Abstract
Diabetic nephropathy (DN) affects both glomerular cells and the extracellular matrix (ECM), yet the pathogenic mechanisms involving cell-matrix interactions are poorly understood. Glycation alters integrin-dependent cell-ECM interactions, and perturbation of these interactions results in severe renal pathology in diabetic animals. Here, we investigated how chemical modifications of the ECM by hyperglycemia and carbonyl stress, two major features of the diabetic milieu, affect mesangial cell functions. Incubation of collagen IV with pathophysiological levels of either the carbonyl compound methylglyoxal (MGO) or glucose resulted in modification of arginine or lysine residues, respectively. Mouse mesangial cells plated on MGO-modified collagen IV showed decreased adhesion and migration. Cells plated on glucose-modified collagen IV showed reduced proliferation and migration and increased collagen IV production. Inhibiting glucose-mediated oxidative modification of collagen IV lysine residues rescued the alterations in cell growth, migration, and collagen synthesis. We propose that diabetic ECM affects mesangial cell functions via two distinct mechanisms: modification of arginine residues by MGO inhibits cell adhesion, whereas oxidative modification of lysine residues by glucose inhibits cell proliferation and increases collagen IV production. These mechanisms may contribute to mesangial cell hypertrophy and matrix expansion in DN.
Diabetic nephropathy (DN) is the most common cause of ESRD in the developed world, yet the underlying pathogenic mechanisms are poorly understood. As DN progresses, extracellular matrix (ECM), which includes collagen IV, is deposited in the mesangium, causing gradual obliteration of glomerular capillary loops and progressive decline in glomerular filtration. Hyperglycemia induces these changes by several mechanisms, including increased oxidative stress and excessive formation of advanced glycation end products (AGEs).1
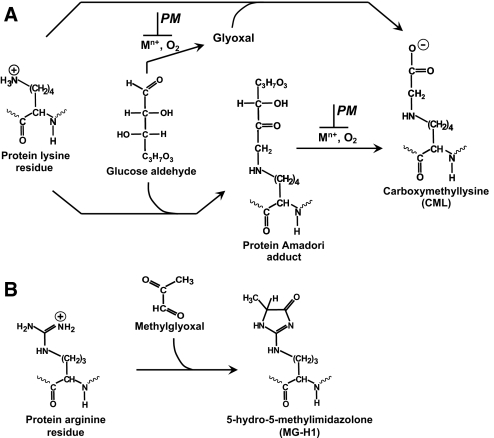
AGE formation in renal ECM proceeds through several major nonenzymatic reactions. Glucose reacts with the protein amino groups via a nonoxidative rearrangement to form Amadori adducts (Figure 1A). Free glucose and Amadori protein adducts can undergo oxidative degradation to form carboxymethyllysine (CML)2,3 (Figure 1A). Pyridoxamine (PM), which blocks oxidative pathways, inhibits formation of CML but not the Amadori adduct4 (Figure 1A). Another source of AGEs is carbonyl stress, which is characterized by an increase in circulating and tissue levels of reactive carbonyl compounds such as methylglyoxal (MGO)5 (Figure 1B). Unlike glucose, which reacts with protein lysine residues, MGO preferentially forms arginine adducts (e.g., hydroimidazolone MG-H16) (Figure 1B). In diabetes, modifications of ECM are significantly increased, with CML and MG-H1 being among the most abundant detected AGE adducts.3,7
Figure 1.
Schematic representation of major pathways of modification of lysine and arginine protein residues by glucose and MGO found in diabetes. (A) Modification of lysine to CML by glucose under oxidative conditions. Blocking of oxidative pathways (e.g., with PM), inhibits CML formation. (B) Modification of arginine residue to MG-H1 by MGO. Electrostatic charge at physiologic pH is shown for charged moieties.
Glucose can directly affect mesangial cells by altering integrin receptor expression8 or modulating various signaling cascades.9 Circulating AGE-albumin also causes mesangial cell damage by affecting cell growth and collagen expression.10 However, the cellular effects of ECM modifications may be different from the direct cellular toxicity of glucose, reactive carbonyl species, or circulating glycated albumin. Moreover, because these modifications accumulate on long-lived ECM proteins such as collagen IV, their effects would continue long after the levels of glucose and other circulating pathogenic factors are normalized. Thus, AGE modification of ECM proteins may contribute independently to DN pathogenesis. There are only a few studies describing the effects of glycated ECM on glomerular cell functions, and the mechanisms involved are poorly understood.11–13 In this manuscript, we report the distinct cellular effects of different AGE modifications of ECM for the first time and propose the underlying mechanisms.
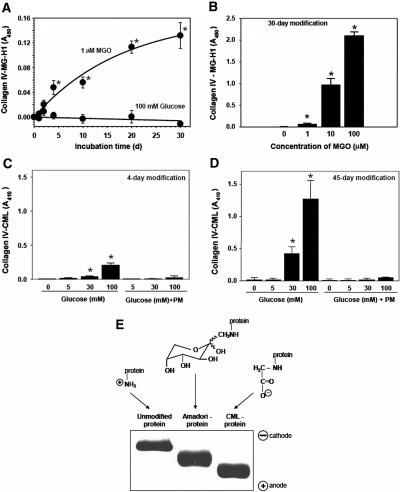
We initially prepared and characterized MGO- or glucose-modified collagen IV. When collagen IV was incubated with 1 μM MGO, modification of arginine residues to MG-H1 was detected at 4 d and significantly increased at 30 d (Figure 2A). MG-H1 formation directly correlated with MGO concentrations between 1 and 100 μM MGO (Figure 2B). Importantly, incubation with 100 mM glucose did not produce any MG-H1 modifications (Figure 2A), indicating that no significant MGO accumulation occurred because of potential oxidative glucose degradation. In contrast, exposure of collagen IV to 30 and 100 mM glucose for either 4 or 45 d resulted in modification of collagen lysine residues to CML (Figure 2, C and D). CML formation was almost completely inhibited in the presence of PM (Figure 2, C and D), which is consistent with the ability of PM to inhibit post-Amadori oxidative steps of CML formation.4 Thus, we prepared three physiologically relevant modifications of collagen IV: nonoxidative early glycation modification (Amadori adduct), oxidative AGE modification (CML), and MGO-derived modification (MG-H1).
Figure 2.
Modification of collagen IV by MGO or glucose and electrophoretic mobility of glucose-modified albumin. Collagen-IV-coated 96-well plates were incubated (A) with either 1 μM MGO or 100 mM d-glucose, (B) with different concentrations of MGO, (C) d-glucose, or (D) d-glucose and 20 mM PM in 200 mM sodium phosphate buffer, pH 7.5 at 37°C. Plates were washed and either MG-H1 or CML modification of collagen IV was determined by ELISA as described in the Concise Methods section. In all figures, data are shown as a mean ± SD (n = 4). *P < 0.05, MGO versus no MGO or glucose versus no glucose. (E) Modified albumin was prepared as described in the Concise Methods section and subjected to nondenaturing PAGE followed by Coomassie blue staining. The scheme shows the ε-amino group of lysine and its modifications by glycation and glycoxidation reactions in the corresponding samples; the theoretical electrostatic charge of each group at physiologic pH is also shown. Amadori intermediate is depicted in the most prevalent pyranose configuration.
Because glycation affects the overall charge of protein macromolecules, we determined whether Amadori and CML-modified proteins demonstrated these characteristics. Because the high molecular weight of collagen IV makes it difficult to visualize charge alterations, we made Amadori- and CML-modified albumin and subjected them to nondenaturing gel-electrophoresis, which generally separates protein isoforms according to their surface charge density. As expected, negative charge and corresponding electrophoretic mobility increased from unmodified albumin to Amadori-albumin to CML-albumin (Figure 2E).
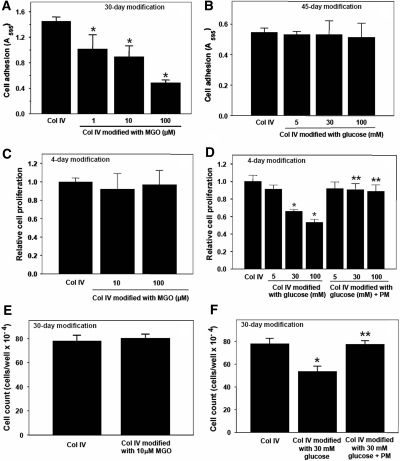
To determine the effects of these modifications on specific mesangial cell functions, modified collagen IV was prepared using pathophysiological concentrations of either glucose (5 to 30 mM) or MGO (1 to 10 μM),5,14 and supraphysiological concentrations to confirm our results. Cell adhesion to MGO-modified collagen IV was significantly inhibited compared with unmodified collagen (Figure 3A). The degree of inhibition correlated with the degree of modification of arginine residues to MG-H1 (Figure 2B). In contrast, cell adhesion to glucose-modified collagen IV, which contained lysine modifications to CML, was not affected even after 45 d of collagen exposure to high concentrations of glucose (Figure 3B).
Figure 3.
Effect of MGO and glucose-modified collagen IV on mesangial cell adhesion and proliferation. Ninety-six-well plates were coated with collagen IV and modified with (A, C, and E) MGO, or with (B, D, and F) glucose or glucose and 20 mM PM at 37°C as described in Figure 2. After washing, (A and B) cell adhesion or (C through F) cell proliferation was determined as described in the Concise Methods section. *P < 0.05 (n = 4), MGO versus no MGO, or glucose versus no glucose; **P < 0.05 (n = 4), glucose + PM versus glucose.
The role of modified collagen IV on cell proliferation was analyzed by either thymidine incorporation or direct cell counts. In contrast to the cell adhesion results, MGO-modified collagen did not alter mesangial cell proliferation (Figure 3, C and E); however, cell proliferation was inhibited on CML-collagen after only 4 d of modification (Figure 3, D and F). The presence of the CML moiety was critical, because no inhibition was observed when CML formation was blocked by PM (Figure 3, D and F). Next, we determined whether the cell growth inhibition is due to induction of cytotoxic oxidative stress or increased apoptosis. Cells grown on unmodified collagen IV had a very low level of apoptosis, which was unchanged when cells were grown on any of the modified collagens (Supplemental Figure S1A). Similarly, none of the collagen IV modifications altered cellular reactive oxygen species (ROS) production (Supplemental Figure S1B).
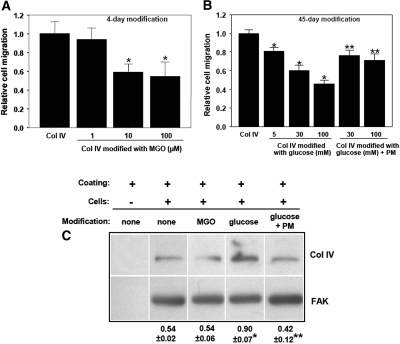
Compared with unmodified collagen, collagen IV modified with MGO for only 4 d significantly inhibited cell migration (Figure 4A). Cell migration was also inhibited on CML-collagen, albeit only after a 45-d modification with glucose (Figure 4B). Blocking CML formation using PM ameliorated this inhibitory effect (Figure 4B). Because elevated glucose levels can directly affect ECM deposition/production by mesangial cells,15 we determined whether glucose- and/or MGO-modified collagen IV alter collagen IV production by mesangial cells. Cells plated on glucose-modified collagen IV produced more collagen IV than cells plated on unmodified or MGO-modified collagen IV (Figure 4C). This effect was prevented when CML formation was inhibited using PM (Figure 4C).
Figure 4.
Cell migration on MGO- or glucose-modified collagen IV and effect of collagen modifications on endogenous collagen IV expression in mesangial cells. Transwells were coated with collagen IV and modified with (A) MGO, and (B) d-glucose or d-glucose and 20-mM PM at 37°C. After washing and blocking, mesangial cells (1 × 104) were plated on the top of Transwell membranes and cell migration was determined after 6 h at 37°C as described in the Concise Methods section. (C) Mesangial cells were plated on unmodified or modified collagen IV for 72 h. The levels of intracellular collagen IV were determined in total cell lysates by Western blot using anti-collagen IV antibody. Membranes were subsequently incubated with anti-FAK antibody to verify equal loading. Collagen IV and FAK bands were quantified by densitometry analysis, and the collagen IV signal was expressed as the collagen IV-to-FAK ratio. *P < 0.05 (n = 3), MGO versus no MGO, or glucose versus no glucose; **P < 0.05 (n = 3), glucose + PM versus glucose.
The specific mechanisms by which AGE modification of ECM can contribute to DN are unknown. The classical hypothesis suggests gradual loss of proteolytic digestibility of renal matrices due to glucose-derived AGE crosslinking.16,17 More recently, we proposed that perturbation of glomerular cell interactions with the underlying ECM may play an important role in the development of diabetic renal pathology.18,19 In this context, cell adhesion to collagen IV can be inhibited by MGO-induced non-crosslink modifications of specific arginine residues in the integrin binding sites of this ECM protein.18 The disruption of these integrin-ECM interactions is important in DN pathogenesis because diabetic mice lacking integrin α1β1, a major collagen IV receptor, develop more severe glomerular scarring than diabetic wild-type mice.19 This mechanism is consistent with inhibition of mesangial cell adhesion and migration by MGO-modified collagen (Figures 3A and 4A).
In contrast, the fact that glucose-modified collagen IV inhibited cell proliferation and induced endogenous collagen synthesis but did not affect cell adhesion suggests a different mechanism, which is dependent upon modifications of lysine residues to CML (Figure 3, B, D, and F and Figure 4C). Integrin α1β1 is unlikely to be involved because glucose-modified collagen IV equally affected the growth of wild-type and integrin α1-null mesangial cells.19 This notion is also consistent with our observation that modification of collagen arginine residues by MGO had no effect on either cell proliferation or collagen synthesis (Figure 3, C and E and Figure 4C).
The inhibition of cell proliferation observed in this study may be due to changes in the electrostatic charge density on collagen upon glycation because increased negative charge of the underlying substrata can inhibit cell proliferation.20 The mechanism is unknown and may involve receptors that can be activated by anionic ligands.10,21 Thus, we propose that increased negative charge because of increased CML content in glycated collagen IV results in inhibition of mesangial cell proliferation. Blocking CML formation using PM diminished the negative charge of glycated collagen and protected cell proliferation. Because MG-H1, like the Amadori adduct, is an electrostatically neutral moiety, modification of arginine residues by MGO does not affect cell proliferation.
Interestingly, we did not detect any effects of collagen IV modifications on apoptosis or ROS levels in mesangial cells. This is contrary to the reported effects of free glucose, free MGO, or soluble AGE-albumin on these parameters in mesangial cells.19,22–24 The absence of these effects in our study suggests that these diabetic factors induce their cellular effects independently of their ability to modify collagen IV.
In conclusion, we suggest that in the diabetic milieu, glucose- and MGO-derived modifications of ECM proteins make distinct contributions to development of characteristic DN glomerular lesions via perturbation of glomerular cell-matrix interactions. It has been suggested that hyperglycemia causes early but transient mesangial cell proliferation followed by a decrease in proliferation and development of cell hypertrophy.25 We propose that the anti-proliferative effect observed after the exposure of mesangial cells to high glucose for prolonged periods is due in part to oxidative modification of ECM lysine residues to CML and the increase in overall anionic matrix properties. This modification also induces collagen synthesis by mesangial cells, thus contributing to mesangial matrix expansion. Furthermore, MGO modification of ECM arginine residues disrupts integrin-dependent cell-matrix adhesions,18 thus exacerbating mesangial cell dysfunction. The same mechanism may potentially induce podocyte detachment from the glomerular basement membrane, a condition thought to contribute to proteinuria in DN.26
Concise Methods
Materials
Glucose was purchased from Invitrogen (Carlsbad, CA). MGO, mouse type IV collagen, PM dihydrochloride, and BSA were purchased from Sigma-Aldrich (St. Louis, MO. Dihydroethidium was purchased from Molecular Probes (Eugene, OR).
Cell Culture
Conditionally immortalized mouse mesangial cells were propagated in DME medium supplemented with 10% FBS, 100-U/ml penicillin, 100-μg/ml streptomycin, and 100-U/ml and interferon-γ at 33°C.19 For experiments, cells were cultured in the above medium without interferon-γ at 37°C at least for 4 d before use, because this is the optimal time for immortalized mesangial cells to acquire a phenotype similar to that of freshly isolated primary mesangial cells (i.e., elongated vimentin-positive cells).19
Preparation and Characterization of Glucose- and MGO-Modified Proteins
Ninety-six-well plates (Nunc, Rochester, NY) or Transwell filters (8-μm pores, Corning Ware) were incubated overnight with collagen IV (20 μg/ml in PBS) at 4°C. Plates or Transwells were washed twice with 150 mM sodium phosphate buffer, 0.05% sodium azide (pH 7.5) and incubated in the same buffer with or without different concentrations of either MGO or d-glucose. To prepare Amadori-modified collagen IV, incubation buffer was supplemented with 20 mM PM to trap protein-Amadori intermediate and prevent oxidative CML formation in the presence of d-glucose.4 Incubations were carried out for 4, 30, or 45 d in the dark at 37°C. No protein desorption was detected after different treatments as determined by alkaline phosphatase competition assay27 (data not shown). Plates were then washed to remove free reagents, and CML residues were determined by ELISA using polyclonal anti-AGE antibody R618, which recognizes protein-CML as an antigenic epitope.28 Modification of collagen arginine residues to MG-H1 was determined using monoclonal antibody 1H7G5, (a gift from Dr. Michael Brownlee, Albert Einstein University, Bronx, NY29).
Albumin-Amadori intermediate was prepared in the presence of 100 mM glucose and 20 mM PM as described earlier;4 albumin-CML was prepared using glyoxylic acid under reducing conditions.3 A degree of albumin modification was determined using previously described analytical methods4,30 and was similar in both samples (0.40 and 0.36 mol/mol of lysine for albumin-Amadori and albumin-CML, respectively).
Adhesion Assay
Ninety-six-well plates coated with either d-glucose- or MGO-modified collagen IV were prepared as described above. Plates were washed with PBS and incubated with 1% BSA in PBS for 60 min to block nonspecific adhesion. Adhesion assays using 100 μl/well of single-cell suspensions (5 × 104 mesangial cells/ml) were performed as described previously.19
Proliferation Assay
Ninety-six-well plates coated with either d-glucose or MGO-modified collagen IV were prepared as described above. Mesangial cells (5 × 103/well) were plated in DME medium containing 2% FCS. The cells were incubated with 3H-thymidine (1 μCi/well) 2 d after plating and then pulsed for an additional 48 h. Cells were then processed as described previously.19 Alternatively, 2 × 105 cells were plated onto 6-well plates coated as described above in DMEM containing 2% FCS. The cells were trypsinized 2 d later, washed once with complete medium, suspended in 1 ml of DME medium containing 2% FCS, and counted using a hematocrit chamber.
Migration Assay
Transwell filters (8-μm pores, Corning Ware) coated with either d-glucose- or MGO-modified collagen IV were prepared as described above. The filters were subsequently blocked with 1% BSA in PBS to inhibit nonspecific migration, and the bottom well was filled with 500 μl of serum-free medium. Mesangial cells (5 × 104 in 300 μl of serum-free medium) were added to the upper well and incubated for 6 h at 37°C. Cell migration was determined as described previously.19
Apoptosis Assay
Cells were seeded onto 96-well plates (104 cells per well) coated with either unmodified or modified collagen IV and incubated for 24 h at 37°C. In separate incubations, cells were treated with 100 μM cisplatin for 24 h and used as a positive control for apoptosis. Apoptosis was quantitated using Roche Cell Death Detection ELISAPLUS kit following the manufacturer's protocol. Briefly, plates were centrifuged at 200 × g for 10 min and supernatant was carefully removed. Cells were then lysed with 200 μl of Roche lysis buffer for 30 min at room temperature and centrifuged again at 200 × g for 10 min. Aliquots of the supernatant (20 μl) were transferred to the Roche streptavidin-coated plates, and fragmented DNA was detected by ELISA using appropriate controls according to the manufacturer's protocol.
Detection of ROS
ROS in mesangial cells were measured using dihydroethidium probe. Briefly, 1.5 × 105 mesangial cells were plated in 6-well plates coated with either unmodified or modified collagen IV in DME medium containing 1% FCS. After 48 h, cells were incubated for 2 h in serum-free medium containing 1 μM dihydroethidium. Cells then were washed, trypsinized, and dihydroethidium fluorescence (emission 510 nm/excitation 590 nm) was analyzed by FACS as described previously.19
Analysis of Endogenous Collagen Synthesis
Mesangial cells (1.5 × 105) were plated in 6-well plates coated with either unmodified or modified collagen IV in DMEM containing 1% FCS. After 72 h, the cells were washed with PBS and incubated with 100 μl of lysis buffer (50 mM Tris, pH 7.4; 150 mM sodium chloride; 1% Triton-X-100; 1% deoxycholate) for 10 min at 4°C. The cell lysates were gently collected and used for Western blot analysis as described below. Plates coated with collagen IV but without cells were incubated with lysis buffer as negative controls.
Western Blot Analysis
An equal amount (20 μg/lane) of cell lysates from cells incubated on unmodified or modified collagen IV as described above were run onto a 10% SDS gel and subsequently transferred to nitrocellulose membranes. Membranes were incubated with mouse anti-collagen IV (Biodesign) and anti-FAK antibodies (Cell Signaling) followed by the appropriate horseradish-peroxidase-conjugated secondary antibodies. Immunoreactive bands were identified using enhanced chemiluminescence.
Statistical Analysis
Data were expressed as means ± SD, and statistical analysis was performed using the t test for unpaired samples or ANOVA followed by post hoc Newman–Keuls comparisons. Differences were considered statistically significant if P values were less than 0.05.
Disclosures
Dr. Billy Hudson owns stock of NephroGenex, a company which develops PM for treatment of DN.
Acknowledgments
The authors thank Dr. Michael Brownlee (Albert Einstein University, Bronx, NY) for the generous gift of monoclonal antibody 1H7G5. This work was supported in part by the research grants from the National Institutes of Health DK066415 (P.V.), DK065123 (B.H.), DK065138 (B.H.), DK074359 (A.P.), DK 69921 (R.Z.), DK075594 (R.Z.), and a merit award from the Department of Veterans Affairs (R.Z. and A.P.). This work was presented in part at Renal Week 2008, sponsored by the American Society of Nephrology.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.Brownlee M: The pathobiology of diabetic complications: A unifying mechanism. Diabetes 54: 1615–1625, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Glomb MA, Monnier VM: Mechanism of protein modification by glyoxal and glycolaldehyde, reactive intermediates of the Maillard reaction. J Biol Chem 270: 10,017–10,026, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Ahmed MU, Thorpe SR, Baynes JW: Identification of N epsilon-carboxymethyllysine as a degradation product of fructoselysine in glycated protein. J Biol Chem 261: 4889–4894, 1986 [PubMed] [Google Scholar]
- 4.Voziyan PA, Khalifah RG, Thibaudeau C, Yildiz A, Jacob J, Serianni AS, Hudson BG: Modification of proteins in vitro by physiological levels of glucose: Pyridoxamine inhibits conversion of Amadori intermediate to advanced glycation end products through binding of redox metal ions. J Biol Chem 278: 46,616–46,624, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Odani H, Shinzato T, Matsumoto Y, Usami J, Maeda K: Increase in three alpha,beta-dicarbonyl compound levels in human uremic plasma: Specific in vivo determination of intermediates in advanced Maillard reaction. Biochem Biophys Res Commun 256: 89–93, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Lo TW, Westwood ME, McLellan AC, Selwood T, Thornalley PJ: Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. J Biol Chem 269: 32,299–32,305, 1994 [PubMed] [Google Scholar]
- 7.Thornalley PJ, Battah S, Ahmed N, Karachalias N, Agalou S, Babaei-Jadidi R, Dawnay A: Quantitative screening of advanced glycation end products in cellular and extracellular proteins by tandem mass spectrometry. Biochem J 375: 581–592, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Setty S, Anderson SS, Wayner EA, Kim Y, Clegg DO, Tsilibary EC: Glucose-induced alteration of integrin expression and function in cultured human mesangial cells. Cell Adhes Commun 3: 187–200, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Haneda M, Koya D, Isono M, Kikkawa R: Overview of glucose signaling in mesangial cells in diabetic nephropathy. J Am Soc Nephrol 14: 1374–1382, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Brizzi MF, Dentelli P, Rosso A, Calvi C, Gambino R, Cassader M, Salvidio G, Deferrari G, Camussi G, Pegoraro L, Pagano G, Cavallo-Perin P: RAGE- and TGF-beta receptor-mediated signals converge on STAT5 and p21waf to control cell-cycle progression of mesangial cells: A possible role in the development and progression of diabetic nephropathy. FASEB J 18: 1249–1251, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Crowley ST, Brownlee M, Edelstein D, Satriano JA, Mori T, Singhal PC, Schlondorff DO: Effects of nonenzymatic glycosylation of mesangial matrix on proliferation of mesangial cells. Diabetes 40: 540–547, 1991 [DOI] [PubMed] [Google Scholar]
- 12.Anderson SS, Kim Y, Tsilibary EC: Effects of matrix glycation on mesangial cell adhesion, spreading and proliferation. Kidney Int 46: 1359–1367, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Skolnik EY, Yang Z, Makita Z, Radoff S, Kirstein M, Vlassara H: Human and rat mesangial cell receptors for glucose-modified proteins: Potential role in kidney tissue remodeling and diabetic nephropathy. J Exp Med 174: 931–939, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagaraj RH, Sarkar P, Mally A, Biemel KM, Lederer MO, Padayatti PS: Effect of pyridoxamine on chemical modification of proteins by carbonyls in diabetic rats: Characterization of a major product from the reaction of pyridoxamine and methylglyoxal. Arch Biochem Biophys 402: 110–119, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Ziyadeh FN, Sharma K, Ericksen M, Wolf G: Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-beta. J Clin Invest 93: 536–542, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mott JD, Khalifah RG, Nagase H, Shield CF, III, Hudson JK, Hudson BG: Nonenzymatic glycation of type IV collagen and matrix metalloproteinase susceptibility. Kidney Int 52: 1302–1312, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Monnier VM, Mustata GT, Biemel KL, Reihl O, Lederer MO, Zhenyu D, Sell DR: Cross-linking of the extracellular matrix by the Maillard reaction in aging and diabetes: An update on “a puzzle nearing resolution”. Ann N Y Acad Sci 1043: 533–544, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Pedchenko VK, Chetyrkin SV, Chuang P, Ham AJ, Saleem MA, Mathieson PW, Hudson BG, Voziyan PA: Mechanism of perturbation of integrin-mediated cell-matrix interactions by reactive carbonyl compounds and its implication for pathogenesis of diabetic nephropathy. Diabetes 54: 2952–2960, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Zent R, Yan X, Su Y, Hudson BG, Borza DB, Moeckel GW, Qi Z, Sado Y, Breyer MD, Voziyan P, Pozzi A: Glomerular injury is exacerbated in diabetic integrin alpha1-null mice. Kidney Int 70: 460–470, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Pugliese F, Mene P, Cinotti GA: Glomerular polyanion and control of cell function. Am J Nephrol 10[Suppl 1]: 14–18, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Pugliese G, Pricci F, Romeo G, Pugliese F, Mene P, Giannini S, Cresci B, Galli G, Rotella CM, Vlassara H, Di Mario U: Upregulation of mesangial growth factor and extracellular matrix synthesis by advanced glycation end products via a receptor-mediated mechanism. Diabetes 46: 1881–1887, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Liu BF, Miyata S, Hirota Y, Higo S, Miyazaki H, Fukunaga M, Hamada Y, Ueyama S, Muramoto O, Uriuhara A, Kasuga M: Methylglyoxal induces apoptosis through activation of p38 mitogen-activated protein kinase in rat mesangial cells. Kidney Int 63: 947–957, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Yamagishi S, Inagaki Y, Okamoto T, Amano S, Koga K, Takeuchi M, Makita Z: Advanced glycation end product-induced apoptosis and overexpression of vascular endothelial growth factor and monocyte chemoattractant protein-1 in human-cultured mesangial cells. J Biol Chem 277: 20,309–20,315, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Tuttle KR, Johnson EC, Cooney SK, Anderberg RJ, Johnson EK, Clifton GD, Meek RL: Amino acids injure mesangial cells by advanced glycation end products, oxidative stress, and protein kinase C. Kidney Int 67: 953–968, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Wolf G, Ziyadeh FN: Molecular mechanisms of diabetic renal hypertrophy. Kidney Int 56: 393–405, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Ziyadeh FN, Wolf G: Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev 4: 39–45, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Steinitz M, Baraz L: A rapid method for estimating the binding of ligands to ELISA microwells. J Immunol Methods 238: 143–150, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Voziyan PA, Metz TO, Baynes JW, Hudson BG: A post-Amadori inhibitor pyridoxamine also inhibits chemical modification of proteins by scavenging carbonyl intermediates of carbohydrate and lipid degradation. J Biol Chem 277: 3397–3403, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Kilhovd BK, Giardino I, Torjesen PA, Birkeland KI, Berg TJ, Thornalley PJ, Brownlee M, Hanssen KF: Increased serum levels of the specific AGE-compound methylglyoxal-derived hydroimidazolone in patients with type 2 diabetes. Metabolism 52: 163–167, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Chetyrkin SV, Zhang W, Hudson BG, Serianni AS, Voziyan PA: Pyridoxamine protects proteins from functional damage by 3-deoxyglucosone: Mechanism of action of pyridoxamine. Biochemistry 47: 997–1006, 2008 [DOI] [PubMed] [Google Scholar]