Abstract
Oxidative stress and inflammation predict cardiovascular events in chronic hemodialysis patients. Hemodialysis activates the kallikrein-kinin system, increasing bradykinin. Bradykinin promotes inflammation but also stimulates endothelial release of tissue-plasminogen activator and inhibits platelet aggregation. Understanding the detrimental and beneficial effects of endogenous bradykinin during hemodialysis has implications for the treatment of cardiovascular disease in the hemodialysis population. To test the hypothesis that bradykinin contributes to the inflammatory and fibrinolytic responses to dialysis, we conducted a double-blind, randomized, placebo-controlled crossover study comparing the effect of the bradykinin B2 receptor blocker HOE-140 with vehicle on markers of oxidative stress, inflammation, fibrinolysis, and coagulation in nine hemodialysis patients without coronary artery disease. Bradykinin receptor antagonism did not affect the mean arterial pressure or heart rate response to dialysis. Monocyte chemoattractant protein 1 (MCP-1) peaked postdialysis; HOE-140 blunted the increase in MCP-1 (5.9 ± 5.9 versus 25.6 ± 20.1 pg/ml, P = 0.01). HOE-140 also abolished the increase in plasminogen activator inhibitor 1 (PAI-1) antigen observed at the end of dialysis. In contrast, HOE-140 significantly accentuated the effect of dialysis on F2-isoprostanes and P-selectin. Taken together, these results suggest that endogenous bradykinin contributes to increases in MCP-1 and PAI-1 antigen after hemodialysis via its B2 receptor. Factors that increase the production of bradykinin or decrease its degradation may enhance the inflammatory response to hemodialysis.
ESRD patients face a 3.4-fold risk of cardiovascular events compared with the general population,1 and chronic hemodialysis (CHD) patients face a 100-fold higher risk of cardiovascular death compared with the general population in patients age 45 yr or younger.2 Traditional risk factors for atherosclerosis do not account for the increased incidence of cardiovascular events.3 On the other hand, biomarkers of oxidative stress and inflammation predict cardiovascular events in CHD patients.2,4
During hemodialysis, contact of the blood with the dialyzer and dialysate activates the kallikrein-kinin system (KKS) and induces a systemic inflammatory response characterized by complement activation, leukocyte activation, and the generation of cytokines.5 For example, hemodialysis increases leukocyte expression of interleukin (IL)-1β, IL-8, and TNF-α, and circulating concentrations of IL-6.6,7 Increased inflammation may, in turn, contribute to derangements of the fibrinolytic system in CHD patients. Cytokines such as TNF-α, IL-1β, and IL-6 stimulate expression of plasminogen activator inhibitor-1 (PAI-1), the major physiologic inhibitor of fibrinolysis in vivo.8 Tissue-type plasminogen activator (t-PA) concentrations and activity increase transiently during hemodialysis, whereas circulating PAI-1 concentrations are increased in the CHD population.9–11
Activation of the KKS during hemodialysis could have favorable or deleterious effects on inflammation and the risk of cardiovascular events. On the one hand, bradykinin causes vasodilation and stimulates the release of t-PA from the vascular endothelium via bradykinin B2 receptor-dependent mechanisms, and infused bradykinin inhibits platelet aggregation in healthy subjects.12–14 On the other hand, bradykinin also stimulates activation of nuclear factor κB and cytokine expression via the B2 receptor.15,16 Understanding the contribution of endogenous bradykinin to the inflammatory response to hemodialysis has important implications for the prevention of atherothrombotic events.
Specifically, angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) decrease mortality in patients at risk for cardiovascular events in the general population,17,18 but studies in the CHD population provide conflicting data and often do not discriminate between the effects of ACE inhibitors and ARBs.19–22 The only prospective, randomized clinical trial did not show a beneficial effect of ACE inhibition in patients undergoing hemodialysis.23 Whereas ACE inhibitors and ARBs decrease angiotensin II formation or action, ACE inhibitors also potentiate the effects of bradykinin.24–26 Indeed, during hemodialysis with a polysulfone membrane, plasma bradykinin concentrations are higher during treatment with an ACE inhibitor compared with during treatment with an ARB.27 In light of differences in mechanism of action between ACE inhibitors and ARBs, understanding the contribution of endogenous bradykinin to the proinflammatory effects of hemodialysis could affect the use of these agents in the CHD population.
This study tested the hypothesis that activation of the KKS and the formation of endogenous bradykinin contribute to hypotension, increased inflammation, increased oxidative stress, and increased fibrinolysis in response to hemodialysis. To accomplish this, we compared the effect of continuous infusion of the bradykinin B2 receptor-specific antagonist HOE-140 versus vehicle on the acute responses to hemodialysis.
Results
Baseline Patient Characteristics
Baseline characteristics appear in Table 1. Causes of ESRD were hypertensive nephropathy (six of nine subjects), diabetic nephropathy (one subject), focal segmental glomerulosclerosis (one subject), and posterior urethral valves with chronic obstruction (one subject).
Table 1.
Subject characteristics
| Parameter | Value |
|---|---|
| Age (yr) | 40 ± 3.0 |
| Body mass index (kg/m2) | 30.7 ± 2.6 |
| Race (African-American:Caucasian) | 8:1 |
| Gender (male:female) | 6:3 |
| Hypertension (yes:no) | 9:0 |
| Smoker (yes:no) | 2:7 |
| ACE inhibitor use (yes:no) | 2:7 |
| Calcium × phosphate producta (mg2/dl2) | 53.3 ± 6.7 |
| Parathyroid hormone level (mg/dl) | 811.2 ± 188.3 |
| Heparin dose (units) | 4611 ± 462 |
| Erythropoietin dose (units) | 5511 ± 2522 |
Effects of Dialysis and Bradykinin Receptor Blockade on Hemodynamics
Mean arterial pressure (MAP) decreased during hemodialysis, from 115.4 ± 7.0 to 90.1 ± 8.9 mmHg during placebo and from 100.6 ± 5.7 to 89.2 ± 5.1 mmHg during HOE-140. Heart rate increased significantly during hemodialysis (supplemental data). Neither forearm blood flow (FBF) nor forearm vascular resistance (FVR) changed after hemodialysis (supplemental data). There was no effect of bradykinin receptor blockade on baseline MAP, heart rate, FBF, and FVR or on the hemodynamic response to hemodialysis (all P ≥ 0.05, supplemental data).
Effects of Dialysis and Bradykinin Receptor Blockade on Oxidative Stress and Inflammatory Biomarkers
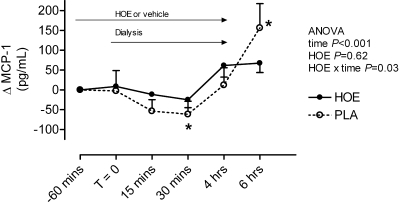
F2-isoprostanes increased during hemodialysis during HOE-140 (from 59.6 ± 7.8 to 80.0 ± 12.7 pg/ml at the end of dialysis and 67.7 ± 8.6 pg/ml 2 h postdialysis, P = 0.007) but not during vehicle (from 54.1 ± 6.1 to 58.5 ± 6.9 pg/ml at the end of dialysis and 43.5 ± 3.4 pg/ml 2 h postdialysis, P = 0.49); F2-isoprostane concentrations were significantly higher postdialysis during administration of the bradykinin receptor antagonist HOE-140 (P = 0.02 for effect of HOE-140) (supplemental data). MCP-1 concentrations tended to decrease during the first 30 min of hemodialysis but were increased at the end of hemodialysis and continued to increase 2 h postdialysis (Figure 1). HOE-140 significantly blunted the increase in MCP-1 after hemodialysis (change from end dialysis of 5.9 ± 5.9 versus 25.6 ± 20.1 pg/ml, P = 0.01).
Figure 1.
Effect of study drug and hemodialysis on MCP-1. *P < 0.05 versus T = 0 in post hoc analysis.
IL-6 (from 5.3 ± 1.1 to 6.9 ± 0.9 pg/ml at the end of dialysis during placebo, P = 0.045), IL-8 (from 17.7 ± 3.0 to 20.2 ± 3.7 pg/ml postdialysis, P = 0.08), and IL-10 (from 3.2 ± 0.7 to 3.7 ± 0.6 pg/ml at the end of dialysis, P = 0.02) all increased or tended to increase during and after hemodialysis (supplemental data). For both the proinflammatory cytokine IL-8 (P = 0.02 for HOE-140 by time interaction) and the anti-inflammatory cytokine IL-10 (P = 0.03), bradykinin receptor blockade enhanced this effect. There was no effect of HOE-140 on IL-6 concentrations. TNF-α, IL-1β, and IL-12p70 did not change during or after hemodialysis and there was no effect of HOE-140 on these cytokines (supplemental data).
Effects of Dialysis and Bradykinin Receptor Blockade on Markers of Fibrinolysis and Coagulation
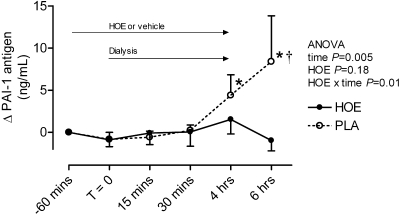
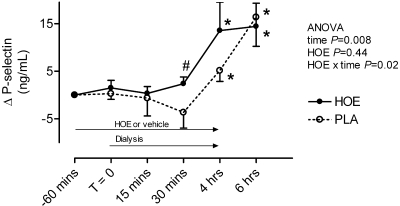
PAI-1 antigen increased significantly during (from 6.1 ± 1.1 to 10.4 ± 2.1 ng/ml at the end of hemodialysis, P = 0.04) and after hemodialysis (14.6 ± 6.2 ng/ml 2 h postdialysis, P = 0.01 versus baseline) during placebo (Figure 2). The bradykinin receptor antagonist HOE-140 prevented this effect (PAI-1 2 h postdialysis 6.9 ± 1.5 ng/ml HOE-140 arm). When the two patients taking an ACE inhibitor were excluded from the analysis, PAI-1 antigen increased from 5.9 ± 1.5 ng/ml predialysis to 16.9 ± 9.2 ng/ml 2 h post dialysis (P = 0.03) during placebo but not during HOE-140 (7.5 ± 2.1 to 7.7 ± 1.8 ng/ml). PAI-1 activity decreased during and after hemodialysis, whereas t-PA antigen and activity increased (Table 2). There was no effect of HOE-140 on PAI-1 activity or t-PA antigen or activity (Table 2). However, in post hoc analysis, PAI-1 activity decreased significantly during hemodialysis during HOE-140 and not during placebo. d-dimer concentrations increased during hemodialysis, and there was a significant interactive effect of hemodialysis and HOE-140 on d-dimer concentrations (Table 2). P-selectin, a marker of platelet activation, increased during and after hemodialysis, an effect that occurred earlier during bradykinin receptor antagonism (Figure 3).
Figure 2.
Effect of study drug and hemodialysis on PAI-1. *P < 0.05 versus T = 0. †P = 0.02 for vehicle versus HOE-140 in post hoc analysis.
Table 2.
Effect of treatment on biomarkers of fibrinolysis
| T = 0 | T = 15 min | T = 30 min | T = 4 h | T = 6 h | P Value Time | P Value Drug | P Value Drug × Time | |
|---|---|---|---|---|---|---|---|---|
| PAI-1 activity (IU/ml) | ||||||||
| vehicle | 4.12 ± 1.06 | 3.67 ± 0.84 | 3.52 ± 0.78 | 2.24 ± 0.24 | 2.71 ± 0.53 | 0.02 | 0.99 | 0.18 |
| HOE-140 | 5.34 ± 1.89 | 4.74 ± 1.65 | 4.47 ± 1.59a | 3.13 ± 0.91a | 3.26 ± 0.73 | |||
| t-PA antigen (ng/ml) | ||||||||
| vehicle | 3.79 ± 0.83 | 4.16 ± 0.84 | 4.43 ± 0.65 | 7.67 ± 1.18a | 6.45 ± 1.12a | 0.049 | 0.26 | 0.17 |
| HOE-140 | 4.60 ± 0.39 | 5.94 ± 0.93 | 6.33 ± 0.88a | 8.57 ± 1.28a | 5.87 ± 0.84 | |||
| t-PA activity (IU/ml) | ||||||||
| vehicle | 0.28 ± 0.05 | 0.38 ± 0.02a | 0.41 ± 0.03a | 0.79 ± 0.09a | 0.58 ± 0.07a | 0.04 | 0.94 | 0.34 |
| HOE-140 | 0.39 ± 0.07 | 0.33 ± 0.07 | 0.48 ± 0.10 | 0.72 ± 0.14a | 0.55 ± 0.12 | |||
| d-dimer (ng/ml) | ||||||||
| vehicle | 131.19 ± 27.73 | 147.23 ± 26.64 | 151.82 ± 25.80 | 161.00 ± 27.31a | 164.71 ± 32.04 | 0.03 | 0.72 | 0.04 |
| HOE-140 | 134.82 ± 29.67 | 126.66 ± 19.90 | 145.38 ± 32.39 | 189.15 ± 35.46a | 162.35 ± 32.09 |
Mean ± SEM.
aP < 0.05 versus T = 0 in post hoc analysis.
Figure 3.
Effect of study drug and hemodialysis on P-selectin. *P < 0.05 versus T = 0 in post hoc analysis. #P = 0.05 for vehicle versus HOE-140 in post hoc analysis.
Discussion
Using the bradykinin B2 receptor blocker HOE-140 to dissect the role of endogenous bradykinin, this study tested the hypothesis that activation of the KKS and the formation of endogenous bradykinin contribute to the hypotensive, proinflammatory, profibrinolytic, and anti-coagulant responses to hemodialysis. The major finding is that endogenous bradykinin contributes to increased MCP-1 and PAI-1 concentrations after hemodialysis via a B2-receptor dependent mechanism. Given that PAI-1 antigen and activity independently predict cardiovascular mortality and thrombotic events in CHD patients,10,11,28 the data suggest that factors that increase the formation of bradykinin or decrease its degradation would increase cardiovascular risk in the CHD population.
CHD patients exhibit abnormalities of platelet function, fibrinolysis, and coagulation.29,30 Fibrinolytic activity increases acutely during hemodialysis, largely related to an increase in t-PA, but falls precipitously after hemodialysis.9 Most investigators have reported no change in PAI-1 antigen or activity during hemodialysis31,32 but have not measured PAI-1 in the postdialysis period. During cardiopulmonary bypass, a form of extracorporeal circulation similar to hemodialysis, t-PA and fibrinolytic activity rise during bypass, whereas PAI-1 increases during the early postoperative period in response to inflammation.33 In the study presented here, we observed a similar stimulatory effect of hemodialysis on PAI-1 antigen in the vehicle-treated group.
Bradykinin may be predicted to increase PAI-1 expression through its proinflammatory effects or to decrease PAI-1 expression by increasing the formation of nitric oxide (NO), which downregulates PAI-1 expression through a cGMP-dependent mechanism.34 Studies in cultured cells provide conflicting information regarding the role of bradykinin in the regulation of PAI-1 expression. Kimura et al. demonstrated that bradykinin increases PAI-1 mRNA and protein expression in rat vascular endothelial cells and that the effect is negatively affected by NO.35 In contrast, Okada et al. reported that bradykinin decreases PAI-1 expression in murine renal tubulointerstitial cells during ACE inhibition.36 The finding that bradykinin B2 receptor blockade abolished the rise in PAI-1 after hemodialysis in the study presented here suggests that in CHD patients that exhibit endothelial dysfunction,37 the proinflammatory effects of bradykinin predominate in regulating PAI-1, at least during hemodialysis. PAI-1 antigen is both an acute-phase reactant and an inhibitor of fibrinolysis. During hemodialysis, increased t-PA antigen would complex with PAI-1 antigen to decrease its activity; however, in post hoc analysis PAI-1 activity decreased only during HOE-140. Taken together with the observation that d-dimer concentrations were higher during HOE-140, these data suggest that endogenous bradykinin receptor blockade attenuates fibrinolysis.
As reported previously,9–11 t-PA antigen and activity increased during and after hemodialysis in this study. Although bradykinin stimulates the release of t-PA from the vascular endothelium through a B2-receptor-dependent mechanism,9,10 our data do not support a significant contribution of bradykinin to increased t-PA during hemodialysis. Bradykinin-stimulated t-PA release is attenuated in conditions associated with endothelial dysfunction such as smoking, obesity, and hypertension,38 and ESRD is characterized by endothelial dysfunction.37 During hemodialysis, factors other than bradykinin (e.g., direct endothelial damage from high shear stress39 or from the effects of extracorporeal circulation40) may contribute to increased t-PA release.
Concentrations of the proinflammatory cytokines IL-6 and IL-8, the anti-inflammatory cytokine IL-10, and chemotactic factors such as MCP-1 are chronically elevated in CHD patients.41,42 We found that hemodialysis increased the concentrations of these cytokines and chemokines, consistent with previously published reports by our laboratory and others.6,7 That bradykinin B2 receptor blockade attenuated the rise in MCP-1 after hemodialysis concurs with data from cell culture studies. Thus, bradykinin stimulates lung fibroblasts, type II alveolar cells, macrophages, and bronchial epithelial cells to release inflammatory cytokines and chemotactic factors such as MCP-1, whereas B2 receptor antagonism decreases cytokine-induced neutrophil migration.16,43 On the basis of these data, ACE inhibitors, which potentiate bradykinin, could have a proinflammatory effect during hemodialysis.
Whereas bradykinin B2 receptor blockade decreased MCP-1 and PAI-1 after hemodialysis, bradykinin receptor blockade was associated with an increase in F2-isoprostanes postdialysis, suggesting that endogenous bradykinin decreases oxidative stress in patients undergoing hemodialysis. This concurs with recent in vitro and animal model data suggesting that bradykinin reduces oxidative stress via its B2 receptor44 but conflicts with other studies showing that bradykinin augments production of reactive oxygen species after ischemia-reperfusion injury.45
In addition, we found that as measured by circulating P-selectin concentrations, platelet aggregation increased earlier during dialysis when the bradykinin B2 receptor was blocked. Endogenous bradykinin stimulates the release of two potent inhibitors of platelet aggregation via its B2 receptor—prostacyclin and NO.46 Both of these endothelial-derived factors modulate platelet-vessel wall interactions, inhibit aggregation, and promote disaggregation in vitro and in animal models.47 Studies in bradykinin B2 receptor knockout mice indicate that the anti-aggregatory effects of bradykinin occur via the B2 receptor,48 although bradykinin and its metabolite BK1–5 can also inhibit thrombin-induced platelet aggregation via interactions with protease-activated receptor 1.13,49
Bradykinin causes vasodilation via the B2 receptor-mediated release of prostacyclin, NO, and endothelium-derived hyperpolarizing factor;46 however, the lack of effect of HOE-140 on the hypotensive response to hemodialysis suggests that endogenous bradykinin does not substantially contribute to the hemodynamic response to hemodialysis. Blood pressure (BP) is volume-dependent in patients with ESRD, and BP decreases during hemodialysis because of decreased intravascular plasma volume, whereas heart rate rises because of activation of the baroreflex.50
Two study limitations merit notation. First, we excluded subjects with evidence of coronary artery disease because we were studying an investigational drug that had the potential to increase BP or cause coronary vasoconstriction. Thus, in our subjects, concentrations of inflammatory biomarkers were lower than have been reported previously.6 Second, our small study included subjects who tended to be young, obese, predominantly African American, and male. Therefore, the results may not be readily generalizable to a large population of CHD patients.
In conclusion, despite a favorable effect on oxidative stress, endogenous bradykinin contributes to increases in MCP-1 and PAI-1 antigen during and after hemodialysis via a B2-receptor dependent mechanism. These data support a net proinflammatory role of endogenous bradykinin during hemodialysis and suggest that ACE inhibitors, which prevent the degradation of bradykinin,24 could have a proinflammatory effect in CHD patients. The results of our study provide a plausible biologic mechanism for the lack of a beneficial effect of ACE inhibitors in patients with ESRD and left ventricular hypertrophy in trials such as FOSIDIAL.23
Concise Methods
Study Population
Nine CHD patients without evidence of CAD or congestive heart failure completed the study. The study was performed according to the Declaration of Helsinki after being approved by the Vanderbilt University Institutional Review Board, and all patients gave written informed consent. The subjects were on a three-times-weekly hemodialysis schedule with polysulphone membrane (Optiflux 160) for at least 6 mo before the study. Dialysis adequacy was determined based on the total clearance (Kt) to total body water or urea distribution space (V) ratio (Kt/V) of 1.2 or greater. Hemodialysis was carried out for 4 h on each study day with the same flow rates (400 ml/min blood flow rate and 800 ml/min dialysis flow rate) and ultrafiltration (varied for each subject) on the two study days for each subject. Subjects received heparin (4611 ± 462 U) as a bolus per typical hemodialysis protocol at the beginning of each hemodialysis session with no requirement for continuous infusion (Table 1). Subjects also received erythropoietin (5511 ± 2522 U) three times weekly per typical hemodialysis protocol (Table 1). The study drug constituted the only varied condition for each subject between the two study days. Antihypertensive medications in use at the time of the study and the numbers of subjects taking each one included beta blockers (metoprolol: one, labetalol: one); ACE inhibitors (lisinopril: one, enalapril: one); calcium channel blockers (nifedipine: four); and α2-adrenergic agonists (clonidine: two).
Subjects with documented coronary artery disease were excluded, and all subjects had a negative dobutamine echo before study. Individuals with a body mass index (BMI) greater than 35 mg/kg;2 history of kidney transplant less than 6 mo before study; use of anti-inflammatory medications other than aspirin less than 325 mg per day; use of immunosuppressive drugs within 1 mo before study; history of active connective tissue disease; history of acute infectious disease within 1 mo before study; AIDS; history of myocardial infarction or cerebrovascular event within 3 mo; advanced liver disease; gastrointestinal dysfunction requiring parenteral nutrition; active malignancy excluding basal cell carcinoma of the skin; anticipated live donor kidney transplant; use of vitamin E greater than 60 IU per day or vitamin C greater than 500 mg per day; pregnancy, breastfeeding, or childbearing potential; history of poor adherence to hemodialysis or medical regimen; or inability to provide consent were excluded.
Study Protocol
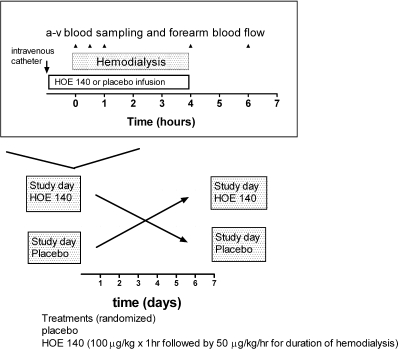
Subjects participated in a prospective, randomized, double-blind, placebo-controlled, crossover trial of the effects of placebo versus HOE-140, a bradykinin B2 receptor antagonist, during hemodialysis. Subjects were randomized to treatment with vehicle or HOE-140 on each of two study days separated by 1 wk. Subjects reported to the Vanderbilt General Clinical Research Center for study drug administration, hemodialysis, hemodynamic monitoring, and plasma sampling (Figure 4). HOE-140 (or the equivalent volume of vehicle) was infused intravenously at 100 μg/kg over 1 h before dialysis and at 50 μg/kg per h for the duration of dialysis. This dose has been shown to significantly blunt the vasodilatory response to intra-arterial bradykinin.51 BP was determined using an automated oscillometric recording device every 5 min during the study (Dinamap, Critikon, Carlsbad, CA), using the average of three readings at each time point. Blood for biochemical analysis was collected 1 h before dialysis (T = −60), at initiation of dialysis (T = 0), 15 and 30 min after initiation of dialysis (T = 15 and 30 min), at termination of dialysis (T = 4 h), and 2 h after termination of dialysis (T = 6 h). In the first two subjects studied, blood was also obtained at 48 h, but this collection was discontinued because all parameters had returned to baseline at this time point. FBF was measured using strain-gauge venous occlusion plethysmography (D.E. Hokanson, Bellevue, WA), as described previously,14,52 at each of the six timepoints outlined above. FBF was measured in milliliters per 100 ml of volume tissue per minute. FVR was calculated as the ratio of MAP to FBF and expressed as arbitrary units (AU).
Figure 4.
Study design.
Laboratory Procedures
Blood was centrifuged immediately after collection and stored at −80°C until assay. TNF-α, IL-1β, IL-6, IL-8, IL-10, IL-12p70, and MCP-1 were measured using Luminex immunoassay technology and commercially available kits (Linco Research, St. Charles, MO). Coefficients of variation (interassay and intra-assay, respectively) and limits of detection for the inflammatory markers are as follows: 0.037, 0.020, and 0.7 pg/ml for TNF-α; 0.083, 0.030, and 2.3 pg/ml for IL-1β; 0.067, 0.027, and 1.6 pg/ml for IL-6; 0.037, 0.023, and 1.2 pg/ml for IL-8; 0.090, 0.037, and 0.13 mg/ml for IL-10; 0.030, 0.033, and 0.6 pg/ml for IL-12p70; and 0.080, 0.037, and 1.3 pg/ml for MCP-1. Blood for F2-isoprostanes was collected in chilled tubes containing EDTA, and F2-isoprostane levels were measured using negative-ion gas-chromatography mass spectroscopy.53 Blood for PAI-1, t-PA, and d-dimer assays was collected in vacutainer tubes containing acidified 0.105 M sodium citrate (Biopool, Umea, Sweden). PAI-1 and t-PA antigen were determined using two-site ELISAs (Elisa, Immulyse, and TintElize). PAI-1 and t-PA activity were measured using chromogenic substrates and standardized commercial kits (Chromolize, Biopool), with results expressed as units per milliliter (IU/ml). d-dimer was measured using a standardized commercial kit (TintElize, Biopool), with results expressed as nanograms per milliliter. P-selectin was measured using a commercially available ELISA kit (R&D, Minneapolis, MN). Coefficients of variation (interassay and intra-assay, respectively) and limits of detection for the coagulation and fibrinolysis markers are as follows: 0.019, 0.024, and 20 ng/ml for PAI-1; 0.055, 0.035, and 6 ng/ml for t-PA; 0.040, 0.040, and 40 ng/ml for d-dimer; and 0.049, 0.088, and 0.5 ng/ml for P-selectin.
Statistical Methods
Data are presented as mean ± SEM. Missing variables in the data were replaced using series means. Baseline characteristics on the two study days were compared using a paired t test or Wilcoxon signed-rank test. General linear model repeated measure ANOVA was used to evaluate the effect of hemodialysis and drug (HOE-140 versus placebo) over time. Gender or race was included as a between-subject variable if there was evidence for a direct or interactive effect of these variables. A two-sided P value less than 0.05 was considered significant. Statistical analyses were performed using SPSS software v.15.0 (SPSS, Inc., Chicago, IL).
Disclosures
Dr. Brown has consulted for Jerini AG and Baxter in the past year but received neither financial support for nor intellectual input in the study presented here. None of the other authors have any relationships to report.
Acknowledgments
The authors wish to thank Ms. Loretta Byrne for her assistance in carrying out the research protocol and data entry.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Stenvinkel P: Interactions between inflammation, oxidative stress, and endothelial dysfunction in end-stage renal disease. J Ren Nutr 13: 144–148, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Coresh J, Eustace JA, Longenecker JC, Jaar B, Fink NE, Tracy RP, Powe NR, Klag MJ: Association between cholesterol level and mortality in dialysis patients: Role of inflammation and malnutrition. JAMA 291: 451–459, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Himmelfarb J, Hakim RM: Oxidative stress in uremia. Curr Opin Nephrol Hypertens 12: 593–598, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Horl WH: Hemodialysis membranes: Interleukins, biocompatibility, and middle molecules. J Am Soc Nephrol 13[Suppl 1]: S62–S71, 2002 [PubMed] [Google Scholar]
- 6.Caglar K, Peng Y, Pupim LB, Flakoll PJ, Levenhagen D, Hakim RM, Ikizler TA: Inflammatory signals associated with hemodialysis. Kidney Int 62: 1408–1416, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Friedrich B, Alexander D, Janessa A, Haring HU, Lang F, Risler T: Acute effects of hemodialysis on cytokine transcription profiles: Evidence for C-reactive protein-dependency of mediator induction. Kidney Int 70: 2124–2130, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Lijnen HR: Pleiotropic functions of plasminogen activator inhibitor-1. J Thromb Haemost 3: 35–45, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Nakamura Y, Tomura S, Tachibana K, Chida Y, Marumo F: Enhanced fibrinolytic activity during the course of hemodialysis. Clin Nephrol 38: 90–96, 1992 [PubMed] [Google Scholar]
- 10.Segarra A, Chacon P, Martinez-Eyarre C, Argelaguer X, Vila J, Ruiz P, Fort J, Bartolome J, Camps J, Moliner E, Pelegri A, Marco F, Olmos A, Piera L: Circulating levels of plasminogen activator inhibitor type-1, tissue plasminogen activator, and thrombomodulin in hemodialysis patients: Biochemical correlations and role as independent predictors of coronary artery stenosis. J Am Soc Nephrol 12: 1255–1263, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Tomura S, Nakamura Y, Doi M, Ando R, Ida T, Chida Y, Ootsuka S, Shinoda T, Yanagi H, Tsuchiya S, Marumo F: Fibrinogen, coagulation factor VII, tissue plasminogen activator, plasminogen activator inhibitor-1, and lipid as cardiovascular risk factors in chronic hemodialysis and continuous ambulatory peritoneal dialysis patients. Am J Kidney Dis 27: 848–854, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Brown NJ, Gainer JV, Murphey LJ, Vaughan DE: Bradykinin stimulates tissue plasminogen activator release from human forearm vasculature through B(2) receptor-dependent, NO synthase-independent, and cyclooxygenase-independent pathway. Circulation 102: 2190–2196, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Murphey LJ, Malave HA, Petro J, Biaggioni I, Byrne DW, Vaughan DE, Luther JM, Pretorius M, Brown NJ: Bradykinin and its metabolite bradykinin 1–5 inhibit thrombin-induced platelet aggregation in humans. J Pharmacol Exp Ther 318: 1287–1292, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Pretorius M, Rosenbaum D, Vaughan DE, Brown NJ: Angiotensin-converting enzyme inhibition increases human vascular tissue-type plasminogen activator release through endogenous bradykinin. Circulation 107: 579–585, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Brunius G, Domeij H, Gustavsson A, Yucel-Lindberg T: Bradykinin upregulates IL-8 production in human gingival fibroblasts stimulated by interleukin-1β and tumor necrosis factor alpha. Regul Pept 126: 183–188, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Santos DR, Calixto JB, Souza GE: Effect of a kinin B2 receptor antagonist on LPS- and cytokine-induced neutrophil migration in rats. Br J Pharmacol 139: 271–278, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J: Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: The CHARM-Preserved Trial. Lancet 362: 777–781, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G: Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 342: 145–153, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Berger AK, Duval S, Krumholz HM: Aspirin, beta-blocker, and angiotensin-converting enzyme inhibitor therapy in patients with end-stage renal disease and an acute myocardial infarction. J Am Coll Cardiol 42: 201–208, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Efrati S, Zaidenstein R, Dishy V, Beberashvili I, Sharist M, Averbukh Z, Golik A, Weissgarten J: ACE inhibitors and survival of hemodialysis patients. Am J Kidney Dis 40: 1023–1029, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Ishani A, Herzog CA, Collins AJ, Foley RN: Cardiac medications and their association with cardiovascular events in incident dialysis patients: Cause or effect? Kidney Int 65: 1017–1025, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Winkelmayer WC, Charytan DM, Levin R, Avorn J: Poor short-term survival and low use of cardiovascular medications in elderly dialysis patients after acute myocardial infarction. Am J Kidney Dis 47: 301–308, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Zannad F, Kessler M, Lehert P, Grunfeld JP, Thuilliez C, Leizorovicz A, Lechat P: Prevention of cardiovascular events in end-stage renal disease: Results of a randomized trial of fosinopril and implications for future studies. Kidney Int 70: 1318–1324, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Erdos EG: Kinins, the long march—A personal view. Cardiovasc Res 54: 485–491, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Gainer JV, Morrow JD, Loveland A, King DJ, Brown NJ: Effect of bradykinin-receptor blockade on the response to angiotensin-converting-enzyme inhibitor in normotensive and hypertensive subjects. N Engl J Med 339: 1285–1292, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Witherow FN, Helmy A, Webb DJ, Fox KA, Newby DE: Bradykinin contributes to the vasodilator effects of chronic angiotensin-converting enzyme inhibition in patients with heart failure. Circulation 104: 2177–2181, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Ball AM, Murphey LJ, Pascual TT: Bradykinin metabolism in hemodialysis: Comparison of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers. J Am Soc Nephrol 14: 712A–713A, 2003 [Google Scholar]
- 28.Haaber AB, Eidemak I, Jensen T, Feldt-Rasmussen B, Strandgaard S: Vascular endothelial cell function and cardiovascular risk factors in patients with chronic renal failure. J Am Soc Nephrol 5: 1581–1584, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Kawabata K, Nagake Y, Shikata K, Fukuda S, Nakazono H, Takahashi M, Ichikawa H, Makino H: Soluble P-selectin is released from activated platelets in vivo during hemodialysis. Nephron 78: 148–155, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Sloand JA, Sloand EM: Studies on platelet membrane glycoproteins and platelet function during hemodialysis. J Am Soc Nephrol 8: 799–803, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Martin-Malo A, Velasco F, Rojas R, Castillo D, Rodriguez M, Torres A, Aljama P: Fibrinolytic activity during hemodialysis: A biocompatibility-related phenomenon. Kidney Int Suppl 41: S213–S216, 1993 [PubMed] [Google Scholar]
- 32.Sabovic M, Salobir B, Preloznik Zupan I, Bratina P, Bojec V, Buturovic Ponikvar J: The influence of the haemodialysis procedure on platelets, coagulation and fibrinolysis. Pathophysiol Haemost Thromb 34: 274–278, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Chandler WL, Fitch JC, Wall MH, Verrier ED, Cochran RP, Soltow LO, Spiess D: Individual variations in the fibrinolytic response during and after cardiopulmonary bypass. Thromb Haemost 74: 1293–1297, 1995 [PubMed] [Google Scholar]
- 34.Bouchie JL, Hansen H, Feener EP: Natriuretic factors and nitric oxide suppress plasminogen activator inhibitor-1 expression in vascular smooth muscle cells. Role of cGMP in the regulation of the plasminogen system. Arterioscler Thromb Vasc Biol 18: 1771–1779, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Kimura S, Tsuji H, Nishimura H, Kato H, Ukimura N, Yano S, Kunieda Y, Kawano H, Nakagawa K, Nakagawa M: Bradykinin enhances in vitro procoagulant and antifibrinolytic properties of rat vascular endothelial cells. Thromb Res 106: 41–50, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Okada H, Watanabe Y, Kikuta T, Kobayashi T, Kanno Y, Sugaya T, Suzuki H: Bradykinin decreases plasminogen activator inhibitor-1 expression and facilitates matrix degradation in the renal tubulointerstitium under angiotensin-converting enzyme blockade. J Am Soc Nephrol 15: 2404–2413, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Miyazaki H, Matsuoka H, Itabe H, Usui M, Ueda S, Okuda S, Imaizumi T: Hemodialysis impairs endothelial function via oxidative stress: Effects of vitamin E-coated dialyzer. Circulation 101: 1002–1006, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Brown NJ: Blood pressure reduction and tissue-type plasminogen activator release. Hypertension 47: 648–649, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Papadaki M, Ruef J, Nguyen KT, Li F, Patterson C, Eskin SG, McIntire LV, Runge MS: Differential regulation of protease activated receptor-1 and tissue plasminogen activator expression by shear stress in vascular smooth muscle cells. Circ Res 83: 1027–1034, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Opatrny K, Jr, Opatrny K, Vit L, Racek J, Valek A: What are the factors contributing to the changes in tissue-type plasminogen activator during haemodialysis? Nephrol Dial Transplant 6[Suppl 3]: 26–30, 1991 [PubMed] [Google Scholar]
- 41.Pawlak K, Pawlak D, Mysliwiec M: Impaired renal function and duration of dialysis therapy are associated with oxidative stress and proatherogenic cytokine levels in patients with end-stage renal disease. Clin Biochem 40: 81–85, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Sertic J, Slavicek J, Bozina N, Malenica B, Kes P, Reiner Z: Cytokines and growth factors in mostly atherosclerotic patients on hemodialysis determined by biochip array technology. Clin Chem Lab Med 45: 1347–1352, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Koyama S, Sato E, Numanami H, Kubo K, Nagai S, Izumi T: Bradykinin stimulates lung fibroblasts to release neutrophil and monocyte chemotactic activity. Am J Respir Cell Mol Biol 22: 75–84, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Chao J, Li HJ, Yao YY, Shen B, Gao L, Bledsoe G, Chao L: Kinin infusion prevents renal inflammation, apoptosis, and fibrosis via inhibition of oxidative stress and mitogen-activated protein kinase activity. Hypertension 49: 490–497, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Chiang WC, Chien CT, Lin WW, Lin SL, Chen YM, Lai CF, Wu KD, Chao J, Tsai TJ: Early activation of bradykinin B2 receptor aggravates reactive oxygen species generation and renal damage in ischemia/reperfusion injury. Free Radic Biol Med 41: 1304–1314, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Vanhoutte PM: Endothelium and control of vascular function. State of the Art lecture. Hypertension 13: 658–667, 1989 [DOI] [PubMed] [Google Scholar]
- 47.Radomski MW, Palmer RM, Moncada S: The anti-aggregating properties of vascular endothelium: Interactions between prostacyclin and nitric oxide. Br J Pharmacol 92: 639–646, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Labonte J, Brochu I, Honore JC, D'Orleans-Juste P: Role of ETB and B2 receptors in the ex vivo platelet inhibitory properties of endothelin and bradykinin in the mouse. Br J Pharmacol 132: 934–940, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hasan AA, Warnock M, Nieman M, Srikanth S, Mahdi F, Krishnan R, Tulinsky A, Schmaier AH: Mechanisms of Arg-Pro-Pro-Gly-Phe inhibition of thrombin. Am J Physiol Heart Circ Physiol 285: H183–H193, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Maeda K, Fujita Y, Shinzato T, Vega BV, Nakane K, Morita H, Kobayakawa H, Inoue I, Miyazaki T, Emoto Y, Takai I: Change in sympathetic activity before, during, and after dialysis-induced hypotension. ASAIO Trans 36: M462–M464, 1990 [PubMed] [Google Scholar]
- 51.Cockcroft JR, Chowienczyk PJ, Brett SE, Bender N, Ritter JM: Inhibition of bradykinin-induced vasodilation in human forearm vasculature by icatibant, a potent B2-receptor antagonist. Br J Clin Pharmacol 38: 317–321, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Guilder GP, Hoetzer GL, Smith DT, Irmiger HM, Greiner JJ, Stauffer BL, DeSouza CA: Endothelial t-PA release is impaired in overweight and obese adults but can be improved with regular aerobic exercise. Am J Physiol Endocrinol Metab 289: E807–E813, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ, II: A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A 87: 9383–9387, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]