Abstract
Advanced age is associated with decline in many areas of cognition as well as increased frequency of vascular disease. Well-described risk factors for vascular disease such as diabetes and arterial hypertension have been linked to cognitive deficits beyond those associated with aging. To examine whether vascular health indices such as fasting blood glucose levels and arterial pulse pressure can predict subtle deficits in age-sensitive abilities, we studied 104 healthy adults (age 18 to 78 years) without diagnoses of diabetes or hypertension. Whereas results revealed a classic pattern of age-related differences in cognition, pre-prandial blood glucose level and pulse pressure independently and differentially affected cognitive performance. High-normal blood glucose levels were associated with decreased delayed associative memory, reduced accuracy of working memory processing among women, and slower working memory processing among men. Elevated pulse pressure was associated with slower perceptual-motor processing. Results suggest that blood glucose levels and pulse pressure may be sensitive indicators of cognitive status in healthy adults however longitudinal research is needed to determine whether such relatively mild elevations in this select group predict age-related cognitive declines.
Keywords: aging, glucose, memory, executive functions, speed of processing
Advanced age is associated with moderate reduction of performance in multiple areas of cognition, with only few being spared (Horn, 1986). In nondemented older adults, measurable declines are noted in declarative memory (Verhaeghen, Marcoen, & Goossens, 1993), executive functions (West, 1996), and speed of processing (Salthouse, 1993, 1996), although verbal reasoning skills may remain intact well into the late part of the lifespan (Alwin & McCammon, 2001). The cumulative research of the past decade indicates that cognitive change is not attributable exclusively to “old age,” but is affected and modified by many factors associated with aging. One of the most significant modifiers of course of cognitive aging is vascular disease. Multiple studies indicate that cardiovascular risk factors become more prominent with age and exacerbate age-related cognitive declines (Elias et al., 1997; Fontbonne, Berr, Ducimetière, & Alpérovitch, 2001; Hiltunen, Keinänen-Kiukaanniemi, & Läärä, 2001; Kuusisto et al., 1993; Meyer, Rauch, Rauch, & Haque, 2000). Hyperglycemia (Kaplan, Greenwood, Winocur, & Wolever, 2000; Rolandsson, Backeström, Eriksson, Hallmans, & Nilsson, 2008; Vanhanen et al., 1997) and hypertension (Elias, Elias, Robbins, & Budge, 2004; Elias, Elias, Sullivan, Wolf, & D’Agostino, 2003; Raz, Rodrigue, & Acker, 2003; Singh-Manoux & Marmot, 2005) are the most common vascular risk factors associated with cognitive deficits in ostensibly healthy individuals.
Although vascular risk may be assessed through multiple indices, increased arterial stiffness has been identified as a major contributor to cardiovascular mortality as well as cerebrovascular morbidity (Franklin, Jacobs, Wong, L’Italien, & Lapuerta, 2001; O’Rourke, 2007; Schiffrin, 2004). One of the more convenient and valid indices of arterial stiffness is arterial pulse pressure (Cameron, 2007; Cameron & Cruickshank, 2007; Franklin et al., 1997), which may represent a surrogate end point for cardiovascular disease (Laurent & Boutouyrie, 2007). Notably, age-related increases in arterial pulse pressure are steeper than those in systolic blood pressure (Franklin et al., 1997; Safar, Lajemi, Rudnichi, Asmar, & Benetos, 2004), and recently, elevated pulse pressure has been linked to declines in select age-sensitive cognitive functions (Waldstein et al., 2008).
Most of the previous research on the cognitive effects of vascular risk has been based on populations with clinically identifiable conditions such as diabetes mellitus or hypertension. Although such diagnostic labels are assigned on the basis of multiple indicators and clinical findings, they are categories imposed on continua of biological functions. It is unclear, therefore, if blood glucose and blood pressure are linked to cognitive performance at levels below those that are required for diagnosis of diabetes and hypertension.
The goal of this study was to examine the cognitive correlates of normal variation in two common indices of vascular health, blood glucose concentration and arterial pulse pressure. In selecting the cognitive measures, we reasoned that cognitive skills that are most vulnerable to aging would experience additional reduction due to increased arterial stiffness and other vascular risks as measured by elevated blood glucose levels and pulse pressure. Thus, we hypothesized that in addition to the negative effects of age, performance on tests of age-sensitive cognitive abilities, such as speed of processing, episodic memory, and executive functions, would be reduced by elevated glucose levels and pulse pressure.
Method
Participants
Participants were recruited from the community of a large Midwestern metropolitan area in the United States through posted flyers and media announcements. A written questionnaire and telephone interview were used to exclude participants with a history of cardiovascular, neurological, endocrinological, metabolic, and psychiatric disease, head trauma associated with a loss of consciousness, drug or alcohol abuse, uncorrected visual or hearing impairments, and hypertension. In addition, participants were screened for depression and dementia, using the Geriatric Depression Questionnaire from the Center for Epidemiologic Studies-Depression Scale ([CES-D], Radloff, 1977) and the Mini Mental State Examination ([MMSE], Folstein, Folstein, & McHugh, 1975) respectively. Only those participants who scored below 16 on the CES-D and above 26 on the MMSE were enrolled. Both inventories are efficient screening instruments. As reported in a recent large-sample investigation, the MMSE cut-off used in selecting this sample is highly sensitive and specific in discriminating between demented and non-demented adults with a college education (O’Bryant et al., 2008). Participants who reported the use of glucose lowering agents, anti-hypertensive medication, anxiolytics, antidepressants, or anticonvulsants were also excluded. All participants were Caucasian, native English speakers, with a minimum of a high school education, right-hand dominant for basic manual activities (75% or greater on the Edinburgh Handedness Questionnaire; Oldfield, 1971), corrected visual acuity of 50/20 or better (assessed by Optic 2000 apparatus, Stereo Optic), and hearing of 40 dB or better for frequencies of 500-4000 Hz (Maico, MA27). All participants were given informed consent in compliance with Institutional Review Board and American Psychological Association requirements, and were monetarily compensated for their efforts. The sample consisted of 104 persons (69 women) with an age range of 19-78 years. Sample’s descriptive characteristics are reported in Table 1.
Table 1.
Sample Characteristics: Means, Standard Deviations and t-tests for Sex Differences
| Variable | Men | Women | t | p |
|---|---|---|---|---|
| Age (years) | 55.31 ± 12.92 | 53.45 ± 13.36 | 0.68 | .50 |
| Education (years) | 16.74 ± 2.32 | 15.90 ± 2.38 | 1.73 | .09 |
| MMSE | 28.69 ± 1.16 | 28.94 ± 1.00 | -1.17 | .24 |
| Glucose blood levels (mg/dl) | 90.03 ± 9.08 | 86.19 ± 7.92 | 2.22 | .03 |
| Systolic pressure (mmHg) | 121.57 ± 9.04 | 118.20 ± 10.94 | 1.57 | .12 |
| Diastolic pressure (mmHg) | 76.37 ± 5.74 | 72.72 ± 6.36 | 2.85 | .01 |
| Pulse pressure (mmHg) | 45.20 ± 7.37 | 45.48 ± 7.20 | -0.19 | .85 |
Measures of Vascular Health
Blood glucose
Following a 12-hr overnight fast, whole blood glucose levels were assessed by standard enzymatic glucose oxidase method. Only those persons whose blood glucose levels were above 70 mg/dl (3.9 mmol/L) and below 126 mg/dl (< 7.0 mmol/L) were retained for further analysis.
Blood pressure
Blood pressure was measured on three separate days at the start of each cognitive test session by a mercury sphygmomanometer (BMS 12-S25) with a standard blood pressure cuff (Omron Professional) on the left arm with participants seated, and the systolic and diastolic means averaged for each individual. Only those persons with a mean systolic of 140 mmHg and a mean diastolic of 90 mmHg and below were retained for further analyses. Pulse pressure was also computed as the difference between systolic blood pressure and diastolic blood pressure.
Cognitive Tasks and Procedures
The following tasks were administered to each participant individually in well-lit, quiet rooms on three non-consecutive days, with strict adherence to task and session order for all participants. For tests requiring the administration of more than one block of trials, block order was counterbalanced across subjects. For tests requiring a delay within administration, every effort was made to maintain an equal period of delay across subjects. The cognitive tests were administered by extensively trained graduate students.
Declarative memory
Two declarative memory functions were assessed. Associative memory was measured by the Memory for Names subtest from the Woodcock-Johnson Psychoeducational Battery-Revised ([WJ-R], Woodcock & Johnson, 1989), and free recall by the Logical Memory subtest from the Wechsler Memory Scale-Revised ([WMS-R], Wechsler, 1987). In Memory for Names, participants are shown cartoons of “space creatures” and read aloud creatures “names” (nonsense one- or two-syllable words). After presentation of name-picture pairs, participants are asked to point to the named space creature first alone, then among other space creatures. With each successive trial, participants are asked to learn the identity of a new space creature and retain the identity of all the preceding space creatures until all twelve are presented. Corrections are provided throughout acquisition. After a 20 min delay, participants are given three trials with no correction to identify the named space creature by pointing. The split-half reliability of the immediate and delayed recognition scores is .91 (Woodcock & Mather, 1989).
In Logical Memory, participants are read two short stories of just a few lines each and are asked to retell the story, verbatim. After a 20 min delay, participants are asked to repeat the stories. If they are unable to do so, a simple reminder cue is provided for one or both stories. The split-half reliability of this task is .74 for immediate and .75 for delayed presentation (Wechsler, 1987).
Working memory
Participants performed two computerized versions of the nback task modeled after Dobbs and Rule (1989). In the verbal version, a series of digits (1-9) of variable length was presented. After the presentation, participants were asked to name the digit shown 1-, 2-, or 3-back in blocked trials of the same nback. Participants were aware of the condition being tested and were familiarized with each condition before the test trials. In the nonverbal version, a series of abstract drawings was presented, and at the end of each presentation, the participants were asked to indicate the drawing shown 1-, 2-, or 3-back. Trials were blocked and the participants were aware of the condition prior to starting. The test-retest reliability for the verbal version of the task is .68, .88, and .91, for 1-, 2-, and 3-back conditions respectively (Salthouse, Hancock, Meinz, & Hambrick, 1996).
Executive functions
Two executive functions were assessed: inhibition of pre-potent response and task switching. The response inhibition measures were in paper-and-pencil format, whereas administration of the task switching tasks was computerized.
Two variants of a modified Stroop task (Stroop, 1935) modeled after Salthouse and Meinz (1995) were administered. In the color version, participants read aloud a page of words rendered in colors that were congruent or incongruent with the written word meaning, e.g., the word red printed in red ink (compatible) or in green ink (incompatible). The second Stroop task involved viewing a page of boxes with a word printed in each and separated by either a horizontal or vertical line. The task was to identify the position of the word relative to the internal line. The position of the word was either consistent or inconsistent with its meaning, e.g., the word above could appear above (compatible) or below (incompatible) the line. Split-half reliability estimates of these tasks as a measure of interference are .72 for the color version and .70 for the position version (Salthouse & Meinz, 1995).
Two variations of a Task Switching test modeled after Salthouse, Fristoe, McGuthry, and Hambrick (1998) were administered. In the Right-Left version, participants viewed two numbers and were asked to indicate in turn the number on the right, the number on the left, and switching between indicating the number on the right and the number on the left, depending on which was in parentheses. In the More-Odd version of the test, participants viewed a single number and were asked to indicate whether the number was more or less than five, odd or even, or switching between the “more or less” and “odd or even” task, depending on whether the number was in parentheses or not. The participants were familiarized with each condition before beginning the test trials. Split-half reliability of the condition presented singly is .96 for the Right-Left version, and .86 for the More-Odd version. Split-half reliability based on switch costs (average of pre- and post-switch trials subtracted from reaction time on switch trials) is .71 for the Right-Left version, and .89 for the More-Odd version (Salthouse et al., 1998).
Psychomotor speed
Psychomotor speed was assessed by two comparison tasks, both of which were modeled after Salthouse and Meinz (1995). In Letter Comparison, participants are presented with two columns of letter strings, three to nine letters each. Letter strings are either the same in both columns or differ by one letter. For each set of letter strings, participants are required to indicate whether the strings are the same or different. Pattern Comparison has two columns of abstract patterns that are either the same in both columns, or with one line having a different orientation. For each set of abstract patterns, participants are required to indicate whether the patterns are the same or different. Participants are instructed to work as quickly and accurately as possible on each of two pages, with a time limit of 30 s per page. Test-retest reliability estimates for the Letter Comparison task have ranged from .77 to .80, and between .76 and .87 for Pattern Comparison (Salthouse & Meinz, 1995; Salthouse et al., 1998).
Results
All independent variables and covariates correlated significantly, with the exception of diastolic pressure, which did not correlate with age and pulse pressure. Pearson product moment correlations among independent variables and covariates are reported in Table 2, and Pearson correlations among cognitive variables are included in the Appendix.
Table 2.
Correlations Among Vascular Health Indicators
| Variable | Age | Systolic | Diastolic | Pulse |
|---|---|---|---|---|
| Systolic pressure (mm Hg) | .35** | |||
| Diastolic pressure (mm Hg) | .14 | .73** | ||
| Pulse pressure (mm Hg) | .39** | .80** | .17 | |
| Blood Glucose (mg/dL) | .40** | .38** | .26* | .31** |
p < .01
p < .001 not adjusted for multiple comparisons.
With adjustment for multiple comparisons, r ≤ .31 is significant at p < .05.
Data Conditioning and Analyses
In computer-administered reaction time tasks (i.e., task switching, nback), trials resulting in errors were removed from analysis, as were trials in which reaction time was less than 500 ms or in excess of 10,000 ms as these were deemed to reflect chance responding or motivational problems. The application of these criteria produced missing data for two cases on task switching and three cases on nback.
Distributions of the reaction time values on both versions on the nback task, as well as for processing speed were skewed. Hence, logarithmic transformation was applied to those variables. The mean and standard deviations of reaction times across switch tasks did not differ between pre- and post-switch trials, whereas reaction time on switch trials was considerably slower. To gauge individual switch costs, a cost index was computed as the average of pre- and post-switch trials subtracted from reaction time on switch trials and divided by the average pre- and post-time.
Pearson product moment correlations were used to assess potential relationships among variables, and the General Linear Model approach was used to assess the relationship between age, sex, vascular health indicators, and cognitive functions. In such analyses, the respective cognitive function(s) served as the dependent variable (or vector of dependent variables, if more than one index was available) whereas age, glucose levels, and pulse pressure were centered at their sample means and entered as continuous independent variables. Sex was a categorical independent variable.
We elected to use only pulse pressure as an index of vascular risk because of its high correlation with systolic blood pressure, which in turn is highly correlated with diastolic blood pressure, and entering variables with correlations exceeding r = .70 would result in multicollinearity. Variations and conditions applied to the cognitive tests (e.g., difficulty or delay time) were entered as repeated measures (within-subject) factors. Unless otherwise specified, all data are expressed as mean ± SD and all p values for interactions involving repeated measures are reported after Huynh-Feldt correction.
Cognitive Performance Analyses
Significant effects for all analyses are summarized in Table 3, and therefore omitted from the narrative. The details of the analyses are presented below.
Table 3.
General Linear Model Analysis: Summary of Significant Effects
| Task | Effect | df | F | p |
|---|---|---|---|---|
| Declarative Memory | ||||
| Age | 1, 99 | 6.01 | < .01 | |
| Delay | 1, 99 | 2773.56 | < .001 | |
| Glucose × Task × Delay | 1, 99 | 4.38 | < .05 | |
| Working Memory Accuracy | ||||
| Age | 1, 95 | 10.53 | < .01 | |
| Task | 1, 95 | 69.70 | < .001 | |
| Glucose × Sex | 1, 95 | 6.42 | < .05 | |
| Working Memory Speed | ||||
| Age | 1, 94 | 21.58 | < .001 | |
| Task | 1, 94 | 1602.30 | < .001 | |
| Glucose × Sex | 1, 94 | 5.60 | < .05 | |
| Age × Task × Difficulty | 2, 188 | 7.55 | < .01 | |
| Age × Sex × Difficulty | 2, 188 | 7.55 | < .01 | |
| Task Switch | ||||
| Sex × Task | 1, 98 | 8.31 | < .01 | |
| Stroop Inhibition | ||||
| Age | 1, 99 | 5.10 | < .05 | |
| Age × Task | 1, 99 | 7.08 | < .01 | |
| Psychomotor Speed | ||||
| Age | 1, 99 | 37.68 | < .001 | |
| Age × Task | 1, 99 | 4.68 | < .05 | |
| Pulse Pressure × Task | 1, 99 | 4.86 | = .05 | |
Declarative Memory
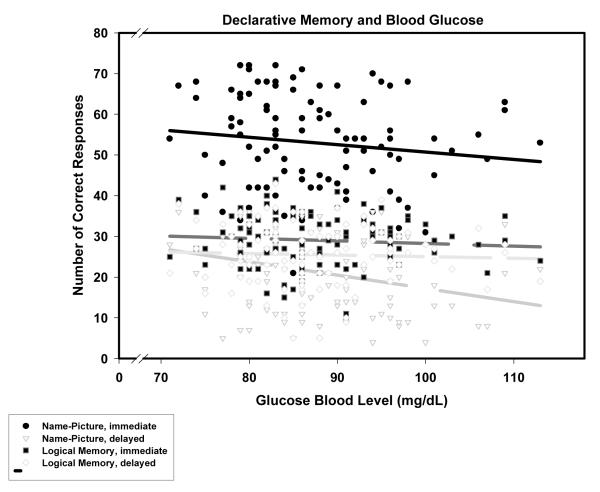
In the general linear model analysis for declarative memory, task type (Logical Memory versus Memory for Names) and delay time (immediate versus delayed) were two-level within-subject repeated measures factors. Interactions between sex and the other variables were not significant (all p > .15) and were, therefore, removed from the model. The analyses of the reduced linear model revealed a significant main effect of age reflective of poorer performance of older participants (β = -.28, p < .01). The expected main effect of delayed versus immediate recall (Delay) was also observed. The main effect of glucose level was not significant, but the Glucose Level × Task × Delay interaction was. As depicted in Figure 1, higher glucose levels were associated with lower scores on the association memory task but only in the delayed recall condition: β = -.28, p < .01; for all the other memory tests and conditions β’s, p > .18, ns.
Figure 1.
Memory and fasting blood glucose levels. Regression lines: dark — Name-Picture recognition, solid —immediate, long dash - delayed; light — Logical memory; short dash — immediate, long dash - delayed. The only significant slope is for delayed name-picture association recognition: long-dash dark regression line and empty circles observation symbols.
Working Memory
Working memory performance can be assessed by measuring accuracy (the number of errors) and speed of processing (the time that elapses between presentation of the stimulus and response, i.e., reaction time [RT]). Both working memory indices were analyzed.
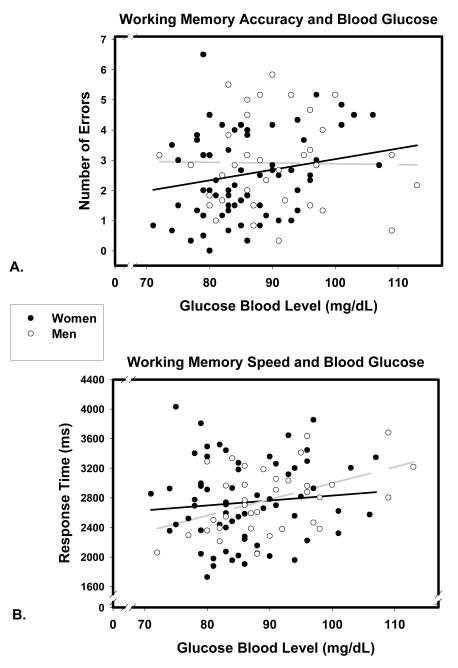
In the general linear model for accuracy, task type (verbal versus non-verbal) was a two level within-subject factor. Although the nback task has three levels of difficulty, levels one and two were excluded from further analysis due to the extremely narrow range in number of errors (0.08 ± 0.30 and 0.43 ± 0.87 for 1- and 2-back verbal and 0.33 ± 0.69 and 1.39 ± 2.23 for 1- and 2-back nonverbal) in the lower-difficulty conditions. Significant main effects of age and task, reflected a greater number of errors made by older participants (β = .39, p < .001), and a significantly greater number of errors made in the nonverbal task (8.78 ± 4.70 versus 4.71 ± 3.35). A significant Glucose Level × Sex interaction (depicted in Figure 2a) was attributable to the association between higher glucose levels and errors in women: β=.32, p <.05 or β = .41, p <.01 after removal of one outlier, a 66-year old woman with the highest number of errors and moderate-low glucose levels. No significant effect was observed for men: β = .02, ns.
Figure 2.
Association of working memory (n-back) accuracy (A) and speed (B) with fasting blood glucose levels in men (empty circles, light dashed regression line) and women (filled circles, dark solid regression line).
Working memory speed of processing was analyzed in a 2 × 3 repeated measures general linear model where task type (verbal versus nonverbal) was a two-level factor, and task difficulty (1-back, 2-back, and 3-back) a three-level factor. The analysis revealed significant main effects of age and task. Younger age was associated with faster response times (β = .37, p < .001) and responses to verbal stimuli were faster than those of nonverbal ones across all levels of difficulty (1882.27 ± 461.99 ms versus 3623.88 ± 662.75 ms). However, a significant Age × Task × Difficulty interaction reflected a linear decrease in the age-RT association as task difficulty increased. The effect was more pronounced for the verbal task (β = .61, .44, and .27 for verbal 1-, 2- [both p < .001], and 3-back [p < .01] respectively; β = .66, .55, and .38 for nonverbal 1-, 2-, and 3-back respectively, [all p < .001]).
The main effects of sex, glucose level, and pulse pressure were not significant (all F < 1). However, a significant Glucose Level × Sex interaction was observed (depicted in Figure 2b). Higher glucose levels were associated with slower response times for men (β = .46, p <.01) but not women (β = -.08, ns). There was also a significant Age × Sex × Difficulty interaction. Among men, the association between processing speed and age decreased with increased task difficulty (β = .71, p < .001 for 1-back, to β = .35, p < .05 for 2-back, to β = .13, ns for 3-back), whereas among women, longer response times were associated with advanced age regardless of difficulty (β’s ranging between .51 and .60, all p <.001).
Executive Functions
Task switching costs were analyzed in a repeated measures general linear model where task type (Right-Left versus More-Odd) was a two-level factor and switch costs expressed as response time difference, was the dependent variable. Interactions between sex and continuous variables were nonsignificant (all F ≤ 1.5) and were not retained in the model. In the reduced model, there were no significant main effects (all F ≤ 1). The nosignificant Age × Task interaction, F(1, 98) = 3.70, p = .06, reflected a trend for age-related increase in switching costs on a difficult (More-Odd) but not an easy (Right-Left) task: β = .21, p < .05 versus β = -.06, ns. A significant Sex × Task interaction was due to the fact that women incurred slightly greater costs than men on the Right-Left switch task (11.73 ± 6.55% versus 8.78 ± 6.57%) but smaller costs on the More-Odd task (48.21 ± 28.27% versus 60.73 ± 37.65%).
For the Stroop task, a ratio of response time on the incompatible trials to response on the neutral trials for each task was analyzed as a two-level repeated measures factor for task type (color versus position). The main effect of age was significant and was qualified by a significant Age × Task interaction. Age was associated with an increase in interference costs on the color but not the position Stroop task: β = .32, p < .01 versus β = -.09, ns, respectively.
Psychomotor Speed
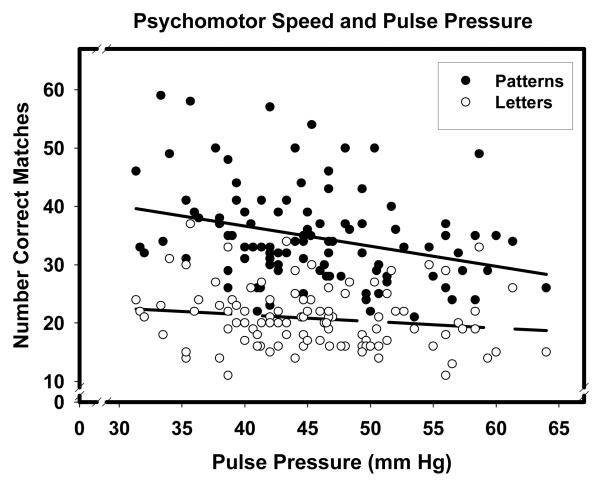
In this model, log-transformed scores were the dependent variable and task type (Letter versus Pattern Comparison) a two-level repeated measures factor. A significant main effect of age was due to slower processing speed in older participants (β = -.54, p < .001). A significant Age × Task interaction was due to the somewhat steeper slope of age-related slowing on the Letter Comparison task than the Pattern Comparison task: β = -.59 versus β = -.43 both p < .001. A significant Pulse Pressure × Task interaction (graphically presented in Figure 3) was due to the fact that higher pulse pressure was associated with slower processing speed on the Pattern Comparison task (β = -.31, p < .01), but not the Letter Comparison task (β = -.17, ns).
Figure 3.
Pulse pressure and psychomotor speed on two tasks. Pattern comparison: filled circles, solid regression line; letter comparison: empty circles, dashed regression line.
Discussion
The results of this study indicate that the influence of vascular risk factors adds to age-related differences in cognitive performance. In accordance with the cognitive aging literature, negative associations with age were observed in most cognitive domains. Advanced age was associated with reduced psychomotor speed, poorer declarative memory, increased costs of interference on color-naming response, increased switching costs on a more difficult switching task, slower working memory processing and reduced efficiency (accuracy) with high working memory loads.
The effects of vascular health indicators varied according to the cognitive domain. In some areas of cognition, the effect was restricted to more difficult tasks. Higher pre-prandial blood glucose level was associated with poorer delayed recall of name-picture associations but not with scores on easier memory tasks. In other cognitive domains, the effect of glucose was conditioned on the participant’s sex. Thus, slower processing time on working memory tasks was associated with elevated glucose in men, whereas the link between high-normal glucose levels and reduced working memory accuracy was observed only in women. The negative effect of elevated pulse pressure was noted only on the Pattern Comparison task, for which it served as an additive negative modifier of the age effect.
The observed domain-specific effects are in accordance with the findings reported in the extant literature. Reduced psychomotor speed is frequently cited as an area of greatest vulnerability both in aging and hypertension, and accounts for a significant amount of variability in neuropsychological test performance in general (Salthouse, 1993, 1996). Several studies have found a negative association between systolic blood pressure and psychomotor speed among hypertensives (Gupta, Solanki, & Pathak, 2007; van Boxtel, Gaillard, Houx, Buntinx, de Leeuw, & Jolles, 1997) and normotensives (Hakamada-Taguchi, Uehara, Haebara, Negoro, & Toko-Oka, 2002) in the absence of a similar relationship for diastolic blood pressure or other cognitive domains. Although we focused on pulse pressure as a preferred surrogate for vascular stiffness, a post-hoc analysis of the systolic and diastolic blood pressure effects replicated the abovementioned observation (data not shown). Deficits in verbal memory are frequently reported as a specific focus of vulnerability in diabetics, with decrements in executive functions and psychomotor speed less frequently observed (Strachan, Deary, Ewing, & Frier, 1997). Moreover, abnormal glucose metabolism has been linked to the development of neurological disorders (e.g., Alzheimer’s disease) characterized by memory deficits (Convit, Wolf, Tarshish, & de Leon, 2003). Thus, memory is likely to be more sensitive to mild elevations of blood glucose than other cognitive functions.
The notable feature of this study is that it reflects the best-case scenario as far as vascular risks are concerned. The findings were obtained on a very healthy sample that spanned a wide age range, in contrast to previous studies of vascular risk factors and cognitive aging that have utilized typical older adults (i.e., Hassing et al., 2004; Hiltunen et al., 2001, Convit et al. 2003). Thus, we show that even in healthy and not very old adults, normal but elevated values of recognized vascular risk factors are negatively associated with performance in age-sensitive cognitive tasks. Whereas in persons diagnosed with diabetes and hypertension, synergistic effects of multiple vascular indices on cognition are observed (Pavlik, Hyman, & Doody, 2005), in the normal range, we found no evidence of such effects.
Sex differences in several executive functions and their modification by the effects of glucose and pulse pressure were observed. Although sex differences on a variety of cognitive tasks in general and within the context of aging have been reported before (e.g., Basso, Harrington, Matson, & Lowery, 2000), the stability of those differences and their mechanisms are far from clear (Hogervorst, De Jager, Budge, & Smith, 2004; Wolf & Kirschbaum, 2002). The relationship between vascular health indices and cognition is in addition complicated by sex differences in glucose and blood pressure regulation that are mediated by multiple endocrine factors (e.g., Ding, Song, Malik, & Liu, 2006). Those important hormonal factors were not measured in this study and their assessment in future studies may provide a better basis for interpretation of the results.
The study has several limitations, and its results should be interpreted within the constraints thereof. First, due to the study’s design focus on healthy adults, the findings have limited generalizability for a typical aging population and results are likely to underestimate true population effects. Second, the differential effect of vascular health and age on cognitive performance could have reflected differential reliability of the tests. If that were the case, tests with lower reliability would be less likely to show significant effects than highly reliable tests. Although this concern may be relevant for some measures, in general it is unlikely to affect the results. Whereas on Task Switching, the more reliable task (More-Odd) showed stronger effects of age, among Stroop tasks of equivalent reliability, differential age effects were observed. The same is true with regards to memory tests of equivalent reliability that evidenced differential effects of age and vascular health. On the other hand, reliability estimates may vary according to ability levels for the tests that show ceiling and floor effects. This is unlikely because in this study, indices with ceiling effects (e.g., error rates under low load conditions on nback tasks) were excluded from the analyses. Third, because this is a cross-sectional study, it is possible that the observed results reflect the influence of other variables not specified in the model. Sorting out such influences as well as confounding individual differences is possible only in the longitudinal framework.
Fourth, the findings reported here are essentially correlational, and as such they cannot be taken as evidence of causality. For example, the directionality of the link between cognitive performance and vascular risk cannot be determined on the basis of statistical inference. However, the extant findings provide a context for interpretation of the observed associations. On the one hand, longitudinal studies have shown that elevated vascular risk at middle age predicts brain deterioration and exacerbates cognitive declines in late adulthood (Beason-Held, Moghekar, Zonderman, Kraut, & Resnick, 2007; Knopman et al., 2001; Launer, Masaki, Petrovitch, Foley, & Havlik, 1995; Swan et al., 1998). On the other hand, it is possible that young persons with lower cognitive abilities choose a less healthy lifestyle, thus exposing themselves to greater burden of risk factors, and the eventual development of hyperglycemia and hypertension. According to several recent reports, lower IQ at childhood predicted elevated vascular risk factors at middle age and increased mortality (Batty, Deary, & Macintire, 2007; Chandola, Deary, Blane, & Batty, 2006). It must be kept in mind, however, that those findings come from population samples with a broad range of cognitive abilities, educational and socio-economic indicators. It is unlikely that in a sample of educated adults with no vascular disease or cognitive impairment such reverse effects would be strong, although they cannot be ruled out. As most of the participants of this study are part of a longitudinal follow-up, it will be interesting to determine if the observed associations between vascular variables and cognition predict later declines.
Fifth, the study addressed the effects of only two indices of vascular health. Although those indices are valid, reliable, and widely used, other predictors of arterial function and glycemic control are available. Because of their strong correlation, assessment of separate effects of systolic and diastolic blood pressure would require additional analyses that would inflate the Type I error. Because more is known about the effects of blood pressure on cognition than those of pulse pressure, we chose to focus on the latter. Indices such as glycated hemoglobin HbA1c (Rohlfing et al., 2000) and measures of pulsatile pressure derived from ultrasonography (O’Rourke, 2007) may provide additional and more precise information about early cognitive changes in the healthy population.
Finally, the study does not take into account genetic variations among the participants. Although the magnitude of single nucleotide polymorphisms on cognition and vascular risk is relatively small, some of them, such as angiotensin-converting enzyme (ACE) gene influence vascular risks (Safar et al., 2004) and may thus contribute to age-related differences in cognitive performance. In a study that contained a large part of this sample, we have recently found that speed of processing is reduced by a joint effect of Brain Derived Neurotrophic Factor (BDNF) gene Val66Met polymorphism and hypertension (Raz, Rodrigue, Kennedy, & Land, in press), and presence of BDNF 66Met allele exacerbates the negative influence of high glucose levels on memory (Raz, Dahle, Rodrigue, Kennedy, Land, & Jacobs, in press). Variations in the genes controlling energy balance also affect a person’s risk for developing hypertension or hyperglycemia (Mager et al., 2006) and may modify the strength of association between blood glucose and cognition observed here.
In conclusion, this study showed that in euglycemic and normotensive individuals, high-normal levels of blood glucose and pulse pressure were associated with reduced performance in several age-sensitive domains. Longitudinal research is needed to determine whether such relatively mild elevations predict cognitive status at older age. Randomized controlled trials of optimizing glucose and/or pulse pressure are necessary to determine whether this would contribute to successful cognitive aging.
Acknowledgments
This study was supported in part by the grant R37-AG-11230 from the National Institutes of Health.
Appendix
Appendix.
Correlation Matrix Among Cognitive Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. LMD | .88 | ||||||||||||||||
| 2. Names | .35 | .35 | |||||||||||||||
| 3. NamesD | .39 | .34 | .86 | ||||||||||||||
| 4. NbV3er | -.12 | -.13 | -.33 | -.37 | |||||||||||||
| 5. NbNv3er | -.29 | -.24 | -.43 | -.53 | .48 | ||||||||||||
| 6. NbV1rt | -.08 | -.15 | -.07 | -.08 | .09 | .18 | |||||||||||
| 7. NbV2rt | -.17 | -.21 | -.14 | -.11 | .08 | .21 | .77 | ||||||||||
| 8. NbV3rt | -.19 | -.18 | -.12 | -.11 | -.01 | .19 | .41 | .54 | |||||||||
| 9. NbNv1rt | -.20 | -.26 | -.32 | -.32 | .24 | .29 | .66 | .58 | .43 | ||||||||
| 10. NbNv2rt | -.38 | -.40 | -.37 | -.32 | .23 | .29 | .63 | .64 | .35 | .74 | |||||||
| 11. NbNv3rt | -.13 | -.05 | -.18 | -.18 | .17 | .14 | .40 | .35 | .40 | .38 | .40 | ||||||
| 12. RL Cost | .06 | .10 | -.01 | -.03 | .01 | -.07 | .02 | .06 | -.07 | .03 | -.03 | .03 | |||||
| 13. MO Cost | -.28 | -.26 | -.17 | -.19 | .14 | .22 | .22 | .34 | .23 | .36 | .48 | .19 | .02 | ||||
| 14. ColorInt | -.16 | -.19 | -.32 | -.32 | .30 | .37 | .26 | .17 | .17 | .30 | .29 | .32 | -.11 | .26 | |||
| 15. PositnInt | -.20 | -.25 | -.31 | -.24 | .09 | .12 | .08 | .01 | .12 | .14 | .14 | .11 | -.11 | .15 | .48 | ||
| 16. LetComp | .30 | .41 | .36 | .41 | -.34 | -.29 | -.44 | -.37 | -.21 | -.52 | -.49 | -.17 | .01 | -.24 | -.25 | -.05 | |
| 17. PatComp | .25 | .34 | .23 | .28 | -.20 | -.27 | -.43 | -.35 | -.29 | -.47 | -.48 | -.32 | -.07 | -.29 | -.28 | -.09 | .70 |
All r ≥ .32 significant at p < .001, r ≥ .26 significant at p < .01, and r ≥ .20 significant at p < .05. After adjustment for multiple comparisons, only r ≥ .36 significant. CFIT = Culture Fair Intelligence Test, Vocab = Vocabulary, LM & LMD Logical Memory Immediate & Delayed, Names & NamesD = Memory for Names Immediate & Delayed, NbV3er = Nback Verbal 3-back Errors, NbNv3er = Nback Nonverbal 3-back Errors, NbV1rt, NbV2rt, & NbV3rt = Nback Verbal 1 -, 2-& 3 -back Reaction Time, NbNv1rt, NbNv2rt, & NbNv3rt = Nback Nonverbal 1 -, 2-, & 3-back Rea ction Time, RL Cost & MO Cost = More/Odd and Right/Left Switch Costs, ColorInt & PositnInt = Color & Position Stroop Interference, LetComp & PatComp = Letter & Pattern Comparison.
References
- Alwin DF, McCammon RJ. Aging, cohorts, and verbal ability. Journal of Gerontology B: Psychological and Social Sciences. 2001;56:S151–S161. doi: 10.1093/geronb/56.3.s151. [DOI] [PubMed] [Google Scholar]
- Basso MR, Harrington K, Matson M, Lowery N. Sex differences on the WMS-III: Findings concerning verbal paired associates and faces. Clinical Neuropsychology. 2000;14:231–235. doi: 10.1076/1385-4046(200005)14:2;1-Z;FT231. [DOI] [PubMed] [Google Scholar]
- Batty GD, Deary IJ, Macintyre S. Childhood IQ in relation to risk factors for premature mortality in middle-aged persons: the Aberdeen Children of the 1950s study. Journal of Epidemiology and Community Health. 2007;61:241–247. doi: 10.1136/jech.2006.048215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beason-Held LL, Moghekar A, Zonderman AB, Kraut MA, Resnick SM. Longitudinal changes in cerebral blood flow in the older hypertensive brain. Stroke. 2007;38:1766–1173. doi: 10.1161/STROKEAHA.106.477109. [DOI] [PubMed] [Google Scholar]
- Cameron J. Ageing and central aortic pulse wave analysis. Commentary on “Is augmentation index a good measure of vascular stiffness in the elderly?” by Fantin et al. Age and Ageing. 2007;36:3–5. doi: 10.1093/ageing/afl142. [DOI] [PubMed] [Google Scholar]
- Cameron JD, Cruickshank JK. Glucose, insulin, diabetes and mechanisms of arterial dysfunction. Clinical and Experimental Pharmacology and Physiology. 2007;34:677–682. doi: 10.1111/j.1440-1681.2007.04659.x. [DOI] [PubMed] [Google Scholar]
- Chandola T, Deary IJ, Blane D, Batty GD. Childhood IQ in relation to obesity and weight gain in adult life: the National Child Development (1958) Study. International Journal of Obesity. 2006;30:1422–1432. doi: 10.1038/sj.ijo.0803279. [DOI] [PubMed] [Google Scholar]
- Convit A, Wolf OT, Tarshish C, de Leon MJ. Reduced glucose tolerance is associated with poor memory performance and hippocampal atrophy among normal elderly. Proceedings of the National Academy of Sciences. 2003;100:2019–2022. doi: 10.1073/pnas.0336073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding EL, Song Y, Malik VS, Liu S. Differences of endogenous sex hormones and risk of type 2 diabetes: A systematic review and meta-analysis. Journal of the American Medical Association. 2006;295:1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- Dobbs AR, Rule BG. Adult age differences in working memory. Psychology and Aging. 1989;4:500–503. doi: 10.1037//0882-7974.4.4.500. [DOI] [PubMed] [Google Scholar]
- Elias PK, Elias MF, Robbins MA, Budge MM. Blood pressure-related cognitive decline: Does age make a difference? Hypertension. 2004;44:631–636. doi: 10.1161/01.HYP.0000145858.07252.99. [DOI] [PubMed] [Google Scholar]
- Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agnostino RB. Lower cognitive function in the presence of obesity and hypertension: The Framingham Heart Study. International Journal of Obesity and Related Metabolic Disorders. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- Elias PK, Elias MF, D’Agostino RB, Cupples LA, Wilson PW, Silbershatz H, et al. NIDDM and blood pressure as risk factors for poor cognitive performance: The Framingham Study. Diabetes Care. 1997;20:1388–1395. doi: 10.2337/diacare.20.9.1388. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fontbonne A, Berr C, Ducimetière P, Alpérovitch A. Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: Results of the epidemiology of Vascular Aging Study. Diabetes Care. 2001;24:366–370. doi: 10.2337/diacare.24.2.366. [DOI] [PubMed] [Google Scholar]
- Franklin SS, Gustin W, IV, Wong ND, Larson MG, Weber MA, Kannel WB, et al. Hemodynamic patterns of age-related changes in blood pressure: The Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle aged and elderly US hypertensives: Analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37:869–874. doi: 10.1161/01.hyp.37.3.869. [DOI] [PubMed] [Google Scholar]
- Gupta R, Solanki RK, Pathak V. Blood pressure is associated with cognitive impairment in young hypertensives. The World Journal of Biological Psychiatry. 2008;9:43–50. doi: 10.1080/15622970601187784. [DOI] [PubMed] [Google Scholar]
- Hakamada-Taguchi R, Uehara Y, Haebara T, Negoro H, Toyo-Oka T. The relationship between changes in normal-range systolic blood pressure and cognitive function in middle-aged healthy women. Hypertension Research. 2002;25:565–569. doi: 10.1291/hypres.25.565. [DOI] [PubMed] [Google Scholar]
- Hassing LB, Hofer SM, Nilsson SE, Berg S, Pedersen NL, McClearn G, et al. Comorbid type 2 diabetes mellitus and hypertension exacerbates cognitive decline: Evidence from a longitudinal study. Age and Ageing. 2004;33:355–361. doi: 10.1093/ageing/afh100. [DOI] [PubMed] [Google Scholar]
- Hiltunen LA, Keinänen-Kiukaanniemi SM, Läära EM. Glucose tolerance and cognitive impairment in an elderly population. Public Health. 2001;115:197–201. doi: 10.1038/sj/ph/1900758. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, De Jager C, Budge M, Smith AD. Serum levels of estradiol and testosterone and performance in different cognitive domains in healthy elderly men and women. Psychoneuroendocrinology. 2004;29:405–421. doi: 10.1016/s0306-4530(03)00053-2. [DOI] [PubMed] [Google Scholar]
- Horn JL. Intellectual stability concepts. In: Steinberg RJ, editor. Advances in psychology of human intelligence. Erlbaum; Hillsdale, NJ: 1986. [Google Scholar]
- Kaplan RJ, Greenwood CE, Winocur G, Wolever TMS. Cognitive performance is associated with glucose regulation in healthy elderly persons and can be enhanced with glucose and dietary carbohydrates. American Journal of Clinical Nutrition. 2000;72:825–836. doi: 10.1093/ajcn/72.3.825. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Boland LL, Mosley T, Howard G, Liao D, Szklo M, et al. Cardiovascular risk factors and longitudinal cognitive changes in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- Kuusisto J, Koivisto K, Mykkänen L, Helkala E-L, Vanhanen M, Hänninen T, et al. Essential hypertension and cognitive function: The role of hyperinsulinemia. Hypertension. 1993;22:771–779. doi: 10.1161/01.hyp.22.5.771. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. Journal of the American Medical Association. 1995;274:1846–1851. [PubMed] [Google Scholar]
- Laurent S, Boutouyrie P. Arterial stiffness: A new surrogate end point for cardiovascular disease? Journal of Nephrology. 2007;20:S45–S50. [PubMed] [Google Scholar]
- Mager U, Kolehmainen M, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, et al. Finnish Diabetes Prevention Study Group Association between ghrelin gene variations and blood pressure in subjects with impaired glucose tolerance. American Journal of Hypertension. 2006;19:920–926. doi: 10.1016/j.amjhyper.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Rauch G, Rauch RA, Haque A. Risk factors for cerebral hypoperfusion, mild cognitive impairment, and dementia. Neurobiology of Aging. 2000;21:161–169. doi: 10.1016/s0197-4580(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- O’Bryant SE, Humphreys JD, Smith GE, Ivnik RJ, Graff-Radford NR, Petersen RC, et al. Detecting dementia with the Mini-Mental State Examination in highly educated individuals. Archives of Neurology. 2008;65:963–967. doi: 10.1001/archneur.65.7.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke MF. Arterial aging: Pathophysiological principles. Vascular Medicine. 2007;12:329–341. doi: 10.1177/1358863X07083392. [DOI] [PubMed] [Google Scholar]
- Pavlik VN, Hyman DJ, Doody R. Cardiovascular risk factors and cognitive function in adults 30-59 years of age (NHANES III) Neuroepidemiology. 2005;24:42–50. doi: 10.1159/000081049. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: Vulnerability of the prefrontal regions and executive functions. Behavioral Neuroscience. 2003;117:1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Land S. Genetic and vascular modifiers of age-sensitive cognitive skills: Effects of COMT, BDNF, ApoE and hypertension. Neuropsychology. doi: 10.1037/a0013487. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Dahle CL, Rodrigue KM, Kennedy KM, Land SJ, Jacobs BS. Brain-Derived Neurotrophic Factor Val66Met and blood glucose: A Synergistic effect on memory. Frontiers in Human Neuroscience. doi: 10.3389/neuro.09.012.2008. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfing CL, Little RR, Wiedmeyer HM, England JD, Madsen R, Harris MI, et al. Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population. Diabetes Care. 2000;23:187–191. doi: 10.2337/diacare.23.2.187. [DOI] [PubMed] [Google Scholar]
- Rolandsson O, Backeström A, Eriksson S, Hallmans G, Nilsson L-G. Increased glucose levels are associated with episodic memory in nondiabetic women. Diabetes. 2008;57:440–443. doi: 10.2337/db07-1215. [DOI] [PubMed] [Google Scholar]
- Safar ME, Lajemi M, Rudnichi A, Asmar R, Benetos A. Angiotensin-converting enzyme D/I gene polymorphism and age-related changes in pulse pressure in subjects with hypertension. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24:782–786. doi: 10.1161/01.ATV.0000119354.41615.33. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Speed and knowledge as determinants of adult age differences in verbal tasks. Journal of Gerontology. 1993;48:P29–P36. doi: 10.1093/geronj/48.1.p29. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Fristoe N, McGuthry KE, Hambrick DZ. Relation of task switching to speed, age, and fluid intelligence. Psychology and Aging. 1998;13:445–461. doi: 10.1037/0882-7974.13.3.445. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Hancock HE, Meinz EJ, Hambrick DZ. Interrelations of age, visual acuity, and cognitive functioning. Journal of Gerontology: Psychological Sciences. 1996;51B:P317–P330. doi: 10.1093/geronb/51b.6.p317. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Meinz EJ. Aging, inhibition, working memory, and speed. Journal of Gerontology: Psychological Sciences. 1995;50B:P297–P306. doi: 10.1093/geronb/50b.6.p297. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL. Vascular stiffening and arterial compliance. American Journal of Hypertension. 2004;17:39S–48S. doi: 10.1016/j.amjhyper.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Marmot M. High blood pressure was associated with cognitive function in middle-age in the Whitehall II study. Journal of Clinical Epidemiology. 2005;58:1308–1315. doi: 10.1016/j.jclinepi.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Strachan MW, Deary IJ, Ewing FM, Frier BM. Is type II diabetes associated with a increased risk of cognitive dysfunction? A critical review of published studies. Diabetes Care. 1997;20:438–445. doi: 10.2337/diacare.20.3.438. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, et al. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51:986–993. doi: 10.1212/wnl.51.4.986. [DOI] [PubMed] [Google Scholar]
- van Boxtel MPJ, Gaillard C, Houx PJ, Buntinx F, de Leeuw PW, Jolles J. Can the blood pressure predict cognitive task performance in a healthy population sample? Journal of Hypertension. 1997;15:1069–1076. doi: 10.1097/00004872-199715100-00004. [DOI] [PubMed] [Google Scholar]
- Vanhanen M, Koivisto K, Karjalainen L, Helkala E-L, Laakso M, Soininen H, et al. Risk for non-insulin dependent diabetes in the normoglycaemic elderly is associated with impaired cognitive function. NeuroReport. 1997;8:1527–1530. doi: 10.1097/00001756-199704140-00041. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Marcoen A, Goossens L. Facts and fiction about memory aging: A quantitative integration of research findings. Journal of Gerontology: Series B: Psychological and Social Sciences. 1993;48:P157–P171. doi: 10.1093/geronj/48.4.p157. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51:99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for Wechsler Memory Scale-Revised. Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Kirschbaum C. Endogenous estradiol and testosterone levels are associated with cognitive performance in older women and men. Hormones and Behavior. 2002;41:259–266. doi: 10.1006/hbeh.2002.1770. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, Johnson MB. Manual for Woodcock-Johnson Psychoeducational Battery-Revised. Riverside Publishing; Itasca, IL: 1989. [Google Scholar]
- Woodcock RW, Mather N. Woodcock-Johnson tests of cognitive ability- Standard and supplemental batteries: Examiner’s manual. In: Woodcock RW, Johnson MB, editors. Woodcock-Johnson Psychoeducational Battery-Revised. Riverside Publishing; Itasca, IL: 1989. [Google Scholar]