Abstract
Leishmania braziliensis infections are often associated with exaggerated immune responses that can sometimes lead to severe disease associated with high levels of IFN-γ and TNF-alpha. To explore the role played by dendritic cells (DCs) in these responses, we characterized DCs that were exposed to L. braziliensis. We found that DCs cultured with L. braziliensis parasites upregulated DC activation markers and produced IL-12 and TNF-alpha. However, not all DCs in the culture became infected, and an analysis of infected and uninfected DCs demonstrated that the upregulation of activation markers and IL-12 production was primarily confined to the uninfected (bystander) DCs. Further studies with transwell chambers and parasite fractions indicated that the activation of bystander DCs was mediated by a soluble parasite product, in a type 1 IFN and Myd88-independent but TNF-alpha-dependent fashion, and that the activated DCs were more efficient at presenting antigen than control DCs. In contrast, L. braziliensis infected DCs failed to upregulate activation markers, but exhibited a dramatic enhancement in their ability to produce TNF-alpha in response to LPS as compared to uninfected DCs. These findings uncover a dual role for DCs in L. braziliensis infection: T cell activation by bystander DCs due to enhanced antigen presenting capacity following exposure to soluble parasite products, and increased production of TNF-alpha by infected cells that may contribute to the local control of the parasites but concomitantly induce immunopathology.
Keywords: Dendritic cells, Parasitic-protozoan, Cell activation, Cytokine
Introduction
Leishmaniasis is caused by several different species of protozoan parasites, and the clinical manifestations of the infection vary widely depending upon the Leishmania species involved and the immune response of the host. In all cases, control of the parasites is associated with the expansion of CD4+ Th1 cells that produce IFN-γ, which promotes destruction of the parasites within infected cells (1-4). In the case of Leishmania braziliensis infections, however, an exaggerated Th1 response can lead to a severe immunopathologic form of leishmaniasis, known as mucosal (ML) disease, characterized predominantly by severe tissue damage in the nasopharyngeal region and disfiguring facial lesions. The pathogenesis of this infection remains poorly understood, although TNF-alpha is thought to play a contributing role in the tissue damage seen in these patients (5-10). Thus, following stimulation peripheral blood mononuclear cells (PBMC) from ML patients secrete higher levels of TNF-alpha than those from individuals with simple cutaneous leishmaniasis (6, 7). In addition, combining drug therapy with pentoxifylline–a drug that down-modulates TNF-alpha production–enhances resolution of mucosal lesions (8, 9).
DCs play a pivotal role in promoting resistance to leishmaniasis, both by activating CD4+ T cells and promoting their differentitation into Th1 cells by producing IL-12 (11, 12). Thus, a reasonable place to start investigating the pathogenesis of L. braziliensis infections would be to study the interactions of these parasites with DCs. The response of DCs to infection with several other Leishmania parasites has been examined, although the results of these studies have often yielded conflicting results. For example, L. major amastigotes, but not promastigotes, were reported to activate murine DCs, while activation of human DCs required an additional stimulation through CD40 (11, 13-16). In the case of New World Leishmania species, some studies have indicated that the parasites activate DCs, while others fail to see DC activation by the parasites (17-22). Differences in the parasite stage, parasite species or strain, and source of DCs may explain some of these divergent results. Moreover, most previous studies did not differentiate between infected DCs within the cultures and those that were exposed to the parasites but not infected.
In the present study, we investigated whether interactions between DCs and L. braziliensis might contribute to the pathogenesis of ML. We found that, while DCs infected with L. braziliensis were not activated, uninfected DCs in the same culture (bystander DCs) were stimulated by a secreted product from L. braziliensis. This stimulation led to the upregulation of costimulatory molecules, production of IL-12 and TNF-alpha, and the enhanced ability of DCs to activate T cells. We found that bystander DC activation was TNF-alpha-dependent but MyD88 and type 1 IFN-independent. On the other hand, L. braziliensis infected DCs failed to upregulate DC activation markers, although they produced TNF-alpha. Further studies found that L. major and L. mexicana also activated bystander DCs, but in contrast to L. braziliensis these bystander DCs produced much lower levels of TNF-alpha. Taken together, these results demonstrate that Leishmania parasites can activate bystander DCs, and indicate that one factor contributing to the development of ML following L. braziliensis infection may be the ability of these parasites to induce bystander DCs to produce high levels of TNF-alpha.
Material and Methods
Animals
Female wild type C57BL/6 and C3H mice, and C3H type 1 IFN receptor deficient and OTII transgenic mice were purchased from The Jackson Laboratory (Bar Harbor, ME). MyD88-deficient mice were kindly provided by Dr. Larry Turka. Animals were maintained in a specific-pathogen-free environment and tested negative for pathogens in routine screening. All experiments were conducted following the guidelines of the University of Pennsylvania institutional animal care and use committee.
Parasites and DC culture
L. braziliensis parasites were isolated from a mucosal leishmaniasis patient from the endemic area of Corte de Pedra, Brazil and typed by RAPD PCR (23). L. braziliensis, L mexicana (MNYC/BZ/62/M379) and L. major parasites (MHOM/IL/80/Friedlin) were grown until stationary phase in Grace’s insect culture medium (Life Technologies, Gaithersburg, MD)supplemented with 20% heat-inactivated FBS (HyClone Laboratories, Logan, UT), 2 mM L-glutamine. Soluble Leishmania Ag (SLA) was prepared as previously described (24), tested for endotoxin using the Limulus amebocyte lysate test and used at a concentration of 10 μg/ml. Endotoxin levels were below the level of detection. Anti-TNF-alpha (R&D systems) monoclonal antibody was used in some cultures in a concentration of 10 μg/ml. LPS was used at a concentration of 10 ng/ml. Bone marrow-derived dendritic cells (DC) were cultured as previously described (25). Briefly, bone marrow DC precursors were differentiated for 8–10 days in the presence of 20 ng/ml GM-CSF in RPMI 1640 containing 10% FBS, 100 U/ml penicillin/streptavidin, 0.05 mM 2-ME, and 2 mM L-glutamine. On days 8–10 of culture, DCs were harvest and infected with stationary phase promastigotes at different parasite:DC ratios. After 2 hours, cells were washed twice to eliminate extracellular parasites, and incubated for 18 hours at 37°C. For the transwell experiments, DCs (1 million) were placed on one side of the chamber and Leishmania (1, 2 or 5 million) were placed on the other side. After an incubation period of 18 hours at 37°C, DCs were harvested and analyzed by flow cytometry as described below. For these experiments, we used 0.4μm pore size filters (Corning, NY) to impair the ability of parasites to infect DCs but allow the passage of soluble factors through the pores.
CFSE labeling of parasites and flow cytometric analysis of DCs
Parasite labeling with CFSE (Invitrogen) was performed as previously described (26). Briefly, parasites were washed twice in PBS and resuspended at 5 × 107/mL in 1 mL of PBS, with 5mM of CFSE, and incubated at 37°C for 10 minutes in the dark. Parasites were then washed in 10 mL of PBS, 10% fetal bovine serum and resuspended in RPMI. For flow cytometry, DCs were harvested, stained with fluorochrome-conjugated antibodies for surface markers (CD11c, CD80, CD86, class II (e-Bioscience)) and fixed by using 2% formaldehyde. For intracellular staining, the fixed cells were permeabilized with a solution of saponin and stained for 30 min at 4°C using fluorochrome-conjugated antibodies against IL-12 p40, TNF-alpha or isotype control antibodies.
Samples were acquired on a FACSCalibur flow cytometer (BD Pharmingen), and analysis was performed using FlowJo software (Tree Star). Analysis gates were based live cells, and in some cases live CD11c+ cells, as indicated in the figure legends.
DC and T cell co-culture and proliferation assays
Naive OTII CD4+ T cells were isolated by negative depletion using MACS columns, labeled with CFSE as previously described (27), and then cultured with DCs that had been pulsed overnight with OVA (100 μg/ml) or OVA plus SLA (10 μg/ml), at a ratio of 10 CD4+ cells/DC. Cells were cultured at 37°C for 4 days and then collected and stained for CD4. CFSE dilution was assessed by flow cytometry as described above. For the transwell experiments, immune cells were obtained from the spleens of infected C57BL/6 mice (1 million parasites/footpad) for 20 weeks. Immune CD4+ T cells were isolated by negative depletion and cultured with DCs that had been exposed overnight to parasite secreted products in 0.4 μm pore size transwells (Corning, NY) at a ratio of 10 CD4+ cells/DC. After 4 days, supernatants were harvested and IL-2 and IFN-gamma concentrations were measured by sandwich ELISAs as previously described (28).
Statistics
Statistical analysis was performed using a two-tailed Student’s t test. Differences were considered significant at p< 0.05.
Results
L. braziliensis parasites infect and activate DCs
We first examined whether L. braziliensis parasites could infect and survive within murine DCs. Bone-marrow derived DCs were cultured with L. braziliensis promastigotes and after 2 hours we observed (by optical microscopy) that approximately 70% of the DCs were infected. The percentage of infected DCs remained the same over 96 hours, while the number of parasites per DC increased (Fig. 1A). These results indicate that L. braziliensis parasites are able to infect and multiply within murine DCs. We next examined the activation status of the DCs that were cultured with L. braziliensis, by analyzing the expression of DC activation markers (class II, CD80 and CD86) and the production of proinflammatory cytokines (IL-12 and TNF-alpha). We observed a slight upregulation of class II and CD86 on DCs taken from cultures infected with L. braziliensis (Fig. 1B). Moreover, there was increase in the frequency of IL-12 and in TNF-alpha producing DCs in cultures infected with L. braziliensis (Fig. 1C).
Figure 1. Infection and activation of DCs by L. braziliensis.
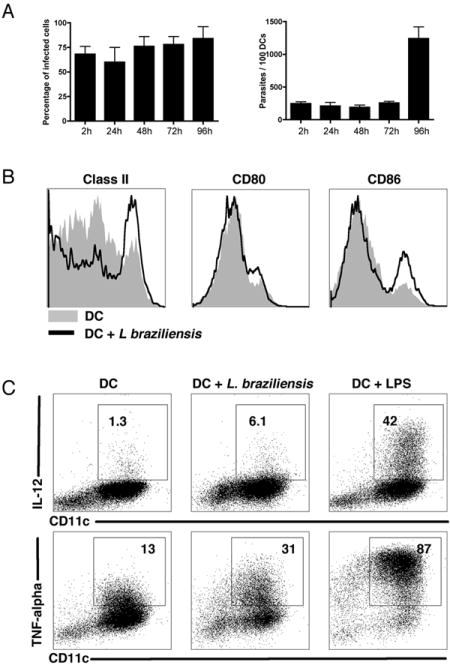
DCs from B6 mice were infected with L. braziliensis (5:1 parasites:DC ratio) for 2 hours, washed and cells were then incubated for the period of time indicated. A, Percentage of infected cells (A, left panel) and number of parasites /100 DCs (A, right panel) assessed by optical microscopy (representative of 2 experiments). Each data point represents the mean +/- SD. B, DC activation status (class II, CD80 and CD86) 18 hours post infection with L. braziliensis assessed by flow cytometry. Histograms are gated on CD11c+ cells. C, IL-12 (upper panel) and TNF-alpha (lower panel) production 18 hours post infection with L. braziliensis. Cytokines gates were determined by isotype controls and the numbers represents the frequency of positive cells within the CD11c+ population. Results are from one experiment and representative of three independent experiments.
Bystander DCs, but not infected DCs, upregulate class II and costimulatory molecules
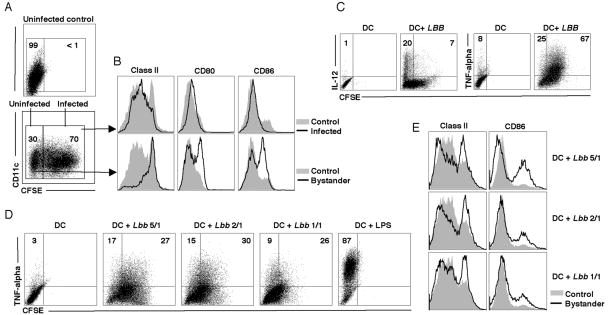
As not all of the DCs were infected with L. braziliensis, we next wanted to focus specifically on those DCs that were infected. Therefore, we infected DCs with parasites that had been labeled with CFSE, which allowed us to analyze DC activation in both infected (CFSE bright) and bystander (CFSE dim) DC populations within the same culture (Fig. 2, A and B). Surprisingly, slightly lower expression of class II and CD86 was observed in infected DCs when compared to DCs from control cultures that were not exposed to parasites (Fig. 2, A and B). On the other hand, uninfected bystander DCs expressed higher levels of class II, CD80 and CD86. Furthermore, when the ability of infected and bystander DCs to produce IL-12 was tested, it was the bystander DC population that contained cells positive for IL-12, while TNF-alpha producing cells were observed in both the infected and bystander populations (Fig. 2C). Part of the difference seen in activation markers between infected cells and control DCs may be due to preferential infection by the parasites of slightly more immature DCs, which would be consistent with decreased phagocytosis by activated DCs (29, 30). To see whether the activation of bystander cells was related to infected cell frequency within a culture, a dose response curve was performed where DCs were exposed to different numbers of parasites. The ability of bystander DCs to upregulate class II, CD86 and produce TNF-alpha was found to be related to the frequency of infected cells within the cultures (Fig. 2D and E).
Figure 2. Bystander DCs are activated by L. braziliensis.
DCs were infected with CFSE-labeled L. braziliensis and cultured for 18 hours. A, Percentage of infected (CFSE bright) and uninfected (bystander) (CFSE dim) DCs 18 hours after infection at a ratio of 5:1 parasites/DC. B, DC activation status (class II, CD80 and CD86) in infected DCs (upper panel) and bystander DCs (lower panel). Histograms are gated on CD11c+ cells. C, Pro-inflammatory cytokine production by infected (CFSE bright) and bystander (CFSE dim) cells at 18 hours. Numbers in C represent percentage of cells positive for IL-12 and TNF-alpha within the bystander and infected population. Dot plots are gated on CD11c+ population. D and E, Influence of altering the parasite:DC ratio on TNF-alpha production (D) and class II and CD86 expression (E). DCs were infected in at 5:1, 2:1 and 1:1 (parasite:DCs) for 2 hours, washed and cells were then incubated for 18 hours. TNF-alpha, class II and CD86 expression was assessed by flow cytometry. Numbers in D represent the percentage of cells positive for TNF-alpha within the bystander and infected population. Dot plots and histograms are gated on CD11c+ populations. Data are from one experiment and representative of six independent experiments.
L. braziliensis secretes a molecule that activates DCs and enhances their antigen-presenting capability
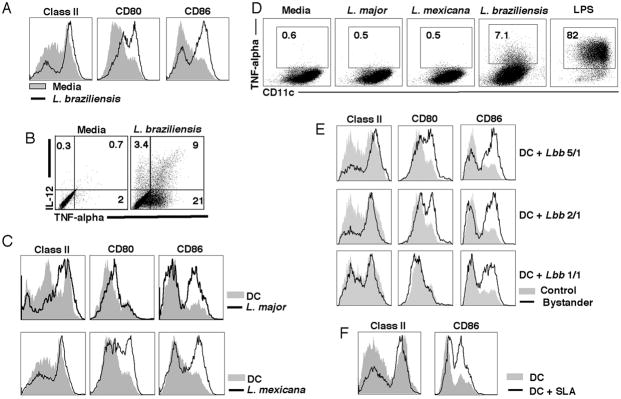
The activation of the bystander DCs that we observed could be due to the secretion of a factor from the infected DCs, or due to a parasite molecule present in the culture. To differentiate between these possibilities we used transwell chambers, where uninfected DCs could be separated from parasites by a membrane that allowed passage of soluble molecules but not the parasites. When DCs were cultured in a transwell chamber without parasites, we saw no DC activation. However, the presence of L. braziliensis parasites in the chamber separated from the DCs by a membrane led to increased expression of activation markers and the production of IL-12 and TNF-alpha (Fig. 3, A and B). In order to determine if the bystander DCs were activated by other species of Leishmania, we performed transwell assays with L. major and L. mexicana. We found that similar to L. braziliensis, DCs exposed to L. major and L. mexicana exhibited an increase in activation markers (Fig. 3C). However, when we examined TNF-alpha production we found that neither L. major nor L. mexicana stimulated bystander DCs to produce detectable levels of TNF-alpha (Fig 3D). The ability of secreted products from the parasites to activate DCs was decreased as the number of parasites added to the transwell was decreased (Fig. 3E). These results suggest that a soluble parasite factor can activate DCs in the absence of any infection. To confirm this result we made a soluble fraction of L. braziliensis (SLA) and tested its ability to activate DCs. As seen in Fig. 3F L. braziliensis SLA induced upregulation of class II and CD86.
Figure 3. L. braziliensis secreted products activate DCs.
A, L. braziliensis promastigotes (5 million) were placed in the bottom and DCs (1 million) in the top of a transwell and cultured for 18 hours. Class II, CD80 and CD86 expression (assessed by flow cytometry) in cells not exposed to parasites (gray solid) and cells exposed to L. braziliensis (black line). Histograms are gated on the CD11c+ population. B, Frequency of IL-12 and TNF-alpha positive cells (incubation as in A). Numbers in B represent percentages of cells in each quadrant. Plots are gated on CD11c+ cells. C and D, L. major, L. mexicana or L. braziliensis (5 million) parasites were placed in the bottom and DCs (1 million) in the top of transwell chambers and cultured for 18 hours. C, Class II, CD80 and CD86 expression (assessed by flow cytometry) in cells not exposed to parasites (gray solid) and cells exposed to L. major and L. mexicana (black line). Histograms are gated on CD11c+ cells. D, Frequency of TNF-alpha positive DCs after 18 hours exposure to parasite products in transwell chambers. Plots are gated on a live cell gate. E, Influence of parasite:DC ratio on class II, CD80 and CD86 expression in transwell experiments. Transwell experiments were performed as in A with parasite:DC ratio of 5:1, 2:1 or 1:1. After 18 hours incubation, class II, CD80 and CD86 expression levels were assessed by flow cytometry. Histograms are gated on the CD11c+ population. F, DCs were incubated with soluble Leishmania antigen (SLA) at 10 μg/ml and class II and CD86 expression was assessed by flow cytometry after 18 hours incubation. Histograms are gated on the CD11c+population. Data are from one experiment and representative of five independent experiments.
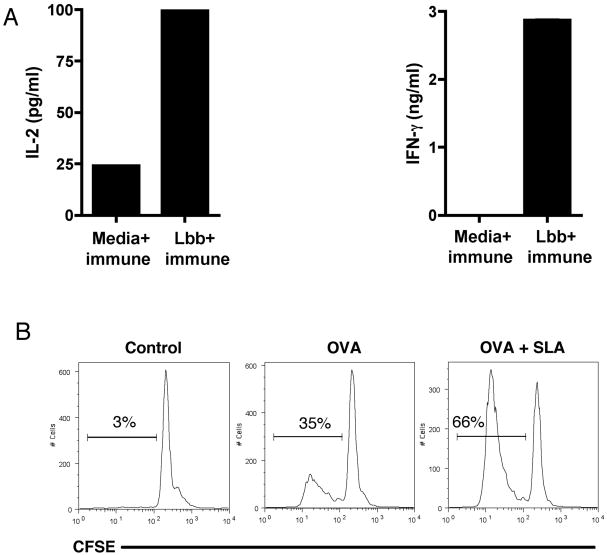
We next asked if the bystander DCs had taken up leishmanial antigens and could stimulate T cells. To test this, we assayed the ability of DCs exposed to L. braziliensis through the transwell membrane to activate T cells from Leishmania-immune mice. DCs were cultured in a transwell chamber with or without L. braziliensis parasites in the other compartment. After 18 hours, CD4 T cells from immune (infected for 20 weeks) or naïve mice were added and 4 days later the production of IL-2 and IFN-γ was assessed. DCs not exposed to parasites failed to stimulate the immune cells to produced IL-2 or IFN-γ (Fig. 4A), and naïve T cells failed to respond (data not shown). In contrast, there was a significant increase in the production of IL-2 and IFN-γ by immune CD4 T cells added to DCs that were cultured in a transwell with L. braziliensis (Fig. 4A).
Figure 4. DCs exposed to L. braziliensis secreted products are better antigen presenting cells.
A, DCs (1 million) exposed to L. braziliensis (5 million) secreted products in transwells for 18 hours, were cultured together with immune CD4+ T cells at a ratio of 10 CD4+ T cells to 1 DC. After 4 days, the levels of IL-2 and IFN-gamma in the supernatants were measured by ELISA. B, DCs (1 million) were pulsed with OVA (100 μg/ml) in the presence or absence of SLA (10 μg/ml) and incubated for 18 hours. CFSE labeled CD4+ OTII cells were then cultured together with DCs at a ratio of 10 CD4+ T cells to 1 DC. After 4 days the percentage of cells that diluted CFSE was determined by flow cytometry. Numbers represent percentages of CFSE-dim cells. Histograms are gated on CD4+ populations. Data are from one experiment and representative of three independent experiments.
In order to determine if the bystander DCs activated by L. braziliensis exhibited an increased capacity to present antigen compared with DCs that were not exposed to the parasites, we tested the ability of these two populations to present antigen to T cells. CFSE labeled TCR transgenic OTII T cells, which recognize ovalbumin (OVA), were cultured with OVA pulsed DCs or OVA pulsed DCs that had been exposed to a soluble fraction of L. braziliensis (SLA). After 4 days in culture the proliferation of the OTII cells was assessed by flow cytometry. As expected, we found that DCs exposed to OVA stimulated a significant increase in the proliferation of OTII cells. However, DCs that were exposed to OVA and SLA stimulated significantly better T cell proliferation (Fig. 4B).
Infected DCs make high levels of TNF-alpha
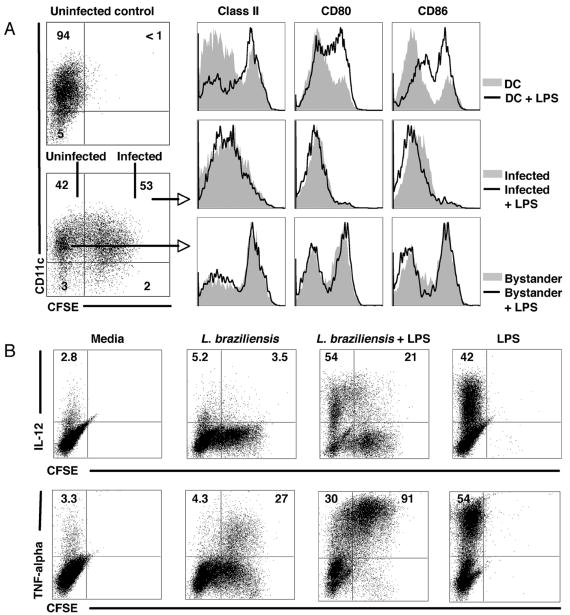
Leishmania infection of macrophages has been associated with a loss of the ability of the infected cells to respond to other activating agents, such as TLR ligands. Therefore, we wanted to know if L. braziliensis infected DCs were able to respond to LPS. To test this, we infected DCs with CFSE labeled parasites for 2 hours, and then pulsed some of the cultures with LPS for 6 hours. We found that infected DCs were not able to upregulate class II, CD80 or CD86 in response to LPS (Fig. 5A). Bystander DCs, which already expressed higher levels of class II, CD80 and CD86, did not further upregulate these molecules in response to LPS. There was an LPS-induced increase in IL-12 production by infected DCs, although this increase was substantially less than that observed by uninfected DCs (Fig. 5B). On the other hand, there was a dramatic increase in the ability of infected DCs to produce TNF-alpha in response to LPS. While 30% of the bystander DCs produced TNF-alpha, over 90% of the infected DCs did so after LPS stimulation (Fig. 5B). These results demonstrate that while infected DCs may fail to increase the expression of activation markers, they not only can make TNF-alpha, but make more TNF-alpha than bystander DCs following exposure to a TLR ligand.
Figure 5. Infected DCs retain immature characteristics after stimulation with LPS, but produce high amounts of TNF-alpha.
DCs were infected with L. braziliensis (5:1 parasite:DC ratio) for 2 hours and pulsed with LPS for 6 hours. A, class II, CD80 and CD86 expression (assessed by flow cytometry) in infected DCs and infected DCs plus LPS (top panel), bystander DCs and bystander DCs plus LPS (middle panel) and DCs alone (not exposed to Leishmania) and DCs plus LPS (lower panel). Numbers in A represent the percentage of cells in each quadrant. Dot plots were gated on live cells and histograms were gated in the CD11c+ cell population. B, IL-12 and TNF-alpha production (assessed by flow cytometry) in infected (CFSE bright) versus bystander (CFSE dim) DCs in the presence or absence of LPS. Infection and incubation as in A. Numbers in B represent the percentage of cells positive for IL-12 or TNF-alpha within the bystander and infected population. Dot plots are gated on the CD11c+ population. Data are from one experiment and representative of four independent experiments.
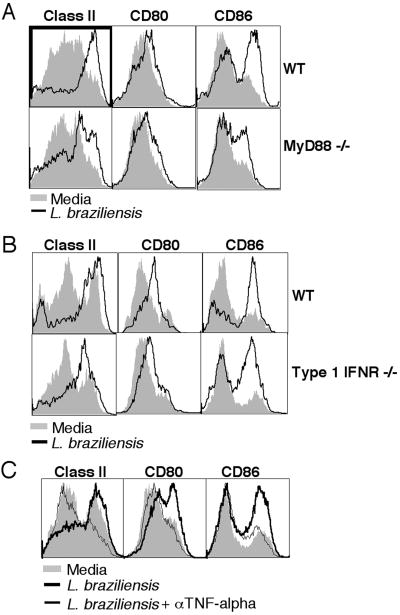
In order to study the mechanism by which Leishmania secreted products activate bystander DCs, we investigated the role of MyD88 and type 1 IFN by using MyD88-/- and type 1 IFN-receptor deficient DCs in transwell experiments, as described above. We found that activation proceeded independently of Myd88 and type 1 IFN (Fig 6A and B). Since the data above show that both bystander and infected DCs produce TNF-alpha and TNF-alpha is known to induce upregulation of class II, CD80 and CD86 (31, 32), we next evaluated whether TNF-alpha plays a role in bystander DC activation. By blocking TNF-alpha activity with anti-TNF-alpha monoclonal antibody, we found that the upregulation of class II, CD80 and CD86 on bystander DCs was prevented (Fig 6C). Thus, while neither Myd88 nor type 1 IFN is required for bystander DC activation, both infected and bystander DCs produce TNF-alpha that is essential for activation of the bystander DCs.
Figure 6. DC activation by L. braziliensis secreted products is TNF-alpha-dependent, but MyD88- and Type 1 IFN-independent.
L. braziliensis parasites (5 million) were placed in the bottom and MyD88-/- (A), type 1 IFN receptor-/- (B) and WT C57BL/6 (C) DCs (1 million) in the top chambers of a transwell plate, and after 18 hours class II, CD80 and CD86 expression was assessed by flow cytometry. Anti-TNF-alpha monoclonal antibody (10 μg/ml) or isotype control were used (C). Histograms are gated on the CD11c+ population. Data are from one experiment and representative of five independent experiments.
Discussion
The activation state of DCs can be distinguished based on the expression of class II, co-stimulatory molecules, as well as cytokine production (33). Prior to infection, DCs survey the tissues and the lymphoid organs as ‘immature’ cells. However, when exposed to pathogens, DCs upregulate expression of surface markers associated with antigen presentation–such as class II, CD80 and CD86–and produce proinflammatory cytokines–such as IL-12 and TNF. In leishmaniasis, DCs not only play a key role in the development of a protective immune response to Leishmania, but also act as a host cell for the parasites. This dual role means that DCs will need to be activated to initiate the immune response, but at the same time the parasites will presumably want to inhibit activation to promote parasite survival. Many studies have examined the response of DCs to Leishmania parasites, but the use of different parasite species and life-cycle stages, as well as different sources of DCs, has led to a wide range of results (11, 17-21, 34-37). In addition, most of these studies examined DC activation in cultures that contained both infected and uninfected DCs. Here, we monitored the response of infected versus bystander DCs, and found that while infected DCs appear immature, bystander DCs upregulate class II, CD80 and CD86 expression and secrete IL-12 and TNF-alpha. Our data suggest that infected DCs are relatively immature, and in other contexts these semi-mature DCs have been reported to be more likely to induce tolerance, than promote an immune response (33). Thus, it is likely that the bystander DCs may be more important for the initial T cell response, which is consistent with our finding that the bystander DCs were capable of stimulating T cell responses. We also found that bystander DC activation was not unique to L. braziliensis, and was observed with L. major and L. mexicana, although bystander DCs exposed to L. braziliensis made significantly more TNF-alpha. Consistent with these results, non-infected DCs appear to mature in vivo following infection and present leishmanial antigens (21, 38, 39). Taken together, these data indicate infected and bystander DCs play distinct roles in leishmaniasis.
Leishmania is known to inhibit several macrophage functions, such as activation, cytokine release and antigen presentation (17, 40-43). For instance, down-regulation of class II expression and the inability to produce IL-12 has been observed in several studies (16, 43-45). Also, TLR-induced up-regulation of co-stimulatory molecules, as well as TNF-alpha and IL-12 production, was impaired in L. major, L. chagasi, L. donovani and L. mexicana-infected macrophages, and in the case of L. mexicana, disruption of NF-κB signaling was observed (44, 46-48). Less well understood are the interactions between Leishmania and DCs. While some studies documented impairment in DC function, others found increased DC activation, IL-12 production and NF-κB signaling after Leishmania infection (11, 15, 18, 19, 21, 36, 37). We found not only that infected DCs remain immature after Leishmania infection, but that they are also impaired in the upregulation of class II, costimulatory molecules and IL-12 after stimulation by LPS. Our results show that infected and bystander DCs respond differently to infection, indicating that understanding the response of DCs to Leishmania requires analyzing both infected and non-infected cells within a culture. Some of the conflicting results addressing the question of whether DCs are activated by Leishmania may be due to the fact that the impact of Leishmania on DC activation is often assessed on populations that contain both infected and uninfected cells. Here we show that infected DCs are less responsive to the TLR-ligand LPS as measured by their ability to up-regulate expression of class II, CD80, CD86 and IL-12, but remain responsive as assessed by their ability to produce high levels of TNF-alpha upon LPS stimulation. In another study it was shown that concomitant activation with LPS and IFN-gamma may overcome this inhibition, although even under these conditions, fewer infected cells made IL-12 when compared with DCs from uninfected cultures (22).
Of particular interest is the increased percentage of TNF-alpha producing cells within the L. braziliensis infected DC population. TNF-alpha is a key cytokine mediating T cell-mediated inflammation. It is involved in leukocyte recruitment by increasing expression of adhesion molecules on vascular endothelium and increasing angiogenesis (49, 50). Although TNF-alpha promotes increased macrophage activation, and contributes to control of Leishmania parasites, deleterious consequences of excessive TNF-alpha production have been reported in many conditions, such as rheumatoid arthritis or psoriasis (51-55). In humans, TNF-alpha plays an important role in the pathogenesis of mucosal and cutaneous leishmaniasis due to L. braziliensis infection (6, 7). The high levels of TNF-alpha and IFN-gamma secreted by mononuclear cells from these patients is positively correlated with lesion size (56) and the use of drugs that down modulate production of TNF-alpha in combination with antimony increases the rate of healing and allows the cure of refractory cases of mucosal and cutaneous disease (8, 9). While monocytes, CD4+ and CD8+ T cells are known to contribute to TNF-alpha production in patients with mucosal leishmaniasis (6), the role of DCs in this process has not been explored. Our data show that both bystander and infected DCs are another source of TNF-alpha following infection with L. braziliensis. Future studies with DCs from both patients and uninfected individuals will be required to extend these studies to human leishmaniasis.
DCs can be activated by several pathways, including MyD88-dependent TLR signaling and via type 1 IFN- and TNF-alpha-stimulated pathways (31-33, 57). The adaptor molecule MyD88 is required for resistance to Leishmania, and type 1 IFN has been shown to upregulate expression of nitric oxide synthase in vivo and ameliorate cutaneous leishmaniasis lesions (21, 58, 59). Therefore, we tested whether MyD88 and type 1 IFN would mediate DC activation by Leishmania secreted products. We found that while MyD88 and type 1 IFN are not involved in bystander DC activation, products secreted from the parasites induce TNF-alpha production, and in an autocrine manner TNF-alpha induces up-regulation of class II and costimulatory molecules on bystander DCs. DCs activated by TNF-alpha have been reported to gain a semi-mature phenotype, characterized by upregulation of class II and costimulatory molecules, but a failure to make proinflammatory cytokines (33). These DCs have been thought to be tolerogenic rather than inflammatory. However, in our studies bystander DCs not only upregulate class II and costimulatory molecules, but also produce TNF-alpha and IL-12. Moreover, they are able to process and present Leishmania antigens to CD4 T cells. We hypothesize that the differences between the semi-mature tolerogenic DCs induced by TNF-alpha alone and the DCs activated by Leishmania secreted products might be due to additional signals induced by the parasite. Interestingly, while the bystander DCs respond to TNF-alpha, Leishmania infected DCs were inhibited in their ability to respond. Why this is the case is unknown, although the inability of Leishmania infected-cells to respond to other signals (such as LPS) has been described before. For example, NF-κB signaling is compromised in Leishmania-infected macrophages and since TNF-alpha-mediated DC activation is dependent on NF-κB, this is one possible mechanism that could be involved (48).
Finally, the parasite molecule(s) that activates bystander DCs is unknown. The most well-described Leishmania surface molecule is a lipophosphoglycan (LPG), which covers the surface of the parasite and can be released (60, 61). Many functions have been ascribed to LPG, both within the sandfly and the mammalian host (62, 63). Specifically, LPG can activate DCs by binding to TLR2, although LPG has also been reported to inhibit activation (64, 65). However, since the products secreted by Leishmania activate DCs in a MyD88-independent fashion, it may be unlikely that LPG would be involved in the bystander DC activation process. Similarly, another TLR ligand known to contribute to the activation of DCs is Leishmania DNA (58, 66). However, Leishmania DNA activates DCs by binding to TLR9 and as this process is also dependent on MyD88, we can rule out the possibility that DNA is involved in the bystander DC activation that we are observing. Thus, further studies will be required to define the parasite products that can stimulate bystander DCs.
Our findings show a dual role for DCs following infection with L. braziliensis. While infected DCs remain immature and secrete TNF-alpha, bystander DCs respond to parasites factors by producing TNF-alpha, which in turn upregulates class II and co-stimulatory molecules. These bystander DCs also produce IL-12, and thus are able to present antigen to CD4+ T cells and promote the generation of CD4+ Th1 cells. On the other hand, the inability of infected DCs to upregulate costimulatory molecules or produce IL-12 suggests that these cells are not efficient at antigen presentation. However, these DCs produced high levels of TNF-alpha, and at the same time provide a site for the parasites to survive, suggesting that they may promote increased immunopathology. Future studies are ongoing to characterize the mechanisms by which L. braziliensis modulates DC function, as well as identification of Leishmania products involved in DC activation. A better understanding of how Leishmania parasites inhibit DC activation will be useful in the design of new immunotherapies for leishmaniasis.
Acknowledgments
We thank Euihye Jung and Carlos Rodriguez for technical assistance and Christopher Hunter, David Artis and members of the Pathobiology Department for comments and criticisms.
This work was supported by National Institutes of Health (NIH) Tropical Infectious Diseases Training Grant D43 TW007127 and grants AI35914 and AI053825.
References
- 1.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 2.Scott P, Farrell JP. Experimental cutaneous leishmaniasis: induction and regulation of T cells following infection of mice with Leishmania major. Chem Immunol. 1998;70:60–80. doi: 10.1159/000058698. [DOI] [PubMed] [Google Scholar]
- 3.Scott P, Natovitz P, Coffman RL, Pearce E, Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988;168:1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locksley RM, Heinzel FP, Holaday BJ, Mutha SS, Reiner SL, Sadick MD. Induction of Th1 and Th2 CD4+ subsets during murine Leishmania major infection. Res Immunol. 1991;142:28–32. doi: 10.1016/0923-2494(91)90007-6. [DOI] [PubMed] [Google Scholar]
- 5.Da-Cruz AM, de Oliveira MP, De Luca PM, Mendonca SC, Coutinho SG. Tumor necrosis factor-alpha in human american tegumentary leishmaniasis. Mem Inst Oswaldo Cruz. 1996;91:225–229. doi: 10.1590/s0074-02761996000200019. [DOI] [PubMed] [Google Scholar]
- 6.Bacellar O, Lessa H, Schriefer A, Machado P, Ribeiro de Jesus A, Dutra WO, Gollob KJ, Carvalho EM. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun. 2002;70:6734–6740. doi: 10.1128/IAI.70.12.6734-6740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribeiro-de-Jesus A, Almeida RP, Lessa H, Bacellar O, Carvalho EM. Cytokine profile and pathology in human leishmaniasis. Braz J Med Biol Res. 1998;31:143–148. doi: 10.1590/s0100-879x1998000100020. [DOI] [PubMed] [Google Scholar]
- 8.Machado PR, Lessa H, Lessa M, Guimaraes LH, Bang H, Ho JL, Carvalho EM. Oral pentoxifylline combined with pentavalent antimony: a randomized trial for mucosal leishmaniasis. Clin Infect Dis. 2007;44:788–793. doi: 10.1086/511643. [DOI] [PubMed] [Google Scholar]
- 9.Lessa HA, Machado P, Lima F, Cruz AA, Bacellar O, Guerreiro J, Carvalho EM. Successful treatment of refractory mucosal leishmaniasis with pentoxifylline plus antimony. Am J Trop Med Hyg. 2001;65:87–89. doi: 10.4269/ajtmh.2001.65.87. [DOI] [PubMed] [Google Scholar]
- 10.Cabrera M, Shaw MA, Sharples C, Williams H, Castes M, Convit J, Blackwell JM. Polymorphism in tumor necrosis factor genes associated with mucocutaneous leishmaniasis. J Exp Med. 1995;182:1259–1264. doi: 10.1084/jem.182.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Stebut E, Belkaid Y, Jakob T, Sacks DL, Udey MC. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: implications for the initiation of anti-Leishmania immunity. J Exp Med. 1998;188:1547–1552. doi: 10.1084/jem.188.8.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemos MP, Esquivel F, Scott P, Laufer TM. MHC class II expression restricted to CD8alpha+ and CD11b+ dendritic cells is sufficient for control of Leishmania major. J Exp Med. 2004;199:725–730. doi: 10.1084/jem.20030795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDowell MA, Marovich M, Lira R, Braun M, Sacks D. Leishmania priming of human dendritic cells for CD40 ligand-induced interleukin-12p70 secretion is strain and species dependent. Infect Immun. 2002;70:3994–4001. doi: 10.1128/IAI.70.8.3994-4001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woelbing F, Kostka SL, Moelle K, Belkaid Y, Sunderkoetter C, Verbeek S, Waisman A, Nigg AP, Knop J, Udey MC, von Stebut E. Uptake of Leishmania major by dendritic cells is mediated by Fcgamma receptors and facilitates acquisition of protective immunity. J Exp Med. 2006;203:177–188. doi: 10.1084/jem.20052288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prina E, Abdi SZ, Lebastard M, Perret E, Winter N, Antoine JC. Dendritic cells as host cells for the promastigote and amastigote stages of Leishmania amazonensis: the role of opsonins in parasite uptake and dendritic cell maturation. J Cell Sci. 2004;117:315–325. doi: 10.1242/jcs.00860. [DOI] [PubMed] [Google Scholar]
- 16.Marovich MA, McDowell MA, Thomas EK, Nutman TB. IL-12p70 production by Leishmania major-harboring human dendritic cells is a CD40/CD40 ligand-dependent process. J Immunol. 2000;164:5858–5865. doi: 10.4049/jimmunol.164.11.5858. [DOI] [PubMed] [Google Scholar]
- 17.Bennett CL, Colledge L, Richards HE, Reay PA, Blackburn CC, Aebischer T. Uncompromised generation of a specific H-2DM-dependent peptide-MHC class II complex from exogenous antigen in Leishmania mexicana-infected dendritic cells. Eur J Immunol. 2003;33:3504–3513. doi: 10.1002/eji.200323425. [DOI] [PubMed] [Google Scholar]
- 18.Bennett CL, Misslitz A, Colledge L, Aebischer T, Blackburn CC. Silent infection of bone marrow-derived dendritic cells by Leishmania mexicana amastigotes. Eur J Immunol. 2001;31:876–883. doi: 10.1002/1521-4141(200103)31:3<876::aid-immu876>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 19.Aebischer T, Bennett CL, Pelizzola M, Vizzardelli C, Pavelka N, Urbano M, Capozzoli M, Luchini A, Ilg T, Granucci F, Blackburn CC, Ricciardi-Castagnoli P. A critical role for lipophosphoglycan in proinflammatory responses of dendritic cells to Leishmania mexicana. Eur J Immunol. 2005;35:476–486. doi: 10.1002/eji.200425674. [DOI] [PubMed] [Google Scholar]
- 20.Vasquez RE, Xin L, Soong L. Effects of CXCL10 on dendritic cell and CD4+ T-cell functions during Leishmania amazonensis infection. Infect Immun. 2008;76:161–169. doi: 10.1128/IAI.00825-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Trez C, Brait M, Leo O, Aebischer T, Torrentera FA, Carlier Y, Muraille E. Myd88-dependent in vivo maturation of splenic dendritic cells induced by Leishmania donovani and other Leishmania species. Infect Immun. 2004;72:824–832. doi: 10.1128/IAI.72.2.824-832.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vargas-Inchaustegui DA, Xin L, Soong L. Leishmania braziliensis infection induces dendritic cell activation, ISG15 transcription, and the generation of protective immune responses. J Immunol. 2008;180:7537–7545. doi: 10.4049/jimmunol.180.11.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schriefer A, Schriefer AL, Goes-Neto A, Guimaraes LH, Carvalho LP, Almeida RP, Machado PR, Lessa HA, de Jesus AR, Riley LW, Carvalho EM. Multiclonal Leishmania braziliensis population structure and its clinical implication in a region of endemicity for American tegumentary leishmaniasis. Infect Immun. 2004;72:508–514. doi: 10.1128/IAI.72.1.508-514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott P, Pearce E, Heath S, Sher A. Identification of T-cell-reactive antigens that protect BALB/c mice against Leishmania major. Ann Inst Pasteur Immunol. 1987;138:771–774. doi: 10.1016/s0769-2625(87)80036-3. [DOI] [PubMed] [Google Scholar]
- 25.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 26.Kamau SW, Nunez R, Grimm F. Flow cytometry analysis of the effect of allopurinol and the dinitroaniline compound (Chloralin) on the viability and proliferation of Leishmania infantum promastigotes. BMC Pharmacol. 2001;1:1. doi: 10.1186/1471-2210-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 28.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 29.Reis e Sousa C, Stahl PD, Austyn JM. Phagocytosis of antigens by Langerhans cells in vitro. J Exp Med. 1993;178:509–519. doi: 10.1084/jem.178.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato K, Nagayama H, Tadokoro K, Juji T, Takahashi TA. Extracellular signal-regulated kinase, stress-activated protein kinase/c-Jun N-terminal kinase, and p38mapk are involved in IL-10-mediated selective repression of TNF-alpha-induced activation and maturation of human peripheral blood monocyte-derived dendritic cells. J Immunol. 1999;162:3865–3872. [PubMed] [Google Scholar]
- 33.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 34.von Stebut E, Belkaid Y, Nguyen BV, Cushing M, Sacks DL, Udey MC. Leishmania major-infected murine langerhans cell-like dendritic cells from susceptible mice release IL-12 after infection and vaccinate against experimental cutaneous Leishmaniasis. Eur J Immunol. 2000;30:3498–3506. doi: 10.1002/1521-4141(2000012)30:12<3498::AID-IMMU3498>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 35.Gorak PM, Engwerda CR, Kaye PM. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur J Immunol. 1998;28:687–695. doi: 10.1002/(SICI)1521-4141(199802)28:02<687::AID-IMMU687>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 36.Konecny P, Stagg AJ, Jebbari H, English N, Davidson RN, Knight SC. Murine dendritic cells internalize Leishmania major promastigotes, produce IL-12 p40 and stimulate primary T cell proliferation in vitro. Eur J Immunol. 1999;29:1803–1811. doi: 10.1002/(SICI)1521-4141(199906)29:06<1803::AID-IMMU1803>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 37.Jayakumar A, Donovan MJ, Tripathi V, Ramalho-Ortigao M, McDowell MA. Leishmania major Infection Activates NF{kappa}B, IRF-1 and IRF-8 in Human Dendritic Cells. Infect Immun. 2008 doi: 10.1128/IAI.01252-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Misslitz AC, Bonhagen K, Harbecke D, Lippuner C, Kamradt T, Aebischer T. Two waves of antigen-containing dendritic cells in vivo in experimental Leishmania major infection. Eur J Immunol. 2004;34:715–725. doi: 10.1002/eji.200324391. [DOI] [PubMed] [Google Scholar]
- 39.Iezzi G, Frohlich A, Ernst B, Ampenberger F, Saeland S, Glaichenhaus N, Kopf M. Lymph node resident rather than skin-derived dendritic cells initiate specific T cell responses after Leishmania major infection. J Immunol. 2006;177:1250–1256. doi: 10.4049/jimmunol.177.2.1250. [DOI] [PubMed] [Google Scholar]
- 40.Fruth U, Solioz N, Louis JA. Leishmania major interferes with antigen presentation by infected macrophages. J Immunol. 1993;150:1857–1864. [PubMed] [Google Scholar]
- 41.Weinheber N, Wolfram M, Harbecke D, Aebischer T. Phagocytosis of Leishmania mexicana amastigotes by macrophages leads to a sustained suppression of IL-12 production. Eur J Immunol. 1998;28:2467–2477. doi: 10.1002/(SICI)1521-4141(199808)28:08<2467::AID-IMMU2467>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 42.Feng GJ, Goodridge HS, Harnett MM, Wei XQ, Nikolaev AV, Higson AP, Liew FY. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J Immunol. 1999;163:6403–6412. [PubMed] [Google Scholar]
- 43.Carrera L, Gazzinelli RT, Badolato R, Hieny S, Muller W, Kuhn R, Sacks DL. Leishmania promastigotes selectively inhibit interleukin 12 induction in bone marrow-derived macrophages from susceptible and resistant mice. J Exp Med. 1996;183:515–526. doi: 10.1084/jem.183.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olivier M, Gregory DJ, Forget G. Subversion mechanisms by which Leishmania parasites can escape the host immune response: a signaling point of view. Clin Microbiol Rev. 2005;18:293–305. doi: 10.1128/CMR.18.2.293-305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Almeida MC, Cardoso SA, Barral-Netto M. Leishmania (Leishmania) chagasi infection alters the expression of cell adhesion and costimulatory molecules on human monocyte and macrophage. Int J Parasitol. 2003;33:153–162. doi: 10.1016/s0020-7519(02)00266-7. [DOI] [PubMed] [Google Scholar]
- 46.Descoteaux A, Matlashewski G. c-fos and tumor necrosis factor gene expression in Leishmania donovani-infected macrophages. Mol Cell Biol. 1989;9:5223–5227. doi: 10.1128/mcb.9.11.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaye PM, Rogers NJ, Curry AJ, Scott JC. Deficient expression of co-stimulatory molecules on Leishmania-infected macrophages. Eur J Immunol. 1994;24:2850–2854. doi: 10.1002/eji.1830241140. [DOI] [PubMed] [Google Scholar]
- 48.Cameron P, McGachy A, Anderson M, Paul A, Coombs GH, Mottram JC, Alexander J, Plevin R. Inhibition of lipopolysaccharide-induced macrophage IL-12 production by Leishmania mexicana amastigotes: the role of cysteine peptidases and the NF-kappaB signaling pathway. J Immunol. 2004;173:3297–3304. doi: 10.4049/jimmunol.173.5.3297. [DOI] [PubMed] [Google Scholar]
- 49.Pober JS, Bevilacqua MP, Mendrick DL, Lapierre LA, Fiers W, Gimbrone MA., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986;136:1680–1687. [PubMed] [Google Scholar]
- 50.Munro JM, Pober JS, Cotran RS. Tumor necrosis factor and interferon-gamma induce distinct patterns of endothelial activation and associated leukocyte accumulation in skin of Papio anubis. Am J Pathol. 1989;135:121–133. [PMC free article] [PubMed] [Google Scholar]
- 51.Ettehadi P, Greaves MW, Wallach D, Aderka D, Camp RD. Elevated tumour necrosis factor-alpha (TNF-alpha) biological activity in psoriatic skin lesions. Clin Exp Immunol. 1994;96:146–151. doi: 10.1111/j.1365-2249.1994.tb06244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 53.Maioli TU, Takane E, Arantes RM, Fietto JL, Afonso LC. Immune response induced by New World Leishmania species in C57BL/6 mice. Parasitol Res. 2004;94:207–212. doi: 10.1007/s00436-004-1193-6. [DOI] [PubMed] [Google Scholar]
- 54.Kanaly ST, Nashleanas M, Hondowicz B, Scott P. TNF receptor p55 is required for elimination of inflammatory cells following control of intracellular pathogens. J Immunol. 1999;163:3883–3889. [PubMed] [Google Scholar]
- 55.Nashleanas M, Kanaly S, Scott P. Control of Leishmania major infection in mice lacking TNF receptors. J Immunol. 1998;160:5506–5513. [PubMed] [Google Scholar]
- 56.Antonelli LR, Dutra WO, Almeida RP, Bacellar O, Carvalho EM, Gollob KJ. Activated inflammatory T cells correlate with lesion size in human cutaneous leishmaniasis. Immunol Lett. 2005;101:226–230. doi: 10.1016/j.imlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 57.Gautier G, Humbert M, Deauvieau F, Scuiller M, Hiscott J, Bates EE, Trinchieri G, Caux C, Garrone P. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med. 2005;201:1435–1446. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Veer MJ, Curtis JM, Baldwin TM, DiDonato JA, Sexton A, McConville MJ, Handman E, Schofield L. MyD88 is essential for clearance of Leishmania major: possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur J Immunol. 2003;33:2822–2831. doi: 10.1002/eji.200324128. [DOI] [PubMed] [Google Scholar]
- 59.Mattner J, Wandersee-Steinhauser A, Pahl A, Rollinghoff M, Majeau GR, Hochman PS, Bogdan C. Protection against progressive leishmaniasis by IFN-beta. J Immunol. 2004;172:7574–7582. doi: 10.4049/jimmunol.172.12.7574. [DOI] [PubMed] [Google Scholar]
- 60.Ilg T, Stierhof YD, Wiese M, McConville MJ, Overath P. Characterization of phosphoglycan-containing secretory products of Leishmania. Parasitology. 1994;108(Suppl):S63–71. doi: 10.1017/s0031182000075739. [DOI] [PubMed] [Google Scholar]
- 61.Spath GF, Garraway LA, Turco SJ, Beverley SM. The role(s) of lipophosphoglycan (LPG) in the establishment of Leishmania major infections in mammalian hosts. Proc Natl Acad Sci U S A. 2003;100:9536–9541. doi: 10.1073/pnas.1530604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Turco SJ, Descoteaux A. The lipophosphoglycan of Leishmania parasites. Annu Rev Microbiol. 1992;46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- 63.Beverley SM, Turco SJ. Lipophosphoglycan (LPG) and the identification of virulence genes in the protozoan parasite Leishmania. Trends Microbiol. 1998;6:35–40. doi: 10.1016/S0966-842X(97)01180-3. [DOI] [PubMed] [Google Scholar]
- 64.Frankenburg S, Leibovici V, Mansbach N, Turco SJ, Rosen G. Effect of glycolipids of Leishmania parasites on human monocyte activity. Inhibition by lipophosphoglycan. J Immunol. 1990;145:4284–4289. [PubMed] [Google Scholar]
- 65.Descoteaux A, Matlashewski G, Turco SJ. Inhibition of macrophage protein kinase C-mediated protein phosphorylation by Leishmania donovani lipophosphoglycan. J Immunol. 1992;149:3008–3015. [PubMed] [Google Scholar]
- 66.Liese J, Schleicher U, Bogdan C. TLR9 signaling is essential for the innate NK cell response in murine cutaneous leishmaniasis. Eur J Immunol. 2007;37:3424–3434. doi: 10.1002/eji.200737182. [DOI] [PubMed] [Google Scholar]