Abstract
Background
There is little information available concerning trichobezoars in the nonhuman primate literature.
Methods
We evaluated 118 cases of trichobezoar in baboons over a 29 year period at the Southwest National Primate Research Center.
Results
The anatomic locations affected in decreasing order were the stomach, small intestine, cecum, esophagus, and colon. The most common clinical history was weight loss. The most frequent associated pathology included gastrointestinal inflammation and ulceration, emaciation, peritonitis, intussusception, pneumonia, and aspiration. Trichobezoars were the cause of death in 9 baboons and the reason for euthanasia in 12. Females were 2.14 times more likely than males to be affected. The greater the percentage of group housing time, the more likely the baboon was to develop trichobezoars.
Conclusions
The baboon may present a useful model to evaluate the etiology, genetic predisposition, physiopathology, neurobiology, and treatment response of trichobezoars.
Keywords: Stomach, hairball, trichophagia, trichotillomania, hair pulling, nonhuman primate, Papio
Introduction
Pulling and swallowing hair (trichophagia) has been described in humans and in dogs, cats, rabbits, mice, guinea pigs, sheep, musk ox, and nonhuman primates [7, 9]. This uncommon behavior has been associated with confinement conditions in large groups of animals [11, 12]. The Institute for Laboratory Animal Research considers hair pulling a “maladaptive behavior” that smoothes over the stress from chronic confinements [12]. Hair pulling in nonhuman primates has been classified as a pathological behavior that seems quite similar to trichotillomania in humans [11].
The clinical signs can include patches of alopecia (in self pickers or the cagemates of hair pullers). In extreme cases, animals can become completely bald. The ingested hair accumulates in the gastrointestinal tract, resulting in a nondigestible mass called a trichobezoar [3]. Trichobezoars are conglomerates of hair, fur or wool, and have been reported in man, cats, dogs, pigs, sheep, rats, rabbits and nonhuman primates [1]. Trichobezoars in the digestive tract of nonhuman primates pose a clinical problem, causing anorexia, gradual weight loss and occasionally regurgitation and vomiting [13]. Severe cases may lead to complications such as obstruction, intussusception, or gastric perforation and hemorrhage [4, 12]. Trichobezoars in humans and animals are resistant to treatment (mineral oils, laxatives) often necessitating surgical intervention [3, 5, 7].
We evaluated 118 cases of trichobezoar in baboons over a 29 year period at the Southwest National Primate Research Center at the Southwest Foundation for Biomedical Research.
Materials and methods
Animals
The baboons were housed in indoor-outdoor metal and concrete cages in groups, or singly-housed in metal cages for a specified period if enrolled in research projects or for acute clinical care. Some baboons were group-housed in two six-acre corrals. They were fed commercial monkey diets supplemented with grains, vegetables and fruits. Water was supplied ad libitum. The baboons were usually members of the breeding colony. All animal care and procedures were approved by the Southwest Foundation for Biomedical Research Institutional Animal Care and the Use Committee.
A computer search was performed using an internal anatomic pathology database (apath) and identified 118 baboons necropsied over a 29 year period with a diagnosis of trichobezoar. We did not include animals in our search under the age of 182 days, which are still nursing and considered not to be at risk. An additional 4 animals were removed from statistical analyses due to incomplete housing location records, which were used to classify housing type and duration of stay. The final statistical data set included 4,456 baboons of which 114 were trichobezoar positive and 4,342 were trichobezoar negative.
The housing location data included 15,495,070 days of life divided into four categories: single housing, gang housing, corral housing and undefined housing. Single housing indicates that an animal was in an individual indoor cage so that physical contact with other baboons was prevented. Gang housing indicates that the animals were housed with other baboons, either indoor or outdoor. No effort was made to distinguish between gang cages that included animals of both sexes and those that did not. Corral housing indicates that the animal was located in one of two six acre outdoor corrals. Animals of both sexes are always present within either corral. Undefined housing consisted of locations that changed housing types (modifications from single to group housing or vice-versa) one or more times during the study period and records of when those changes occurred were not available.
Statistical evaluation
Generalized Linear Model analyses were used to estimate effects of a categorical predictor variable (sex) and continuous predictor variables (age, total days alive, days in single housing, days in gang housing, days in corral housing, and days in undefined housing). In addition to these continuous predictors, the predictive power of the following ratios was also estimated: single housing days/total days alive, gang housing days/total days alive, corral days/total days alive, undefined housing days/total days alive, and group housing days/total days alive. Group housing days was defined as gang housing days plus corral housing days. Fitted models included a binomial with five different link functions (logit, log, complementary log-log, probit, and cauchit) and a Poisson with a log link function. Models with and without interaction were evaluated.
In addition to using genearlized linear model regression, the effect of age was analyzed using a permutation test with Monte Carlo estimation.
The R statistical package was used for all calculations. Generalized linear model analyses were performed using the function “glm”, which is part of the stats package that comes with R [10].
Results
The results are summarized in Table 1. One hundred forty six trichobezoars were identified in 118 baboons throughout the digestive tract: stomach (n=103), small intestine (n=14), cecum (14), esophagus (n=8), colon (6); one baboon had a large trichobezoar in the oral cavity resulting in asphyxiation (Figures 1A–D). Twenty two baboons had trichobezoars in more than one location. They ranged from small accumulations of densely matted hair to large accumulations that filled, distended and obstructed the affected organ. Trichobezoars were considered the cause of death in 9 baboons and the reason for euthanasia in another 12.
Table 1.
Trichobezoars in 118 baboons.
| Animal ID | Sex | Age (yr) | Clinical history | Trichobezoar Location(s) | Associated lesions |
|---|---|---|---|---|---|
| 1 | F | 0.75 | Found dead | Stomach, colon | None |
| 2 | F | 2 | Found dead | Esophagus | Gastric dilatation, esophageal and gastric necrosis |
| 3 | F | 3 | Found dead | Stomach | Gastric ulcer, emaciation |
| 4 | F | 3 | Found dead | Stomach | Gastritis |
| 5 | F | 3 | None | Stomach | None |
| 6 | F | 3 | None | Stomach | Small intestine intussusception |
| 7 | F | 3 | Weight loss | Stomach | Gastritis |
| 8 | F | 4 | Anemia | Stomach | Colitis |
| 9 | F | 4 | Found dead | Stomach | Gastritis |
| 10 | F | 4 | Found dead | Stomach | None |
| 11 | F | 4 | Found moribund, bloated abdomen | Stomach | Duodenal intussusception |
| 12 | F | 4 | Hairball | Stomach | None |
| 13 | F | 4 | None | Stomach | Emaciation |
| 14 | F | 4 | None | Stomach | Emaciation, enteritis, colitis |
| 15 | F | 4 | None | Stomach | Peritonitis |
| 16 | F | 5 | None | Stomach | None |
| 17 | F | 5 | Bloat | Stomach, small intestine | Peritonitis |
| 18 | F | 5 | Painful abdomen | Stomach, small intestine | Gastritis, enteritis, colitis, peritonitis, small intestine infarct |
| 19 | F | 5 | None | Stomach, small intestine, cecum | Pneumonia |
| 20 | F | 6 | Found dead | Stomach | None |
| 21 | F | 6 | Found dead | Stomach | None |
| 22 | F | 6 | Found dead | Stomach | None |
| 23 | F | 6 | None | Stomach | Gastritis, gastric ulcer, emaciation |
| 24* | F | 6 | None | Stomach | Peritonitis |
| 25 | F | 6 | Weight loss, dehydration, impaction | Stomach | Emaciation, colonic atony |
| 26 | F | 6 | None | Stomach, cecum | Cecal ulcer, peritonitis |
| 27 | F | 6 | None | Stomach, cecum | None |
| 28 | F | 6 | Found dead | Stomach, colon | None |
| 29 | F | 7 | Weight loss | Esophagus | Intussusception, jejunum |
| 30 | F | 7 | Found dead | Mouth | Asphyxiation |
| 31 | F | 7 | Anemia, possible hairball | Stomach | Emaciation |
| 32 | F | 7 | Found dead | Stomach | Emaciation |
| 33 | F | 7 | Found dead | Stomach | Emaciation, peritonitis |
| 34 | F | 7 | None | Stomach | None |
| 35 | F | 7 | Palpable abdominal mass, possible hairball | Stomach | None |
| 36 | F | 7 | Painful abdomen | Stomach, small intestine | Peritonitis |
| 37 | F | 8 | Found dead | Cecum, colon | None |
| 38 | F | 8 | None | Esophagus, stomach | Gastric and esophageal ulcers, pneumonia, tracheitis |
| 39 | F | 8 | Found dead | Small intestine | Emaciation, colitis |
| 40 | F | 8 | Found dead | Stomach | Peritonitis |
| 41 | F | 8 | Found dead | Stomach | Peritonitis, small intestinal ulcer |
| 42 | F | 8 | None | Stomach | None |
| 43 | F | 8 | None | Stomach | None |
| 44 | F | 8 | Weight loss | Stomach | Gastric hyperplasia |
| 45 | F | 8 | Weight loss | Stomach | None |
| 46 | F | 9 | None | Esophagus, stomach, small intestine | Peritonitis |
| 47 | F | 9 | Found dead | Stomach | Emaciation |
| 48 | F | 9 | Found moribund | Stomach | None |
| 49 | F | 10 | Found dead | Stomach | Gastritis, emaciation |
| 50 | F | 10 | None | Stomach | Steatosis, liver |
| 51* | F | 11 | Weight loss, abdominal mass | Cecum | Amyloidosis |
| 52 | F | 11 | Found dead | Small intestine | Steatosis, liver |
| 53 | F | 11 | Hairball | Stomach | Gastric ulcer, gastric hyperplasia |
| 54 | F | 11 | Obese | Stomach | Amyloidosis, stomach |
| 55 | F | 11 | Renal failure | Stomach | Esophagitis, gastritis |
| 56 | F | 11 | Weight loss | Stomach | Enteritis |
| 57 | F | 12 | Renal failure, possible hairball | Cecum, colon | Colitis |
| 58 | F | 12 | Found moribund | Stomach | Gastritis, cholecystitis, gall bladder fibrosis |
| 59 | F | 12 | Weight loss | Stomach | None |
| 60 | F | 12 | Weight loss | Stomach | Peritonitis |
| 61 | F | 12 | Weight loss, anemia. diarrhea | Stomach | Hyperplasia, gastric mucosa, foreign body (wire) in intestinal tract, emaciation |
| 62 | F | 14 | Found moribund | Small intestine | Steatosis, liver |
| 63 | F | 14 | Found dead | Stomach | Emaciation |
| 64 | F | 14 | None | Stomach | Gastric ulcer, gastritis, candida albicans |
| 65 | F | 15 | Weight loss | Cecum | Cecal adenocarcinoma |
| 66 | F | 15 | Found dead | Esophagus | Esophagitis |
| 67 | F | 15 | Found dead | Stomach | None |
| 68* | F | 15 | Found dead | Stomach | None |
| 69 | F | 16 | None | Stomach | None |
| 70 | F | 16 | Renal failure | Stomach | Amyloidosis, colitis |
| 71 | F | 16 | Weight loss | Stomach | Small intestine, intussusception |
| 72 | F | 16 | Hairball removal | Stomach, cecum, colon | None |
| 73 | F | 17 | Weight loss, abdominal mass | Stomach | None |
| 74 | F | 17 | Anemia | Stomach | Alopecia, emaciation, gastric ulcer |
| 75 | F | 17 | None | Stomach | Obese |
| 76 | F | 17 | Weight loss, anemia, bloody diarrhea | Stomach | Emaciation |
| 77 | F | 18 | Weight loss, abdominal mass | Stomach | Gastritis, emaciation |
| 78 | F | 19 | Renal failure | Stomach | Alopecia |
| 79 | F | 20 | Weight loss | Stomach | Gastritis |
| 80 | F | 20 | Weight loss, possible hairball | Stomach | None |
| 81 | F | 20 | Found dead | Stomach, small intestine | None |
| 82 | F | 22 | Found dead | Colon | Steatosis, liver |
| 83 | F | 23 | Weight loss | Cecum | None |
| 84 | F | 23 | Weight loss | Stomach | None |
| 85 | F | 26 | Weight loss | Cecum | Colitis, Campylobacter sp. |
| 86 | F | 26 | None | Stomach | Obese, gastric lipidosis |
| 87 | F | 26 | Weight loss, anemia | Stomach | Emaciation |
| 88 | M | 2 | None | Stomach | None |
| 89 | M | 3 | Weight loss | Small intestine | Peritonitis, small and large intestinal ulcers |
| 90 | M | 3 | Found dead | Stomach | None |
| 91 | M | 3 | Found dead | Stomach, small intestine | Emaciation |
| 92 | M | 4 | None | Stomach | Aspiration |
| 93 | M | 5 | Hairball removal | Stomach | None |
| 94 | M | 5 | Weight loss | Stomach | Emaciation |
| 95 | M | 5 | Weight loss | Stomach | None |
| 96 | M | 5 | None | Stomach, small intestine | Gastritis, enteritis, duodenal intussusception, pneumonia |
| 97 | M | 6 | Found dead | Esophagus, stomach | Esophagitis, peritonitis |
| 98 | M | 6 | Weight loss | Esophagus, stomach, cecum | Gastric erosion, emaciation |
| 99 | M | 6 | Found dead | Stomach | Foreign body |
| 100 | M | 6 | None | Stomach | None |
| 101 | M | 6 | Possible hairball | Stomach | None |
| 102 | M | 7 | None | Stomach | None |
| 103 | M | 8 | Painful abdomen, hairball | Stomach, small intestine, cecum | Nematodiasis, colon |
| 104 | M | 8 | Found dead | Stomach | Esophagitis, cecal intussusception |
| 105 | M | 8 | None | Stomach | None |
| 106 | M | 8 | None | Stomach | None |
| 107 | M | 8 | None | Stomach | Peritonitis, duodenal rupture |
| 108 | M | 9 | Hairball removed from esophagus | Stomach | Esophagitis |
| 109 | M | 9 | None | Stomach | None |
| 110 | M | 10 | None | Esophagus, stomach, small intestine | None |
| 111 | M | 10 | Weight loss, diarrhea, abdominal mass | Stomach, cecum | Peritonitis, colitis, cholecystitis, gall bladder fibrosis |
| 112 | M | 11 | None | Stomach | None |
| 113* | M | 13 | None | Stomach | None |
| 114 | M | 15 | Weight loss | Stomach | Colitis, gastric ulcer, steatosis, liver |
| 115 | M | 16 | None | Stomach | Lymphosarcoma |
| 116 | M | 16 | Weight loss, anemia, abdominal mass | Stomach | Amyloidosis |
| 117 | M | 19 | None | Stomach | None |
| 118 | M | 24 | None | Stomach | None |
= Not used for statistical analyses due to incomplete housing location records
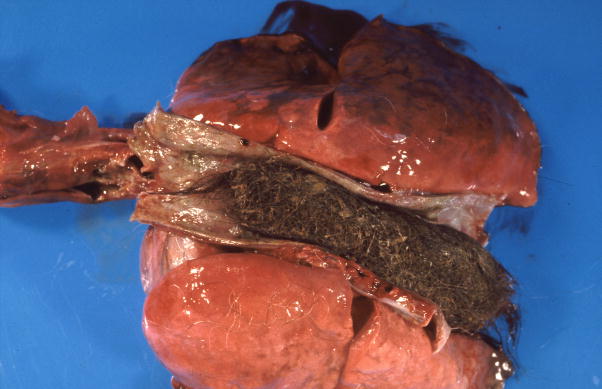
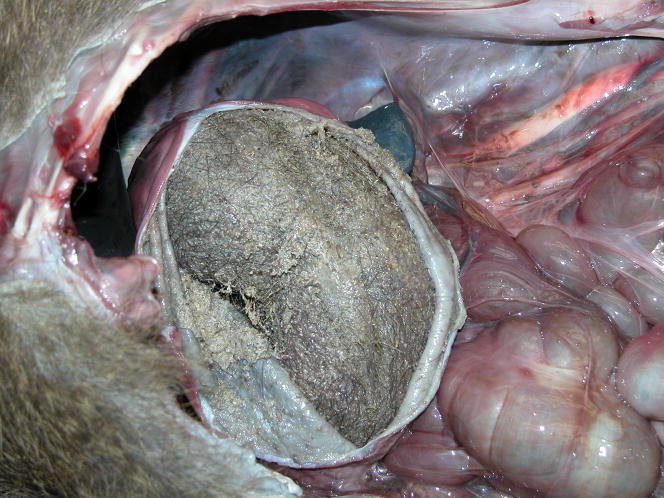
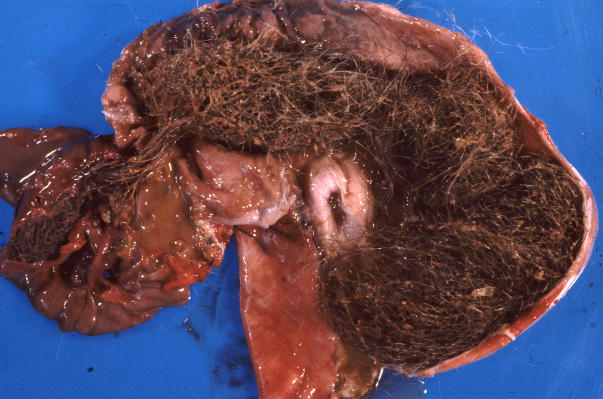
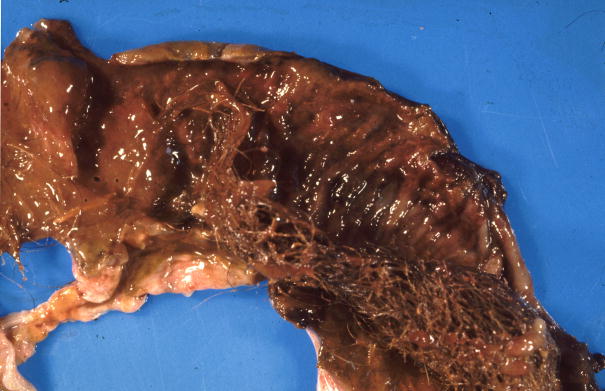
Figure 1.
Gross appearance of trichobezoars in the baboon. A. An obstructive trichobezoar occluding the lumen of the esophagus. B. A mature, compact, trichobezoar completely filling the stomach. C. A less compact trichobezoar partially filling and occluding the stomach with extension into the duodenum. D. A trichobezoar partially filling and occluding the small intestine.
Thirty six baboons arrived at necropsy without any clinical history; another 35 were either found dead or moribund. Of the 47 baboons with clinical histories, findings included: weight loss (n=28); palpable or radiographic abdominal mass (n=6); anemia (n=6); diarrhea (n=3); painful abdomen (n=3); bloat (n=2); impaction (n=1); and obesity (n=1). Trichobezoars were diagnosed antemortem in 11 baboons. Behavioral data was only available for 3 baboons with an identified trichobezoar; there was a history of self-biting in one, trichophagia and regurgitation in one, and one with regurgitation only.
There were several trichobezoar associated findings. Twenty two baboons had gastric lesions, with three baboons each having two gastric lesions (gastritis, n=12; ulcerations, n=8; hyperplasia, n=3; necrosis, n=1; and extensive submucosal lipidosis, n=1). Emaciation was seen in 20 and peritonitis in 16 baboons. Eleven baboons had colonic lesions (colitis, n=9; and ulcerations, (n=2). Seven had esophageal lesions (esophagitis, n=5; necrosis, n=1; and ulcerations, n=1); Seven had small intestinal lesions (enteritis, n=4; ulcerations, n=2; and rupture, n=1). Intussusception or infarction was seen in 7 baboons; pneumonia, aspiration, or asphyxiation in 5; hepatic steatosis in 5; and amyloidosis in 4. Alopecia, foreign body, cholecystitis and fibrosis, and obesity were each observed in 2 baboons. One baboon each had lymphosarcoma, cecal adenocarcinoma, nematodiasis, and bloat. Forty two baboons lacked associated lesions.
One hundred fourteen of the trichobezoar positive baboons and 4342 of the trichobezoar negative baboons were evaluated statistically. Analyses of binomial and Poisson models resulted in identifying the binomial regression model with the probit link function to be the optimal model when the categorical variable, sex, and the continuous predictor variable, proportion of days in group housing, were included. This model was found to be a better predictor of trichobezoar status than the null model (log-likelihood test, p=2e-15). Both the categorical predictor (sex) and the continuous predictor (group housing) had significant effects (p=0.0018 and p=0.0000001 respectively) in this model.
There were 30 positive males and 84 positive females resulting in a frequency of 0.74 females in the trichobezoar positive animals and there were 1899 negative males and 2443 negative females resulting in frequency of 0.56 females in the trichobezoar negative animals. Females had a probability of 0.033 of being trichobezoar positive while males had a probability of 0.016 of being trichobezoar positive. Thus, females were 2.14 times more likely than males to be trichobezoar positive.
All continuous predictor variables had significant affects as individual predictors except for corral days and days alive. However, comparative analysis of alternate linear models demonstrated that the proportion of life in group housing (ratio of gang plus corral housing days/total days alive) had the highest predictive power and that other continuous predictor variables did not contribute to the predictive power of the model. The greater the percentage of group housing time, the more likely the animal was to be trichobezoar positive.
Age did not have a significant effect in the generalized binomial regression analysis. However, it should be noted that only 1 of 872 baboons (0.1%) under the age of 2.5 years was found to have a trichobezoar at necropsy while 113 of 3,584 baboons (3.15%) at or above 2.5 years was found to have a trichobezoar at necropsy. These proportions were found to be very highly significantly different (p<0.0000001) using a permutation test with Monte Carlo estimation (1,000,000 iterations).
Discussion
We evaluated 118 cases of trichobezoars in baboons over a 29 year period and describe significant associations with sex, and group housing. Although age did not appear to be significant, trichobezoars appear to be uncommon in baboons less than 2.5 years old. The stomach was the most common organ affected. The most frequent associated findings included gastric lesions, emaciation, peritonitis, colonic lesions, esophageal lesions, small intestinal lesions, intussusception, pneumonia, aspiration, and hepatic steatosis.
Nutritional deficiency and exposure to environmental stressors are considered the most common causes of hair pulling and trichophagia in laboratory animals [9, 12, 13]. However, since all baboons were fed a nutritionally balanced ration with supplemental grains, vegetables and fruits, a nutritional deficiency was unlikely to be responsible for these cases.
In primates, hair pulling and trichophagia are considered social behaviors [11]. Similar to trichotillomania disorder in humans, hair pulling in nonhuman primates is seen more often in females [5, 12]. In this study females were 2.14 times more likely than males to be trichobezoar positive, compatible the literature on social behavior. Captive-bred baboons may develop excessive grooming more frequently than other primates species [3] and there is a predilection for group housed animals to have trichobezoars [11]. These data confirm that group housing, probably through increased grooming, increases the likelihood of a baboon developing trichobezoars. Behavioral data and observational studies will also be required for a better understanding of the causes of trichobezoars in baboons.
Spontaneously dysfunctional behaviors in animals have been proposed to validate new models for human psychiatric disorders. Animal models for trichotillomania include feather picking in birds, barbering in mice, feline psychogenic alopecia, and canine acral lick dermatitis [2, 8]. The similarities of natural excessive grooming in animals and trichotillomania in humans are evident. Hence, it is reasonable to suggest that nonhuman primates should be considered as appropriate natural models for this maladaptive disorder in humans. The baboon may present a useful model to evaluate the etiology, genetic predisposition, physiopathology, neurobiology, and treatment response of trichobezoars.
Acknowledgments
The authors thank Marie Silva, Michaelle Hohmann and Denise Trejo for pathology support and the expert assistance of the Technical Publications personnel. In addition, the authors would like to thank Mr. Heath Nevill and Dr Corrine Lutz of the SNPRC Behavioral Services, for their review of the animal behavioral records and this manuscript, respectively.
This research was funded in part by NIH/NCRR grant P51 RR013986 to the Southwest National Primate Research Center and conducted in facilities constructed with support from Research Facilities Improvement Program Grant C06 RR014578 and C06 RR015456.
References
- 1.Cary ME, Chavez MS, Wolf RF, Kosanke SD, White GL. Jejunal intussusception and small bowel transmural infarction in a baboon (Papio hamadryas anubis) Laboratory Animal Science. 2006;45:41–44. [PubMed] [Google Scholar]
- 2.Garner JP, Weisker SM, Dufour B, Mench JA. Barbering (fur and whisker trimming) by laboratory mice as a model of human trichotillomania and obsessive-compulsive spectrum disorders. Comparative Medicine. 2004;54:216–224. [PubMed] [Google Scholar]
- 3.Gillin AG, Phippard AF, Thompson JF, Harewood WJ, Waugh RC, Horvath JS. Gastric haemorrhage and perforation caused by a trichobezoar in a baboon (Papio hamadryas) Lab Anim. 1990;24:180–182. doi: 10.1258/002367790780890176. [DOI] [PubMed] [Google Scholar]
- 4.Gozalo AS, Montoya E, Nolan TE. Trichobezoars in two saddleback tamarins (Saguinus fuscicollis) J Med Primatol. 1990;19:151–153. [PubMed] [Google Scholar]
- 5.Graber J, Arndt WB. Trichotillomania. Compr Psychiatry. 1993;34:340–346. doi: 10.1016/0010-440x(93)90021-u. [DOI] [PubMed] [Google Scholar]
- 6.Hahn NE, Lau D, Eckert K, Markowitz H. Environmental enrichment-related injury in a macaque (Macaca fascicularis): intestinal linear foreign body. Comp Med. 2000;50:556–558. [PubMed] [Google Scholar]
- 7.Mook DM. Gastric trichobezoars in a rhesus macaque (Macaca mulatta) Comp Med. 2002;52:560–562. [PubMed] [Google Scholar]
- 8.Moon-Fanelli AA, Dodman NH, O’Sullivan RL. Veterinary models of compulsive self grooming: Parallels with trichotillomania. In: Stein Dan J, Christenson Gary A, Hollander Eric., editors. Trichotillomania: New Developments. Chapter 3. American Psychiatric Press; 1999. pp. 63–86. [Google Scholar]
- 9.Nolan TE, Schaffer L, Conti PA. A gastric trichobezoar in a chimpanzee. J Med Primatol. 1988;17:63–65. [PubMed] [Google Scholar]
- 10.R Development Core Team. R Foundation for Statistical Computing. Vienna, Austria: R: A language and environment for statistical computing. URL http://www.R-project.org. [Google Scholar]
- 11.Reinhardt V, Reinhardt A, Houser D. Hair pulling and eating in captive rhesus monkey troops. Folia Primatol. 1986;47:158–164. doi: 10.1159/000156272. [DOI] [PubMed] [Google Scholar]
- 12.Reinhardt V. Hair pulling: a review. Lab Anim Sci. 2005;39:361–369. doi: 10.1258/002367705774286448. [DOI] [PubMed] [Google Scholar]
- 13.Wagner JL, Hackel DB, Samsell AG. Spontaneous deaths in rabbits resulting from gastric trichobezoars. LAB Anim Sci. 1974;24:826–830. [PubMed] [Google Scholar]