Abstract
Endothelial dysfunction contributes to the increased cardiovascular risk that accompanies CKD. We hypothesized that the soluble VEGF receptor 1 (sFlt-1), a VEGF antagonist, plays a role in endothelial dysfunction and decreased angiogenesis in CKD. We enrolled 130 patients with CKD stages 3 to 5 and 56 age- and gender-matched control patients. Plasma sFlt-1 levels were higher in patients with CKD and, after multivariate regression analyses, exclusively associated with renal function and levels of vWF, a marker of endothelial dysfunction. Compared with serum from control patients, both recombinant sFlt-1 and serum from patients with CKD had antiangiogenic activity in the chick chorioallantoic membrane (CAM) assay, induced endothelial cell apoptosis in vitro, and decreased nitric oxide generation in two different endothelial cell lines. Pretreating the sera with an antibody against sFlt-1 abrogated all of these effects. Furthermore, we observed increased sFlt1 levels in 5/6-nephrectomized rats compared with sham-operated animals. Finally, using real-time PCR and ELISA, we identified monocytes as a possible source of increased sFlt-1 in patients with CKD. Our findings show that excess sFlt-1 associates with endothelial dysfunction in CKD and suggest that increased sFlt-1 may predict cardiovascular risk in CKD.
Chronic kidney disease (CKD) is a prevalent health problem associated with increased cardiovascular morbidity and mortality. It is now well established that the impairment of renal function is independently associated with endothelial dysfunction and that endothelial dysfunction is involved in this renal function–associated cardiovascular mortality.1 In addition, the functional changes in vascular endothelium observed in patients with CKD are associated with increased atherosclerosis, ischemic heart disease, and vascular stiffening.2,3 Moreover, animal models of uremic disease generally present decreased angiogenesis and arteriogenesis, i.e., the formation of new vessels from pre-existing ones and opening of collateral vessels in the heart, kidney, and hind-limb.4–6
Hypertension and shear stress, inflammation, diabetes-associated factors such as advanced glycated end products, and uremic toxins are some of the prevalent risk factors of endothelial dysfunction in CKD.7–9 However, other unknown risk factors may be present.
Soluble vascular endothelial growth factor (VEGF) receptor 1, also known as soluble Flt-1 (sFlt-1), is a splice variant of the VEGF receptor lacking the transmembrane and cytoplasmic domains. As a potent antagonist of VEGF, sFlt-1 specifically binds VEGF. It is known to regulate its action negatively on two levels: first, it binds and sequesters VEGF in the circulation; second, by occupying the VEGF receptor, it prevents VEGF occupancy and subsequent signal transduction.10 Of note, VEGF is a well-known promoter of angiogenesis and an endogenous regulator of endothelial integrity. Anti-VEGF compounds, such as sFlt-1, have been found to cause endothelial dysfunction, decrease angiogenesis, impair capillary repair, and increase proteinuria.11 Furthermore, sFlt-1 has been described as a risk factor for pre-eclampsia, the major renal complication of pregnancy.12 Endothelial cells, monocytes, and placenta are the major sources of sFlt-1.13,14
Serum sFlt-1 levels have not been determined in patients with CKD yet. Because these patients are characterized by endothelial dysfunction and impaired angiogenesis, we speculated that sFlt-1 levels might be increased in these patients, ultimately constituting a novel cardiovascular risk factor in CKD. The aim of this study was to investigate this hypothesis and to study possible mechanisms of sFlt-1–induced effects on endothelial function.
Results
We studied 130 patients (average age, 64 ± 14 yr; 65% men) presenting estimated GFR (eGFR) <60 ml/min/1.73 m2 and 56 controls (average age, 61 ± 19 yr; 61% men) with eGFR ≥60 ml/min/1.73 m2. Characteristics of all participants are shown in Table 1. Patients had different underlying causes of renal insufficiency. Several risk factors were present, which were (in part) responsible for the renal disease, particularly diabetes and hypertension.
Table 1.
Baseline characteristics of all participantsa
| Estimated GFR (ml/min/1.73 m2) |
P Value | |||||
|---|---|---|---|---|---|---|
| ≥60 (n = 56) | 45 to 59 (n = 17) | 30 to 44 (n = 31) | 15 to 29 (n = 44) | <15 (n = 38) | ||
| Age (yr) | 61 ± 19 | 69 ± 14 | 69 ± 14 | 66 ± 14 | 60 ± 12 | 0.042b |
| Male gender [% (n)] | 61 (34) | 71 (12) | 58 (18) | 59 (26) | 66 (25) | 0.886c |
| Creatinine (mg/dl) | 0.8 (0.8 to 0.9) | 1.3 (1 to 1.4) | 1.6 (1.4 to 1.8) | 2.6 (2.3 to 3) | 6.6 (5 to 7.6) | <0.001b |
| Proteinuria (g/24h) | 0.0 (0.0 to 0.1) | 0.1 (0.0 to 0.2) | 0.2 (0.0 to 0.4) | 0.2 (0.0 to 0.7) | 0.2 (0.0 to 0.6) | <0.001d |
| History of hypertension [% (n)] | 55 (31) | 82 (14) | 81 (25) | 77 (34) | 74 (28) | 0.038c |
| SBPf (mmHg) | 130 (125 to 150) | 130 (120 to 145) | 130 (124 to 140) | 130 (120 to 140) | 130 (129 to 140) | 0.439b |
| DBP (mmHg) | 80 (80 to 80) | 80 (75 to 80) | 80 (70 to 80) | 80 (75 to 80) | 80 (80 to 81) | 0.020b |
| Smokers [% (n)] | 29 (16) | 35 (6) | 26 (8) | 27 (12) | 26 (10) | 0.963c |
| Diabetes [% (n)] | 9 (5) | 35 (6) | 29 (9) | 34 (15) | 41 (15) | 0.005c |
| HbA1c (%) | 4.9 (4.7 to 5.4) | 5.2 (5.0 to 6.0) | 5.3 (4.9 to 6.3) | 5.2 (4.9 to 5.9) | 5.3 (5.0 to 5.8) | 0.002b |
| BMI (kg/m2) | 25 (23 to 28) | 27 (24 to 30) | 26 (22 to 29) | 25 (23 to 31) | 25 (23 to 28) | 0.481b |
| Cholesterol (mg/dl) | 185 ± 66 | 178 ± 58 | 192 ± 50 | 174 ± 48 | 165 ± 50 | 0.271b |
| HDL-cholesterol (mg/dl) | 48 (12 to 56) | 43 (39 to 53) | 49 (42 to 72) | 43 (38 to 49) | 41 (36 to 45) | 0.001b |
| Calculated-LDL cholesterol (mg/dl) | 140 ± 51 | 126 ± 48 | 107 ± 32 | 112 ± 45 | 96 ± 50 | <0.001b |
| Cardiovascular events | ||||||
| Myocardial infarction [% (n)] | 2 (4) | 0 (0) | 5 (17) | 8 (19) | 5 (15) | 0.075e |
| Stroke [% (n)] | 0 (0) | 1 (7) | 5 (17) | 3 (7) | 5 (15) | 0.016e |
| Current medication | ||||||
| ACE inhibitors [% (n)] | 38 (21) | 59 (10) | 68 (21) | 48 (21) | 26 (9) | 0.005c |
| AT1 blockers [% (n)] | 29 (11) | 41 (7) | 23 (7) | 30 (13) | 14 (5) | 0.203c |
| Statins [% (n)] | 18 (10) | 41 (7) | 52 (16) | 41 (18) | 24 (9) | 0.007c |
| Erythropoietin [% (n)] | 0 (0) | 0 (0) | 3 (1) | 16 (7) | 44 (16) | <0.001e |
| Heparin [% (n)] | 25 (14) | 6 (1) | 13 (4) | 16 (7) | 58 (22) | <0.001c |
| sFlt-1 (pg/ml) | 62 (49 to 83) | 83 (62 to 165) | 112 (89 to 156) | 99 (84 to 152) | 134 (100 to 270) | <0.001b |
| vWF (U/ml) | 0.2 (0.2 to 0.5) | 0.3 (0.2 to 0.6) | 0.5 (0.2 to 0.9) | 0.5 (0.3 to 1.0) | 0.9 (0.4 to 1.4) | <0.001b |
| sVCAM (pg/ml) | 731 (574 to 915) | 741 (667 to 966) | 911 (721 to 1254) | 1000 (764 to 1270) | 1760 (1356 to 2233) | <0.001b |
aValues are mean ± SD. ACE, angiotensin-converting enzyme; AT1, Angiotensin-II receptor; BMI, body mass index; DBP, diastolic blood pressure; HbA1c, glycohemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure.
bUnivariate ANOVA, post hoc test (Scheffe). In case of a normal distribution, variables are presented as mean (±SD). In case of a skewed distribution, variables are presented as median (interquartile range).
cχ2 test.
dKruskall-Wallis test.
eFisher's exact test.
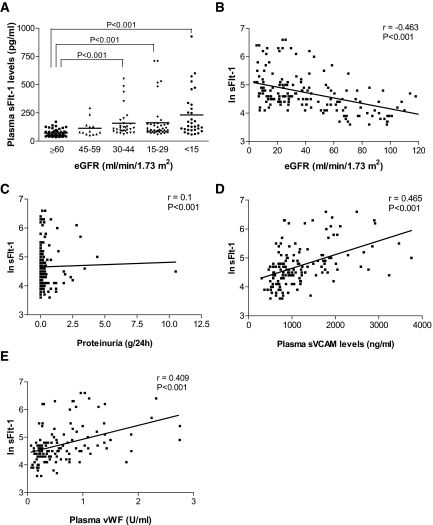
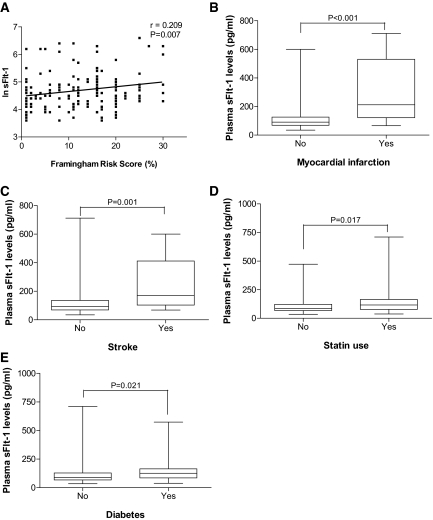
We divided patients with respect to the eGFR and plotted them against plasma concentration of sFlt-1. Patients with decreased eGFR presented increased plasma levels of sFlt-1, showing a significant negative correlation between them (Figure 1, A and B, respectively). Table 2 shows baseline characteristics of all participants according to the sFlt-1 levels. Increased sFlt-1 levels correlated positively with proteinuria (Figure 1C). Because we expected increased sFlt-1 concentration was associated with endothelial dysfunction, we assessed plasma levels of soluble vascular adhesion molecule-1 (sVCAM-1) and von Willebrand factor (vWF), well-known markers of endothelial dysfunction. Plasma sVCAM-1 and vWF levels were increased at low eGFR ranges and presented a positive correlation with sFlt-1 levels (Figure 1, D and E, respectively). Supplementary Figure S1 confirms the association between inflammatory response (C-reactive protein) and endothelial dysfunction (sVCAM and vWF). Framingham risk score was also positively correlated with sFlt-1 levels (Figure 2A), showing that patients presenting higher levels of sFlt-1 also present higher cardiovascular risk. Increased cardiovascular events, such as myocardial infarction and stroke, were also increased at high plasma sFlt-1 levels (Figure 2, B and C, respectively). Univariate analysis also showed an association between sFlt-1 levels and the presence of diabetes mellitus (Figure 2D) and glycohemoglobin A1c (HbA1c). Statin use was also positively associated with sFlt-1 levels (Figure 2E).
Figure 1.
Plasma soluble VEGF receptor 1 (sFlt-1) levels in patients with CKD. (A) Plasma sFlt-1 concentrations of patients with CKD grouped according to their eGFR. (B) Univariate analysis showed a negative correlation of sFlt1 levels with eGFR and (C) a positive correlation with proteinuria and (D) plasma vWF and (E) plasma sVCAM levels, both markers of endothelial dysfunction. In Panel A, sFlt-1 values are expressed as median and range (unadjusted). In Panels B through E, values are expressed as natural logarithm (ln). r, correlation coefficient.
Table 2.
Baseline characteristics of all participants according to plasma sFlt-1 levelsa
| Tertiles of sFlt-1 (pg/ml) |
P Value | |||
|---|---|---|---|---|
| First (<79.4)(n = 55) | Second (79.4 to 124.7)(n = 56) | Third (>124.7)(n = 55) | ||
| eGFR (ml/min/1.73 m2) | 68.3 (40.8 to 92.0) | 28.1 (17.2 to 43.9) | 23.7 (10.3 to 38.8) | <0.001b |
| Age (yr) | 61 ± 18 | 64 ± 16 | 66 ± 13 | 0.246b |
| Male gender [% (n)] | 51 (28) | 68 (38) | 64 (35) | 0.161c |
| Creatinine (mg/dl) | 0.9 (0.8 to 1.4) | 1.9 (1.3 to 3.1) | 2.5 (1.6 to 5.1) | <0.001b |
| Proteinuria (g/24 h) | 0.0 (0.0 to 0.2) | 0.1 (0.0 to 0.5) | 0.3 (0.1 to 0.6) | <0.001d |
| History of hypertension [% (n)] | 64 (35) | 66 (37) | 82 (45) | 0.079c |
| SBP (mmHg) | 130 (128 to 150) | 130 (125 to 140) | 130 (125 to 140) | 0.251b |
| DBP (mmHg) | 80 (80 to 80) | 80 (75 to 80) | 80 (75 to 80) | 0.109b |
| Smokers [% (n)] | 29 (16) | 18 (10) | 36 (20) | 0.092c |
| Diabetes [% (n)] | 17 (9) | 23 (13) | 44 (24) | 0.005c |
| HbA1c (%) | 5.0 (4.7 to 5.4) | 5.1 (4.8 to 5.7) | 5.4 (5.0 to 5.9) | 0.008b |
| BMI (kg/m2) | 24.5 (22.3 to 28.3) | 25.7 (22.1 to 29.0) | 25.1 (23.0 to 29.5) | 0.521b |
| Cholesterol (mg/dl) | 179.0 (±49.8) | 178.9 (±54.3) | 183.8 (±66.2) | 0.874b |
| HDL (mg/dl) | 48.0 (43.0 to 56.0) | 44.5 (40.3 to 52.5) | 42.0 (38.5 to 49.8) | 0.120b |
| LDL (mg/dl) | 132.0 (109 to 165) | 109.0 (87 to 132) | 111.0 (70 to 160) | 0.027b |
| Cardiovascular events | ||||
| Myocardial infarction [% (n)] | 2 (1) | 5.5 (3) | 28.3 (15) | <0.001c |
| Stroke [% (n)] | 2 (1) | 7.3 (4) | 17 (9) | 0.021e |
| Current medication | ||||
| ACE inhibitors [% (n)] | 42 (23) | 46 (26) | 48 (25) | 0.817c |
| AT1 blockers [% (n)] | 13 (14) | 20 (11) | 25 (13) | 0.757c |
| Statins [% (n)] | 20 (11) | 34 (19) | 40 (22) | 0.071c |
| Erythropoietin [% (n)] | 7 (4) | 7 (8) | 7 (8) | 0.397c |
| Heparin [% (n)] | 22 (12) | 16 (9) | 33 (18) | 0.107c |
| vWF (U/ml) | 0.3 (0.2 to 0.5) | 0.3 (0.2 to 0.7) | 0.8 (0.5 to 1.4) | <0.001b |
| sVCAM (pg/ml) | 718 (570 to 886) | 979 (783 to 1303) | 1415 (883 to 1918) | <0.001b |
| Framingham risk score (%) | 11 (2 to 17) | 12 (4 to 20) | 16 (8 to 20) | 0.013b |
aAnalyses were based on raw and partly transformed data (unadjusted). Data are presented as mean (±SD), median (interquartile range), or actual numbers (%). Significance of differences: P < 0.05.
bUnivariate ANOVA; post hoc test (Scheffe). In case of a normal distribution, variables are presented as mean (±SD). In case of a skewed distribution, variables are presented as median (interquartile range).
cχ2 test.
dKruskall-Wallis test.
eFisher's exact test.
Figure 2.
High plasma sFlt-1 levels are correlated with (A) increased cardiovascular risk and incidence of cardiovascular events such as (B) myocardial infarction and (C) stroke. The presence of (D) diabetes mellitus and (E) statin use was also positively associated with sFlt-1 levels. In Panel A, values are expressed as natural logarithm (ln). In Panels B through E, values are expressed as median and range (unadjusted). r, correlation coefficient.
Table 3 shows that, after multivariate regression analyses, eGFR was independently associated with plasma sFlt-1 levels in both models 1 and 4. In models 2 and 3, there was no significant positive association found between sFlt-1 levels and any other analyzed parameter. The final model (model 4) confirms that sFlt-1 levels are associated with renal function (eGFR) and with the endothelial dysfunction (vWF and sVCAM).
Table 3.
Relation of different parameters with plasma sFlt-1 levelsa
| Model | sFlt-1 |
|
|---|---|---|
| Stand. B | 95% CI | |
| 1. Age, gender | ||
| eGFR | −0.0098b | −0.01266 to −0.00694 |
| 2. Model 1 + diabetes mellitus, HbA1c, statins, triglycerides, vWF, sVCAM | ||
| eGFR | −0.0039 | −0.00831 to 0.00060 |
| 3. diabetes mellitus, HbA1c, statins, triglycerides, vWF, sVCAM | ||
| eGFR | −0.00353 | −0.00777 to 0.00070 |
| 4. Full model 3 (stepwise selection) | ||
| eGFR | −0.00427c | −0.00825 to −0.00029 |
| sVCAM | 0.00029 | −0.000006 to 0.00059 |
| vWF | 0.31550c | 0.00756 to 0.62346 |
asFlt-1 was transformed to natural logarithm. B, regression coefficient β; CI, confidence interval.
bP < 0.001.
cP < 0.05
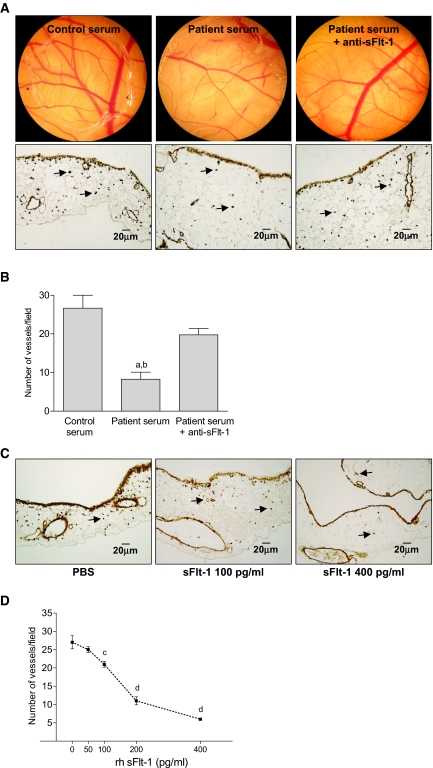
To check the direct effect of patient serum on angiogenesis and endothelial cell function, we performed a series of in vivo/in vitro assays. First, we functionally tested serum for antiangiogenic activity by using the in vivo CAM assay. Patient serum inhibited angiogenesis (Figure 3, A and B; n = 3 to 5 per group). To prove that sFlt-1 is directly involved in the increased antiangiogenic activity found in serum of patients with CKD, we performed two independent experiments: (1) we neutralized sFlt-1 in sera by using a specific antibody and immunoprecipitation; and (2) we applied recombinant sFlt-1 directly onto the CAM. As also shown in Figure 3, A and B, neutralization of sFlt-1 prevented the loss of vessels observed when serum was incubated with a nonimmune IgG (n = 5 per treatment), whereas recombinant sFlt-1 decreased angiogenesis in a dose-dependent manner (Figure 3, C and D; n = 4 per tested concentration).
Figure 3.
CKD is associated with an antiangiogenic state. (A) Effect of control and patient sera (10 μl) on the CAM after 24-h incubation. Top panels show macroscopic images of the CAM, whereas bottom panels represent capillary vessels labeled with Sambucus nigra lectin (magnification, ×20). A great density of vessels is observed in control serum-treated CAM, whereas patient serum-treated CAM presented a significant reduction in the number of vessels (n = 3 control sera; n = 5 patient sera). The antiangiogenic activity of patient serum was antagonized by immunoprecipitation of sFlt-1 after incubation with a specific monoclonal antibody (anti-sFlt-1, 40 μg/ml). (B) The graphic represents the number of vessels/field. (C) Panels represent CAM treated with human recombinant sFlt-1. (D) Dose–response curve of hr sFlt-1 (n = 4 per tested concentration). Arrows indicate capillary vessels. Results are expressed as mean, and bars are SEM. aP < 0.001, control serum × patient serum; bP < 0.05, patient serum × patient serum + anti-sFlt-1; cP < 0.05 and dP < 0.001 compared with vehicle (PBS).
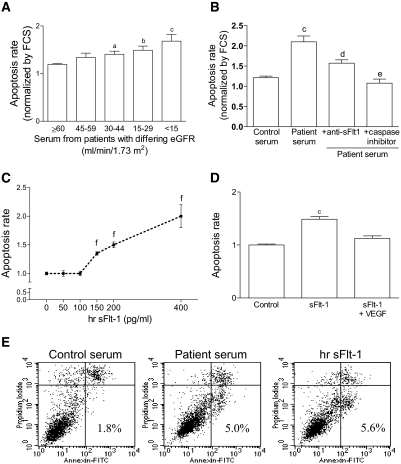
Because endothelial cell apoptosis is associated with decreased angiogenesis and impaired endothelial function, we further analyzed the effect of patient sera on an endothelial cell line (EAhy926) in culture. Figure 4A shows that patient sera induce apoptosis compared with control sera and that the apoptotic response is negatively correlated with eGFR (n = 13 to 54 per group). Figure 4B shows that neutralization of sFlt-1 decreases the apoptotic rate induced by undiluted patient sera, whereas Z-VAD-FMK, a caspase inhibitor, completely abolishes apoptosis (n = 9 controls and n = 12 patients: 4 with eGFR 30 to 59 ml/min/1.73 m2, 3 with eGFR 15 to 29 ml/min/1.73 m2, and 5 with eGFR <15 ml/min/1.73 m2). Human recombinant sFlt-1 induced EAhy926 cell apoptosis in a dose-dependent manner (Figure 4C; n = 8 per concentration). In addition, its effect is abrogated in the presence of exogenous VEGF, a sFlt-1 antagonist (Figure 4D; n = 8 per treatment). The sFlt-1 property to induce apoptosis of an endothelial cell line was confirmed by means of the terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling assay (Supplementary Figure S2).
Figure 4.
Sera from a patient with CKD induce apoptosis of an endothelial cell line (EAhy926 cells). Apoptosis was measured after incubation of the human endothelial cell line with 10% and 100% serum and human recombinant sFlt-1 (hr sFlt-1) in culture medium for 24 h. (A) EAhy926 cells present increased apoptosis when incubated with 10% of serum from patients with differing eGFR. (B) The graphic represents the effect of the neutralization of sFlt-1 and the caspase inhibitor on protecting cells against patient serum–induced apoptosis (undiluted serum). Immunoprecipitation of sFlt-1 was performed after incubation of sera with a specific monoclonal antibody (anti-sFlt-1, 40 μg/ml). For caspase inhibition, the cells were incubated with 100 μM Z-VAD-FMK 45 min before and during treatment with sera. (C) Human recombinant sFlt-1 (hr sFlt-1) directly induces EAhy926 cell apoptosis in a dose-dependent manner, and (D) its effect is abrogated in the presence of exogenous VEGF (hr sFlt-1 = 200 pg/ml; VEGF = 50 ng/ml). For the experiment with hr sFlt-1, cells treated with fetal calf serum (FCS) were set as control, and sFlt-1 was added to the medium in the presence or absence of VEGF. (E) Representative density plots showing the percentage of cells presenting positive staining for annexin-V and negative staining for propidium iodide. All data obtained for control and patient sera were normalized by values obtained with cells incubated with medium containing 10% FCS. Subjects presenting eGFR ≥60 ml/min/1.73 m2 were set as control. Each serum sample was analyzed individually. Results are mean ± SEM. aP < 0.05, bP < 0.01, and cP < 0.001 compared with control sera; dP < 0.01 and eP < 0.001 compared with patient sera. In Panel C, each concentration analyzed was compared with vehicle (fP < 0.05).
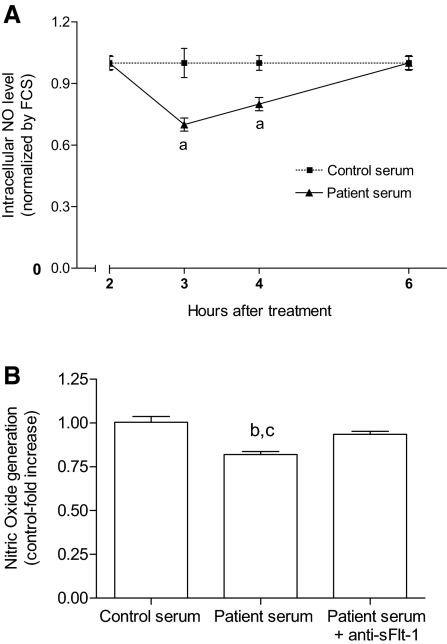
Next, we determined intracellular nitric oxide (NO) generation as a surrogate marker of endothelial function. Patient serum decreased NO production in an aortic endothelial cell line (GM7373) compared with controls. Figure 5 shows that, in the presence of undiluted patient sera, the relative levels of NO decrease compared with cells treated with control sera and that this decline is time dependent (n = 6 for each group). NO levels decrease between 3 and 4 h after incubation, returning to basal levels at 6 h (n = 8 for each group). Immunoprecipitation of sFlt-1 using a specific antibody (anti-sFlt-1, 40 μg/ml) before incubation abolished the deleterious effect of patient's serum on NO generation. As a control, serum was incubated and immunoprecipitated with nonimmune IgG. Supplementary Figure S3 presents additional information concerning the effect of hr sFlt-1 on NO bioavailability.
Figure 5.
Endothelial cell NO generation is decreased under incubation with serum from patients with CKD. (A) Time course analyses of NO generation after incubation of an aortic endothelial cell line, the GM7373 cells, with control or patient sera. NO was assessed by DAF-2 staining followed by flow cytometry. (B) Undiluted patient sera decreased NO bioavailability after 3-h incubation in comparison to undiluted control sera. Neutralization of sFlt-1 by using a specific antibody (anti-sFlt-1, 40 μg/ml) and immunoprecipitation abolished this effect. Each serum sample was analyzed individually. Results are mean ± SEM. aP < 0.01 and bP < 0.001 compared with control serum; cP < 0.05 compared with patient serum + anti-sFlt-1.
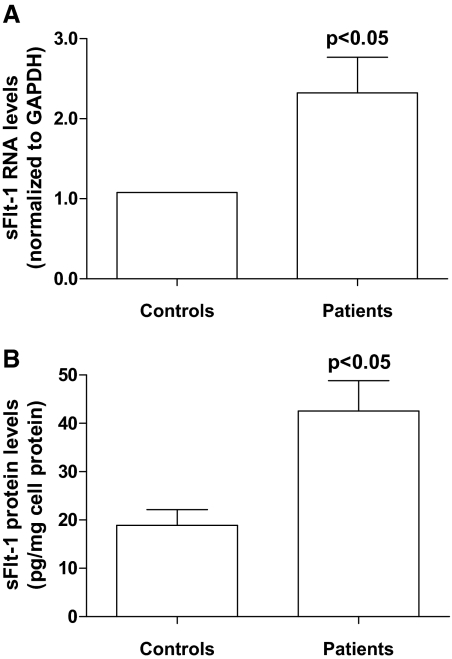
Peripheral blood mononuclear cells (PBMCs) express sFlt-1. Therefore, and for their implication in renal and endothelial damage, we tested the hypothesis that CKD increases sFlt-1 expression in monocytes. As analyzed by real-time PCR, sFlt-1 expression levels were higher in monocytes isolated from patients than in those isolated from controls (Figure 6A; n = 9 controls and n = 20 patients: 6 with eGFR 30 to 59 ml/min/1.73 m2, 5 with eGFR 15 to 29 ml/min/1.73 m2, and 10 with eGFR <15 ml/min/1.74 m2). Moreover, sFlt-1 concentration in conditioned medium from patient monocytes was higher than in that from control monocytes. The amount of sFlt-1 secreted by human monocytes was 18 ± 3 and 45 ± 6 pg/mg cell protein for controls and patients, respectively (Figure 6B; n = 9 controls and n = 14 patients: 4 patients with eGFR 30 to 59 ml/min/1.73 m2, 5 with eGFR 15 to 29 ml/min/1.73 m2, and 5 with eGFR <15 ml/min/1.74 m2). In these experiments, we did not observe any differences concerning statin use and the presence or absence of diabetes.
Figure 6.
Soluble Flt-1 expression and secretion by monocytes from patients with CKD. Freshly patient and control monocytes were cultured and allowed to condition the medium for 24 or 72 h. (A) Gene expression was analyzed by real-time PCR using specific primer pairs. Relative changes were evaluated using the 2-ΔΔCt method. (B) Secretion of sFlt-1 in the conditioned medium (72 h) was evaluated by ELISA. Data are presented as mean ± SEM values.
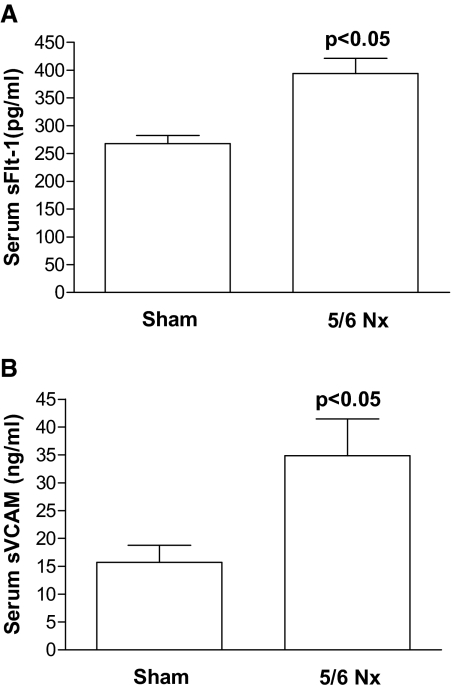
To confirm our results and avoid potential confounders present in human patients, such as concomitant diseases and medications, we decided to use an animal model of kidney disease/uremia, the 5/6 nephrectomy. Physiologic data assessed 14 d after surgery are given in Table 4. Increased plasma sFlt1 and sVCAM levels were observed in the nephrectomized rats (n = 6) compared with sham-operated animals (n = 5; Figure 7, A and B, respectively). These results corroborate with our previous findings observed in human renal patients, indicating that increased sFlt1 levels are directly associated with a decrease in GFR and with endothelial dysfunction.
Table 4.
Effects of 5/6 nephrectomy on whole animal functional data (day 14 after surgery)a
| Sham(n = 5) | 5/6-Nx(n = 6) | |
|---|---|---|
| Body weight, day 14 after surgery (g) | 332 ± 4 | 222 ± 17b |
| Weight gain after surgery (% body weight) | 43 ± 2 | −10 ± 6b |
| Urine volume (ml/24 h) | 16 ± 1 | 45 ± 6b |
| Na+ in serum (mM) | 134 ± 3 | 135 ± 6 |
| Na+ in urine (mM) | 112 ± 11 | 58 ± 11b |
| FENa+ (%) | 0.24 ± 0.03 | 1.05 ± 0.17b |
| K+ in urine (mM) | 245 ± 16 | 124 ± 23b |
| Protein excretion (mg/mg creatinine) | 0.35 ± 0.05 | 2.64 ± 0.62b |
| Serum creatinine (mg/dl) | 0.19 ± 0.01 | 0.34 ± 0.01b |
| Blood urea nitrogen (mg/dl) | 16.2 ± 1.1 | 36.5 ± 7.5b |
| CrCl (ml/min/100g) | 1.15 ± 0.09 | 0.54 ± 0.03b |
| BUN-Cl (ml/min/100g) | 0.46 ± 0.05 | 0.36 ± 0.06 |
| (CrCl + BUN)/2-Cl (ml/min/100g) | 0.81 ± 0.05 | 0.45 ± 0.04b |
aSham, sham-operated animals; 5/6 Nx, 5/6 nephrectomy; CrCl, creatinine clearance; BUN, blood urea nitrogen; FENa+, fractional excretion of Na+. Data are presented as mean values ± SEM.
bSignificantly different to CTR (P < 0.05).
Figure 7.
Serum sFlt-1 and sVCAM levels in 5/6-nephrectomized rats. CKD was induced in Sprague-Dawley rats by resection of renal tissue. After 14 d, serum samples were analyzed for (A) sFlt-1 and (B) sVCAM using ELISA kits. Sham, sham-operated animals; 5/6 Nx, 5/6-nephrectomy. Results are mean ± SEM.
Discussion
Even mild to moderate impairment of renal function is associated with an increased risk of death and of cardiovascular events. Endothelial dysfunction, which characterizes early stages of renal insufficiency, is one of the initial steps in the development of atherosclerosis and is associated with impaired angiogenesis.1
We showed for the first time that sFlt-1, the soluble form of the VEGF receptor 1, is increased in patients with CKD. Moreover, sFLT-1 concentrations are not only positively correlated with plasma vWF and sVCAM-1 levels, both markers of endothelial dysfunction, but even more important with the Framingham risk score. We next showed that sera from patients directly impair angiogenesis in an in vivo model. By using two different endothelial cell lines, we showed that these sera also induce endothelial cell apoptosis and decrease NO bioavailability. We suggest that sFlt-1 is directly involved in these effects by showing that human recombinant sFlt-1 had similar, dose-dependent effects and that neutralization of sFlt-1 in the serum with a monoclonal antibody had the reverse effect. Furthermore, we identified circulating monocytes as one possible source of increased sFlt-1 levels in CKD.
We are aware that Framingham risk score is not totally appropriate to predict cardiac events in individuals with CKD, because the equation does not accurately weight the present risk factors.15 However, we confirmed in our study that sFlt-1 levels are directly associated with the incidence of myocardial infarction and stroke.
We investigated patients receiving standard treatment, including statins, and presenting different risk factors, such as diabetes and hypertension. Statins are already known to increase sFlt-1 levels in patients with acute myocardial infarction16; however, their precise mechanisms remain unclear. In contrast, because it has been described that diabetes and hyperglycemia do not influence sFlt-1 levels,17,18 one can assume that the observed effects are directly related to the impaired renal function of these patients.18 Baseline characteristics of patients also differ from controls regarding LDL-cholesterol levels. This might be related to the intensified use of statin in this population. The patients were also characterized by the use of erythropoietin, a proangiogenic factor, and heparin and the presence of hypertension. Nevertheless, regression analysis did not show any association with sFlt-1 concentration in our study. Notably, multivariate analysis was the final confirmation of the exclusive positive association between sFlt-1 levels and renal and endothelial dysfunctions.
It has been extensively described that sFlt-1 excess antagonizes VEGF and, consequently, contributes to endothelial dysfunction.11,19,20 Moreover, endothelial dysfunction is associated with CKD, linking impaired renal function to cardiovascular disease.1,21 Therefore, we chose to focus on this aspect of sFlt-1 function in our study.
We showed that sVCAM-1 and vWF, well-known markers of endothelial dysfunction, were not simply increased in patient plasma as described previously,1 but more important, significantly correlated with sFlt-1 levels. Using multivariate analyses that adjusted for potential confounders, sFlt-1 levels were exclusively associated with renal function (decreased GFR) and endothelial dysfunction (sVCAM and vWF levels). Additionally, we specified the effect of sera of patients with CKD and sFlt-1 on endothelial cell function in multiple in vitro experiments.
First, we showed that patient sera exhibited a direct antiangiogenic activity in the chick chorioallantoic membrane (CAM) model. Abnormal vascular remodeling in renal failure, especially concerning heart capillarization, has been well documented.2,4 However, despite some hypotheses, the pathomechanisms causing faulty angiogenesis still remain unclear. Thus, it is conceivable that sFlt-1 has direct implications on the process of impaired angiogenesis.
Second, we observed that patient sera had a proapoptotic activity on an endothelial cell line (EAhy926 cells). Endothelial cell apoptosis is undoubtedly evidence of endothelial dysfunction. Many observations led to the suggestion that endothelial cell apoptosis is crucially involved in the pathogenesis of atherosclerosis,22,23 which is, in turn, a hallmark of CKD.2 However, there is only indirect evidence for the role of sFlt-1 on endothelial cell apoptosis because the authors used sFlt-1 gene transfer in hepatoma and renal cell carcinoma models.24,25 Herein, we proved this effect by using recombinant protein and showed that the proapoptotic effect was reversed by neutralization of sFLt-1. The proapoptotic activity found in patient serum and shown by recombinant sFlt-1 probably contributes to the antiangiogenic activity described above. Indeed, the main function of sFlt-1 is to bind and decrease the concentration of bioactive VEGF, the major angiogenic factor. This is the central putative mechanism responsible for pre-eclampsia in women presenting increased concentration of sFlt-1 during pregnancy.11,26
Third, we observed decreased intracellular NO bioavailability when aortic endothelial cells (GM-7373 cells) were treated with both patient serum and human recombinant sFlt-1. Endothelial NO synthesis is known to be impaired in individuals with decreased renal function, an effect that is likely to play an important role in the pathogenesis of their accelerated vascular disease.1,27,28 Moreover, our data are in line with previous studies that have shown that plasma from uremic patients reduces both NO synthase activity and l-arginine transport in cultured vascular endothelial cells, which, consequently, leads to decreased NO production.29–31 It has been described that VEGF stimulates production of NO in endothelial cells. Furthermore, VEGF promotes endothelial cell survival and angiogenesis through activation of the protein kinase Akt, which, in turn, activates endothelial NO synthase and increases NO generation.32 Herein, we confirmed that recombinant sFlt-1 antagonizes VEGF action and inhibits Akt phosphorylation, thereby decreasing NO bioavailability.
Concerning the possible sources of increased sFlt-1 in CKD, we showed that monocytes isolated from patients express higher levels of sFlt-1 than cells isolated from control subjects. These results are in line with previous studies that have also described monocytes as an important alternate source of sFlt-1 in pre-eclampsia.33,34
Finally, we confirmed that increased sFlt-1 levels are directly associated with the impairment of renal function by using an animal model of CKD.
The results of this study may be clinically relevant because endothelial dysfunction is potentially reversible,35 and antagonizing endogenous sFlt-1 may be a therapeutic approach for these patients to improve cardiovascular prognosis. Moreover, because sFlt-1 levels increase early in CKD and are positively correlated with Framingham risk score, sFlt-1 might serve as a diagnostic marker in patients at high risk for cardiovascular disease.
In conclusion, our findings strongly suggest that excess sFlt-1 is an important determinant of CKD-associated endothelial dysfunction. Moreover, we present evidence that PBMCs/monocytes isolated from patients are a source of sFlt-1 levels in these patients. Although our results do not provide a definitive mechanism and role for the increased sFlt-1 levels in patients with CKD, they suggest that increased sFlt-1 may constitute a novel cardiovascular risk factor in CKD.
Concise Methods
Study Population
We performed a cross-sectional study in patients with CKD recruited from the University Clinics, Münster, Germany. Consecutively attending patients were included. Exclusion criteria were current infection and malignancy, a history of organ transplantation, and pregnancy. All patients were maintained on their regular medication.
The clinical definition of diabetes included fasting glucose ≥126 mg/dl, oral hypoglycemic medication, or insulin use. Hypertension was defined as systolic blood pressure ≥140 mmHg and diastolic blood pressure ≥90 mmHg or antihypertensive therapy use. The Framingham risk score of each patient was calculated to assess the risk of developing hard coronary heart disease outcomes, i.e., myocardial infarction and coronary death.
We used the abbreviated Modification of Diet in Renal Disease equation to estimate the GFR (eGFR) as described previously by Go et al.36 In addition, we used a modified National Kidney Foundation classification of CKD, which stratifies eGFR in the following ranges: ≥60 ml/min/1.73 m2 (control and stages 1 and 2), 45 to 59 ml/min/1.73 m2 (stage 3a), 30 to 44 ml/min/1.73 m2 (stage 3b), 15 to 29 ml/min/1.73 m2 (stage 4), and <15 ml/min/1.73 m2 (stage 5). Subjects with eGFR ≥60 ml/min/1.73 m2 served as controls.36
The control group was composed of patients attending the Department of Surgery and Orthopedics, University Clinics Münster, and healthy volunteers. The subjects were chosen according to their eGFR (≥60 ml/min/1.73 m2). Because a large number of patients were hypertensive, we also selected subjects with essential hypertension (average eGFR = 89 ± 22 ml/min/1.73 m2) to serve as control and to minimize the possible implications of BP as well as of antihypertensive treatment. Diabetic patients without nephropathy (average eGFR = 86 ± 12 ml/min/1.73 m2) were also included. All subjects were maintained on their regular medication, including statin use and antihypertensive drugs.
Serum and EDTA plasma were obtained from all patients.
The protocol was approved by the medical ethical committee of the University Clinics Münster. Written informed consent was obtained from all patients and control subjects.
ELISAs
We determined plasma sFlt-1, sVCAM (R&D Systems), and vWF (vWF;Ag; Technoclone) using commercial ELISA kits and according to the manufacturer's specifications.
CAM Angiogenesis Assay
The antiangiogenic activity from patient serum was determined by using the CAM model.37,38
sFlt-1 Immunoprecipitation
To determine whether sFlt-1 was directly associated with the increased antiangiogenic activity found in serum of patients, we neutralized this protein in the serum using a specific antibody for immunoprecipitation.39 A nonimmune IgG was used as negative control.
Endothelial Cell Culture and Treatments
To study the effect of serum from patients with CKD on endothelial function in vitro, we used two different cell lines: a human endothelial cell line (EAhy 926) and bovine aortic endothelial cells (GM-7373) cultured as described previously.8,40
Most of control and patient sera were analyzed in vitro to imitate the patient population. The patients were only grouped according to their eGFR; if not, we provide the specific conditions in the text.
Endothelial Cell Apoptosis
Apoptotic cells were measured by staining with 5 μl annexin-V-FITC (BD Biosciences Pharmingen) and 5 μg/ml propidium iodide (Sigma) and subsequent flow cytometry (Becton-Dickinson) as described previously.8 All data obtained for control and patient sera were normalized using values obtained from cells incubated with medium containing 10% FCS (internal control for the experiment).
NO Measurement in Endothelial Cells
To measure changes in intracellular NO level, a proprietary membrane-permeable NO sensor (DAF-2 diacetate; Cayman Chemical) was used according to the manufacturer's instructions and followed by flow cytometry analysis as described previously by She et al.41
Western Blotting Analysis of Endothelial Cell Lysates
After 24-h incubation with human recombinant sFlt-1, endothelial cells were submitted to immunoblotting analysis by using specific antibodies against Akt or phospho-Akt (Cell Signaling).
Human PBMC/Monocyte Culture and Production of sFlt-1 In Vitro
Human PBMCs were obtained by Ficoll-Hypaque density gradient centrifugation from patients with CKD and control subjects. Soluble Flt-1 expression was analyzed by both real-time PCR and ELISA.
Soluble Flt-1 Expression by Monocytes: Real-Time PCR Analysis
Real-time PCR was performed using the SYBR Green PCR Master Mix (Applied Biosystems) with the ABI PRISM 7700 Sequence Detection System. Relative gene expression values were evaluated with the 2-ΔΔCt method using gapdh as a housekeeping gene. Human sFlt1 was amplified using the following primer sequences: forward, 5′-GGC TGT TTT CTC TCG GAT CTC-3′; reverse, 5′-CAT CTC CTC CGA GCC TGA AAG-3′ (product size: 158 bp; accession number U01134). The PCR product was confirmed by sequencing.
Animal Model: 5/6-Nephrectomized Rat
CKD was induced in Sprague-Dawley rats by 5/6 resection of renal tissue. Male Sprague-Dawley rats (Charles River) were randomly assigned to surgical 5/6 nephrectomy. Experiments were approved by a governmental committee on animal welfare and were performed in accordance with national animal protection guidelines.
Statistical Analysis
All analyses were performed using SPSS version 15.0. Non-normal data are presented as median and range and were analyzed on the log-transformed data by ANOVA followed by Scheffes's post hoc test. Data found to be normally distributed are presented as means ± SD and were analyzed by ANOVA. Variables based on proportions were analyzed by χ2 test and Fisher's exact test. For descriptive purposes, the group was divided in tertiles according to the sFlt-1 levels. P value for trend over tertiles was calculated with univariate ANOVA. Continuous data were subjected to the Kolmogorov-Smirnov test to determine its distribution. For continuous variables, univariate regression and correlation analyses were performed. The Pearson test was used to assess correlation coefficients based on the log-transformed data. Multivariate regression analyses were performed to assess associations between sFlt-1 and other parameters with regard to potential confounding factors. Variables that did not have a normal distribution were transformed into their natural logarithm (ln) for a better fit of the data. Results are described as regression coefficient β with 95% confidence interval. Two-sided P < 0.05 was considered to reflect statistical significance. In the first model multivariate regression analysis was performed with adjustment for age and gender. In the second model, we additionally adjusted for potential confounders, i.e., diabetes mellitus, HbA1c, statins, vWF, sVCAM, and triglycerides. In the third model, we adjusted only for variables that were significant after univariate analysis. Based on the full model, stepwise selection was applied to create a final model.
For experimental data, data are presented as mean ± SEM. Comparison among groups was performed by Kruskal-Wallis test or χ2 test for percent data. The t test or nonparametric Mann-Whitney test was used when appropriate. A level of P < 0.05 was accepted as statistically significant. Analyses were performed using GraphPad Prism version 4.0.
Disclosures
None.
Acknowledgments
M.B. and this study were supported by Stifterverband für die Deutsche Wissenschaft und der Simon-Claussen-Stiftung (Project H1405409999915626). E.B. is supported by a Heisenberg professorship (Br1589/8-1).
We thank Katrin Beul and Rita Schröter for indispensable technical assistance.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Stam F, van Guldener C, Becker A, Dekker JM, Heine RJ, Bouter LM, Stehouwer CD: Endothelial dysfunction contributes to renal function-associated cardiovascular mortality in a population with mild renal insufficiency: The Hoorn study. J Am Soc Nephrol 17: 537–545, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Amann K, Ritz E: Microvascular disease—The Cinderella of uraemic heart disease. Nephrol Dial Transplant 15: 1493–1503, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Blacher J, Safar ME, Pannier B, Guerin AP, Marchais SJ, London GM: Prognostic significance of arterial stiffness measurements in end-stage renal disease patients. Curr Opin Nephrol Hypertens 11: 629–634, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Amann K, Wiest G, Zimmer G, Gretz N, Ritz E, Mall G: Reduced capillary density in the myocardium of uremic rats—A stereological study. Kidney Int 42: 1079–1085, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Jacobi J, Porst M, Cordasic N, Namer B, Schmieder RE, Eckardt KU, Hilgers KF: Subtotal nephrectomy impairs ischemia-induced angiogenesis and hindlimb re-perfusion in rats. Kidney Int 69: 2013–2021, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson RJ: Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol 12: 1448–1457, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Al AZ, Edwards JC: Vascular biology in uremia: Insights into novel mechanisms of vascular injury. Adv Chronic Kidney Dis 11: 310–318, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Di Marco GS, Hausberg M, Hillebrand U, Rustemeyer P, Wittkowski W, Lang D, Pavenstadt H: Increased inorganic phosphate induces human endothelial cell apoptosis in vitro. Am J Physiol Renal Physiol 294: F1381–F1387, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Wu-Wong JR: Endothelial dysfunction and chronic kidney disease: Treatment options. Curr Opin Investig Drugs 9: 970–982, 2008 [PubMed] [Google Scholar]
- 10.Kendall RL, Wang G, Thomas KA: Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem Biophys Res Commun 226: 324–328, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA: Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA: Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350: 672–683, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Barleon B, Reusch P, Totzke F, Herzog C, Keck C, Martiny-Baron G, Marme D: Soluble VEGFR-1 secreted by endothelial cells and monocytes is present in human serum and plasma from healthy donors. Angiogenesis 4: 143–154, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Hornig C, Barleon B, Ahmad S, Vuorela P, Ahmed A, Weich HA: Release and complex formation of soluble VEGFR-1 from endothelial cells and biological fluids. Lab Invest 80: 443–454, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, Sarnak MJ: The Framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol 50: 217–224, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Kodama Y, Kitta Y, Nakamura T, Takano H, Umetani K, Fujioka D, Saito Y, Kawabata K, Obata JE, Mende A, Kobayashi T, Kugiyama K: Atorvastatin increases plasma soluble Fms-like tyrosine kinase-1 and decreases vascular endothelial growth factor and placental growth factor in association with improvement of ventricular function in acute myocardial infarction. J Am Coll Cardiol 48: 43–50, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Blann AD, Belgore FM, McCollum CN, Silverman S, Lip PL, Lip GY: Vascular endothelial growth factor and its receptor, Flt-1, in the plasma of patients with coronary or peripheral atherosclerosis, or type II diabetes. Clin Sci (Lond) 102: 187–194, 2002 [PubMed] [Google Scholar]
- 18.Kim NH, Oh JH, Seo JA, Lee KW, Kim SG, Choi KM, Baik SH, Choi DS, Kang YS, Han SY, Han KH, Ji YH, Cha DR: Vascular endothelial growth factor (VEGF) and soluble VEGF receptor FLT-1 in diabetic nephropathy. Kidney Int 67: 167–177, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Maynard SE, Venkatesha S, Thadhani R, Karumanchi SA: Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia. Pediatr Res 57: 1R–7R, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Xia L, Zhou XP, Zhu JH, Xie XD, Zhang H, Wang XX, Chen JZ, Jian S: Decrease and dysfunction of endothelial progenitor cells in umbilical cord blood with maternal pre-eclampsia. J Obstet Gynaecol Res 33: 465–474, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Amann K, Wanner C, Ritz E: Cross-talk between the kidney and the cardiovascular system. J Am Soc Nephrol 17: 2112–2119, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Inoue T, Node K: Vascular failure: A new clinical entity for vascular disease. J Hypertens 24: 2121–2130, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Littlewood TD, Bennett MR: Apoptotic cell death in atherosclerosis. Curr Opin Lipidol 14: 469–475, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Schmidt K, Hoffend J, Altmann A, Strauss LG, Mitrakopoulou-Strauss A, Engelhardt B, Koczan D, Peter J, Vorwald S, Eskerski H, Eisenhut M, Metz J, Kinscherf R, Haberkorn U: Transfer of the sFLT-1 gene in Morris hepatoma results in decreased growth and perfusion and induction of genes associated with stress response. Clin Cancer Res 11: 2132–2140, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Yoshimura I, Mizuguchi Y, Miyajima A, Asano T, Tadakuma T, Hayakawa M: Suppression of lung metastasis of renal cell carcinoma by the intramuscular gene transfer of a soluble form of vascular endothelial growth factor receptor I. J Urol 171: 2467–2470, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Maharaj AS, Walshe TE, Saint-Geniez M, Venkatesha S, Maldonado AE, Himes NC, Matharu KS, Karumanchi SA, D'Amore PA: VEGF and TGF-beta are required for the maintenance of the choroid plexus and ependyma. J Exp Med 205: 491–501, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Annuk M, Zilmer M, Fellstrom B: Endothelium-dependent vasodilation and oxidative stress in chronic renal failure: impact on cardiovascular disease. Kidney Int Suppl 84: S50–S53, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Wever R, Boer P, Hijmering M, Stroes E, Verhaar M, Kastelein J, Versluis K, Lagerwerf F, van RH, Koomans H, Rabelink T: Nitric oxide production is reduced in patients with chronic renal failure. Arterioscler Thromb Vasc Biol 19: 1168–1172, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Xiao S, Schmidt RJ, Baylis C: Plasma from ESRD patients inhibits nitric oxide synthase activity in cultured human and bovine endothelial cells. Acta Physiol Scand 168: 175–179, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao S, Wagner L, Schmidt RJ, Baylis C: Circulating endothelial nitric oxide synthase inhibitory factor in some patients with chronic renal disease. Kidney Int 59: 1466–1472, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao S, Wagner L, Mahaney J, Baylis C: Uremic levels of urea inhibit L-arginine transport in cultured endothelial cells. Am J Physiol Renal Physiol 280: F989–F995, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dimmeler S, Dernbach E, Zeiher AM: Phosphorylation of the endothelial nitric oxide synthase at ser-1177 is required for VEGF-induced endothelial cell migration. FEBS Lett 477: 258–262, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE: Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med 203: 2165–2175, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, Thadhani R, Wolf M, Harger G, Markovic N: Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta 26: 563–573, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Hsueh WA, Lyon CJ, Quinones MJ: Insulin resistance and the endothelium. Am J Med 117: 109–117, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Brand M, Lamande N, Larger E, Corvol P, Gasc JM: Angiotensinogen impairs angiogenesis in the chick chorioallantoic membrane. J Mol Med 85: 451–460, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Cid MC, Hernandez-Rodriguez J, Esteban MJ, Cebrian M, Gho YS, Font C, Urbano-Marquez A, Grau JM, Kleinman HK: Tissue and serum angiogenic activity is associated with low prevalence of ischemic complications in patients with giant-cell arteritis. Circulation 106: 1664–1671, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Ahmad S, Ahmed A: Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res 95: 884–891, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Oberleithner H, Riethmuller C, Schillers H, MacGregor GA, de Wardener HE, Hausberg M: Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci U S A 104: 16281–16286, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.She MR, Li JG, Guo KY, Lin W, Du X, Niu XQ: Requirement of reactive oxygen species generation in apoptosis of leukemia cells induced by 2-methoxyestradiol. Acta Pharmacol Sin 28: 1037–1044, 2007 [DOI] [PubMed] [Google Scholar]