parameter. The mutation L59P itself may also change the whole structure of the receptor and explain why the agonist cannot interact anymore with the binding site. Finally, different conformational changes induced by agonists rather than antagonists may explain the differences observed in terms of rescuing.18 Altogether, a more exhaustive series of cNDI-hV2Rs should be investigated and several other agonist pharmacochaperones should be developed to rescue a larger panel of cNDI-hV2Rs.
In terms of signaling, contrary to the antagonist pharmacochaperones, the MCF compounds directly activated membrane-targeted L44P and A294P cNDI-hV2Rs but not the R337X, despite restoration of membrane trafficking. Poor functional recovery of R337X, potentially reflecting an impairment of this mutant in its ligand affinity and/or its ability to interact with Gs, has been previously described.11 For L44P and A294P mutants, a single molecule having both pharmacochaperone and agonist properties seems highly beneficial and may represent an alternative to antagonists for cNDI treatment.
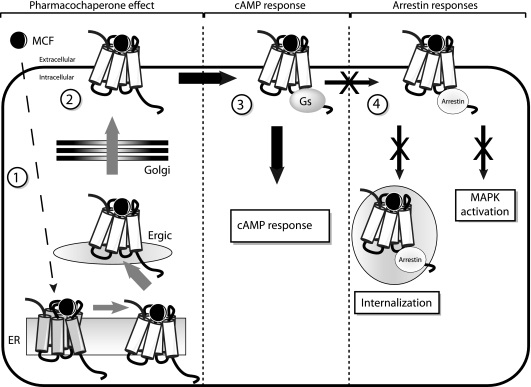
A drawback of most GPCR agonists regarding long-term efficacy is their propensity to trigger internalization of target receptors, leading to desensitization and decrease in the intensity of cell response. Arrestins are key players regarding desensitization/internalization of GPCRs or MAPK activation.31 These phenomena were shown to occur upon AVP stimulation of the hV2R.32,33 In contrast to AVP, MCF compounds were unable to induce arrestin mobilization (both β-arrestin 1 and 2). Together with their Gs agonism, they thus displayed functional selectivity properties. Moreover, they could antagonize the strong and long-lasting interaction of hV2R with arrestins upon AVP binding. Accordingly, these molecules could not promote hV2R internalization and MAPK stimulation, confirming their cAMP selectivity. A schematic view of the transduction pathways induced or inhibited by the pharmacochaperone MCFs after binding to cNDI-hV2Rs is shown in Figure 12. Functional selectivity or biased agonism describes the capacity of ligands to induce selectively one or several pathways among all those usually activated by the endogenous related agonist.10 It is of major importance for future drug development. Indeed, the μ-opioid agonist herkinorin activates Gi protein coupling and MAPKs but not arrestin recruitment and receptor internalization.34 Herkinorin represents a promising opiate analgesic with limited adverse effects such as tolerance. J-2156, a peptidomimetic analogue of somatostatin is a super-agonist of the somatostatin receptor 4 subtype but does not cause desensitization.35 This makes it clinically useful for controlling cellular proliferation in tumorous tissues. The OT antagonist atosiban, which is used clinically for preventing preterm birth, inhibits Gq and concomitantly stimulates Gi, leading to MAPK activation and cell proliferation.36 The hV2R agonist pharmacochaperones, devoid of arrestin recruitment and acting as noninternalizing ligands, seem to be particularly adapted for the treatment of the cNDI, potentially providing a long-lasting cellular response during drug administration.
Figure 12.
Various transduction pathways induced or inhibited by the biased agonist pharmacochaperones. The nonpeptide pharmacochaperone compounds (MCFs) are able to cross the various cellular membranes and interact with intracellularly ER-retained cNDI-hV2Rs (step 1), enabling their maturation and translocation to the plasma membrane through the ER-Golgi intermediate compartment (Ergic) and the Golgi systems (step 2). Once the cNDI-hV2Rs are at the cell surface, the agonist pharmacochaperones activate the cAMP pathway through interaction between receptors with the Gs protein and stimulation of AC (step 3). Contrary to the hormone AVP, the MCF ligands do not recruit arrestins and are unable to internalize hV2Rs and to activate the MAPK pathway (step 4).
Concise Methods
Cell Culture and Transfection
Human embryonic kidney HEK293 T cells and the SV40-transformed HEK293 cell line tsA201 were used and transfected as described in the Supplemental Concise Methods.
[3H]AVP Binding Assays
[3H]AVP binding to whole cells was performed as follows: 10 × 106 tsA201 cells were electroporated (20 μg of total DNA) with the N-terminally c-myc–tagged receptors hV2R, hV1aR, hV1bR, and hOTR (100, 250, 500, and 6000 ng, respectively) and seeded at a density of 3.3 × 105 cells per well in 24-well plates pretreated with polyornithine. Forty-eight hours after transfection, the plates were placed on ice and cells were washed three times with 250 μl of ice-cold buffer A (PBS [pH 7.4], 5 mM MgCl2, 1 mg/ml BSA, and 0.1 mM phenylalanine). For competition studies, cells were transfected with the receptors and incubated with buffer A containing approximately 1.5 nM [3H]AVP and increasing concentrations (10−10 to 10−5 M) of the various compounds (MCF14, MCF18, and MCF57). An excess of unlabeled AVP (1 μM) was also added in the medium to determine the nonspecific binding. All of the determinations were made in the presence of 0.1% DMSO. After four washings with ice-cold buffer A, the cells were lysed for 15 min at 37°C with 400 μl of 0.1 M NaOH. Then 100 μl of 0.4 M acetic acid was added. The lysates were transferred into scintillation vials, and their radioactivity was determined. The specific binding was calculated as the difference between the total binding and the nonspecific binding and expressed as percentage of maximum [3H]AVP binding in control conditions. The ligand-binding data were analyzed by nonlinear least-squares regression using the software Ligand (Elsevier Biosoft, Cambridge, UK) and GraphPad Prism (GraphPad Software, Inc., San Diego, CA).
cAMP Accumulation Assays
For measuring the cAMP accumulation triggered by each nonpeptide compound (Gs/AC agonist activity), tsA201 cells were electroporated with 100 ng of the PRK5-c-myc-hV2R plasmid and seeded at a density of 75 × 103 cells per well into 96-well plates pretreated with polyornithine. Forty-eight hours after transfection, cells were treated for 30 min at 37°C in the cAMP buffer with or without increasing compound concentrations (10−12 to 10−5 M) in the presence of the phosphodiesterase inhibitor RO 201724 0.1 mM (Sigma). The cAMP production for each condition was expressed in nM and compared with the maximal AVP response obtained under the same conditions.
For the evaluation of the functional activity of cNDI rescued receptors by nonpeptide pharmacochaperones, tsA201 cells were transfected with 50 ng of DNA plasmids and seeded at a density of 50 × 103 cells per well into polyornithine-treated Costar 96-well plates. Twenty-four hours after transfection, cells were treated with 10−5 M MCF compounds in serum-free DMEM (final DMSO concentration of 0.1%). Eighteen hours later, cells were washed for 2 min three times with PBS 1× at 37°C. Then, cells were stimulated for 30 min at 37°C with AVP in the cAMP buffer (serum-free DMEM, 0.5% BSA) supplemented with 0.1 mM RO 201724. For evaluation of the direct agonist effect of nonpeptide compounds on cNDI receptors, tsA201 cells were treated with 10−5 M MCF compounds in serum-free DMEM (final DMSO concentration of 0.1%). Eighteen hours later, the medium was supplemented with RO 201724 0.1 mM, the concentration of the MCF being constant at 10−5 M. Then, cells were incubated for 30 min at 37°C.
For both types of experiments, the accumulated cAMP was quantified using the cAMP Dynamic 2 Kit from Cisbio Int. (Bagnols-sur-Cèze, France) based on a homogeneous time-resolved fluorescence-fluorescence resonance energy transfer technology.37 The principle of the kit is described in the Supplemental Concise Methods.
ELISA Experiments
TsA201 cells were electroporated as described already with 100 and 250 ng of DNA plasmids coding for N-terminally c-myc–tagged hV2R and cNDI receptors, respectively, and seeded at a density of 75 × 103 per well in polyornithinilated Greiner 96-well plates. Twenty-four hours after transfection, cells were treated with MCF compounds (10 μM in 0.1% DMSO) in serum-free DMEM or with 0.1% DMSO at 37°C. All subsequent steps were done at room temperature. Eighteen hours later, cells were fixed for 5 min with 4% formaldehyde diluted in PBS 1× and then washed with PBS 1×. Cells were blocked for 30 min with PBS 1× containing 1% FCS. Then, anti–c-myc antibody (9E10; 1 μg/ml) was added for 30 min. After blocking buffer washes, anti-mouse horseradish peroxidase (HRP)-coupled secondary antibody (0.55 μg/ml) was added for 30 min. After blocking buffer and PBS washes, the Super signal reagent was added and luminescence was recorded using the ANALYST apparatus. The cell surface receptor expression was expressed as a percentage of c-myc-hV2R expression (maximal expression) in the same cells.
Immunocytochemistry
TsA201 cells were seeded on polyornithine-precoated glass coverslips at a density of 1.5 × 105 cells per 18-mm dish. Twenty-four hours after transfection, the medium was removed and replaced by serum-free medium containing compounds at 10 μM in 0.1% DMSO. Control cells were incubated at 37°C in medium containing only 0.1% DMSO. Eighteen hours later, cells were first rinsed once with PBS at room temperature. All subsequent steps were done at room temperature. The cells were fixed for 5 min in 4% paraformaldehyde-PBS and rinsed once rapidly with PBS and twice again with PBS (10 min each time). Then, cells were washed twice with PBS-BSA 1% (blocking buffer) for 10 min and incubated for 2 h with the rabbit anti–c-myc antibody (A14) at 1 μg/ml. Then, the cells were washed twice with blocking buffer, and Cy3-labeled secondary anti-rabbit antibody (0.62 10−10 g/ml) was added for 1 h. The cells were rinsed twice with PBS and H2O. Slides were mounted in Fluomount (Interchim), and images were collected on a Bio-Rad fluorescence confocal microscope.
Immunoprecipitation and Analysis of Glycosylation Pattern of the Receptors
After transfection of the cells with 250 ng of c-myc–tagged L44P, A294P, and R337X or 100 ng of c-myc-hV2R, tsA201 cells were seeded at a density of 15 × 106 cells per 150-mm dish with complete medium. Twenty-four hours after transfection, the medium was removed and replaced by serum-free medium without (control conditions containing only 0.1% DMSO) or with either MCF14 or SR121463 at 10 μM in 0.1% DMSO. Eighteen hours later, cells were rinsed twice with PBS at 4°C and resuspended into lysis buffer (50 mM Tris-HCl [pH 7.4], 1 mM EDTA, 200 mM NaCl, 1 mM Na3VO4, 1% Triton X-100, 10 μg/ml leupeptin, and 10 μg/ml aprotinin) and mixed at 4°C for 30 min. Then the lysate was centrifuged at 21,000 × g to eliminate insoluble cell fragments, and the protein concentration was measured into the supernatant. C-myc antibodies (4 μg) were covalently linked to agarose-protein G (50 μl) using 20 mM dimethyl-pimelimidate reagent (Sigma-Aldrich, St. Louis, MO). After clearing, protein lysate (1 mg/ml) was incubated overnight at 4°C with the c-myc–coupled agarose beads. Immune complexes were collected by centrifugation and washed twice with washing buffer (50 mM Tris-HCl [pH 7.4], 1 mM EDTA, 0.1% Triton X-100, 1 mM Na3VO4, 10 μg/ml leupeptin, and 10 μg/ml aprotinin). The beads were divided into two fractions, and 100 μl of washing buffer containing or not 12.5 mU of EndoH was added to the beads and mixed for 3 h at 37°C. Beads were then centrifuged at 10,000 × g, and 100 μl of 2× Laemmli was added before Western blotting analysis.
Protein samples (30 μl) separated using 12% SDS/PAGE were blotted onto nitrocellulose membranes (Hybond-C; Amersham Biosciences, Buckinghamshire, UK). Nitrocellulose membranes were incubated overnight at 4°C with 5 μg/ml c-myc antibodies. HRP-labeled secondary anti-mouse (from sheep) antibodies (Amersham Biosciences) at 0.22 μg/ml were then used for primary antibody detection. Immunoreactivity was detected using an enhanced chemiluminescence method according to manufacturer's instructions (ECL detection reagent; Amersham Biosciences).
BRET Measurements
TsA201 cells (5 × 106) were electroporated with 5 μg of DNA: 150 ng of hV2R-Rluc and 1 μg of β-arrestin 1–YFP or 800 ng of hV2R-Rluc and 1.8 μg of β-arrestin 2–YFP and empty vector. A total of 2.5 × 106 cells from the electroporation step were subsequently seeded per well of a six-well plate. Forty-eight hours after transfection, the cells were washed twice with KREBS buffer (146 mM NaCl, 4.2 mM KCl, 0.5 mM MgCl2, 1 mM CaCl2, 10 mM HEPES [pH 7.4], and 1 mg/ml glucose) and resuspended in 1 ml of KREBS. BRET measurements were performed into Greiner 96-well plates with a final volume of 50 μl using 30 μl of cellular suspension corresponding to approximately 75,000 cells with 10 μl of the Rluc substrate Coelenterazine-h (final concentration 5 μM) and 10 μl of KREBS buffer containing 5× DMSO, AVP, MCF, or AVP and MCF mixed together (to determine the antagonist effect on arrestin mobilization). The mix suspension was incubated at 37°C, and BRET measurements were acquired on a BertholdTech Mithras LB940 using the MikroWin software. The BRET signal corresponded to the ratio between the light emitted at 530 nm (EYFP) and the light emitted at 480 nm (RLuc) from cells transfected with V2R-Rluc and β-arrestin 1–YFP (or β-arrestin 2–YFP) corrected for the background signal that corresponds to the same ratio from cells transfected with the receptor alone (V2R-Rluc). The BRET net signal was calculated from the difference between the corrected ratio 530 nm/480 nm of agonist-treated cells and vehicle-treated cells. All compounds were diluted in KREBS with a final DMSO concentration of 0.1% as in control conditions.
ERK Activation and Immunoblotting
HEK 293T cells stably expressing c-myc-hV2R were seeded at the density of 150 × 103 in 12-well plates coated with polyornithine into DMEM containing 10% FCS. Approximately 24 h later, cells were treated overnight with serum-free medium. Two hours before ligand stimulation, the medium was replaced with fresh serum-free medium. Cells were then incubated for 3 min at 37°C in serum-free DMEM without (control) or with 10−7 M AVP (maximal effect) or 10−5 M MCF14, MCF18, MCF57, or AVP and MCF mixed together (antagonist effects). Then, the cells were washed twice with cold PBS 1× and lysed using Laemmli buffer 2×. Protein samples were separated onto 12% SDS/polyacrylamide gels and blotted onto nitrocellulose membranes (Hybond-C; Amersham Pharmacia Biotech). The membranes were incubated overnight at 4°C with rabbit anti–p-ERK1/2 primary antibodies (0.4 μg/ml). An anti-rabbit HRP-conjugated antibody (0.1 μg/ml) was added to reveal p-ERK. After stripping of the membranes, total ERK proteins were detected by using a concentration of 0.2 μg/ml rabbit anti-ERK2 antibodies and anti-rabbit HRP-conjugated secondary antibodies (0.1 μg/ml). p-ERK1/2 and ERK2 were visualized using the enhanced chemiluminescence reagent ECL.
Statistical Analysis
Results are reported as group means ± SEM from at least three independent experiments, each performed in duplicate or triplicate. The t test gave us the statistical significance of differences between independent groups with: P < 0.05, P < 0.01, and P < 0.001.
Disclosures
None.
Acknowledgments
This work was supported by CNRS and INSERM and was also made possible by the pharmacology screening platform of Montpellier for luminescence and fluorescence measurements. We thank the Fondation pour la Recherche Médicale for providing financial support to F.J.A. and the Ministère de l'Enseignement Supérieur et de la Recherche for supporting M.-C.F. and S.P.
We thank Dr. Peter Deen (Radboud University Nijmegen Medical Centre, Nijmegen, Netherlands) for providing the plasmid pEGF-N1-L44P-hV2R. We are grateful to Nicole Lautredou-Audouy from the MRI platform for confocal microscopy analysis. Thanks to Dr. Jean-Philippe Pin for critically reading the manuscript, Dr. Mohammed Akli Ayoub for introducing us to BRET measurements, and Muriel Asari-Gien for iconography. We also thank Laure Boudier for her participation in the characterization of the ligands.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.Birnbaumer M: Vasopressin receptors. Trends Endocrinol Metab 11: 406–410, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Robben JH, Knoers NV, Deen PM: Regulation of the vasopressin V2 receptor by vasopressin in polarized renal collecting duct cells. Mol Biol Cell 15: 5693–5699, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thibonnier M, Conarty DM, Preston JA, Wilkins PL, Berti-Mattera LN, Mattera R: Molecular pharmacology of human vasopressin receptors. Adv Exp Med Biol 449: 251–276, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Morello JP, Bichet DG: Nephrogenic diabetes insipidus. Annu Rev Physiol 63: 607–630, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Tsukaguchi H, Matsubara H, Taketani S, Mori Y, Seido T, Inada M: Binding-, intracellular transport-, and biosynthesis-defective mutants of vasopressin type 2 receptor in patients with X-linked nephrogenic diabetes insipidus. J Clin Invest 96: 2043–2050, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen FE, Kelly JW: Therapeutic approaches to protein-misfolding diseases. Nature 426: 905–909, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Chaudhuri TK, Paul S: Protein-misfolding diseases and chaperone-based therapeutic approaches. FEBS J 273: 1331–1349, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Bernier V, Bichet DG, Bouvier M: Pharmacological chaperone action on G-protein-coupled receptors. Curr Opin Pharmacol 4: 528–533, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Ulloa-Aguirre A, Janovick JA, Brothers SP, Conn PM: Pharmacologic rescue of conformationally-defective proteins: Implications for the treatment of human disease. Traffic 5: 821–837, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Galandrin S, Oligny-Longpre G, Bouvier M: The evasive nature of drug efficacy: Implications for drug discovery. Trends Pharmacol Sci 28: 423–430, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Morello JP, Salahpour A, Laperriere A, Bernier V, Arthus MF, Lonergan M, Petaja-Repo U, Angers S, Morin D, Bichet DG, Bouvier M: Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J Clin Invest 105: 887–895, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robben JH, Sze M, Knoers NV, Deen PM: Functional rescue of vasopressin V2 receptor mutants in MDCK cells by pharmacochaperones: Relevance to therapy of nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 292: F253–F260, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Bernier V, Morello JP, Zarruk A, Debrand N, Salahpour A, Lonergan M, Arthus MF, Laperriere A, Brouard R, Bouvier M, Bichet DG: Pharmacologic chaperones as a potential treatment for X-linked nephrogenic diabetes insipidus. J Am Soc Nephrol 17: 232–243, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Caggiano TJ: WAY-VNA-932: Vasopressin V2 agonist for treatment of central diabetes insipidus, nocturnal enuresis, nocturia. Drug Future 27: 248–253, 2002 [Google Scholar]
- 15.Kondo K, Ogawa H, Shinohara T, Kurimura M, Tanada Y, Kan K, Yamashita H, Nakamura S, Hirano T, Yamamura Y, Mori T, Tominaga M, Itai A: Novel design of nonpeptide AVP V(2) receptor agonists: Structural requirements for an agonist having 1-(4-aminobenzoyl)-2,3,4,5-tetrahydro-1H-1-benzazepine as a template. J Med Chem 43: 4388–4397, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Robben JH, Knoers NV, Deen PM: Characterization of vasopressin V2 receptor mutants in nephrogenic diabetes insipidus in a polarized cell model. Am J Physiol Renal Physiol 289: F265–F272, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Oksche A, Leder G, Valet S, Platzer M, Hasse K, Geist S, Krause G, Rosenthal A, Rosenthal W: Variant amino acids in the extracellular loops of murine and human vasopressin V2 receptors account for differences in cell surface expression and ligand affinity. Mol Endocrinol 16: 799–813, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Wuller S, Wiesner B, Loffler A, Furkert J, Krause G, Hermosilla R, Schaefer M, Schulein R, Rosenthal W, Oksche A: Pharmacochaperones post-translationally enhance cell surface expression by increasing conformational stability of wild-type and mutant vasopressin V2 receptors. J Biol Chem 279: 47254–47263, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Robben JH, Sze M, Knoers NV, Deen PM: Rescue of vasopressin V2 receptor mutants by chemical chaperones: Specificity and mechanism. Mol Biol Cell 17: 379–386, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan CM, Nickols HH, Limbird LE: Appropriate polarization following pharmacological rescue of V2 vasopressin receptors encoded by X-linked nephrogenic diabetes insipidus alleles involves a conformation of the receptor that also attains mature glycosylation. J Biol Chem 278: 35678–35686, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Azzi M, Charest PG, Angers S, Rousseau G, Kohout T, Bouvier M, Pineyro G: Beta-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc Natl Acad Sci U S A 100: 11406–11411, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charest PG, Bouvier M: Palmitoylation of the V2 vasopressin receptor carboxyl tail enhances beta-arrestin recruitment leading to efficient receptor endocytosis and ERK1/2 activation. J Biol Chem 278: 41541–41551, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Charest PG, Oligny-Longpre G, Bonin H, Azzi M, Bouvier M: The V2 vasopressin receptor stimulates ERK1/2 activity independently of heterotrimeric G protein signaling. Cell Signal 19: 32–41, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Shayman JA: Thinking about rare kidney diseases. J Am Soc Nephrol 17: 15–16, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Oueslati M, Hermosilla R, Schonenberger E, Oorschot V, Beyermann M, Wiesner B, Schmidt A, Klumperman J, Rosenthal W, Schulein R: Rescue of a nephrogenic diabetes insipidus-causing vasopressin V2 receptor mutant by cell-penetrating peptides. J Biol Chem 282: 20676–20685, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Saliba RS, Munro PM, Luthert PJ, Cheetham ME: The cellular fate of mutant rhodopsin: quality control, degradation and aggresome formation. J Cell Sci 115: 2907–2918, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Janovick JA, Maya-Nunez G, Conn PM: Rescue of hypogonadotropic hypogonadism-causing and manufactured GnRH receptor mutants by a specific protein-folding template: misrouted proteins as a novel disease etiology and therapeutic target. J Clin Endocrinol Metab 87: 3255–3262, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Petaja-Repo UE, Hogue M, Bhalla S, Laperriere A, Morello JP, Bouvier M: Ligands act as pharmacological chaperones and increase the efficiency of delta opioid receptor maturation. EMBO J 21: 1628–1637, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernier V, Lagace M, Lonergan M, Arthus MF, Bichet DG, Bouvier M: Functional rescue of the constitutively internalized V2 vasopressin receptor mutant R137H by the pharmacological chaperone action of SR49059. Mol Endocrinol 18: 2074–2084, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Robben JH, Knoers NV, Deen PM: Cell biological aspects of the vasopressin type-2 receptor and aquaporin 2 water channel in nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 291: F257–F270, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Charest PG, Terrillon S, Bouvier M: Monitoring agonist-promoted conformational changes of beta-arrestin in living cells by intramolecular BRET. EMBO Rep 6: 334–340, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren XR, Reiter E, Ahn S, Kim J, Chen W, Lefkowitz RJ: Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci U S A 102: 1448–1453, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shenoy SK, Lefkowitz RJ: Trafficking patterns of beta-arrestin and G protein-coupled receptors determined by the kinetics of beta-arrestin deubiquitination. J Biol Chem 278: 14498–14506, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, Bohn LM: An opioid agonist that does not induce μ-opioid receptor-arrestin interactions or receptor internalization. Mol Pharmacol 71: 549–557, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engstrom M, Savola JM, Wurster S: Differential efficacies of somatostatin receptor agonists for G-protein activation and desensitization of somatostatin receptor subtype 4-mediated responses. J Pharmacol Exp Ther 316: 1262–1268, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Reversi A, Rimoldi V, Marrocco T, Cassoni P, Bussolati G, Parenti M, Chini B: The oxytocin receptor antagonist atosiban inhibits cell growth via a “biased agonist” mechanism. J Biol Chem 280: 16311–16318, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Maurel D, Kniazeff J, Mathis G, Trinquet E, Pin JP, Ansanay H: Cell surface detection of membrane protein interaction with homogeneous time-resolved fluorescence resonance energy transfer technology. Anal Biochem 329: 253–262, 2004 [DOI] [PubMed] [Google Scholar]