Abstract
The accumulation of extracellular matrix proteins is a common feature of fibrotic kidney diseases. Accumulating evidence suggests that TGF-β and plasminogen activator inhibitor type 1 (PAI-1) promote the development of renal fibrosis by stimulating the generation and inhibiting the removal of matrix proteins. The small heterodimer partner (SHP) represses PAI-1 expression in the liver by inhibiting TGF-β signaling, but whether SHP inhibits renal fibrosis is unknown. Here, unilateral ureteral obstruction (UUO) markedly increased the expression of PAI-1, type I collagen, and fibronectin but decreased SHP gene expression. Moreover, in kidneys of SHP−/− mice, the expression of PAI-1, type I collagen, fibronectin and α-smooth muscle actin (α-SMA) were higher compared with those in kidneys of wild-type mice. In addition, loss of SHP accelerated renal fibrosis after UUO. Adenovirus-mediated overexpression of SHP in cultured rat mesangial cells and renal tubular epithelial cells inhibited TGF-β-stimulated expression of PAI-1, type I collagen, and fibronectin. SHP inhibited TGF-β- and Smad3-stimulated PAI-1 promoter activities as well as TGF-β-stimulated binding of Smad3 to its consensus response element on the PAI-1 promoter. Similarly, in vivo, adenovirus-mediated overexpression of SHP in the kidney inhibited the expression of UUO-induced PAI-1, type I collagen, fibronectin, and α-SMA. In summary, SHP attenuates renal fibrosis in obstructive nephropathy, making its pathway a possible therapeutic target for chronic kidney disease.
The accumulation of extracellular matrix (ECM) proteins is the key feature of chronic fibrotic kidney disease. TGF-β is central to the development of renal fibrosis through its stimulating effect on matrix protein generation and its inhibitory effect on matrix protein removal.1,2 Expression of TGF-β is elevated in multiple forms of experimental and human kidney disease, ranging from diabetic nephropathy and GN to tubulointerstitial nephritis.3–6 Overexpression of TGF-β was reported in experimental GN and the progression of the glomerular disease.7–9 In the postobstructed kidney, TGF-β expression was increased to induce the transcription of genes involved in ECM protein accumulation, including type I collagen and fibronectin.9 In addition, TGF-β stabilizes ECM proteins by stimulating the expression of protease inhibitors, including plasminogen activator inhibitor 1 (PAI-1). Thus, suppression of TGF-β signaling has been included in several therapeutic approaches for preventing renal fibrosis.10,11
PAI-1 is the main physiologic inhibitor of the tissue and urokinase plasminogen activator and is considered to be the most important inhibitor of fibrinolysis.12 However, plasminogen inhibition does not explain the action of PAI-1 in renal fibrosis.13,14 Recent studies suggest that PAI-1 directly promotes tissue fibrosis by promoting the migration of monocytes/macrophages, transdifferentiated tubular epithelia, and myofibroblasts.15–17 It has been implicated in experimental GN,18 chronic renal transplant rejection,19 and pulmonary fibrosis.20 Transgenic mice overexpressing PAI-1 develop significantly greater pulmonary fibrosis when administered bleomycin, whereas similarly treated PAI-1-deficient mice have substantially less fibrosis compared with wild-type mice.21 PAI-1 deficiency also protects against renal interstitial fibrosis induced by unilateral ureteral obstruction (UUO).16
Orphan nuclear receptor small heterodimer partner (SHP) is an atypical member of the orphan nuclear receptor superfamily because it lacks a conventional DNA-binding domain.22 It is a transcriptional repressor and exerts its regulatory functions through protein-protein interactions with other nuclear hormone receptors and possibly other transcription factors that can inhibit or even reverse their transactivation.23 A recent study has shown that the loss of SHP sensitizes mice to liver injury from obstructive cholestasis.24 Previously, we demonstrated that SHP represses hepatic PAI-1 expression by inhibiting TGF-β signaling through the repression of transactivation by Smad3.25 Moreover, Fiorucci et al. have reported that SHP attenuates liver fibrosis by inhibition of hepatic stellate cells by the farnesoid X receptor.26 These studies suggest that direct targeting of SHP may provide promising prospects for the prevention of fibrotic disease; however, the clinical significance of SHP in fibrotic kidney disease remains to be determined. Here, we examined whether SHP plays an important role in renal fibrosis in the UUO model and whether upregulation of SHP prevents renal fibrosis.
Results
SHP Expression in UUO Kidney Is Downregulated and Loss of SHP Increases PAI-1 Expression, ECM Protein Expression, and Renal Fibrosis after UUO
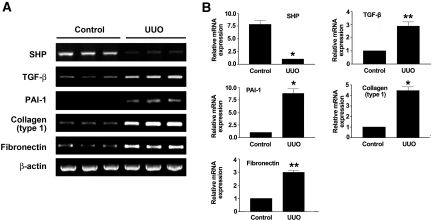
We first examined whether the expression levels of SHP in kidney are altered by UUO. SHP expression in the kidney was examined by β-galactosidase staining (Supplemental Figure 1). A drastic increase in TGF-β expression is a key feature of the UUO kidney. Levels of TGF-β mRNA expression in UUO kidneys were increased at day 7 after ureteral ligation compared with sham-operated animals. As expected, the expression of TGF-β target fibrotic genes including PAI-1, type I collagen, and fibronectin were increased in the kidneys of rats with UUO compared with those of control kidneys. Interestingly, SHP mRNA expression was abundant in control kidneys, but its expression was markedly decreased in UUO kidneys (Figure 1).
Figure 1.
Relative mRNA expression levels of SHP, TGF-β, PAI-1, type I collagen, and fibronectin in kidneys of rats with UUO. (A) Representative RT-PCR analysis of SHP, TGF-β, PAI-1, type I collagen, and fibronectin expression in UUO kidneys. Rats were killed at 7 d after UUO. RT-PCR was performed on RNA from three different normal rat kidneys or UUO rat kidneys. (B) Quantification of RT-PCR results expressed as the mean ± SEM of three independent experiments (n = 9 in each group). β-actin protein levels were analyzed as an internal control. *P < 0.001 and **P < 0.01 compared with control.
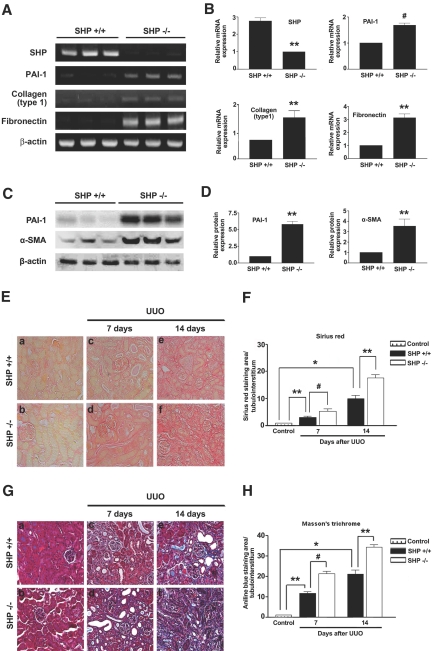
We next examined whether the loss of SHP influences renal expression of PAI-1, type I collagen, fibronectin, and α-SMA as well as renal fibrosis by using SHP−/− mice. Indeed, in the kidneys of SHP−/− mice, PAI-1, type I collagen, and fibronectin mRNA levels were increased compared with those from the kidneys of wild-type mice (Figure 2, A and B). PAI-1 and α-SMA protein expression in the kidneys of SHP−/− mice was examined by Western blot analysis (Figure 2, C and D). Moreover, loss of SHP accelerated renal fibrosis after UUO. On day 7 after UUO, wild-type mice were characterized by widespread renal tubulointerstitial damage and fibrosis, as evidenced by Sirius red and Masson's trichrome staining. In comparison with wild-type mice, the SHP−/− mice exhibited more markedly increased tubulointerstitial damage and fibrosis 7 d after UUO. The differences of tubulointerstitial damage and renal fibrosis between wild-type mice and SHP −/− mice were more evident at day 14 after UUO (Figure 2, E through H). Taken together, these data suggest that SHP plays an important role in ECM accumulation in obstructive nephropathy.
Figure 2.
Effect of the loss of SHP on fibrotic gene expression and renal fibrosis in the UUO model. (A) Representative RT-PCR analysis of PAI-1, type I collagen, and fibronectin expression in kidneys of SHP−/− mice. RT-PCR was performed on RNA from three different wild-type (WT) mouse kidneys or SHP−/− mouse kidneys. (B) Quantification of RT-PCR results expressed as the mean ± SEM of three independent experiments (n = 9 in each group). β-actin mRNA levels were analyzed as an internal control. **P < 0.01 and #P < 0.05 compared with WT mice. (C) Representative Western blot analysis of PAI-1 and α-SMA expression in the kidneys of SHP−/− mice. (D) Quantification of Western blot results expressed as the mean ± SEM of three independent experiments (n = 9 in each group). β-actin mRNA levels were analyzed as an internal control. **P < 0.01 compared with WT mice. (E and G) Representative kidney tissue sections stained with Sirius red and Masson's trichrome. Magnification, ×200. (a) A normal kidney from a WT mouse. (b) A normal kidney from an SHP knockout (KO) mouse. (c) A UUO kidney from WT mouse at 7 d. (d) A UUO kidney from KO mouse at 7 d. (e) A UUO kidney from WT mouse at 14 d. (f) A UUO kidney from KO mouse at 14 d. (F and H) Computer-based morphometric analysis of kidney fibrosis in the UUO model. Data are the mean ± SEM of five independent measurements (n = 5 in each group). **P < 0.01, *P < 0.001, and #P < 0.05.
SHP Inhibits TGF-β-Stimulated PAI-1 and ECM Protein Expression
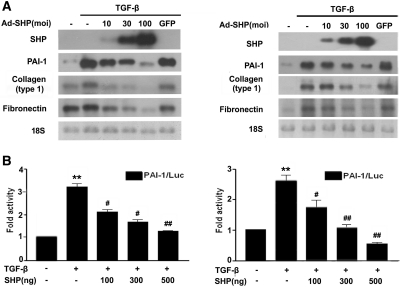
Next, we examined whether SHP inhibits TGF-β-stimulated fibrotic gene expression in cultured renal cells. As shown in Figure 3A, adenovirus-mediated overexpression of SHP in rat mesangial cells (RMCs) and NRK-52E cells inhibited TGF-β-stimulated PAI-1, type I collagen, and fibronectin mRNA expression in a dose-dependent manner (Figure 3A). Transient transfection showed that SHP inhibited the TGF-β-stimulated PAI-1 promoter activity (Figure 3B), suggesting that SHP inhibits fibrotic gene expression through the downregulation of TGF-β-stimulated transcription factor activity.
Figure 3.
Effect of SHP on TGF-β-induced PAI-1, type I collagen, and fibronectin expression in cultured RMCs and NRK-52E cells. (A) Representative Northern blot analysis of the expression of PAI-1, type I collagen, and fibronectin mRNA in TGF-β-stimulated RMCs and NRK-52E cells. Cells were incubated with TGF-β (2 ng/ml) after 24 h serum starvation and then infected with the indicated doses of Ad-SHP. 18S rRNA levels were analyzed as an internal control. (B) Effects of SHP on PAI-1 promoter activity in RMCs and NRK-52E cells. Cells were treated with TGF-β (2 ng/ml) for 3 h with or without pretreatment of the indicated amounts of the SHP expression vector (pcDNA3) for 24 h. Data are the mean ± SEM of three independent measurements. **P < 0.01 compared with control, #P < 0.05, and ##P < 0.01 compared with TGF-β alone.
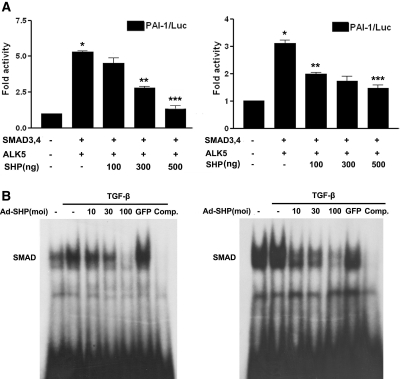
Because Smad3 is a main downstream mediator of TGF-β expression, we examined whether SHP inhibits Smad3 activity. Indeed, RMCs and NRK-52E cells cotransfected with ALK5 and Smad3/4 increased PAI-1 promoter activity, but cotransfection with SHP inhibited this stimulation in a dose-dependent manner (Figure 4A). To test whether SHP reduces Smad's binding to the Smad binding element for the inhibition of TGF-β-stimulated Smad3 activity, gel shift analyses were conducted with the Smad binding element oligonucleotide, multiple copies of which are located in the PAI-1 gene promoter. Smad3 DNA-binding activity was stimulated by TGF-β, and cells infected with adenovirus encoding SHP (Ad-SHP) decreased TGF-β-stimulated Smad3 DNA-binding activity in a dose-dependent manner. The protein-DNA complexes were specific for the Smad sequence because the addition of excess unlabeled Smad binding site oligomer efficiently competed for Smad binding activity (Figure 4B). A supershifted band was observed during an electrophoretic mobility shift assay when the nuclear extract was treated with an antibody against Smad3 (Supplemental Figure 2). Taken together, these data suggest that SHP inhibits fibrotic gene expression through downregulation of the TGF-β/Smad3 signaling pathway.
Figure 4.
Effect of SHP on the TGF-β/Smad3 signaling pathway. (A) Effects of SHP on PAI-1 promoter activity in RMCs and NRK-52E cells. Cells were cotransfected with the PAI-1 promoter and expression vectors for Smad3/4 (pRK5) and ALK5 (pcDNA). Data are the mean ± SEM of three independent measurements. *P < 0.001 compared with control, **P < 0.01, and ***P < 0.001, compared with Smad3/4 and ALK5. (B) Effect of SHP on TGF-β-stimulated Smad DNA-binding activity. Cells were incubated with TGF-β for 30 min and pretreatment of the indicated concentrations of Ad-SHP or Ad-GFP (100 moi) for 24 h.
Adenovirus-Mediated Overexpression of SHP Ameliorates Renal Fibrosis after UUO
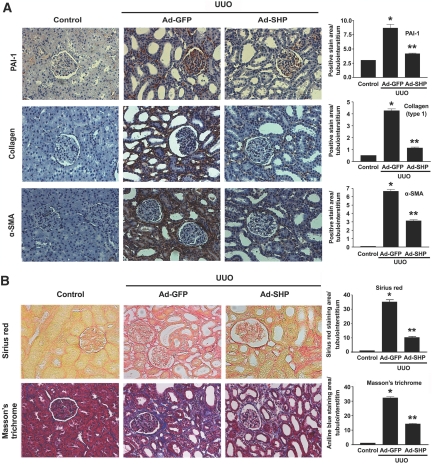
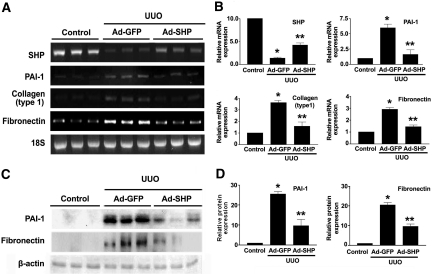
To examine the possible beneficial effects of SHP on UUO-induced renal fibrosis, Ad-SHP or adenovirus encoding green fluorescent protein (Ad-GFP) was infused intrarenally into the left kidneys of rats after ligation of the left ureter. Immunohistochemical staining showed that UUO kidneys were accompanied by increased expression of PAI-1, type I collagen, and α-SMA. In contrast, Ad-SHP-infected UUO kidneys showed decreased expression of all of these proteins (Figure 5A). Moreover, Sirius red and Masson's trichrome staining showed that Ad-SHP markedly prevented UUO-induced renal fibrosis (Figure 5B). The changes in the mRNA expression of fibrotic genes were further examined by reverse transcription (RT)-PCR and Northern blot analysis. In UUO kidneys, SHP mRNA expression was decreased, but PAI-1, type I collagen, and fibronectin gene expression was increased. However, the increase in SHP expression by intrarenal infusion of Ad-SHP decreased UUO-induced renal fibrotic gene expression (Figure 6, A and B). PAI-1 and fibronectin protein expression in response to UUO with or without infection of Ad-SHP was further confirmed by Western blot analysis (Figure 6, C and D). Successful delivery of Ad-SHP was confirmed by immunohistochemical staining (Supplemental Figure 3).
Figure 5.
Effect of SHP on UUO-induced renal interstitial fibrosis. (A) Representative images of immunohistochemistry for PAI-1, type I collagen, and α-SMA expression in UUO kidneys infected with Ad-GFP or Ad-SHP. (B) Sirius red and Masson's trichrome staining in UUO kidneys infected with Ad-GFP or Ad-SHP. Mice were killed 7 d after UUO with Ad-GFP or Ad-SHP. Magnification, ×200. Bar graphs show data obtained by computer-based morphometric analysis on day 7 after UUO. *P < 0.001 compared with control and **P < 0.01 compared with Ad-GFP (n = 5 in each group).
Figure 6.
Effect of SHP on fibrotic gene expression in the UUO model. (A) Representative RT-PCR analysis of PAI-1, type I collagen, and fibronectin expression in UUO kidneys with Ad-GFP or Ad-SHP. Rats were killed at 7 d after UUO. RT-PCR was performed on RNA from three different normal rat kidneys or UUO kidneys with or without Ad-SHP. (B) Quantification of RT-PCR results expressed as the mean ± SEM of three independent experiments (n = 9 in each group). β-actin mRNA levels were analyzed as an internal control. *P < 0.001 compared with control and **P < 0.01 compared with Ad-GFP. (C) Representative Western blot analysis of PAI-1 and fibronectin protein expression in UUO kidneys with Ad-GFP or Ad-SHP. β-actin levels were analyzed as an internal control. (D) Quantification of Western blot results expressed as the mean ± SEM of three independent experiments (n = 9 in each group). *P < 0.001 compared with control and **P < 0.01 compared with Ad-GFP.
Discussion
The study presented here has provided novel insights into the role of SHP in the pathogenesis of renal fibrosis in obstructive nephropathy. We found that the expression of fibrotic genes, including PAI-1, type I collagen, fibronectin, and α-SMA, in rats with complete ureteral obstruction was associated with marked reduction of renal SHP expression. In addition, genetic deficiency of SHP significantly increased the expression of PAI-1 and matrix proteins and accelerated renal fibrosis after UUO. Furthermore, upregulation of SHP by intrarenal infusion of Ad-SHP prevented obstruction-induced PAI-1 and matrix protein expression. SHP appears to downregulate PAI-1 and ECM protein expression via downregulation of TGF-β and the Smad pathway.
TGF-β is increased in human and mouse experimental renal fibrosis models and has been implicated as a major mediator of ECM protein accumulation in diabetic nephropathy and tubulointerstitial fibrosis.3–6 Although several nuclear receptors have been reported to regulate TGF-β signaling,27–31 little is known about the clinical significance of nuclear receptors in fibrotic kidney disease. Here we show the role of orphan nuclear receptor SHP in renal fibrosis induced by complete ureteral obstruction. In this study, we found that in normal conditions, mRNA expression of SHP in kidney is abundant, but PAI-1 and matrix protein gene expression is faint. Permanent obstruction of the ureter leads to increased expression of PAI-1 and the accumulation of ECM proteins in the interstitium; however, the expression of SHP is interestingly decreased after UUO. In addition, the loss of SHP in the kidneys of SHP−/− mice increases PAI-1 and matrix protein expression. Although numerous pathways may induce PAI-1 expression in the UUO kidney, the inverse relationship between SHP expression and fibrotic gene expression suggests that SHP plays, at the least in part, a role in the prevention of fibrotic kidney diseases.
Increased PAI-1 expression has been reported in several progressive renal diseases, including obstructive nephropathy, suggesting a significant functional role for PAI-1 in the development of renal fibrosis.16,18–21,32–34 Oda et al. demonstrated that PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction.16 In addition to its role in mechanical kidney injury, PAI-1 contributes to the development of diabetic nephropathy by regulating TGF-β and ECM production.35 On the basis of these data, we further examined whether upregulation of SHP in experimental renal fibrosis models decreases PAI-1 expression. Indeed, intrarenal delivery of Ad-SHP into UUO kidneys markedly inhibited UUO-induced PAI-1 protein expression. Recent reports have shown that the profibrotic effects of PAI-1 in the tubulointerstitium support its ability to increase the recruitment of inflammatory cells and myofibroblasts and promote the epithelial-to-mesenchymal transition.13,36 Moreover, the study presented here showed that Ad-SHP decreased UUO-induced α-SMA expression, a mesenchymal marker. Therefore, further studies are necessary to elucidate the beneficial effects of SHP on renal fibrosis and to determine whether SHP inhibits proinflammatory cytokine-induced inflammation and epithelial-to-mesenchymal transition.
To elucidate the mechanism by which SHP inhibits PAI-1 and matrix protein expression, we examined the effect of SHP on TGF-β signaling. TGF-β enhances the induction of subsequent downstream genes through Smad signaling, regulates the expression of target genes including PAI-1 and matrix proteins, and subsequently contributes to tubulointerstitial fibrosis.37 Many studies have looked for an improvement of renal fibrosis by the inhibition of TGF-β signaling.11 In this study, we found that adenovirus-mediated overexpression of SHP successfully inhibited TGF-β-stimulated PAI-1 and matrix protein expression in cultured kidney cell lines. Although the mechanism of repression by SHP is not clearly understood, previous reports have suggested that SHP represses transcription factor-mediated transactivation by inhibition of DNA binding,22,38,39 recruitment of unknown corepressors,40–42 and interfering the interaction with coactivator p300.25 In this study, we found that that SHP repressed Smad3 transactivation by inhibition of DNA binding. Taken together, these data suggest that SHP inhibits TGF-β signaling pathway by inhibition of the interaction with coactivator p300 and Smad-DNA binding. However, it is possible that transcriptional inhibition of genes other than Smad, such as redox-sensitive transcription factor AP-1 or the activity of Sp1 on TGF-β target gene promoters, is involved in the prevention of fibrosis by SHP.
In conclusion, this study suggests that downregulated SHP expression in response to ureteral obstruction plays an important role in the pathogenesis of fibrotic renal diseases and that upregulation of SHP in the kidney prevents renal fibrosis in obstructive nephropathy. Our results suggest that an important anti-fibrotic effect of SHP may be a promising therapeutic target for fibrotic renal diseases.
Concise Methods
Materials and Plasmids
Recombinant human TGF-β was purchased from Ramp;D systems (Minneapolis, MN). Anti-PAI-1 antibody was purchased from BD Biosciences (San Jose, CA). Anti-collagen type I antibody and anti-green fluorescent protein (GFP) antibody were purchased from Abcam (Cambridge, U.K.). Anti-α-SMA antibody and anti-actin antibody were purchased from Sigma (St. Louis, MO). Anti-phospho-Smad3 (ser423/425) and anti-Smad3 were purchased from Cell Signaling (Beverly, MA). Radiochemicals ([α-32P]dCTP, [γ-32P]dATP) were purchased from Perkin Elmer (Boston, MA). The pcDNA3HA-ALK5TD plasmids were kind gifts from Dr. Carl-Henrik Heldin (Ludwig Institute for Cancer Research, Sweden). The pRK5-Smad3 and pRK5-Smad4 plasmids were kind gifts from Dr. Rik Derynck (University of California, San Francisco, CA).
Animals
Male 8-wk-old Sprague–Dawley rats weighing 280 g and male 8-wk-old C57BL6 mice were purchased from Samtako (Korea). SHP−/− mice were kind gifts from Dr. David D. Moore (Baylor College of Medicine, Houston, TX). All procedures were performed in accordance with institutional guidelines for animal research.
Cell Culture
RMCs and the NRK-52E rat renal proximal tubular epithelial cell line were purchased from American Type Culture Collection (Manassas, VA). RMCs were cultured in 5% carbon dioxide/95% air at 37°C in DME medium (Life Technologies BRL, Grand Island, NY) containing 4 mmol/L l-glutamine and 25 mmol/l-glucose. The medium was supplemented with 15% FBS and 0.4 mg/ml G418. NRK-52E cells were cultured in 5% carbon dioxide/95% air at 37°C in DME medium containing 4 mmol/L l-glutamine and 25 mmol/l-glucose. The medium was supplemented with 5% FBS. Equal numbers of cells were seeded onto tissue culture plates. RMCs and NRK-52E cells were rendered quiescent by incubation for 24 h in 0.5% serum growth medium. Cells were infected with Ad-SHP in serum-free medium for 2 h and then changed to conditioned medium for 24 h. Cells were subsequently processed for the isolation of RNA, protein, or nuclear protein as described below.
Experimental UUO Animal Model and In Vivo Infection
UUUO surgery was performed as described previously.43 Briefly, after a mid-abdominal incision under anesthesia using pentobarbital (50 mg/kg), the left ureter was ligated with 5.0 silk at two separate points in the UUO groups. A flexible cannula was inserted into the left renal artery via the branch of the femoral artery and then the distal segment of the renal artery was transiently ligated. Adenovirus encoding rat SHP or GFP (3 × 109 plaque forming units/kidney, n = 9/group) was infused into the left kidney and incubated for 5 min. Upon completion, the infusion catheter was removed, blood flow to the kidney was restored by the release of ligatures, and the wound was then closed. Sham-operated rats were used as controls. After 7 d of UUO and adenovirus infection, rats were euthanized and left kidneys were removed, cut in thirds, fixed for 20 h in 4% paraformaldehyde, and embedded in paraffin for histologic examination or frozen in liquid nitrogen for isolation of protein or RNA.
Generation of Recombinant Adenovirus
cDNA encoding full-length rat SHP was inserted into the HindIII/XbaI sites of the shuttle vector pAdTrack-CMV. The vector was then electroporated into BJ5183 cells that contained the adenoviral vector Adeasy to generate a recombinant adenoviral plasmid. Recombinants were amplified in HEK-293 cells and purified by cesium chloride (Sigma) gradient centrifugation. Viral preparations were collected and desalted, and titers were determined using Adeno-X rapid titer (BD Bioscience) according to the manufacturer's instructions. The efficiency of adenoviral infection was assessed using a recombinant adenovirus encoding SHP fused to GFP (data not shown).
RT-PCR
Total RNA was obtained from rat or mouse kidneys using Trizol reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized using a first-strand cDNA kit (Fermentas, Hanover, MD). PCR was carried out using taqpolymerase (Takara, Japan) under the conditions described in Table 1.
Table 1.
Sequences of primers for RT-PCR
| Gene/Primer | Direction | Sequence (5′ to 3′) | Annealing Temperature (Cycle) |
|---|---|---|---|
| Rat | |||
| SHP | Forward | ATG-AGC-TCC-AGC-CAA-TCG-GGG-TC | 60°C (33) |
| Reverse | TCA-CCT-CAA-CAA-AAG-CAT-GTC-TTC-G | ||
| PAI-1 | Forward | AGA-TCA-GTA-CTG-CGG-ACG-CCA-TCT-TTG | 67°C (30) |
| Reverse | GCA-CGG-AGA-TGG-TGC-TAC-CAT-CAG-ACT-TGT | ||
| Mouse | |||
| SHP | Forward | CCG-CAC-CGC-ACC-TGC-AGG-GAG-GCC-TT | 70°C (25) |
| Reverse | ACT-CCA-GGC-AGC-GCT-GCA-GCC-ACT-GAA | ||
| PAI-1 | Forward | CCT-CAT-CCT-GGG-CCT-GGT-TCT-GGT-CT | 67°C (23) |
| Reverse | GGT-TTT-CCC-CGC-TGT-GGT-CAT-CTG-C | ||
| Rat and mouse | |||
| type 1 collagen | Forward | GAG-CGG-AGA-GTA-CTG-GTA-CG | 53°C (25) and 53°C (25) |
| Reverse | TAC-TCG-AAC-GGG-AAT-CCA-TC | ||
| fibronectin | Forward | ACA-CGG-TTT-CCC-ATT-ACG-CCA-T | 55°C (35) and 57°C (25) |
| Reverse | AAT-GAC-CAC-TGC-CAA-AGC-CCA-A |
Northern Blot Analysis
Total RNA was isolated from cells or kidneys using Trireagent (Invitrogen) according to the manufacturer's instructions. The probes for SHP, PAI-1, type I collagen, and fibronectin were labeled with [α-32P]dCTP using a random-primer DNA labeling system (Amersham Bioscience, Buckinghamshire, U.K.).
Western Blot Analysis
Cells were washed twice with PBS and suspended in IPH lysis buffer [50 mM Tris (pH 8.0), 150 mM sodium chloride, 5 mM EDTA, 0.5% NP40, 100 mM phenylmethylsulphonyl fluoride, leupeptin 1 mg/ml, apronitin 1 mg/ml, 1 M dithiothreitol]. Cells were extracted on ice for 30 min. After centrifugation at 12,000 rpm for 10 min, the supernatant was obtained as a cell lysate. Protein quantification was performed using a Bio-Rad protein assay system (Bio-Rad, Richmond, CA). Cell lysates were electrophoresed by SDS-PAGE and electrotransferred to polyvinylidene fluoride membranes (Millipore Corporation, Bedford, MA). The membrane was blocked by incubation in blocking buffer; allowed to react with anti-PAI-1, α-SMA, and fibronectin polyclonal antibodies; and washed. Antibodies were detected by horseradish peroxidase-linked secondary antibody using the enhanced luminol-based chemiluminescent Western blotting detection system, as specified by the manufacturer (Amersham). The membrane was reblotted with anti-actin antibody to verify equal loading of protein in each lane. Densitometric measurements of the bands were made using the digitalized scientific program UN-SCAN-IT (Silk Scientific Corporation, Orem, UT).
In Vitro Transient Transfection and Reporter Assay
RMCs and NRK-52E cells were plated at a density of 1 × 105 cells per well in a 12-well plate and cultured for 1 d in culture medium. Cells were transiently transfected with the p800neo-luc (−800 PAI-1 promoter, 300 ng/well)44 and other DNAs using Lipofectamine 2000 transfection reagent (Invitrogen). Cells were cotransfected with a plasmid encoding β-galactosidase as an internal control. Cells were transfected for 4 h, washed to remove plasmids, and then cultured in conditioned medium. Cells were harvested approximately 24 h after transfection for luciferase and β-galactosidase assays. We analyzed 20 μl of cell lysate using a luciferase assay system according to the manufacturer's instruction (Promega, Madison, WI). Luciferase activity was detected using a SIRUS luminometer (Berthold, Germany), and luciferase activity was normalized to β-galactosidase activity.
Electrophoretic Mobility Shift Assay
Nuclear extracts were isolated from cells using the NucBuster protein extraction kit (Calbiochem, LA Jolla, CA) according to the manufacturer's instructions. After centrifugation, the supernatant (nuclear extract) was collected and the protein concentration was measured using a protein assay kit (Biorad). For supershift analysis, the nuclear extracts were incubated with Smad3 antibody (Cell Signaling) and labeled probe. Nuclear extracts (6 μg) were incubated with approximately 60,000 cpm of a 32P-labeled Smad-binding-site oligomer, 5′-GGG-AGA-GAC-AGA-CAC-AGG-CAG-3′.
Histologic Analysis
Kidneys were fixed with PBS containing 4% paraformaldehyde and then embedded in paraffin; sections (4 μm) were used in immunohistochemical staining, Masson's trichrome, and Sirius red staining. Immunohistochemical staining was performed using anti-PAI-1, type I collagen, and α-SMA primary antibodies and horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG secondary antibodies (Dako, Glostrup, Denmark), according to the manufacturer's instructions. Kidney sections were deparaffinized in xylene and rehydrated through graded ethanol and then stained with Masson's trichrome or Sirius red staining to evaluate interstitial collagen deposition. For Masson's trichrome staining, after mordanting 1 h in Bouin's solution, kidney sections were treated sequentially with hematoxylin for 10 min, Biebrich scarlet-acid fuchsin for 5 min, phosphotungstic acid/phosphomolybdic acid for 10 min, and aniline blue for 15 min. Tissue was destained in 1% acetic acid for 5 min, dehydrated through graded ethanol to xylene, and mounted for examination by light microscopy. For Sirius red staining, slides were stained for 18 h in saturated picric acid with 0.1% Sirius red F3BA (Aldrich Chemicals). The next morning slides were removed, washed in 0.01 N hydrochloric acid for 2 min, and rapidly dehydrated through graded alcohols starting at 70%, then to xylene, and finally cover slipped in Permount. Renal fibrotic areas were quantified by morphometric analysis using a light microscope equipped with an imaging system containing an MRc5 Carl Zeiss microscope (Thornwood, NY) and iSolution DT Ver7.7 (IMT i-Solution, Coquitlam, Canada). The quantification of aniline-blue-positive areas (fibrotic areas) and positive areas of immunostaining for PAI-1, collagen (type 1), and α-SMA for each antibody in the renal fibrotic regions (brown color) were evaluated by computer-based morphometric analysis.
Determination of β-galactosidase Activity in Tissue Samples
Cryostat sections (5 μm) of the kidney were mounted onto glass slides and fixed in 0.5% glutaraldehyde at room temperature for 15 min, washed with PBS, and stained for 14 h at 37°C with fresh 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (Promega) staining solution: 1 mg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal), 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 150 mM sodium chloride, and 2 mM magnesium chloride in 40 mM citric acid/sodium phosphate, pH 6.0. Tissue sections were examined after incubation for 14 h, counterstained with eosin, and mounted for microscopic evaluation.
Statistical Analysis
Data were evaluated using ANOVA followed by a post hoc least significant difference test, and expressed as means ± SEM. Values of P < 0.05 were considered statistically significant. All experiments were performed at least three times.
Disclosures
None.
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation funded by the Ministry of Education, Science and Technology (NRL program M10642140004-06N4214-0040 to K.-U.L. and M10600000271-06J0000-27110 to I.-K.L.), and the Korea Research Foundation Grant founded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2007-313-E00231 to K.-G.P. and F01-2007-000-10182-0 to I.-K.L.) and the Grant of the Korean Ministry of Education, Science and Technology (The Regional Core Research Program/Medical Convergence Technology Development Consortium for Anti-aging and Well-being).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Ha H, Lee HB: Reactive oxygen species and matrix remodeling in diabetic kidney. J Am Soc Nephrol 14: S246–S249, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Baricos WH, Cortez SL, el-Dahr SS, Schnaper HW: ECM degradation by cultured human mesangial cells is mediated by a PA/plasmin/MMP-2 cascade. Kidney Int 47: 1039–1047, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA: Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci U S A 90: 1814–1818, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakamura T, Ebihara I, Fukui M, Osada S, Nagaoka I, Horikoshi S, Tomino Y, Koide H: Messenger RNA expression for growth factors in glomeruli from focal glomerular sclerosis. Clin Immunol Immunopathol 66: 33–42, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Yoshioka K, Takemura T, Murakami K, Okada M, Hino S, Miyamoto H, Maki S: Transforming growth factor-beta protein and mRNA in glomeruli in normal and diseased human kidneys. Lab Invest 68: 154–163, 1993 [PubMed] [Google Scholar]
- 6.Cheng J, Grand JP: Transforming growth factor-beta signal transduction and progressive renal disease. Exp Biol Med 227: 943–956, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto T, Noble NA, Cohen AH, Nast CC, Hishida A, Gold LI, Border WA: Expression of transforming growth factor-β isoforms in human glomerular diseases. Kidney Int 49: 461–469, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Niemer , Stein H., Noronha I.: PDGF and TGF-beta contribute to the natural course of human IgA glomerulonephritis. Kidney Int 48: 1530–1541, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Wright EJ, McCaffrey TA, Robertson AP, Vaughan ED, Jr, Felsen D: Chronic unilateral ureteral obstruction is associated with interstitial fibrosis and tubular expression of transforming growth factor-beta. Lab Invest 74: 528–537, 1996 [PubMed] [Google Scholar]
- 10.Zhou A, Ueno H, Shimomura M, Tanaka R, Shirakawa T, Nakamura H, Matsuo M, Iijima K: Blockade of TGF-beta action ameliorates renal dysfunction and histologic progression in anti-GBM nephritis. Kidney Int 64: 92–101, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Hwang M, Kim HJ, Noh HJ, Chang YC, Chae YM, Kim KH, Jeon JP, Lee TS, Oh HK, Lee YS, Park KK: TGF-beta 1 siRNA suppresses the tubulointerstitial fibrosis in the kidney of ureteral obstruction. Exp Mol Pathol 41: 48–54, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Binder BR, Christ G, Gruber F, Grubic N, Hufnagl P, Krebs M, Mihaly J, Prager GW: Plasminogen activator inhibitor 1: Physiological and pathophysiological roles. News Physiol Sci 17: 56–61, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Zhang G, Kernan KA, Collins SJ, Cai X, López-Guisa JM, Degen JL, Shvil Y, Eddy AA: Plasmin(ogen) promotes renal interstitial fibrosis by promoting epithelial-to-mesenchymal transition: Role of plasmin-activated signals. J Am Soc Nephrol 18: 846–859, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Shultz RW, Mars WM, Wegner RE, Li Y, Dai C, Nejak K, Liu Y: Disruption of tissue-type plasminogen activator gene in mice reduces renal interstitial fibrosis in obstructive nephropathy. J Clin Invest 110: 1525–1538, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y, Haraguchi M, Lawrence DA, Border WA, Yu L, Noble NA: A mutant, noninhibitory plasminogen activator inhibitor type 1 decreases matrix accumulation in experimental glomerulonephritis. J Clin Invest 112: 379–388, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oda T, Jung YO, Kim H, Cai X, Lopez-Guisa J, Ikeda Y, Eddy AA: PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction. Kidney Int 30: 587–596, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Matsuo S, Lopez-Guisa JM, Cai X, Okamura DM, Alpers CE, Bumgarner RE, Peters MA, Zhang G, Eddy AA: Multifunctionality of PAI-1 in fibrogenesis: Evidence from obstructive nephropathy in PAI-1-overexpressing mice. Kidney Int 67: 2221–2238, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Tomooka S, Border WA, Marshall BC, Noble NA: Glomerular matrix accumulation is linked to inhibition of the plasmin protease system. Kidney Int 42: 1462–1469, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Tang WH, Friess H, di Mola FF, Schilling M, Maurer C, Graber HU, Dervenis C, Zimmermann A, Buchler MW: Activation of the serine proteinase system in chronic kidney rejection. Transplantation 65: 1628–1634, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Liu RM: Oxidative stress, plasminogen activator inhibitor 1, and lung fibrosis. Antioxid Redox Signal 10: 303–319, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eitzman DT, McCoy RD, Zheng X, Fay WP, Shen T, Ginsburg D, Simon RH: Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. J Clin Invest 97: 232–237, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seol W., Choi HS., Moore DD: An orphan nuclear hormone receptor that lacks a DNA binding domain and heterodimerizes with other receptors. Science 272: 1336–1339, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Lee YS, Chanda D, Sim J, Park YY, Choi HS: Structure and function of the atypical orphan nuclear receptor small heterodimer partner. Int Rev Cytol 61: 117–158, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Park YJ, Qatanani M, Chua SS, LaRey JL, Johnson SA, Watanabe M, Moore DD, Lee YK: Loss of orphan receptor small heterodimer partner sensitizes mice to liver injury from obstructive cholestasis. Hepatology 47: 1578–1586, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Suh JH, Huang J, Park YY, Seong HA, Kim D, Shong M, Ha H, Lee IK, Lee K, Wang L, Choi HS: Orphan nuclear receptor small heterodimer partner inhibits transforming growth factor-β signaling by repressing Smad3 transactivation. J Biol Chem 281: 39,169–39,178, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Fiorucci S, Antonelli E, Rizzo G, Renga B, Mencarelli A, Riccardi L, Orlandi S, Pellicciari R, Morelli A: The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology 127: 1497–1512, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Kawai T, Masaki T, Doi S, Arakawa T, Yokoyama Y, Doi T, Kohno N, Yorioka N: PPAR-γ agonist attenuates renal interstitial fibrosis and inflammation through reduction of TGF-β. Lab Invest 89: 47–58, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Fiorucci S, Rizzo G, Antonelli E: Cross-talk between farnesoid-X-receptor (FXR) and peroxisome proliferator-activated receptor γ contributes to the antifibrotic activity of FXR ligands in rodent models of liver cirrhosis. J Pharmacol Exp Ther 315: 58–68, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Salbert G, Fanjul A, Piedrafita FJ, Lu XP, Kim SJ, Tran P, Pfahl M: Retinoic acid receptors and retinoid X receptor-alpha down-regulate the transforming growth factor-beta 1 promoter by antagonizing AP-1 activity. Mol Endocrin 7: 1347–1356, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Chipuk JE, Cornelius SC, Pultz NJ, Jorgensen JS, Bonham MJ, Kim SJ, Danielpour D: The androgen receptor represses transforming growth factor-beta signaling through interaction with Smad3. J Biol Chem 277: 1240–1248, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Song CZ, Tian X, Gelehrter TD: Glucocorticoid receptor inhibits transforming growth factor-beta signaling by directly targeting the transcriptional activation function of Smad3. Proc Natl Acad Sci U S A 96: 11,776–11,781, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oikawa T, Freeman M, Lo W, Vaughan DE, Fogo : A modulation of plasminogen activator inhibitor-1 in vivo: A new mechanism for the anti-fibrotic effect of renin-angiotensin inhibition. Kidney Int 51: 164–172, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Tang WW, Feng L, Xia Y, Wilson CB: Extracellular matrix accumulation in immune-mediated tubulointerstitial injury. Kidney Int 45: 1077–1084, 1994 [DOI] [PubMed] [Google Scholar]
- 34.Rerolle JP, Hertig A, Nguyen G, Sraer JD, Rondeau EP: Plasminogen activator inhibitor type 1 is a potential target in renal fibrogenesis. Kidney Int 8: 1841–1850, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Nicholas SB, Aguiniga E, Ren Y, Kim J, Wong J, Govindarajan N, Noda M, Wang W, Kawano Y, Collins A, Hsueh WA: Plasminogen activator inhibitor-1 deficiency retards diabetic nephropathy. Kidney Int 67: 1297–1307, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Eddy AA, Fogo AB: Plasminogen activator inhibitor-1 in chronic kidney disease: Evidence and mechanisms of action. J Am Soc Nephrol 17: 2999–3012, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Das F, Ghosh-Choudhury N, Venkatesan B, Li X, Mahimainathan L, Choudhury GG: Akt kinase targets association of CBP with Smad 3 to regulate TGF-β-induced expression of plasminogen activator inhibitor-1. J Cell Physiol 214: 513–527, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Ourlin JC, Lasserre F, Pineau T, Fabre JM, Sa-Cunha A, Maurel P, Vilarem MJ, Pascussi JM: The small heterodimer partner interacts with the pregnane X receptor and represses its transcriptional activity. Mol Endocrinol 17: 1693–1703, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Kim JY, Kim HJ, Kim KT, Park YY, Seong HA, Park KC, Lee IK, Ha H, Shong M, Park SC, Choi HS: Orphan nuclear receptor small heterodimer partner represses hepatocyte nuclear factor 3/Foxa transactivation via inhibition of its DNA binding. Mol Endocrinol 18: 2880–2894, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Johansson L, Båvner A, Thomsen JS, Färnegårdh M, Gustafsson JA, Treuter E: The orphan nuclear receptor SHP utilizes conserved LXXLL-related motifs for interactions with ligand-activated estrogen receptors. Mol Cell Biol 20: 1124–1133, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee YK, Dell H, Dowhan DH, Hadzopoulou-Cladaras M, Moore DD: The orphan nuclear receptor SHP inhibits hepatocyte nuclear factor 4 and retinoid X receptor transactivation: Two mechanisms for repression. Mol Cell Biol 20: 187–195, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee YK, Moore DD: Dual mechanisms for repression of the monomeric orphan receptor liver receptor homologous protein-1 by the orphan small heterodimer partner. J Biol Chem 277: 2463–2467, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Yamashita S, Maeshima A, Kojima I, Nojima Y: Activin A is a potent activator of renal interstitial fibroblasts. J Am Soc Nephrol 15: 91–101, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Keeton MR, Curriden SA, van Zonneveld AJ, Loskutoff DJ: Identification of regulatory sequences in the type 1 plasminogen activator inhibitor gene responsive to transforming growth factor. J Biol Chem 266: 23,048–23,052, 1991 [PubMed] [Google Scholar]