Abstract
The mechanism by which extracellular hypotonicity stimulates release of renin from juxtaglomerular (JG) cells is unknown. We hypothesized that osmotically induced renin release depends on water movement through aquaporin-1 (AQP1) water channels and subsequent prostanoid formation. We recorded membrane capacitance (Cm) by whole-cell patch clamp in single JG cells as an index of exocytosis. Hypotonicity increased Cm significantly and enhanced outward current. Indomethacin, PLA2 inhibition, and an antagonist of prostaglandin transport impaired the Cm and current responses to hypotonicity. Hypotonicity also increased exocytosis as determined by a decrease in single JG cell quinacrine fluorescence in an indomethacin-sensitive manner. In single JG cells from COX-2−/ − and AQP1−/ − mice, hypotonicity increased neither Cm nor outward current, but 0.1-μM PGE2 increased both in these cells. A reduction in osmolality enhanced cAMP accumulation in JG cells but not in renin-producing As4.1 cells; only the former had detectable AQP1 expression. Inhibition of protein kinase A blocked the hypotonicity-induced Cm and current response in JG cells. Taken together, our results show that a 5 to 7% decrease in extracellular tonicity leads to AQP1-mediated water influx in JG cells, PLA2/COX-2-mediated prostaglandin-dependent formation of cAMP, and activation of PKA, which promotes exocytosis of renin.
Juxtaglomerular (JG) granular cells in the terminal part of the renal afferent glomerular arterioles are the only cells in the organism that synthesize preprorenin, process it to active renin, and store active renin in mature secretory granules. The rate of renin granule exocytosis determines the level of activation of the renin-angiotensin-aldosterone system. Renin secretion from most1,2 but not all3,4 in vitro preparations displays a uniquely high sensitivity to changes in extracellular osmolality such that a moderate reduction in the extracellular osmolality leads to rapid increases in renin secretion. The sensing and transduction events for renin release in response to osmotic perturbations are not known. At the glomerular tuft, the extracellular osmolality may vary depending on sodium chloride (NaCl)transport rate by the adjacent macula densa and thick ascending limb cells, which are relatively water impermeable.5,6 JG cell capacitance (Cm), an index of cell surface area, increased when extracellular osmolality was decreased by 5 to 10% at the single cell level.7 This observation shows exocytotic release of renin in response to decreases in extracellular osmolality.7 Introduction of a pipette solution with slightly increased osmolality to the cell cytoplasm is sufficient to initiate exocytosis of renin in JG cells.7 This indicates that cell swelling, and not granule swelling, is involved in the response and shows that sensing and transduction of the initial change in osmolality is not dependent on an extracellular receptor for the agent used (e.g., sucrose or mannitol), as shown recently to be the case for succinate.4 The estimated number of granules recruited for exocytosis by a hypotonic extracellular challenge corresponded closely to the number that fused after receptor-dependent activation of cAMP formation.7 The existing data predict an involvement of water fluxes across the JG cell membrane, but aquaporin water channels have not been demonstrated in JG cells. Marked cell swelling normally initiates a regulatory volume decrease response whereby the cell, through coordinated activation of ion and organic osmolyte efflux, regains cell volume.8 A distinct role for phospholipase A2 (PLA2) and prostaglandin E2(PGE2) EP2 receptors in swelling-induced activation of regulatory processes in single cells has been demonstrated.9 Prostaglandin E2 and PGI2 enhance outward current and renin secretion from JG cells.10 In the study presented here, we hypothesized that JG cells respond to a decrease in extracellular osmolality by water uptake, Cyclooxygenase (COX)-dependent prostaglandin formation and renin release. The hypotheses were tested using single JG cells subjected to whole-cell patch-clamp analysis and primary cultures enriched in JG cells from rats, wild-type mice, and mice with targeted deletions of COX-2 and aquaporin-1 (AQP1).
Results
Effect of Reduced External Osmolality on Cell Capacitance and Current in Single JG Cells
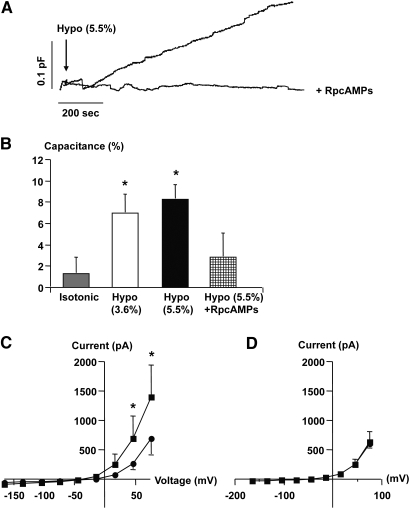
Reductions in extracellular osmolality (3.6 and 5.5%) significantly increased JG cell Cm (Figure 1, A and B) and whole-cell outward current (Figure 1C). The increase in Cm and outward current by reduced external osmolality (5.5%) was prevented by intracellular dialysis with a protein kinase A (PKA) inhibitor, RpcAMPs (5 μmol/L) (Figure 1, A, B, and D). In isolated superfused JG cells there was a rapid and significant increase in renin release to the medium by a 10 ± 1.7 mOsm/kg (approximately 3.3 ± 0.6%) reduction in osmolality of the external medium (Figure 2, A and B), whereas mock changes in superfusate had no effect on renin release (Figure 2B). In primary cultures enriched in JG cells, an acute reduction in extracellular osmolality led to a significant increase in cAMP accumulation after 10-min incubation (Figure 2C).
Figure 1.
Effect of hypotonic extracellular fluid on cell capacitance (Cm) changes in single rat JG cells. (A) Traces were obtained in single JG cells and show the effect of exposure to hypotonic fluid (arrow, −) on Cm without and with concomitant dialysis with a PKA inhibitor, RpcAMPs (+, 5 μmol/L). (B) The columns display average changes of Cm in response to hypotonic stimuli (Hypo) with and without intracellular RpcAMPs ± SEM. *P ≤ 0.05 at t = 0 versus t = 10 min. Isotonic: n = 10, Hypo: 3.6%, n = 5; 5.5%, n = 8; Hypo and RpcAMPs: n = 4. (C) The average [current-voltage (I-V)] relationship was determined immediately after the whole-cell configuration was obtained (filled circles). The measurement was repeated 10 min after introduction of a hypotonic stimulus (filled squares). *P ≤ 0.05 at t = 0 versus t = 10 min. (D) Average (I-V) relationship in JG cells measured immediately after the whole-cell configuration was obtained and after 10 min with RpcAMPs.
Figure 2.
(A) Results from a single experiment where rat renal cortical cells enriched in JG cells were mounted in columns and superfused with rapid collections of superfusate and subsequent measurement of renin concentration. A decrease in superfusate osmolality (−10 mOsmol/kg; open squares) and a mock change (filled circles) was introduced (arrow). (B) Renin release from superfused renal cortical cells enriched in JG cells at 300 s after a change in superfusion fluid to a superfusate with a lower osmolality (Hypo) or the same osmolality (isotonic). Values are mean ± SEM; n = 4 separate experiments. (C) Accumulation of cAMP in rat renal cortical cells enriched in JG cells. Cells were allowed to settle for 3 h and then medium was changed to media with reduced osmolality (Hypo, −5, 10, and 20%) as indicated. All media contained the phosphodiesterase inhibitor IBMX (0.1 mmol/L), n = 6 separate experiments with two wells assigned per condition in one experiment. *P ≤ 0.05 ANOVA and post hoc Dunnetts multiple comparison test.
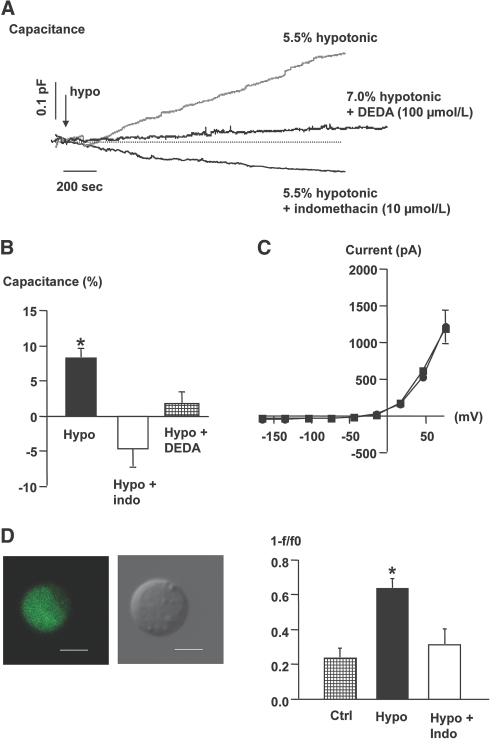
Role of Eicosanoid Pathway for Osmolality-Induced Cm Changes and Granule Trafficking in Single JG Cells
The PLA2 inhibitor 7,7-dimethyleicosadienoic acid (DEDA; 100 μmol/L, n = 4), was dialyzed into single JG cells. DEDA abolished the effect of a hypotonic challenge on Cm (Figure 3, A and B) and whole-cell currents (not shown). Next, JG cells were exposed to the nonselective COX inhibitor, indomethacin, before an osmotic challenge. Indomethacin (10 μmol/L) abolished the stimulatory effect of reduced extracellular osmolality on Cm (Figure 3, A and B) and whole-cell currents in single JG cells (Figure 3C). In a separate series of experiments with single JG cells, indomethacin (10 μmol/L) exposure did not by itself alter Cm significantly (1.3 ± 0.9%, n = 4, not shown). Single JG cells were loaded with quinacrine and fluorescence emission was recorded in response to osmotic perturbations. JG cells rapidly accumulated quinacrine in granules (Figure 3D). A reduced extracellular osmolality (−7%) led to a significant decrease in fluorescence emission from the JG cell compared with exchange of extracellular fluid with identical osmolality (Figure 3D). Pretreatment of JG cells with indomethacin abolished the ability of reduced extracellular osmolality to increase the loss of cellular quinacrine fluorescence as a measure for renin granule exocytosis (Figure 3D).
Figure 3.
(A) Recordings were obtained in single JG cells and show the effect of exposure to hypotonic fluid (arrow, −) on Cm without and with concomitant exposure of the cell to the COX inhibitor indomethacin (10 μmol/L) and a PLA2 inhibitor (DEDA) at 100 μmol/L. (B) The columns display average changes of Cm in response to a hypotonic stimulus (n = 8), a hypotonic stimulus with indomethacin (n = 4), and a hypotonic stimulus with DEDA ± SEM, n = 4. *P ≤ 0.05 at t = 0 versus t = 10 min. (C) The average current-voltage relationship was measured immediately after the whole-cell configuration was obtained (circles). The measurement was repeated 10 min after introduction of a hypotonic stimulus in cells exposed to indomethacin (squares), n = 4. (D) Direct visualization of renin release in single, isolated JG cells. Micrographs display confocal fluorescence image of a quinacrine-stained live JG cell (left) and the same cell as observed by phase-contrast microscopy (right). Bars represent 5 μm. The renin release rate was calculated as the ratio between start fluorescence (F0) and fluorescence after 40 frames (F). Data are shown as 1 − F/F0. The columns show change in quinacrine fluorescence in control cells (n = 6), in cells exposed to an acute reduction in extracellular osmolality (7%) in the absence (n = 5) and in the presence of indomethacin (10 μmol/L, n = 6). Values are mean ± SEM. *P ≤ 0.05 versus control.
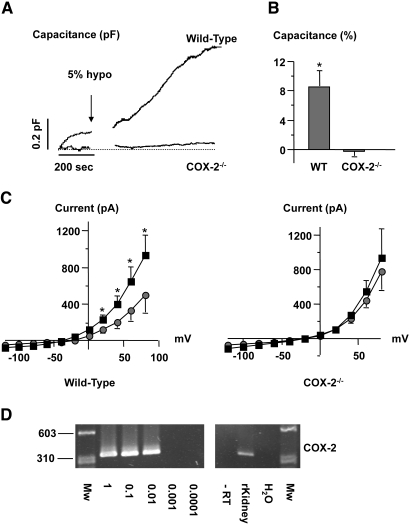
A reduction in extracellular osmolality of the fluid bathing JG cells harvested from COX-2−/− mice had no significant effect on Cm (Figure 4A). Whole-cell current-voltage relationship was also not altered by reduction in extracellular osmolality in JG cells from COX-2−/− mice (Figure 4C, right panel). JG cells were isolated from COX-2+/+ littermate mice. A reduction in external osmolality of 5% lead to a significant increase in Cm and whole-cell current (Figure 4, A and B) similar to the response observed in rat JG cells (Figure 1). In single JG cells sampled from wild-type mice with patch pipettes, PCR analysis showed expression of COX-2 mRNA (Figure 4D). PCR amplification of identical reaction mixtures without cDNA and amplification of RNA samples without reverse transcriptase were negative (Figure 4D). Next, JG cells from COX-2−/− mice were exposed to PGE2 (0.1 μmol/L). PGE2 led to a rapid and significant increase in Cm and whole-cell currents in JG cells from COX-2−/− mice (Figure 5A). An inhibitor of prostaglandin transport, bromcresol green (30 μmol/L), was used. Bromcresol green abolished the ability of reduced extracellular osmolality to alter Cm (Figure 5B). PCR amplification of cDNA from single JG cells sampled with patch pipette showed expression of prostaglandin transporter (PGT) and organic anion-transporting polypeptide D (OATP-D) mRNAs only in the presence of reverse transcriptase (Figure 5C).
Figure 4.
(A) Recordings were obtained in mouse JG cells and show the effect of hypotonic fluid (5%, arrow) on Cm in a single cell isolated from a C57Bl/6 wild-type mouse and a mouse with targeted deletion of COX-2 (COX-2−/−). (B) Columns display mean ± SEM changes of Cm in response to hypotonic stimuli in single JG cells isolated from C57Bl/6 wild-type mice (n = 4) and mice with targeted deletion of COX-2 (n = 4). *P ≤ 0.05 at t = 0 versus t = 10 min. (C) Left: The average (I-V) relationship was measured immediately after the whole-cell configuration was obtained in all JG cells from wild-type mice (filled circles). The measurement was repeated 10 min after introduction of a hypotonic stimulus (filled squares). Right: The average (I-V) relationship was measured immediately after the whole-cell configuration was obtained in all JG cells from COX-2−/− mice (filled circles). The measurement was repeated 10 min after introduction of a hypotonic stimulus (filled squares). (D) PCR amplification of cDNA from single, sampled JG cells for COX-2. Negative controls were omission of reverse transcriptase (−RT) and water instead of cDNA. Size marker is φX174DNA/HaeIII fragments.
Figure 5.
(A) The recording shows Cm in a single JG cell that was obtained from a COX-2−/− mouse and stimulated by PGE2 (0.1 μmol/L) (arrow). The right panel displays average changes of Cm in response to PGE2 in JG cells from COX-2−/− mice ± SEM. *P ≤ 0.05 at t = 0 versus t = 10 min (n = 5). (B) The recordings show Cm in single JG cells exposed to hypotonicity with and without an inhibitor of PGT, bromcresol green, in the bath fluid (30 μmol/L). Right panel displays average changes of Cm in response to hypotonicity (Hypo) with and without bromcresol green ± SEM. *P ≤ 0.05 at t = 0 versus t = 10 min, n = 5. (C) PCR amplification of serially diluted cDNA from single, sampled JG cells for PGT and OATP-D. Negative controls were omission of reverse transcriptase and amplification (−RT) and water instead of cDNA. Size marker is φX174DNA/HaeIII fragments.
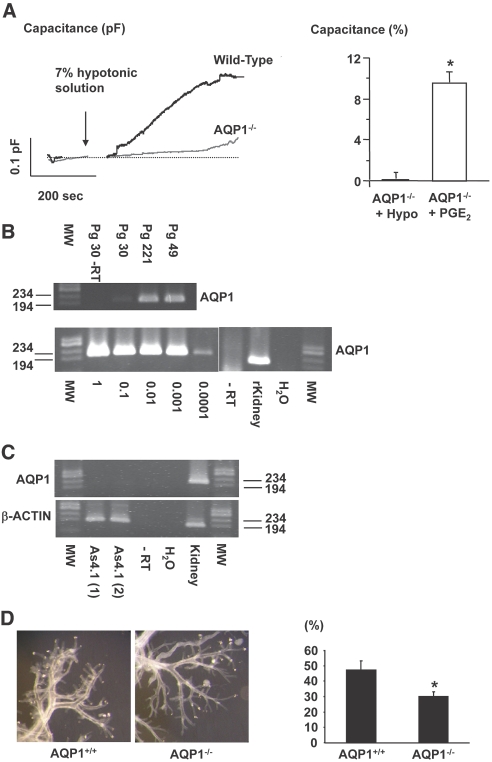
Involvement of AQP1 Water Channels in Osmolality-Induced Cm Changes in Single JG Cells
In JG cells harvested from AQP1−/− mice, a hypotonic challenge had no significant effect on Cm (0.11 ± 0.7%, Figure 6A, n = 4). Whole-cell currents did also not change in response to a reduced extracellular osmolality in JG cells from AQP1−/− (data not shown). Addition of PGE2 (0.1 μmol/L) from the extracellular side to single JG cells harvested from AQP1−/− mice yielded a significant increase in Cm (Figure 6A, n = 5) and whole-cell currents were enhanced (not shown). AQP1 was readily amplified from control mouse kidney and from three separate preparations of microdissected preglomerular vasculature from C57Bl/6 wild-type mice (Figure 6B, upper panel). RNA harvested from single JG cells sampled with patch pipettes was amplified by RT-PCR for AQP1 in a two-step protocol. An AQP1 amplification product with the expected size was detected (Figure 6B, lower panel). Negative controls with omission of reverse transcriptase yielded no product (Figure 6B, both panels). AQP1 was not detectable by PCR analysis in renin-expressing As4.1 cells of various passages and conditions, whereas β-actin was readily amplified (Figure 6C). cAMP (in pmol cAMP/well) was not significantly influenced by reductions in extracellular osmolality in As4.1 cells (control: 0.30 ± 0.03, 5%: 0.42 ± 0.07, 10%: 0.39 ± 0.06, 20%: 0.37 ± 0.07). Forskolin led to a significant (approximately 22 times) accumulation of cAMP in As4.1 cells (control: 0.30 ± 0.03 and forskolin: 6.8 ± 1.9). Dissection of whole renal vascular trees from acid-macerated AQP1−/− and wild-type mouse kidneys revealed no gross abnormalities with respect to overall dimensions, wall thickness, and degree of branching (Figure 6D). Quantitative evaluation of afferent arterioles for granular cells showed a significant reduction in the number of granulated afferent arterioles in AQP1−/− vessels (Figure 6D). Plasma renin concentration was not significantly different in AQP1−/− compared with wild-type mice (AQP1−/−: 1413 × 10−5 ± 423 mGU/ml, n = 5, wild-type: 979 × 10−5 ± 200 mGU/ml, n = 6).
Figure 6.
(A) Original traces of Cm recordings in single JG cells obtained from an AQP1 wild-type mouse and an AQP1−/− littermate mouse subjected to a hypotonic stimulus (arrow). Right panel displays average changes ± SEM of Cm in JG cells from AQP1−/− mice in response to a hypotonic stimulus (+Hypo, n = 4) and to PGE2 (0.1 μmol/L, n = 5) *P ≤ 0.05 at t = 0 versus t = 10 min. (B) PCR amplification of cDNA from microdissected mouse preglomerular vasculature (upper) and single, sampled JG cells for AQP1 (lower). “Pg” denotes preglomerular vasculature. Segments of cortical radial arteries with afferent arterioles were dissected. Numbers are the amount of vascular branching points contained in each preparation. Negative controls were omission of reverse transcriptase (−RT) and water instead of cDNA. Serially diluted cDNA from sampled JG cells (1 = cDNA from 1 JG cell) was amplified for AQP1 (lower). Size marker is φX174DNA/HaeIII fragments. (C) PCR amplification of cDNA from As4.1 cells for AQP1 and β-actin. Negative controls were omission of reverse transcriptase (−RT) and addition of water instead of cDNA. Size marker is φX174DNA/HaeIII fragments. (D) Microphotos display microdissected renal vascular trees from acid-macerated kidneys harvested from AQP1 wild-type and knockout mice. Columns show the proportion of afferent arterioles containing JG cells (in %) in wild-type and AQP1 knockout mice. Columns show mean ± SEM, n = 6. *P < 0.05.
Discussion
The study presented here used whole-cell patch clamp, superfusion with rapid collection and quinacrine fluorescence measurements on single JG cells to provide direct evidence for a novel pathway by which slight reductions in extracellular osmolality (4 to 7%) lead to cAMP accumulation, PKA-dependent enhanced outward current and an increase in cell capacitance (Cm) associated with rapid renin release. The study shows that the osmotically induced exocytotic response in JG cells requires AQP1, PLA2, and COX-2 activity and depends on prostaglandin transport. The set of data is compatible with an AQP1-dependent, water-conductive route that accounts for osmotic water influx and subsequent COX-2-mediated prostaglandin formation, prostaglandin efflux, and autocrine stimulation of renin release. Within the tested hypotonic challenges that ranged from 3.6 to 7% reduction in osmolality, Cm responses did not differ significantly. This probably reflects fusion of a readily releasable granule pool already at a 3% decrease in external osmolality. The recorded changes in Cm after a hypo-osmotic challenge were not different between mice and rat JG cells and correspond to fusion of approximately 10 granules.11 The observations of renin release in response to hypotonic superfusate with similar kinetics as the recorded changes in Cm and the loss of quinacrine fluorescence from single JG cells by slight hypotonicity both corroborate that Cm is a valid measure of granule trafficking that reflects renin release from JG cells.
The data predict involvement of COX-2-derived prostaglandins with Gs-dependent coupling to adenylyl cyclase in the stimulus-secretion pathway initiated by a hypotonic challenge. PGE2 and PGI2 are likely candidates that activate cAMP-coupled EP2, EP4, and IP receptors on JG cells and enhance potassium current carried through the cAMP-sensitive “zero” variant of the calcium-sensitive voltage-activated potassium (BKCa) channels.11,12 The data presented here show that cAMP/PKA is crucial for transduction of the hypotonicity-induced renin secretory and current response. In agreement with this finding, renin release after a hypotonic challenge is not additive to the response elicited by intracellular application of cAMP.7 The existence of a similar pathway was recently demonstrated in murine fibroblasts, in which a cell volume regulatory response involved PGE2-mediated stimulation of an EP2 receptor and cAMP/PKA activity.13 In contrast to several hormone-secreting cells,14 intracellular free calcium concentration is not sensitive to stretch or cell swelling in JG cells.15
An increase in cAMP in JG cells leads to hyperpolarization,7 which appears to be a common feature for stimuli (including a hypotonic challenge) that engage the stimulatory cAMP/PKA pathway. Depolarization of the JG cell membrane potential activates voltage-gated calcium channels and calcium influx, which inhibits cAMP-mediated exocytosis of renin.12 We have shown that hyperpolarization indirectly supports exocytosis of renin by stabilizing membrane voltage in a range far from threshold for calcium channel activation and thus inhibition of renin release.12 The series of events between a hypotonic challenge and COX-2 activity in JG cells is likely to include cell swelling and PLA2 activation as observed in several cell types.9 Hypotonicity-induced stimulation of COX-2 activity in cultured cells has been observed previously.16 Indomethacin inhibits not only COX activity but also prostaglandin transport.17 JG cells from COX-2−/− mice yielded data similar to those obtained with use of indomethacin, which shows that COX-2 activity is necessary for exocytosis initiated by reduced external osmolality in single JG cells. The complete exocytotic machinery and sensitivity to PGE2 was intact in isolated JG cells from COX-2−/− mice as evidenced by an increase of Cm in response to PGE2.10 COX-2-mediated release of prostanoids from the macula densa and cortical thick ascending limb cells is believed to account for stimulation of renin secretion, increased renin mRNA abundance, and renin cell recruitment in several conditions with altered NaCl transport rate.18–22 The data presented here show that very modest changes in JG interstitial fluid osmolality may have a COX-2-dependent effect on renin release directly at the level of the JG cell and independent of the macula densa. This notion of JG cell prostaglandin production and efflux is supported by the present demonstration of COX-2 and PGT mRNAs encoding PGT and OATP-D in single JG cells and previous data, which show COX-2 and PGT in renal microvessels23,24 Do variations in interstitial JG osmolality occur in vivo? In the freshwater salamander Amphiuma, the interstitial concentration of chloride can reach levels significantly above isotonic,5 and mathematical models show that localized variation in interstitial NaCl concentration may occur in the mammalian JG apparatus.25 It is therefore possible that the present mechanism may be relevant for control of renin secretion during physiologic conditions.
The data presented here show that AQP1 is a major route for osmotically induced water flux in JG cells as observed in vascular smooth muscle cells.26 AQP1 was expressed in preglomerular vessels, as reported previously,27,28 and in single JG cells. The finding of fewer granulated arterioles in adult AQP1−/− mice and plasma renin concentration values not different from wild-type mice is opposite to an expected reactive stimulation caused by exaggerated NaCl and water losses in AQP1−/− mice.29 At the subcellular level, AQP1 is associated with granules in secretory cells,30 and the absence of AQP1 impairs secretory granule biogenesis in an array of endocrine cell types and results in lower hormone levels in plasma.31 However, receptor coupling through Gs and cAMP/PKA-mediated exocytosis was intact in isolated JG cells from AQP1−/− mice. In renin-producing As4.1 cells, we found no expression of AQP1 and a hypotonic challenge failed to evoke cAMP formation. It therefore appears that AQP1 is a prerequisite for osmotic sensitivity. In agreement, Peti-Peterdi et al. observed no effect on granule trafficking in live As4.1 cells by a hypotonic challenge and in general, As4.1 cells do not display regulated exocytosis.32 In embryonic and early postnatal life, there is widespread expression of AQP1 along large parts of the preglomerular vasculature28 similar to the distribution of renin. It is an intriguing possibility that lack of AQP1 impairs renin granule processing and release and that AQP1 activity contributes to development and maintenance of the fully differentiated JG cell phenotype.
In summary, the study provides direct evidence for a novel pathway by which modest hypotonic challenges evoke AQP1, PLA2, and COX-2-dependent exocytosis of renin granules from JG cells through stimulation of the cAMP/PKA pathway. This new pathway may be relevant for cell-volume-initiated effects in general and may provide a necessary step in the pathway that leads to physiologic changes in renin release and renin cell recruitment after changes in epithelial NaCl transport rate.
Concise Methods
Animals
All procedures conformed to the Danish national guidelines for the care and handling of animals and the published guidelines from the National Institutes of Health. Male Sprague-Dawley rats (60 to 80 g) were housed at The University of Southern Denmark on a 12:12 h light:dark cycle and had free access to standard pathogen-free rat chow (Altromin-1310, Lage, Germany; Na+ 2 g/kg, Cl− 5 g/kg) and tap water. COX-2 knockout mice were obtained from Jackson Laboratories on a predominant C57bl/6 background and further backcrossed for one generation (C57Bl/6) before experiments. Genotyping was done with DNA from tail biopsies using the REDExtract-N-Amp tissue PCR kit (Sigma). PCR used the following primers: 5′-GCC-CTG-AAT-GAA-CTG-CAG-GAC-G-3′, 5′-CAC-GGG-TAG-CCA-ACG-CTA-TGT-C-3′, 5′-CAC-CAT-AGA-ATC-CAG-TCC-GG-3′ and 5′-ACC-TCT-GCG-ATG-CTC-TTC-c-3′. AQP1 mice were a kind gift from Dr. Alan Verkman.29 They were bred at the University of Aarhus animal facility on a CD1 background and genotyped with the following primers: AQP1_KO_R: 5′-CTC-TAT-GGC-TTC-TGA-GGC-GGA-AAG-3′, AQP1_wt_F: 5′-ACT-CAG-TGG-CTA-ACA-ACA-AAC-AGG-3′, AQP1_wt_R: 5′-AAG-TCA-ACC-TCC-GCT-CAG-CTG-GG-3′.
Cell Culture
JG cells were isolated from rat and mouse renal cortices as described previously.7,11 For patch clamp, cells were transferred to cover slips in RPMI-1640 medium. Superfusion of JG cells and measurement of renin was done as described.11 A specific condition was tested on no more than one cell from one preparation; for example, n = 5 means recordings from five single cells from five different animals. As4.1 cells were grown according to ATCC instructions. As4.1 cell medium was replaced by DMEM containing 2% FCS 24 h before experiments.
Patch-Clamp Experiments
The patch-clamp experiments were performed as described7,11 with the following internal solution (in mmol/L): potassium glutamate 135; NaCl 10; potassium chloride 10; magnesium chloride 1; Hepes-sodium hydroxide 10; magnesium-ATP 0.5; sodium-GTP 0.3; osmolality was 303 ± 2.1 mOsm/kg; pH 7.07 with or without RpcAMPs (5 μmol/L). External solution was Hepes-sodium hydroxide 10; NaCl 140; potassium chloride 2.8; magnesium chloride 1; calcium chloride 2; glucose 11; sucrose 10; osmolality was 300 ± 1.4 mOsm/kg, pH 7.25 with or without agonists/antagonists. Hypotonic solution was prepared as for the external solution with the omission of sucrose (osmolality: 290 ± 4 mOsm/kg).
Laser-Scanning Confocal Microscopy of Quinacrine-Loaded JG Cells
JG cells were loaded with quinacrine (Sigma) essentially as described.32 Confocal laser scanning fluorescence microscopy (Olympus FV1000, Hamburg, Germany) was performed using a 20× (numerical aperture, 0.5) Olympus water immersion objective. The scanning area was set to 512 × 512 pixels with internal zoom. Full-frame imaging was performed at 0.1 Hz using excitation from a laser at 488 nm with fluorescence monitored through a 500- to 600-nm band pass (AOTF). The renin release rate was calculated as ratio between start fluorescence (F0) and fluorescence after 40 frames (F). Data are shown as 1-F/F0.
RT-PCR Analysis of Sampled JG Cells
Single JG cells were sampled with patch pipettes. Total RNA was isolated by phenol-chloroform extraction and used as template for RT-PCR analysis.7 Primer sequences were: AQP1: sense 5′-CGG-GAT-CCG-CAA-CTT-CTC-AAA-CCA-CT-3′ antisense 5′-GGA-ATT-CAT-TTG-GGC-TTC-ATC-TCC-A-3′; PGT: sense 5′-AGA-TGG-TCT-ACA-AGG-CCT-3′ antisense 5′-GAG-GAA-GGC-ATG-TTG-ACA-3′; OATP-D sense 5′-CAG-CCC-TGA-ACT-CAA-3′ antisense 5′-GTA-CAG-GTA-TCT-GTA-GAC-3′. COX-2 primers were as published.33
Determination of cAMP
JG cells and As4.1 cells were cultured as described above. Medium was changed to physiologic saline solution (in mmol/L): NaCl 85, NaHCO3 25, MgSO4 1.2, K2HPO4 2.5, glucose 5.5, sucrose 60, pH 7.4, osmolality 284 mOsm/kg) for 1 h. Then cells incubated for 10 min at 37°C in physiologic saline solution with reduced osmolality (−5%, −10%, −20%) that was obtained by removal of sucrose. In all experiments the phosphodiesterase inhibitor IBMX (100 μmol/L) was included. cAMP was measured as described.11
Quantification of JG Cell Index
Dissection of renal vascular trees was performed as described by Casellas et al.34 Renal vascular trees were microdissected and total afferent arterioles and renin-positive arterioles were counted in four to five vessel trees from each animal in an observer-blinded fashion.
Chemicals
Hepes, Tris-HCl, glucose, sucrose, insulin, penicillin, streptomycin, potassium glutamate, IBMX, magnesium-ATP, sodium-ATP, forskolin, dithiothreitol, PGE2, and trypsin were from Sigma Chemical Co., St. Louis, MO. RPMI 1640 and FCS were from Life Technologies. Collagenase A, sodium-GTP, and cAMP were from Roche. RpcAMPs was from Biomol. DEDA was from Alexis, San Diego, CA. Stock solutions of ligands were made in ethanol or DMSO and kept at −20°C.
Statistics
All values are given as mean ± SEM. Paired t test was used to estimate statistical significant difference from zero in experiments in which Cm was measured. The change in Cm was calculated as the difference (in %) in Cm (at t = 0 min and t = 10 min). ANOVA was used to determine whether there was statistical significance among several groups of data. Post hoc test was performed by unpaired t test. P < 0.05 was considered statistically significant.
Disclosures
None.
Acknowledgments
Dr. Janos Peti-Peterdi is thanked for valuable advice. The authors thank Annette K. Rasmussen, Vibeke Nielsen, Inge Andersen, Gitte Dybmose, and Mette Fredenslund for excellent technical assistance. The present work was supported by grants from Carlsbergfondet, the Danish Research Council for Health and Disease (B.L.J.: 271-07-0635; O.S.: 271-05-0258 and 271-06-0439), the Danish Heart Foundation (UGF0410B151, A229-22200), Helen and Ejnar Bjørnows Foundation, The Foundation for the Promotion of Medical Science, the NOVO Nordisk Foundation, the Lundbeck Foundation, and the Foundation of 17.12.1981.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Frederiksen O, Leyssac PP, Skinner SL: Sensitive osmometer function of juxtaglomerular cells in vitro. J Physiol (Lond) 252: 6669–6679, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skøtt O: Do osmotic forces play a role in renin secretion? Am J Physiol 255: F1–F10, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Kurtz A, Schweda F: Osmolarity-induced renin secretion from kidneys: evidence for readily releasable renin pools. Am J Physiol Renal Physiol 290: F797–F805, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Toma I, Kang JJ, Sipos A, Vargas S, Bansal E, Hanner F, Meer E, Peti-Peterdi J: Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J Clin Invest 118: 2526–2534, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Persson BE, Sakai T, Marsh DJ: Juxtaglomerular interstitial hypertonicity in Amphiuma: Tubular origin-TGF-signal. Am J Physiol 254: F445–F449, 1988 [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez E, Salomonsson M, Müller-Suur C, Persson AEG: Measurements of macula densa cell volume changes in isolated and perfused rabbit cortical thick ascending limb. II. Apical and basolateral cell osmotic water permeabilities. Acta Physiol Scand 133: 159–166, 1988 [DOI] [PubMed] [Google Scholar]
- 7.Friis UG, Jensen BL, Aas JK, Skøtt O: Direct demonstration of exocytosis and endocytosis in single mouse juxtaglomerular cells. Circ Res 84: 929–936, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann EK, Pedersen SF: Sensors and signal transduction in the activation of cell volume regulatory ion transport systems. Contrib Nephrol 123: 50–78, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Lambert IH, Hoffmann EK, Christensen P: Role of prostaglandins and leukotrienes in volume regulation by Ehrlich ascites tumor cells. J Membr Biol 98: 247–256, 1987 [DOI] [PubMed] [Google Scholar]
- 10.Friis UG, Stubbe J, Uhrenholt TR, Svenningsen P, Nüsing R, Skøtt O, Jensen BL: Prostaglandin E2 EP2 and EP4 receptor activation mediates cAMP-dependent hyperpolarization and exocytosis of renin in juxtaglomerular cells. Am J Physiol 289: F989–F997, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Friis UG, Jensen BL, Sethi S, Andreasen D, Hansen PB, Skøtt O: Control of renin secretion from rat juxtaglomerular cells by cAMP-specific phosphodiesterases. Circ Res 90: 996–1003, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Friis UG, Jørgensen F, Andreasen D, Jensen BL, Skøtt O: Molecular and functional identification of cyclic AMP-sensitive BKCa potassium channels (ZERO variant) and L-type voltage-dependent calcium channels in single rat juxtaglomerular cells. Circ Res 93: 213–220, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Heacock AM, Foster DJ, Fisher SK: Prostanoid receptors regulate the volume-sensitive efflux of osmolytes from murine fibroblasts via a cyclic AMP-dependent mechanism. J Pharmacol Exp Ther 319: 963–971, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Strbák V, Greer MA: Regulation of hormone secretion by acute cell volume changes: Ca(2+)-independent hormone secretion. Cell Physiol Biochem 10: 393–402, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Scholz H, Jurk S, Kurtz A: Hypotonic swelling or stretch does not change cytosolic calcium in mouse renal juxtaglomerular cells. Acta Physiol Scand 157: 283–287, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Lundgren DW, Moore RM, Collins PL, Moore JJ: Hypotonic stress increases cyclooxygenase-2 expression and prostaglandin release from amnion-derived WISH cells. J Biol Chem 272: 20,118–20,124, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Bito LZ, Salvador EV: Effects of anti-inflammatory agents and some other drugs on prostaglandin biotransport. J Pharmacol Exp Ther 198: 481–488, 1976 [PubMed] [Google Scholar]
- 18.Traynor TR., Smart A, Briggs JP, Schnermann J: Inhibition of macula densa-stimulated renin secretion by pharmacological blockade of cyclooxygenase-2. Am J Physiol Renal 277: F706–F710, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Yang T, Endo Y, Huang YG, Smart A, Briggs JP, Schnermann J: Renin expression in COX-2-knockout mice on normal or low-salt diets. Am J Physiol Renal Physiol 279: F819–F825, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Yang T, Park JM, Arend L, Huang Y, Topaloglu R, Pasumarthy A, Praetorius H, Spring K, Briggs JP, Schnermann J: Low chloride stimulation of prostaglandin E2 release and cyclooxygenase-2 expression in a mouse macula densa cell line. J Biol Chem 275: 37,922–37,929, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Cheng HF, Wang JL, Zhang MZ, McKanna JA, Harris RC: Role of p38 in the regulation of renal cortical cyclooxygenase-2 expression by extracellular chloride. J Clin Invest 106: 681–688, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peti-Peterdi J, Komlosi P, Fuson AL, Guan Y, Schneider A, Qi Z, Redha R, Rosivall L, Breyer MD, Bell PD: Luminal NaCl delivery regulates basolateral PGE2 release from macula densa cells. J Clin Invest 112: 76–82, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Therland KL, Stubbe J, Thiesson HC, Ottosen PD, Walter S, Sorensen GL, Skott O, Jensen BL: Cyclooxygenase-2 is expressed in vasculature of normal and ischemic adult human kidney and is colocalized with vascular prostaglandin E2 EP4 receptors. J Am Soc Nephrol 15: 1189–1198, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Adachi H, Suzuki T, Abe M, Asano N, Mizutamari H, Tanemoto M, Nishio T, Onogawa T, Toyohara T, Kasai S, Satoh F, Suzuki M, Tokui T, Unno M, Shimosegawa T, Matsuno S, Ito S, Abe T: Molecular characterization of human and rat organic anion transporter OATP-D. Am J Physiol Renal Physiol 285: F1188–F1197, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Moore LC, Iijima K, Rich A, Casellas D, Goligorsky MS: Communication of the tubuloglomerular feedback signal in the JGA. Kidney Int 32: S45–S50, 1991 [PubMed] [Google Scholar]
- 26.Shanahan CM, Connolly DL, Tyson KL, Cary NRB, Osbourn JK, Agre P, Weissberg PL: Aquaporin-1 is expressed by vascular smooth muscle cells and mediates rapid water transport across vascular cell membranes. J Vasc Res 36: 353–362, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Nielsen S, Smith BL, Christensen EI, Agre P: Distribution of aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci U S A 90: 7275–7279, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Kim W-Y, Han K-H, Knepper MA, Nielsen S, Madsen KM: Developmental expression of aquaporin 1 in the rat renal vasculature. Am J Physiol 276: F498–F509, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS: Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J Biol Chem 273: 4296–4299, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Cho SJ, Sattar AK, Jeong EH, Satchi M, Cho JA, Dash S, Mayes MS, Stromer MH, Jena BP: Aquaporin 1 regulates GTP-induced rapid gating of water in secretory vesicles. Proc Natl Acad Sci U S A 99: 4720–4724, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnaoutova I, Cawley NX, Patel N, Kim T, Rathod T, Loh YP: Aquaporin 1 is important for maintaining secretory granule biogenesis in endocrine cells. Mol Endocrinol 22: 1924–1934, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peti-Peterdi J, Fintha A, Fuson AL, Tousson A, Chow RH: Real-time imaging of renin release in vitro. Am J Physiol Renal Physiol 287: F329–F335, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Stubbe J, Madsen K, Nielsen FT, Bonde RK, Skøtt O, Jensen BL: Postnatal adrenalectomy impairs urinary concentrating ability by increased COX-2 and leads to renal medullary injury. Am J Physiol Renal Physiol 293: F780–F7890, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Casellas D, Dupont M, Kaskel FJ, Inagami T, Moore LC: Direct visualization of renin-cell distribution in preglomerular vascular trees dissected from rat kidney. Am J Physiol 265: F151–F156, 1993 [DOI] [PubMed] [Google Scholar]