Abstract
Early events in kidney organogenesis involve reciprocal interactions between the ureteric bud and the metanephric mesenchyme, which lead to remodeling of the extracellular matrix. This remodeling involves matrix metalloproteases (MMPs), but the specific roles of individual MMPs in kidney development are not completely understood. Here, we analyzed MMP9-deficient mice at the first step of kidney development and found that MMP9 deficiency delayed embryonic kidney maturation and increased apoptosis ex vivo by 2.5-fold. These early defects resulted in a 30% decrease in nephron number, a 20% decrease in adult kidney weight, and altered kidney function and morphology at 12 mo. The membrane form of stem cell factor (SCF) increased, whereas the activated form of the SCF receptor, c-kit, decreased in MMP9-deficient embryonic kidneys. In organotypic culture, MMP9-deficient kidneys failed to secrete SCF, and addition of recombinant SCF partially rescued both apoptosis and the branching defect. In conclusion, these data show that MMP9 protects mesenchymal cells from apoptosis during kidney development and stimulates ureteric bud branching morphogenesis, most likely by releasing the soluble form of SCF, suggesting that normal renal development requires MMP9.
The developing kidney is the product of reciprocal inductive interactions between the ureteric bud and its surrounding mesenchyme. These interactions are associated with the expression of extracellular matrix (ECM) components that play a crucial role in the establishment of cell polarity and epithelial phenotype of the mesenchyme and in the branching morphogenesis of the ureteric bud.1 The role of ECM in branching morphogenesis was already pointed out 40 yr ago by Grobstein and Cohen,2 who showed that collagenase inhibited embryonic salivary gland branching.
Among the different families of proteases, special attention has been given to matrix metalloproteases (MMPs) and their inhibitors, the tissue inhibitors of metalloproteases (TIMPs). MMP2 and MMP9, which both belong to the type IV collagenase subfamily, are expressed early in the metanephros.3 We first showed in an organotypic culture model that MMP9, but not MMP2, is required for ureteric bud branching at 11 d, the first step of metanephric development in the mouse.3 However, MMP2 and its receptor MT1-MMP are involved 2 d later, at 13 d of mouse embryonic development.4 In the rat, TIMP2, a MMP2 inhibitor, also inhibits branching morphogenesis at 13 d (corresponding to 12 embryonic d in the mouse).5 In vitro studies using isolated ureteric buds grown in ECM gels confirmed the role of MMPs because ureteric bud branching was markedly reduced by a synthetic inhibitor of MMPs and by TIMP2.6 In addition, Pohl et al.6 showed that the pattern of expression of MMPs and their inhibitors depends on the specific stage of ureteric bud branching.6 Taken together with our findings3 and those of other studies,4,5 these data suggest that distinct MMPs are implicated at different steps of branching morphogenesis, which may account for the absence of obvious kidney developmental defect in MMP9-, MMP2-, and MT1-MMP–deficient mice.7
The principal substrate of MMP9 is type IV collagen, but MMP9 can degrade other basement membrane molecules and nonmatrix components.8 We have previously shown that crescentic proliferative glomerulonephritis and acute kidney injury were more severe in MMP9-deficient mice because of the ability of MMP9 to cleave fibrin9 and to release soluble stem cell factor from kidney cells.10
In this study, we investigated the renal phenotype of MMP9-deficient mice and observed subtle defects of branching morphogenesis associated with a 2.5-fold increase in mesenchymal apoptosis during the first steps of kidney development. These defects induced a 30% reduction in final nephron number in adult, thus providing the first evidence that MMP9 controls nephron formation in vivo. Because MMP9 releases soluble stem cell factor (sSCF), the c-kit ligand,11 and because the SCF/c-kit system has an important antiapoptotic role during acute kidney injury,10 we studied the expression and role of that system. Results suggest that MMP9 favors ureteric bud branching and protects mesenchyme from apoptosis by releasing the soluble form of SCF at the first stage of kidney development.
Results
MMP9 Deficiency Delays Embryonic Kidney Maturation, Decreases Nephron Number in Adult Kidney, and Impairs Renal Function in Old Mice
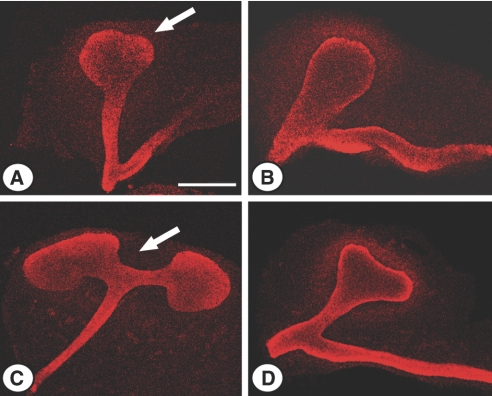
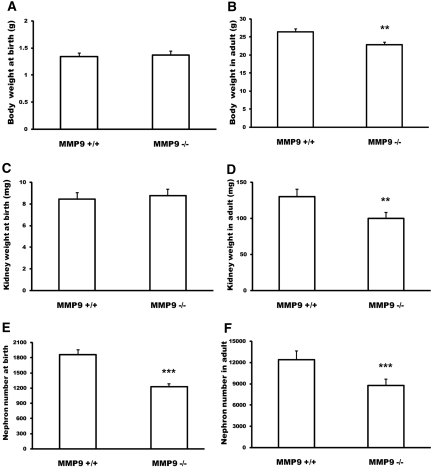
We first compared E11.5 and E12.5 kidneys of control and MMP9-deficient littermates. We observed a delay in MMP9-deficient embryonic kidney maturation. Ureteric bud, stained with calbindin D-28K (Figure 1, arrow), did not initiate division at E11.5 (Figure 1, B versus A) and did not show the T-shaped characteristic aspect at E12.5 (Figure 1, D versus C). At birth, body weight and kidney weight were similar in control and MMP9-deficient mice (Figure 2, A and C, respectively), suggesting no intrauterine growth retardation in MMP9-deficient mice. However, we observed a 30% decrease in the nephron number in newborn MMP9-deficient kidneys (Figure 2E), which was most likely caused by the dysmorphogenesis of ureteric bud branching at the early stages of kidney development (Figure 1). The nephron deficit is definitive because the number of nephrons is also reduced by about 30% in adult MMP9-deficient kidneys (Figure 2F). Adult kidney weight (Figure 2D) and total body weight (Figure 2B) were both decreased by 20% in MMP9-deficient mice, whereas the ratio kidney weight/body weight was similar in both groups of animals (data not shown).
Figure 1.
Effect of MMP9 deficiency on embryonic kidney maturation. Representative photographs of E11.5 (A and B) and E12.5 (C and D) kidneys from MMP9+/+ (A and C) and MMP9−/− mice (B and D) stained with calbindin D-28K to delineate ureteric bud (arrow). Please note the defect in ureteric bud elongation and branching in MMP9−/− embryonic kidney. Bar: 250 μm.
Figure 2.
Effect of MMP9 deficiency on mouse body weight, kidney weight, and nephron number. (A and B) Body weight of newborn (A) and adult (B) MMP9+/+ and MMP9−/− kidneys. (C and D) Kidney weight of newborn (C) and adult (D) MMP9+/+ and MMP9−/− kidneys. Kidney weight and body weight were similar at birth, whereas they were significantly decreased in adult MMP9−/− mice. (E and F) Nephron number in newborn (E) and adult (F) MMP9+/+ and MMP9−/− kidneys. Values are mean ± SEM. ***P < 0.001 and **P < 0.01 versus MMP9+/+ mice.
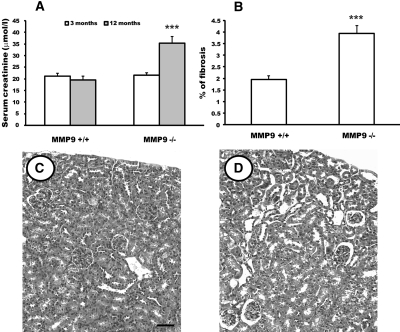
Renal function was normal in control and MMP9-deficient mice at 3 (Figure 3A) and 9 mo (data not shown) of age. However, at 12 mo, MMP9-deficient mice had developed renal failure (Figure 3A), whereas renal function remained unaltered in control mice. Lesions were characterized by increased percentage of fibrosis (Figure 3B) and tubular dilation (Figure 3, D versus C), which might be caused by compensatory mechanisms to the nephron deficit and to the absence of MMP9.
Figure 3.
Effect of MMP9 deficiency on renal function, kidney fibrosis, and kidney morphology. (A) Serum creatinine level of MMP9+/+ and MMP9−/− kidneys at 3 (open bar) and 12 mo (gray bar). (B) Percentage of fibrosis in MMP9+/+ and MMP9−/− kidney sections at 12 mo. Five microphotographs (magnification, ×20) were taken from ten different MMP9−/− and MMP9+/+ kidneys. (C and D) Photomicrographs of representative paraffin kidney sections from MMP9+/+ (C) and MMP9−/− (D) mice stained with hematoxylin and eosin at 12 mo. Note that dilated tubules were observed in MMP9−/− kidney. Values are mean ± SEM. ***P < 0.001 versus MMP9+/+ mice; n = 20. Bar: 170 μm.
MMP9 Deficiency Increases Apoptosis Ex Vivo and In Vitro
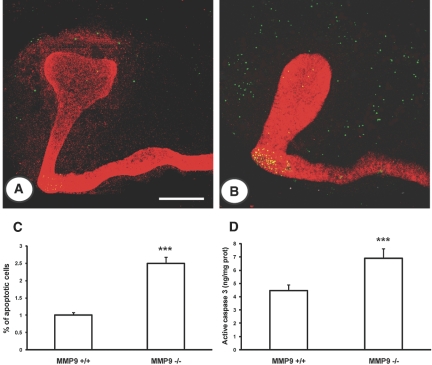
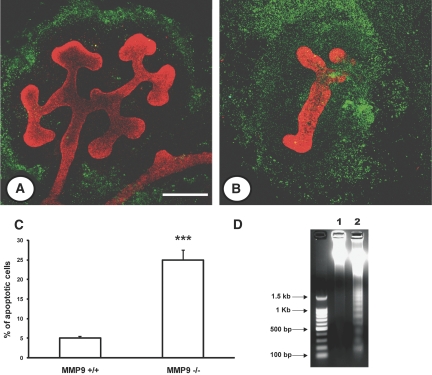
Apoptosis was evaluated ex vivo and in vitro by different techniques. We first used the terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) method and verified that nuclei stained by this technique were morphologically apoptotic with condensed chromatin and that they were not proliferating cell nuclear antigen positive (data not shown). We observed that the defect in ureteric bud branching and delay in maturation was associated with a greater extent of apoptosis in the mesenchyme of E11.5 (Figure 4, B versus A) and E12.5 kidneys (data not shown). The percentage of apoptotic nuclei was increased by 2.5-fold in E11.5 kidneys (Figure 4C). We assessed apoptosis by measuring the concentration of active caspase-3 in E11.5 kidneys and showed that active caspase-3 was increased by almost twofold in MM9-deficient embryonic kidneys compared with controls (Figure 4D).
Figure 4.
Effect of MMP9 deficiency on apoptosis ex vivo. (A and B) Representative photographs of E11.5 kidneys from MMP9+/+ (A) and MMP9−/− mice (B) stained with calbindin D-28K to delineate ureteric bud in red and TUNEL method (apoptotic nuclei in green). (C) Percentage of apoptotic nuclei in E11.5 kidneys. Microphotographs were taken from 10 different MMP9+/+ and MMP9−/− kidneys. (D) Concentration of active caspase-3 in E11.5 kidneys, n = 50. Note that apoptosis assessed by different methods was increased in MMP9-deficient mesenchyme. Values are mean ± SEM. ***P < 0.001 versus MMP9+/+-injected mice. Bar: 250 μm.
The change of maturation and apoptosis of MMP9-deficient kidneys were exacerbated in vitro. The ureteric bud of E11.5 kidney failed to branch after 48 h in culture, whereas a mean of 12 ± 1.2 divisions was observed in normal kidneys. The branching defect was associated with a massive apoptosis (Figure 5, B versus A). The percentage of apoptotic cells was increased by fivefold in the MMP9-deficient kidney compared with control kidney (Figure 5C), and the genomic DNA exhibited a laddering profile characteristic of apoptosis that was not observed in controls (Figure 5D, lane 2 versus lane 1).
Figure 5.
Effect of MMP9 deficiency on apoptosis in vitro. (A and B) Representative photographs of E11.5 kidneys from MMP9+/+ (A) and MMP9−/− mice (B) grown for 48 h in culture. Ureteric bud is stained with calbindin D-28K (red) and apoptotic nuclei by the TUNEL method (green). (C) Percentage of apoptotic nuclei in E11.5 kidneys grown for 48 h. (D) Genomic DNA extracted from E11.5 control (lane 1) and MMP9−/− kidney (lane 2) grown for 48 h in culture. The migration profile in lane 2 is characteristic of apoptosis. Values are mean ± SEM. ***P < 0.001 versus MMP9+/+ mice, n = 20. Bars: (A and B) 250 and (E and F) 25 μm.
Expression of SCF and Its Receptor c-kit Are Modulated by MMP9
We next asked what could be the critical substrate for MMP9 that mediates its antiapoptotic activity.
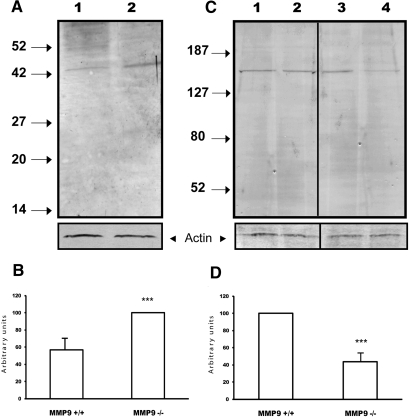
We first examined SCF expression. Data obtained on E11.5 and E12.5 (data not shown) kidney extracts showed that a band, corresponding to the expected molecular weight (45 kD) of the membrane form of SCF, was observed in cell extracts of control and MMP9-deficient kidneys (Figure 6A), but its expression related to β-actin expression was increased by 40% in MMP9-deficient kidneys (Figure 6, A, lane 1 versus lane 2, and B).
Figure 6.
Effect of MMP9 deficiency on SCF (A and B) and c-kit (C and D) expression in E11.5 kidneys. (A) Representative SCF Western blot performed with 20 μg of total proteins from MMP9+/+ (lane 1) and MMP9−/− (lane 2) kidneys. (B) Quantitative analysis of five blots, using β-actin as an internal control, showed a 50% increase of SCF protein expression in MMP9−/− kidneys. (C) Representative Western blots detecting c-kit (lanes 1 and 2) and phospho c-kit (lanes 3 and 4) in 20 μg total proteins extracts from MMP9+/+ (lanes 1 and 3) and MMP9−/− (lanes 2 and 4) kidneys. (D) Phospho c-kit expression related to β-actin expression. Note that c-kit expression is not modified by MMP9 deficiency, whereas phospho c-kit expression is reduced by 60% in MMP9−/− kidneys. Values are mean ± SEM. ***P < 0.001 versus MMP9+/+ mice.
The SCF receptor, c-kit, was observed in equal quantity in E11.5 control (Figure 6C, lane 1) and MMP9-deficient (Figure 6C, lane 2) kidneys. However, the intensity of activated c-kit related to β-actin was lower in MMP9-deficient (Figure 6C, lane 4) kidneys compared with control kidneys (Figure 6C, lane 3). Scanning of the Western blots, using β-actin as an internal control, showed a 60% decrease of activated c-kit in MMP9-deficient kidneys (Figure 6D).
MMP9 Releases the Soluble Form of SCF in Embryonic Kidney
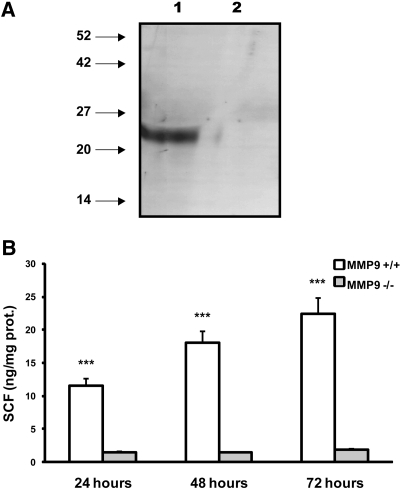
We asked whether MMP9 was able to cleave membranous SCF (Figure 7A). We verified the proteolytic effect of MMP9 on SCF by Western blot and by measuring the concentration of sSCF released in the culture medium of 10 E11.5 kidneys grown for 24 h. Results obtained by Western blotting showed that a band corresponding to the expected molecular weight of sSCF (23 kD) was observed in concentrated media from control kidneys (Figure 7A, lane 1) but not from MMP9-deficient kidneys (Figure 7A, lane 2) or in control concentrated media maintained at the culture temperature in the absence of kidney (data not shown). Similarly, increasing amounts of SCF could be detected in culture media obtained from E11.5 control kidneys grown for 24, 48, and 72 h. MMP9 deficiency almost totally inhibited the release of SCF in culture medium (Figure 7B).
Figure 7.
Sensitivity of membrane SCF to MMP9 proteolysis in vitro. (A) Representative SCF Western blot performed with concentrated medium obtained from 10 E11.5 MMP9+/+ (lane 1) or MMP9−/− (lane 2) kidneys grown for 24 h. A band corresponding to the soluble form of SCF is observed in concentrated media from MMP9+/+ kidney only. (B) ELISA of sSCF in the culture medium of E11.5 MMP9+/+ (white bar) or MMP9−/− (gray bar) kidneys grown for 24, 48, and 72 h. Values are mean ± SEM. ***P < 0.001 versus MMP9+/+ mice, n = 20.
Addition of sSCF to Culture Medium of MMP9-Deficient Mice Inhibits Apoptosis and Restores Ureteric Bud Branching
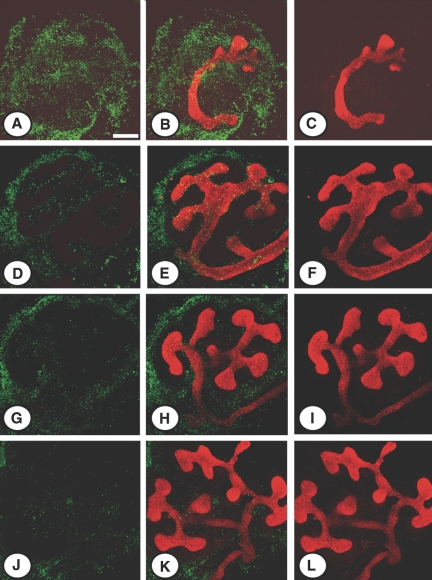
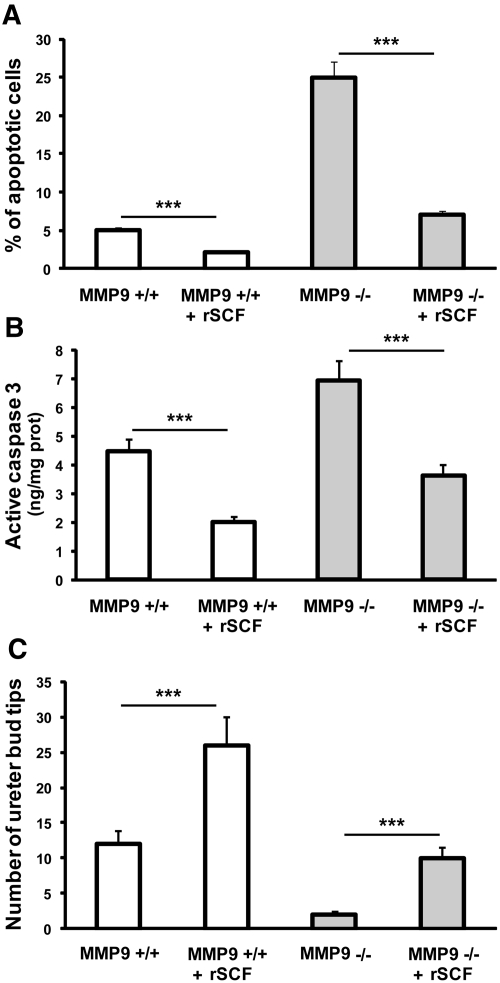
We hypothesized that induction of apoptosis in MMP9-deficient kidneys was the result of the decreased availability of sSCF. To test this hypothesis, we supplemented the culture medium of control (Figure 8, J through L) and MMP9-deficient E11.5 kidneys (Figure 8, D through F) with sSCF for 48 h, at a “physiologic” concentration (0.1 ng/ml) similar to the one observed in mouse plasma.10 We observed that sSCF significantly decreased the number of apoptotic cells detected by the TUNEL method in control (Figures 8, J and K versus G and H, and 9A) and MMP9-deficient (Figures 8, D and E versus A and B, and 9A) kidney. The concentration of active caspase-3 was also decreased in both groups of mice by exogenous sSCF in culture medium (Figure 9B). In addition, ureteric bud branching delineated by calbindin D-28K binding was markedly increased in control (Figures 8, K and L versus H and I, and 9C) and MMP9-deficient (Figures 8, E and F versus B and C, and 9C) kidneys. Moreover, sSCF added to the culture medium of MMP9-deficient E11.5 kidneys for 48 h (Figure 8, D through F) inhibited apoptosis (Figure 9, A and B) and restored ureteric bud branching (Figure 9C) to the level observed in nonsupplemented control E11.5 kidneys (Figure 8, G through I).
Figure 8.
Effect of sSCF on apoptosis in vitro. Representative photographs of E11.5 kidneys from MMP9−/− (A through F) and MMP9+/+ (G through L) kidneys stained with calbindin D-28K to delineate ureteric bud in red and the TUNEL method (apoptotic nuclei in green). Kidneys were grown for 48 h in the absence (A through C and G through I) or the presence (D through F and J through L) of physiologic concentration of sSCF in culture medium. sSCF in culture medium decreased the number of apoptotic cells and increased ureteric bud branching of MMP9+/+ (J through L versus G through I) and MMP9−/− (D through F versus A through C) kidneys. Bar: 250 μm.
Figure 9.
Effect of sSCF on E11.5 MMP9+/+ and MMP9−/− kidney apoptosis and ureteric bud branching. (A) Percentage of apoptotic cells in MMP9+/+ and MMP9−/− kidneys rescued (+SCF) or not with SCF. Microphotographs of ten different kidneys sampled from MMP9+/+ and MMP9−/− mice rescued or not with SCF were analyzed. (B) Concentration of active caspase-3 in MMP9+/+ and MMP9−/− kidneys rescued or not with SCF, n = 50. (C) Number of ureteric bud tips in MMP9+/+ and MMP9−/− kidneys rescued (+SCF) or not with SCF. Microphotographs of ten different kidneys sampled from MMP9+/+ and MMP9−/− mice rescued or not with SCF were analyzed. Note that the percentage of apoptosis, the concentration of active caspase-3 activity, and the number of ureteric bud tips are not statistically different in the MMP9+/+ kidney and in the MMP9−/− kidney supplemented with recombinant SCF. Values are mean ± SEM. ***P < 0.001 versus MMP9+/+-injected mice.
Discussion
We have previously shown that MMP2 and MMP9 are expressed at the first steps of kidney development but that only MMP9 plays a role in kidney organogenesis in organotypic culture.3 Using MMP9-deficient mice, we now show that MMP9 is an important regulator of nephron number in vivo; MMP9 protects mice from apoptosis during kidney development; this effect may be mediated at least in part by MMP9 releasing the soluble form of SCF, a potent antiapoptotic growth factor; and MMP9 protects aging mice from renal impairment.
MMP9-deficient mice are viable, without major organ defects, apart from a 10% reduction of the length of long bones.12 However, studies in MMP9-deficient mice showed that this enzyme is involved in a variety of pathophysiologic processes including reproduction, development, healing, inflammation, and vascular and proliferative diseases.8 We made the important observation that MMP9-deficient adult mice had a 30% reduction of the final nephron number, which most likely results from defects of branching morphogenesis at the first steps of kidney development and from a marked increase in mesenchymal apoptosis.
These data provide the first demonstration that MMP9 is required for nephron mass formation in vivo. The nephron deficit we observed at birth could not be attributed to global intrauterine growth retardation because the body weight and the kidney weight of the MMP9-deficient mice were not different at birth from those of controls. In adult, although the ratio kidney weight to body weight was identical in both groups of animals, the body weight was decreased in MMP9-deficient mice compared with control, possibly because of abnormal postnatal development of growth plates of long bones.12 The decreased kidney weight was most likely related to kidney alterations and particularly to the development of fibrosis. The discrepancy with the results obtained by Andrews et al.,13 who did not observe any kidney phenotype in MMP9-deficient mice, is probably because of a different genetic background of the mice. Although the background was not specified in the study of Andrews et al.,13 our mice were backcrossed 12 times to the C57BL/6J background. Similarly, the lung tumor antimetastatic outcome of MMP9 inhibition is dependent on the C57BL/6 background.14 A major impact of the genetic background on kidney phenotype has also been established in several mutant strains.15
The effect of MMP9 on nephron number may have a strong pathophysiologic relevance. During the last decade, a bulk of studies highlighted the consequence of fetal programming on organ function and adult diseases. In the kidney, multiple animal models have shown the association of low birth weight with later hypertension.16,17 The link between adult hypertension and low birth weight in these animal models seems to be a congenital nephron deficit. Accordingly, hypertension in adult life was observed in some animal models of inborn nephron deficit without hypotrophy at birth, such as exposure to maternal diabetes.18 Consistent with the animal data, human studies also showed an association between congenital nephron number and increased risk of chronic renal disease, hypertension, and accelerated decline in renal function with aging.16,19 Our data clearly support these observations because absence of MMP9 activity results in inborn nephron deficit; impairment of renal function and alteration of kidney morphology in aging mice; and vessel stiffness and increased pulse pressure.20 In addition, they provide a single molecular link to these anomalies.
The clinical relevance of our results is further suggested by the existence of functional MMP9 gene polymorphisms that are strongly associated with disease predisposition. A decreased number of d(CA) repeats in the MMP9 promoter is associated with decreased expression of MMP9 in mesangial cells and glomerulosclerosis susceptibility in mice.21 Analysis of the (CA)n repeats showed strong evidence for an association of shorter alleles with ESRD in whites.22 On the other hand, Gremlich et al.23 failed to show a correlation between intrauterine growth retardation and two polymorphisms that affects MMP9 promoter activity, (CA)n repeats and 1562 C/T substitution, perhaps because of the small size of the studied population. The 1562C/T polymorphism was indeed associated with bone mineral density in Japanese men.24 In patients with cancer, the R279Q gene polymorphism was linked with malignant potential of renal cell carcinoma in humans.25 Because the R279Q gene polymorphism leads to an amino acid exchange (arginine to glycine [Q]) in the catalytic domain of MMP9, it would be of interest to study its effects on nephron number.
We observed a strong antiapoptotic effect of MMP9 during kidney development. In fact, the effect of MMP9 on apoptosis is difficult to predict and may vary according to pathophysiologic conditions. On one hand, MMP9 can be viewed as a proapoptotic molecule: MMP9 degrades ECM components in many tissues including the kidney26–28 and can activate proapoptotic growth factors such as TGFβ and TNFα.29 On the other hand, MMP9 possesses a variety of nonmatrix substrates whose cleavage may protect from apoptosis.8 Decreased apoptosis was observed in MMP9-deficient mice after partial hepatectomy30 and cerebral hypoxia-ischemia,31 although the underlying mechanisms were poorly understood. In contrast, we found that MMP9 was protective in acute kidney injury by markedly reducing apoptosis and thus accelerating renal function recovery.10 This effect was because of the ability of MMP9 to release the soluble form of SCF that inhibits apoptosis by binding to its receptor c-kit in kidney cells,10 as previously observed in other tissues.32
We asked whether this mechanism observed in acute kidney injury could be relevant to normal kidney development. Both c-kit and SCF have been described in the metanephros,33,34 and their localization in the early phases of nephrogenesis was specified by Schmidt-Ott et al.35: SCF was detected in the ureteric bud, and c-kit was localized in a subset of mesenchymal progenitor cells.35 Our data showed that the membrane form of SCF was markedly increased in the MMP9-deficient kidney compared with the control kidney, suggesting that MMP9 cleaves the membrane form of SCF during kidney development as it does in the bone marrow11 and in acute kidney injury.9 Because mesenchymal cells were protected from apoptosis in control mice, whereas they were severely affected in MMP9-deficient mice, we hypothesized that the sSCF released by MMP9 from the ureteric bud had a paracrine effect on mesenchyme by activating c-kit. Our results favor this hypothesis. First, sSCF was detected in metanephric organotypic culture medium from control but not from MMP9-deficient kidneys, which also indicated that other proteases produced by embryonic kidney such as MMP2 and MT-MMP4,36 were not able to cleave SCF and thus could not compensate for MMP9 deficiency. Second, increased mesenchyme apoptosis and delayed kidney maturation could be suppressed by supplementing MMP9-deficient kidney with recombinant SCF. Third, a marked decrease of activated c-kit was detected in MMP9-deficient kidneys by Western blotting. The results imply that the soluble form of SCF, released by MMP9, controls apoptosis in mesenchyme by activating c-kit. They are in full agreement with the antiapoptotic effect of c-kit activation in embryonic kidney reported by Schmidt-Ott et al.,35 who showed that c-kit–positive cells underwent apoptosis when STI-571, an inhibitor of c-kit phosphorylation, was added to the culture medium of embryonic kidneys. sSCF activates PI3 kinase and subsequently Akt pathways, which are known to control the expression of molecules involved in apoptosis such as Bcl-2, Bad, or Bim.37,32 Compared with control mice injected with SCF, we observed incomplete rescue of branching and apoptosis in MMP9-deficient mice, which could be explained by subsaturating concentrations of SCF used for the rescue experiment or by other pathways affected by MMP9 such as those induced by ECM remodeling.
We observed in vivo and in vitro a delay in branching morphogenesis of MMP9-deficient embryonic kidney, which was already noticeable at the time of ureteric bud elongation in mesenchymal cells. Different hypotheses could explain the effect of MMP9 on ureteric bud branching. First, a role for MMP9-released SCF can be considered because it was previously shown that sSCF could stimulate branching in normal kidney,35 probably through expansion of the angioblast pool of c-kit expressing Flk-1–positive cells that were detected in E10–E11 metanephros.38 Second, the massive apoptosis observed in MMP9-deficient kidneys could disrupt the crosstalk between the mesenchymal cells and the ureteric bud, therefore inhibiting transmission of unidentified signal(s) that would induce the ureteric bud to branch. Third, MMP9 could favor branching by degrading basement membrane at the tip or at specific points of the bud or by releasing growth factors from ECM. This hypothesis is further supported by results obtained by Pohl et al.,6 showing in vitro that isolated ureteric buds grown in matrix containing gels had a reduced branching capacity when a synthetic inhibitor of MMPs or TIMP2 was added to the medium.
In conclusion, our results showed that MMP9 is a major regulator of nephron number. MMP9 participates in the control of apoptosis and ureteric bud branching mainly through the release of the soluble form of SCF in the embryonic kidney. A defect in MMP9 expression leads to a reduction in the final nephron number and induces renal impairment in aging mice. The clinical implication of these studies is highlighted by observations that even a moderate inborn nephron deficit represents a potential risk factor of progression in patients with chronic kidney disease.
Concise Methods
Mice
MMP9-deficient 129SV (MMP9−/−) mice were generated as described previously12 and were used after 12 backcrosses to the C57BL/6J background. Control (MMP9+/+) mice were also obtained by backcrossing +/− 129SV mice with wild-type C57BL/6J mice.
Ten-week-old female MMP9−/− and MMP9+/+ mice were used for the study. The animals were maintained with a 12-h light/12-h dark cycle and had free access to food and water. Mouse studies followed the Institutional Animal Care and Use Guidelines, and all protocols were approved by the Institut National de la Santé et de la Recherche Médicale.
Antibodies and Reagents
Rabbit polyclonal anti-SCF, anti c-kit, and their respective blocking peptide were obtained from Santa Cruz (Santa Cruz Biotechnology, Santa Cruz, CA). Rabbit polyclonal antiphosphorylated c-kit antibody was purchased from Biosource International (Clinisciences, Montrouge, France). Mouse monoclonal anti-calbindin D-28k was obtained from Sigma-Aldrich (St. Louis, MO). Anti-rabbit IgG and anti-goat IgG conjugated to alkaline phosphatase, anti-mouse IgG conjugated to tetramethyl rhodamine isothiocyanate, and anti-rabbit IgG conjugated to FITC were purchased from Vector Laboratories (Burlingame, CA). Recombinant mouse SCF and recombinant mouse MMP9 were obtained from Biosource International. The mouse ELISA SCF kit was purchased from Ramp;D Systems (Minneapolis, MN).
Assessment of Renal Function
Serum creatinine was measured using the alkaline picric acid method on an autoanalyzer (Instrumentation Laboratory, Paris, France).
Comparative Studies of Control and MMP9-Deficient Embryonic Kidneys
Heterozygous mice were mated. The morning of the vaginal plug was defined as day 0 of gestation. Embryos located at the extremity of the uterine cord were systematically discarded. We used the legs as a marker of development, and we compared embryos with an identical developmental stage of the legs. Kidneys were dissected from 11.5- (E11.5) and 12.5-d (E12.5) embryos, and the tail of the embryo was used for genotyping as described previously.12
Organ Culture and Experimental Protocols
The morning of the vaginal plug was defined as day 0 of gestation. Kidneys were dissected from MMP9−/− and MMP9+/+ 11.5- (E11.5) and 12.5-d (E12.5) embryos. For some experiments, embryonic kidneys were grown for 24 h (37°C, 5% CO2) on a 3-μm Nucleopore filter (Costar Corp., Cambridge, MA) floating on culture medium (DMEM/Ham F12) supplemented with 30 nM sodium selenate, 50 μg/ml transferrin, and 4 mM glutamine as described previously.3 Rescue experiments were performed by supplementing medium with recombinant mouse SCF (0.1 ng/ml).
Nephron Mass Determination
The number of nephrons was determined in newborn and 3-mo-old MMP9+/+ and MMP9−/− mice as described previously.39 Briefly, kidneys were weighed and incubated in 50% hydrochloric acid for 10 to 45 min at 37°C, the incubation time being dependent on the kidney weight. Kidneys were rinsed with tap water and stored overnight at 4°C in a gauged flask. After mechanical dissociation, tubules and glomeruli were suspended in water. Three 0.5-ml aliquots were taken and placed in a hemocytometer-like chamber, and the glomeruli were counted microscopically by three investigators unaware of the specimen origin. The three results were averaged, and this value was used to determine the total number of glomeruli in the sample and therefore in the kidney.
Histology and Analysis of Fibrosis
Renal tissue was fixed in Dubosq-Brazil's fixative, embedded in paraffin, cut in 3-μm sections, and stained with hematoxylin and eosin. Interstitial fibrosis was semiquantitatively assessed on Sirius red–stained paraffin sections at ×20 magnification under polarized light. Interstitial fibrosis was quantified using computer-based morphometric analysis software (Axionplan; Axiophot 2, Zeiss, Germany). Five fields were randomly selected from each kidney, representing nearly the whole cortex. All scoring was performed in a blinded manner on coded slides. Data are expressed as percentage of positive areas examined.
Assessment of Apoptosis
Apoptotic cells were estimated by different techniques.
First, they were detected by morphologic criteria including cell shrinking, formation of apoptotic bodies, and condensation of chromatin and by the in situ TUNEL method (Apoptag; QBiogene, Irvine, CA) performed on MMP9+/+ and MMP9−/− E11.5 kidneys freshly extracted from the embryo or cultured for 48 h in a medium supplemented or not with 0.1 ng/ml of mouse recombinant SCF. Kidneys were fixed for 30 min at room temperature with 4% paraformaldehyde. Immunofluorescence (FITC) TUNEL technique was performed according to the manufacturer's instruction. To localize the ureteric bud, kidneys were counterstained with a mouse anti-calbindin D-28K antibody (8 μg/ml) followed by a tetramethyl rhodamine isothiocyanate-conjugated anti-mouse IgG antibody. Nuclei were stained with 4′-6-diamino-2-phenylindole (1 μg/ml for 30 s at room temperature). The percentage of apoptotic nuclei was determined on microphotographs taken from ten E11.5 MMP9−/− and MMP9+/+ kidneys cultured or not for 48 h.
In another set of experiments, the genomic DNA from E11.5 MMP9−/− and MMP9+/+ kidneys grown for 24 h in culture was recovered by tissue digestion with proteinase K (0.5 mg/ml), followed by successive extraction by phenol and phenol/chloroform and precipitation by ethanol, and migrated on a 1.5% agarose gel.
Finally, caspase-3 released from tissue was measured in culture media obtained from five sets of ten MMP9+/+ and ten MMP9−/− E12.5 embryos grown for 2 h by an ELISA assay developed by R&D Systems (Quantikine human active caspase-3). The detection limit of the mouse SCF immunoassay was 0.1 ng/ml.
Western Blotting
Western blots were performed with 20 μg kidney lysate proteins to determine expression of c-kit, phospho c-kit, and SCF in E11.5 MMP9+/+ and MMP9−/− kidneys. sSCF was determined on concentrated media from ten MMP9+/+ and ten MMP9−/− E11.5 kidneys grown for 24 h. Proteins were submitted to SDS-PAGE under reducing conditions in a 12 (SCF) or 8% (c-kit and phospho c-kit) polyacrylamide gel and electro-transferred to polyvinylidene fluoride membrane (Immobilon-P; Millipore, Bedford, MA) for 90 min at a constant current of 190 mA. Afterward, a nitrocellulose sheet was saturated with 5% dry milk in TBS-Tween (Tris 50 mM, NaCl 150 mM, Tween 20 0.1%, pH 8.0) for 1 h at 37°C, extensively washed in TBS-Tween, and incubated overnight at 4°C with polyclonal rabbit IgG anti-SCF, anti-c-kit, anti-phospho c-kit (0.2 μg/ml) and polyclonal goat IgG anti-actin (0.5 μg/ml). To assess the specificity of the antibodies, we incubated the anti-SCF and anti-c-kit antibodies with a 50-fold excess of their respective immunogen peptide as a control. The polyvinylidene fluoride membrane was incubated for 2 h at room temperature with anti-rabbit IgG and anti-goat IgG conjugated to alkaline phosphatase (0.02 μg/ml). Alkaline phosphatase activity was shown by adding the nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate complex substrate in 100 mM Tris-HCl, 100 mM NaCl, and 5 mM MgCl2 (pH 9.5). The reaction was stopped in 20 mM Tris-HCl and 5 mM EDTA (pH 8.0). For quantification, blots were analyzed with Image J and converted to a graphical format.
Assessment of Membrane SCF Sensitivity to MMP9 Proteolysis
Five sets of ten MMP9+/+ and ten MMP9−/− E11.5 embryos were cultured for 24 h. Culture media were collected and ultracentrifuged (100,000 × g for 10 min) to remove cell debris. sSCF released from tissue was estimated in the media by Western blotting (see above) and measured by an ELISA assay developed by R&D Systems (Quantikine M mouse SCF). The detection limit of the mouse SCF immunoassay was 5 pg/ml.
Statistics
Results are expressed as mean ± SEM. Significances of differences between different groups of mice were determined by the ANOVA test.
Disclosures
None.
Acknowledgments
This study was funded by grants from the Institut National de la Santé et de la Recherche Médicale, the Université Paris 6, and the Ministère de la Recherche (ACI 1A061G) to P.R. and B.L. We thank Zena Werb for providing us with the MMP9-deficient mice. We are grateful to Adlène Touba for SCF immunohistochemistry and Western blotting and Caroline Martin and Claude Kitou for animal care. We thank Philippe Fontange and Romain Morichon (Station d'Imagerie Cellulaire, IFR65) for confocal microscopy.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Lelongt B, Ronco P: Role of extracellular matrix in kidney development and repair. Pediatr Nephrol 18: 731–742, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Grobstein C, Cohen J: Effect of the morphogenesis of embryonic salivary epithelium in vitro. Science 150: 626–628, 1965 [DOI] [PubMed] [Google Scholar]
- 3.Lelongt B, Trugnan G, Murphy G, Ronco P: Matrix metalloproteinases MMP2 and MMP9 are produced in early stages of kidney morphogenesis but only MMP9 is required for renal organogenesis in vitro. J Cell Biol 136: 1363–1373, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanwar Y, Ota K, Yang Q, Wada J, Kashihara N, Tian Y, Wallner E: Role of membrane-type matrix metalloproteinase 1 (MT-1-MMP), MMP-2, and its inhibitor in nephrogenesis. Am J Physiol 277: F934–F947, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Barasch J, Yang J, Qiao J, Tempst P, Erdjument-Bromage H, Leung W, Oliver J: Tissue inhibitor of metalloproteinase-2 stimulates mesenchymal growth and regulates epithelial branching during morphogenesis of the rat metanephros. J Clin Invest 103: 1299–1307, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pohl M, Sakurai H, Bush K, Nigam S: Matrix metalloproteinases and their inhibitors regulate in vitro ureteric bud branching morphogenesis. Am J Physiol 279: F891–F900, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Sternlicht M, Werb Z: How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17: 463–516, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G: Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit Rev Biochem Mol Biol 37: 375–536, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Lelongt B, Bengatta S, Delauche M, Lund LR, Werb Z, Ronco P: MMP9 protects mice from anti-glomerular basement membrane nephritis through its fibrinolytic activity. J Exp Med 193: 793–802, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bengatta S, Arnould C, Letavernier E, Monge M, Martinan de Préneuf H, Werb Z, Ronco P, Lelongt B: MMP9 and SCF protect from apoptosis in acute kidney injury. J Am Soc Nephrol 20: 787–797, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S: Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell 109: 625–637, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z: MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93: 411–422, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrews KL, Betsuyaku T, Rogers S, Shipley JM, Senior RM, Miner JH: Gelatinase B (MMP-9) is not essential in the normal kidney and does not influence progression of renal disease in a mouse model of alport syndrome. Am J Pathol 157: 303–311, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin M, Carter K, Jean-Philippe S, Chang M, Mobashery S, Thiolloy S, Lynch C, Matrisian L, Fingleton B: Effect of ablation or inhibition of stromal matrix metalloproteinase-9 on lung metastasis in a breast cancer model is dependent on genetic background. Cancer Res 68: 6251–6259, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roselli S, Heidet L, Sich M, Henger A, Kretzler M, Gubler M, Antignac C: Early glomerular filtration defect and severe renal disease in podocin-deficient mice. Mol Cell Biol 24: 550–560, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luyckx V, Brenner B: Low birth weight, nephron number, and kidney disease. Kidney Int Suppl 97: S68–S77, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Merlet-Bénichou C, Gilbert T, Vilar J, Moreau E, Freund N, Lelièvre-Pégorier M: Nephron number: variability is the rule. Causes and consequences. Lab Invest 79: 515–527, 1999 [PubMed] [Google Scholar]
- 18.Duong Van Huyen J, Nehiri T, Viltard M, Fassot C, Heudes D, Freund N, Deschênes G, Houillier P, Bruneval P, Lelièvre-Pégorier M: Exposure to maternal diabetes induces salt-sensitive hypertension and impairs renal function in adult rat offspring. Diabetes 57: 2167–2175, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barker D, Bagby S, Hanson M: Mechanisms of disease: in utero programming in the pathogenesis of hypertension. Nat Clin Pract Nephrol 2: 700–707, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Flamant M, Placier S, Dubroca C, Esposito B, Lopes I, Chatziantoniou C, Tedgui A, Dussaule J, Lehoux S: Role of matrix metalloproteinases in early hypertensive vascular remodeling. Hypertension 50: 212–218, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Fornoni A, Wang Y, Lenz O, Striker L, Striker G: Association of a decreased number of d(CA) repeats in the matrix metalloproteinase-9 promoter with glomerulosclerosis susceptibility in mice. J Am Soc Nephrol 13: 2068–2076, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Hirakawa S, Lange E, Colicigno C, Freedman B, Rich S, Bowden D: Evaluation of genetic variation and association in the matrix metalloproteinase 9 (MMP9) gene in ESRD patients. Am J Kidney Dis 42: 133–142, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Gremlich S, Nguyen D, Reymondin D, Hohlfeld P, Vial Y, Witkin S, Gerber S: Fetal MMP2/MMP9 polymorphisms and intrauterine growth restriction risk. J Reprod Immunol 74: 143–151, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Yamada Y, Ando F, Niino N, Shimokata H: Association of a polymorphism of the matrix metalloproteinase-9 gene with bone mineral density in Japanese men. Metabolism 53: 135–137, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Awakura Y, Ito N, Nakamura E, Takahashi T, Kotani H, Mikami Y, Manabe T, Kamoto T, Habuchi T, Ogawa O: Matrix metalloproteinase-9 polymorphisms and renal cell carcinoma in a Japanese population. Cancer Lett 241: 59–63, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Boudreau N, Sympson CJ, Werb Z, Bissel MJ: Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science 267: 891–893, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stupack DG, Cheresh DA: Apoptotic cues from the extracellular matrix: Regulators of angiogenesis. Oncogene 22: 9022–9029, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Makino H, Sugiyama H, Kashihara N: Apoptosis and extracellular matrix-cell interactions in kidney disease. Kidney Int 77: S67–S75, 2000 [PubMed] [Google Scholar]
- 29.McCawley L, Matrisian L: Matrix metalloproteinases: they're not just for matrix anymore!. Curr Opin Cell Biol 13: 534–540, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Olle EW, Ren X, McClintock SD, Warner RL, Deogracias MP, Johson KJ, Colletti LM: Matrix metalloproteinase-9 is an important factor in hepatic regeneration after partial hepatectomy in mice. Hepatology 44: 540–549, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Svedin P, Haqberq H, Savman K, Zhu C, Mallard C: Matrix metalloproteinase-9 gene knock-out protects the immature brain after cerebral hypoxia-ischemia. J Neurosci 27: 1511–1518, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith MA, Court EL, Smith JG: Stem cell factor: Laboratory and clinical aspects. Blood Rev 15: 191–197, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Bernex F, De Sepulveda P, Kress C, Elbaz C, Delouis C, Panthier JJ: Spatial and temporal patterns of c-kit-expressing cells in WlacZ/+ and WlacZ/WlacZ mouse embryos. Development 122: 3023–3333, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Matsui Y, Zsebo KM, Hogan BL: Embryonic expression of a haematopoietic growth factor encoded by the Sl locus and the ligand for c-kit. Nature 347: 667–669, 1990 [DOI] [PubMed] [Google Scholar]
- 35.Schmidt-Ott KM, Chen X, Paragas N, Levinson RS, Mendelsohn CL, Barasch J: c-kit delineates a distinct domain of progenitors in the developing kidney. Dev Biol 299: 238–249, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Legallicier B, Trugnan G, Murphy G, Lelongt B, Ronco P: Expression of the type-IV collagenase during mouse kidney development and tubule segmentation. J Am Soc Nephrol 12: 2358–2369, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Okayama Y, Kawakami T: Development, migration, and survival of mast cells. Immunol Res 34: 97–115, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woolf A, Loughna S: Origin of glomerular capillaries: Is the verdict in?. Exp Nephrol 6: 17–21, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Amri K, Freund N, Vilar J, Merlet-Bénichou C, Lelièvre-Pégorier M: Adverse effects of hyperglycemia on kidney development in rats: in vivo and in vitro studies. Diabetes 48: 2240–2245, 1999 [DOI] [PubMed] [Google Scholar]