Abstract
Although substantia nigra reticulata (SNR) neurons fire bursts of action potentials during normal movement, excessive burst firing correlates with symptoms of Parkinson’s disease. A major excitatory output from the subthalamic nucleus (STN) to the SNR is thought to provide the synaptic impetus for burst firing in SNR neurons. Using patch pipettes to record from SNR neurons in rat brain slices, we found that a single electrical stimulus delivered to the STN evokes a burst of action potentials. Under voltage-clamp conditions, STN stimulation evokes a complex EPSC that is comprised of an initial monosynaptic EPSC followed by a series of late EPSCs superimposed on a long-lasting inward current. Using varied stimulation frequencies, we found that the initial EPSC was significantly reduced or abolished after 2 s of 50–100 Hz STN stimulation. However, only 4 s of 1 Hz stimulation was required to abolish the late component of the complex EPSC. We suggest that differential effects of repetitive STN stimulation on early and late components of complex EPSCs may help explain the frequency-dependent effects of deep brain stimulation of the STN that is used in the treatment of Parkinson’s disease.
Keywords: subthalamic nucleus, substantia nigra reticulata, patch clamp, synaptic transmission , brain slice, complex EPSC, high-frequency stimulation, low-frequency stimulation
INTRODUCTION
The substantia nigra zona reticulata (SNR) is a major output nucleus of the basal ganglia that plays important roles in normal and abnormal movements (Parent and Hazrati, 1995). Although SNR neurons generate bursts of action potentials during normal limb movements (Magarinos-Ascone et al., 1992), excessive burst firing correlates with the rigidity and bradykinesia that characterize symptoms of Parkinson’s disease (Bergman et al., 1998; Wichmann et al., 1999). Burst firing in the SNR is triggered by activity in the subthalamonigral pathway that originates in the glutamate-containing neurons of the subthalamic nucleus (STN) (Murer et al., 1997; Ryan and Sanders, 1993). As a consequence, there is much interest in developing strategies that reduce activity in STN outflow pathways for the treatment of Parkinson’s disease.
Deep brain stimulation (DBS) of the STN is now recognized as an effective treatment for advanced Parkinson’s disease (Breit et al., 2004). Because DBS mimics the therapeutic effects of lesioning the STN in animal models of Parkinson’s disease (Bergman et al., 1990), it was widely thought that DBS acts to reduce synaptic transmission in STN outflow pathways. However, several in vivo studies have reported that the output of the STN is increased rather than inhibited by DBS (Galati et al., 2006; Hashimoto et al., 2003). Recently, there is growing support for the hypothesis that the therapeutic effect of DBS derives from its ability to disrupt burst firing rather than change absolute firing rate (Wichmann and DeLong, 2006). However, the actual mechanism by which STN DBS is effective in Parkinson’s disease remains unknown.
Using whole-cell patch recordings in rat brain slices, our previous work showed that a single electrical stimulus delivered to the STN evokes a long-lasting, complex excitatory postsynaptic current (EPSC) in SNR neurons (Shen and Johnson, 2006). The complex EPSC begins with an initial EPSC that has a fixed latency and amplitude that is consistent with monosynaptic transmission. This initial EPSC is followed by a series of late EPSCs with variable latencies that are superimposed on a long-duration inward shift in holding current. The late component of complex EPSCs is abolished when ionotropic glutamate antagonists are applied directly to the STN, which suggests it is generated polysynaptically by activation of recurrent collateral axons in the STN (Shen and Johnson, 2006). The present study was undertaken to characterize the influence of varied frequencies of STN stimulation on complex EPSCs that are mediated by synaptic transmission in the subthalamonigral pathway. Results may have implications for mechanisms that underlie the efficacy of STN DBS used in the treatment of Parkinson’s disease.
METHODS
Tissue preparation
Horizontal slices containing diencephalon and rostral midbrain were prepared from young Sprague-Dawley rats (post natal day 8–21; Harlan, Indianapolis, IN) as described previously (Shen and Johnson, 1997). Rats were euthanized under isoflurane anesthesia in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. The brain was removed rapidly and slices (300 µm) were cut in cold physiological saline with a vibratome. A slice containing the STN and SNR was then placed on a supporting net and submerged in a continuously flowing solution (2 ml/min) of the following composition (in mM): NaCl, 126; KCl, 2.5; CaCl2, 2.4; MgCl2, 1.2; NaH2PO4, 1.2; NaHCO3, 19; glucose, 11, gassed with 95% O2 and 5% CO2 (pH 7.4) at 36°C. Using a dissection microscope for visual guidance, the SNR was located 1–2 mm lateral to the substantia nigra zona compacta, whereas the STN was identified as grey matter ~2.7 mm lateral to the midline and 2 mm rostral to the center of the SNR.
Electrophysiological recordings
Whole-cell recordings were made with borosilicate pipettes with an initial resistance of 4–5 MΩ and series resistance < 30 MΩ. Internal pipette solution contained (in mM): cesium or potassium gluconate, 130; MgCl2, 2; CaCl2, 1; EGTA, 11; HEPES, 10; ATP, 1.5; GTP, 0.3 (pH 7.3). Membrane currents were recorded under voltage clamp −70 mV holding potential, filtered at 5 kHz, and amplified fivefold with an Axo-patch-1D amplifier (Molecular Devices, Foster City, CA). Data were sampled at a rate of 10 kHz and acquired using a personal computer with a Digidata 1320A analog/digital interface and analyzed using pCLAMP 9.0 software (Molecular Devices). Holding currents were recorded continuously using a MacLab analog/digital interface, Chart software (AD Instruments, Castle Hill, Australia) and a Macintosh computer. Membrane potentials have been corrected for the liquid junction potential (10 mV).
Complex EPSCs
As we have described previously (Shen and Johnson, 2006), complex EPSCs were evoked in SNR neurons using a bipolar stimulation electrode (2–4 MΩ impedance at 1000 Hz, 10 nA; Frederick Haer & Co., USA) that was placed directly within the STN. Rectangular pulses (0.1 ms duration) of constant current (20–400 µA) were used to evoke complex EPSCs at a rate of 0.05 Hz. In most cases, we successfully avoided the appearance of inhibitory postsynaptic currents (IPSCs) by repositioning the stimulation electrodes, reversing polarity, or by reducing the stimulation intensity. In some experiments, multiple electrical stimuli (2–5 pulses, 10 ms interval) or superfusion with picrotoxin (100 µM) or bicuculline (30µM) were used to increase the amplitude of complex EPSCs. The late component of complex EPSCs was quantified by measuring the integrated area using pCLAMP 9 software, whereas the early component of complex EPSCs was measured as amplitude (pA) with respect to baseline that immediately preceded the initial EPSC.
Drugs
All drugs were dissolved into aqueous stock solutions that were diluted at least 1:1000 to the desired concentration in superfusate immediately before use. Approximately 30 s were required for the drug solution to enter the recording chamber due to passage of the perfusate through a heat exchanger. CGP35348, (RS)-α-methyl-4-carboxyphenylglycine (MCPG), and 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) were obtained from Tocris Cookson (Ellisville, MO). Bicuculline methiodide, sulpiride, and picrotoxin were obtained from Sigma Chemical Co. (St. Louis, MO).
Data analysis
Numerical data in the text and error bars in figures are expressed as mean ± SEM. Statistical significance was tested using two-way analysis of variance (ANOVA) with repeated measures, followed by a Holm-Sidak pairwise multiple comparison test (SigmaStat, Jandel Scientific, San Rafael, CA).
RESULTS
Early and late components of complex EPSCs
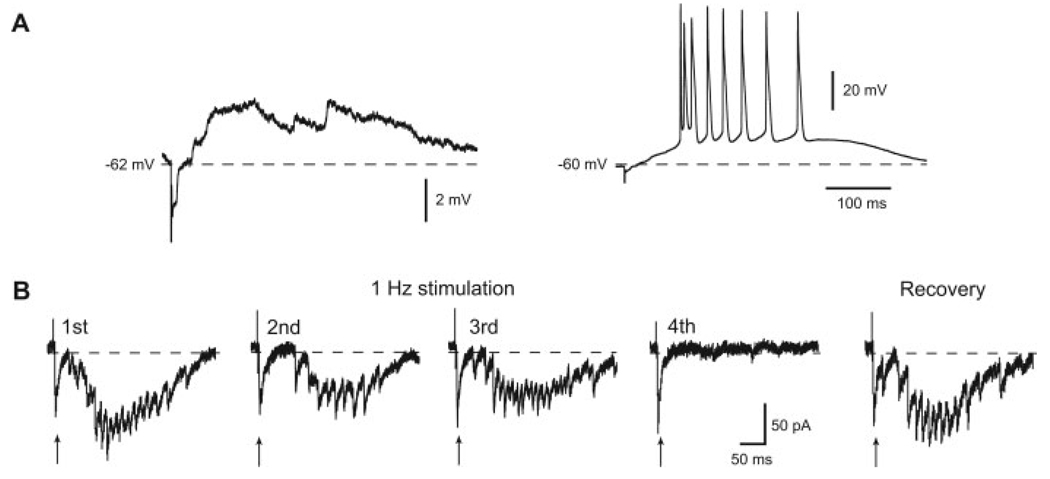
As we have reported previously (Shen and Johnson, 2006), a single electrical stimulus delivered to the STN evokes a long-lasting, complex EPSP when recorded in SNR neurons under current-clamp conditions. When holding current is reduced to allow the cell to fire, STN stimulation evokes a burst of action potentials as shown in Figure 1A. When recording under voltage-clamp, STN stimulation evokes a long-lasting complex EPSC. This response is unique to STN stimulation, because our previous studies showed that stimulating elsewhere in the brain slice failed to evoke complex EPSCs in SNR neurons (Shen and Johnson, 2006). As seen in Figure 1B, the complex EPSC consists of an initial EPSC that is followed by a series of notches and inflections that are superimposed on a slow inward current lasting 200–500 ms. Our earlier work showed that the initial EPSC had a relatively constant latency and amplitude, suggesting monosynaptic transmission. On the other hand, the current inflections in the late component had variable latencies and amplitudes consistent with polysynaptic transmission. When the STN was stimulated at the control rate of once every 20 s, complex EPSCs were evoked reliably with reproducible amounts of current density as measured by the integrated area. However, when the stimulation frequency was increased to 1 Hz, the late component of the complex EPSC was rapidly extinguished, as shown in Figure 1B. In contrast, the initial EPSC easily followed repetitive low-frequency stimulation (LFS) at 1 Hz as would be expected for monosynaptic transmission (Berry and Pentreath, 1976). Although the late component was rapidly extinguished by LFS, the complex EPSC recovered fully 1 min after resuming the control rate of simulation (every 20 s).
Fig. 1. Effects of STN stimulation on synaptic responses recorded in SNR neurons.
A: Current-clamp recording showing that STN stimulation evokes a long-lasting complex EPSP. When holding current is relaxed to allow the cell to fire, STN stimulation evokes a burst of action potentials. B: Recording under voltage-clamp conditions shows that STN stimulation evokes a long-lasting complex EPSC. Repetitive STN stimulation at 1 Hz abolishes the late component of the complex EPSC by the 4th stimulus, whereas the early monosynaptic EPSC remains intact. Arrows indicate the initial, monosynaptic EPSC. The complex EPSC fully recovered 40 sec after the stimulation rate was reduced to 0.05 Hz. Dashed lines in this and subsequent figures indicates 0 pA.
Frequency-and time-dependent effects of STN stimulation on the early component of complex EPSCs
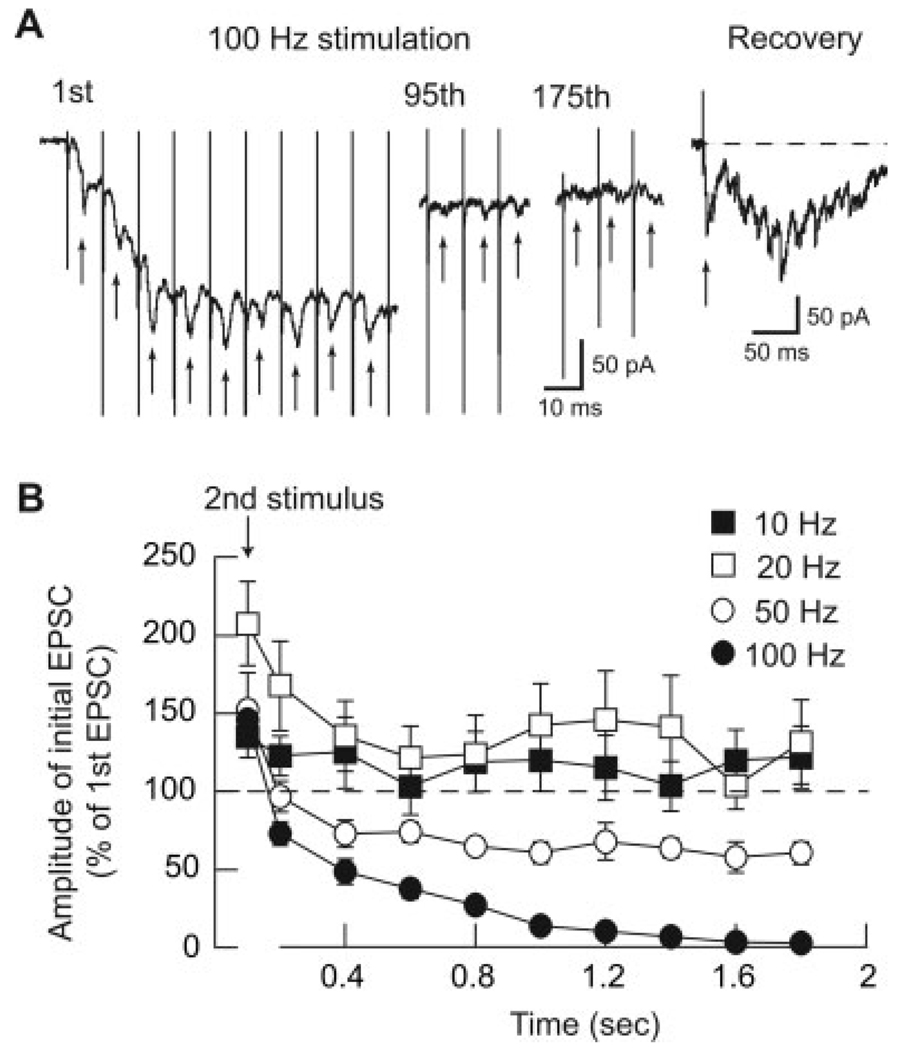
To characterize effects of varied stimulation frequencies, we proceeded to investigate effects of repetitive STN stimulation on the amplitude of the initial, early component of complex EPSCs. An example of the effects of 100 Hz high-frequency stimulation (HFS) on the initial component of a complex EPSC is seen in Figure 2A. Although the early EPSC is able to follow the first 10–20 stimuli at 100 Hz, its amplitude was greatly reduced after 1 s of HFS. Figure 2A also shows that HFS produced a transient inward shift in holding current that was presumably caused by the cumulative summation of long-lasting complex EPSCs. A summary graph for the frequency- and time-dependent effects of repetitive STN stimulation on the early component of complex EPSCs is shown in Figure 2B. Soon after starting repetitive stimulation, there was a transient potentiation of the early component of complex EPSCs that was most pronounced for the 20 Hz stimulation rate (n = 9). However, at stimulation rates of 50 and 100 Hz, there was a progressive reduction in amplitude of initial EPSCs. For example, STN stimulation at 50 Hz reduced the amplitude of initial EPSCs by 35 ± 5% and 39 ± 7% in the middle and at the end of the 2 s train, respectively (P < 0.001; n = 8). Even more dramatically, repetitive stimulation at 100 Hz reduced the amplitude of initial EPSCs by 86 ± 3% and 97 ± 1% in the middle and at the end of the 2-s train, respectively (P < 0.001; n = 6). Stimulation at 100 Hz produced a more significant reduction in amplitude of the initial complex EPSC compared with 50 Hz stimulation (P < 0.05).
Fig. 2. Time-dependent effects of stimulus frequency on amplitude of the initial component of complex EPSCs.
A: HFS at 100 Hz markedly reduces the amplitude of the initial component of complex EPSCs as indicated by arrows. Note that HFS causes an initial shift in holding current that is caused by time-dependent summation of the late components of complex EPSCs. The complex EPSC fully recovers 20 min after cessation of HFS. B: Averaged data showing effects of varied stimulation frequencies on initial components of complex EPSCs. Each data point is the mean ± SEM of 6–9 cells. Note that stimulation at 50 and 100 Hz caused significant reductions in initial EPSC amplitude compared to stimulation at 10 and 20 Hz (P < 0.01). Also note that all stimulation frequencies caused transient increases in amplitudes of initial EPSCs at the beginning of stimulation.
Time-dependent recovery of initial and late components of complex EPSCs from HFS
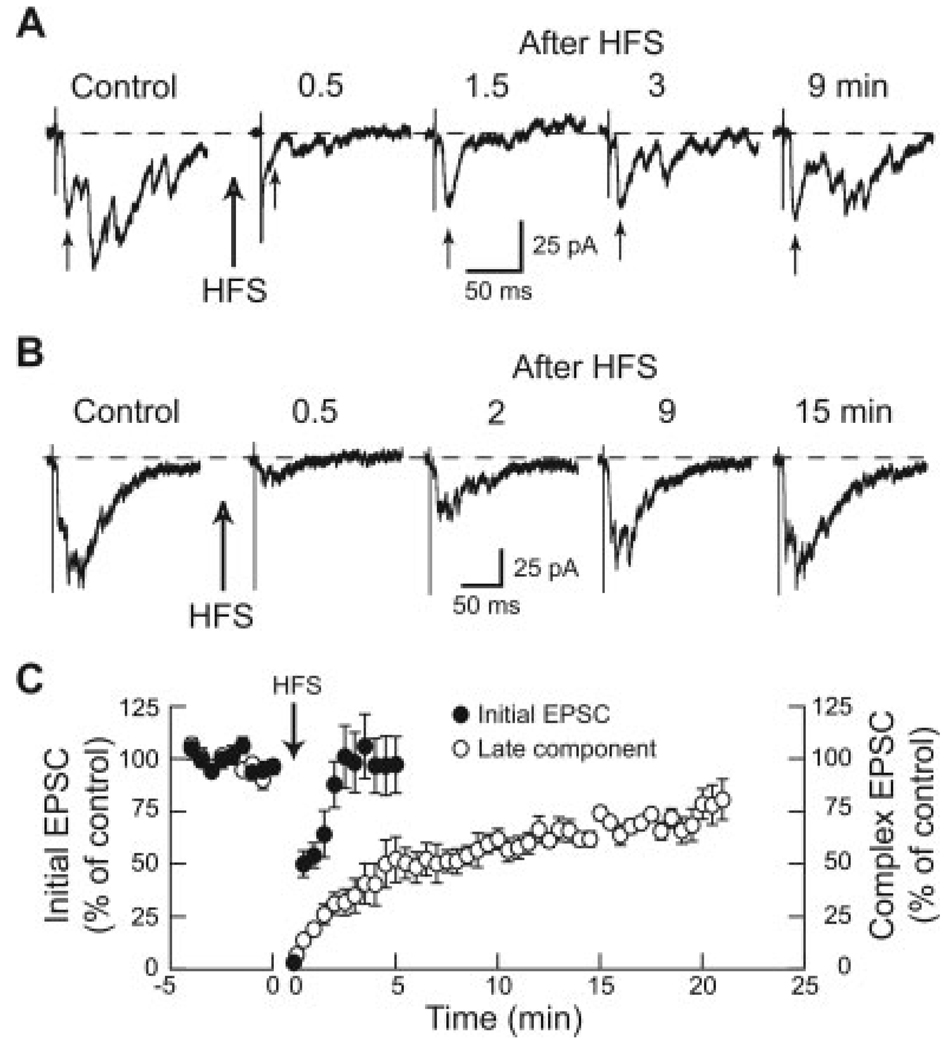
We next examined the time-course for recovery of the complex EPSC following HFS (100 Hz) for 1 min. As seen in Figure 3A, the initial component of the complex EPSC recovered within 2 min of stopping HFS. Summary data (Fig. 3C) show that the average amplitude of the initial EPSC was reduced to 1.3 ± 0.6 pA measured immediately after HFS, from a control amplitude of 42.5 ± 20.3 pA (P < 0.001; n = 8). However, amplitudes increased rapidly to 50 ± 6% of control within 30 s after the termination of HFS, and full recovery of initial EPSCs occurred at 2 min (n = 8). In contrast to the initial component, recovery of the late component of complex EPSCs was much slower, as illustrated in Figure 3B. On average, the integrated area of the late component of complex EPSCs was reduced to 7 ± 1% of control just after the termination of HFS (n = 12). Thereafter, the late component increased to 40 ± 7% of control 3 min after the offset of HFS, and full recovery required more than 20 min (n = 12).
Fig. 3. Early and late components of complex EPSCs recover at different rates following HFS of the STN at 100 Hz for 1 min.
A: Current traces of a complex EPSC that has a clearly recognizable initial EPSC as indicated by small arrows below each trace. Note that the initial EPSC recovers from HFS more quickly than does the late component of the complex EPSC. B: Current traces showing recovery of the late component of a complex EPSC following HFS. In this example, the initial EPSC is not clearly seen. Note that the late component of the complex EPSC requires 15 min to recover fully from HFS. C: Averaged data showing the time courses for recovery of initial (n = 6) and late (n = 12) components of complex EPSCs. Note that the early component recovers within 2 min after stopping HFS, whereas the late component of complex EPSCs requires more than 20 min for full recovery. Initial EPSCs were measured as amplitudes, whereas late components were measured as the integrated area of complex EPSCs.
Blockade of multiple neurotransmitter receptors has no effect on HFS-induced inhibition of complex EPSCs
Multiple subtypes of neurotransmitter receptor have been reported to inhibit glutamate-mediated synaptic transmission in the SNR, including metabotropic glutamate receptors (mGluRs) (Marino et al., 2001), dopamine D2 (Ibañez-Sandoval et al., 2006), GABAB and adenosine A1 receptors (Shen and Johnson, 1997). Therefore, we tested the hypothesis that activation of one or more of these transmitter receptor subtypes may mediate the HFS-induced inhibition of complex EPSCs. HFS was delivered at 100 Hz for 1 min in the presence of either picrotoxin (100 µM) or bicuculline (30 µM) to block GABAA receptors. However, we found that antagonists at group I & II mGluRs (MCPG, 300 µM), dopamine D2 (sulpiride, 10 µM), GABAB (CGP35328, 100 µM), and adenosine A1 receptors (DPCPX, 1 µM) failed to significantly reduce the HFS-induced inhibition of complex EPSCs. Thus, HFS reduced the integrated area of complex EPSCs by 87 ± 5% in MCPG (n = 5), by 88 ± 6% in sulpiride (n = 3), by 91 ± 1% in CGP35348 (n = 6), and by 91 ± 1% in DPCPX (n = 5) as measured 30 s after termination of HFS. These results are not significantly different from the 93 ± 1% reduction in complex EPSCs induced by HFS under control conditions (P > 0.05; n = 12). Consequently, these results suggest that activation of mGluR, dopamine, GABA, or adenosine receptors do not mediate the HFS-induced inhibition of complex EPSCs.
DISCUSSION
Our results show that STN stimulation at 50 or 100 Hz causes rapid failure of the initial, monosynaptic component of complex EPSCs. This demonstrates that HFS of the STN can inhibit synaptic transmission in the subthalamonigral pathway, even though STN neurons are clearly able to fire at frequencies greater than 100 Hz (Hammond et al., 1978). One may speculate that our results might apply also to the use of DBS of the STN in the treatment of Parkinson’s disease. However, conflicting reports in the literature report that STN DBS either drives neuronal activity (Galati et al., 2006; Hashimoto et al., 2003) or inhibits the firing of SNR neurons (Benazzouz et al., 2000; Maurice et al., 2003). Moreover, a study by Windels et al. (2003) has shown that HFS of the STN at rates up to 130 Hz progressively increases glutamate release in the SNR. Thus, controversy remains as to whether DBS of the STN has an excitatory or inhibitory effect on SNR neurons. Our data showing that HFS of the STN exhausts the ability of SNR neurons to generate EPSCs suggest that HFS induces a functional deafferentation of the SNR such as has been described for HFS of afferent inputs to thalamic neurons (Anderson et al., 2004; Iremonger et al., 2006). We would predict that loss of normal afferent input to SNR would alter the firing pattern of SNR neurons, which may be the overriding consequence of STN DBS that benefits patients with Parkinson’s disease (Hashimoto et al., 2003; Maltete et al., 2007).
Compared with the early EPSC, we found that the late component of complex EPSCs was very sensitive to inhibition by low-frequency STN stimulation. Suppression of the complex EPSC is not mediated by stimulation of group I mGluRs, adenosine A1, dopamine D2, or GABAB receptors because we found that antagonists of these receptors failed to prevent stimulation-dependent rundown of complex EPSCs. Although the cause of this synaptic depression is not known, we hypothesize that it may be due to depletion of the readily releasable pool of neurotransmitter vesicles (Dobrunz and Stevens, 1997). Coupled with the fact that polysynaptic pathways are more sensitive to synaptic fatigue than are monosynaptic connections, this may explain the heightened sensitivity of the late component to inhibition by LFS.
Because we have shown that complex EPSCs generate bursts of action potentials when recorded under current-clamp conditions (Shen and Johnson, 2006), one might consider whether or not suppression of complex EPSCs would be expected to improve the parkinsonian symptoms of rigidity and bradykinesia that are known to correlate with burst firing (Bergman et al., 1998; Wichmann et al., 1999). But although excessive burst firing correlates with parkinsonism, it is important to keep in mind that some degree of burst firing is present normally in SNR neurons. For example SNR neurons are known to generate bursts of action potentials during normal limb movements (Magarinos-Ascone et al., 1992). Moreover, it is interesting to note that DBS of the STN at low frequencies (1–10 Hz) has been shown to worsen symptoms of Parkinson’s disease (Moro et al., 2002; Timmermann et al., 2004). Thus, it is possible that selective loss of the late component of complex EPSCs by LFS of the STN interferes with a synaptically-mediated form of burst firing that is important in normal basal ganglia physiology.
In summary, our studies demonstrate that early and late components of complex EPSCs in SNR neurons exhibit differential sensitivities to repetitive STN stimulation. HFS is required to inhibit the early, monosynaptic EPSC, whereas the polysynaptic, late component of complex EPSCs is extinguished by LFS. Therapeutic effects of DBS in the treatment of Parkinson’s disease may be due to HFS-dependent inhibition of excitatory transmission in the subthalamonigral pathway, whereas interference with synaptically mediated burst discharges may mediate the deleterious effects of LFS in Parkinson’s disease patients.
Acknowledgments
Contract grant sponsor: USPHS; Contract grant number: NS 38175; Contract grant sponsor: Portland VA Parkinson’s Disease Research, Education, and Clinical Center.
References
- Anderson T, Hu B, Pittman Q, Kiss ZHT. Mechanisms of deep brain stimulation: An intracellular study in rat thalamus. J Physiol. 2004;559:301–313. doi: 10.1113/jphysiol.2004.064998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benazzouz A, Gao DM, Ni ZG, Piallat B, Bouali-Benazzouz R, Benabid AL. Effect of high-frequency stimulation of the subthalamic nucleus on the neuronal activities of the substantia nigra pars reticulata and ventrolateral nucleus of the thalamus in the rat. Neuroscience. 2000;99:289–295. doi: 10.1016/s0306-4522(00)00199-8. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- Bergman H, Feingold A, Nini A, Raz A, Slovin H, Abeles M, Vaadia E. Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trend Neurosci. 1998;21:32–38. doi: 10.1016/s0166-2236(97)01151-x. [DOI] [PubMed] [Google Scholar]
- Berry MS, Pentreath VW. Criteria for distinguishing between monosynaptic and polysynaptic transmission. Brain Res. 1976;105:1–20. doi: 10.1016/0006-8993(76)90919-7. [DOI] [PubMed] [Google Scholar]
- Breit S, Schulz JB, Benabid AL. Deep brain stimulation. Cell Tissue Res. 2004;318:275–288. doi: 10.1007/s00441-004-0936-0. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Galati S, Mazzone P, Fedele E, Pisani A, Peppe A, Pierantozzi M, Brusa L, Tropepi D, Moschella V, Raiteri M, Stanzione P, Bernardi G, Stefani A. Biochemical and electrophysiological changes of substantia nigra pars reticulata driven by subthalamic stimulation in patients with Parkinson’s disease. Eur J Neurosci. 2006;23:2923–2928. doi: 10.1111/j.1460-9568.2006.04816.x. [DOI] [PubMed] [Google Scholar]
- Hammond C, Deniau JM, Rizk A, Feger J. Electrophysiological demonstration of an excitatory subthalamonigral pathway in the rat. Brain Res. 1978;151:235–244. doi: 10.1016/0006-8993(78)90881-8. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibañez-Sandoval O, Hernández A, Forán B, Galarraga E, Tapia D, Valdiosera R, Erlij D, Aceves J, Bargas J. Control of the subthalamic innervation of substantia nigra pars reticulata by D1 and D2 dopamine receptors. J Neurophysiol. 2006;95:1800–1811. doi: 10.1152/jn.01074.2005. [DOI] [PubMed] [Google Scholar]
- Iremonger KJ, Anderson TR, Hu B, Kiss ZHT. Cellular mechanisms preventing sustained activation of cortex during subcortical high-frequency stimulation. J Neurophysiol. 2006;96:613–621. doi: 10.1152/jn.00105.2006. [DOI] [PubMed] [Google Scholar]
- Magarinos-Ascone C, Buno W, Garcia-Austt E. Activity in monkey substantia nigra neurons related to a simple learned movement. Exp Brain Res. 1992;88:283–291. doi: 10.1007/BF02259103. [DOI] [PubMed] [Google Scholar]
- Maltete D, Jodoin N, Karachi C, Houeto JL, Navarro S, Cornu P, Agid Y, Welter ML. Subthalamic stimulation and neuronal activity in the substantia nigra in Parkinson’s disease. J Neurophysiol. 2007;97:4017–4022. doi: 10.1152/jn.01104.2006. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Wittmann M, Bradley SR, Hubert GW, Smith Y, Conn PJ. Activation of group I metabotropic glutamate receptors produces a direct excitation and disinhibition of GABAergic projection neurons in the substantia nigra pars reticulata. J Neurosci. 2001;21:7001–7012. doi: 10.1523/JNEUROSCI.21-18-07001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice N, Thierry A-M, Glowinski J, Deniau J-M. Spontaneous and evoked activity of substantia nigra pars reticulata neurons during high-frequency stimulation of the subthalamic nucleus. J Neurosci. 2003;23:9929–9936. doi: 10.1523/JNEUROSCI.23-30-09929.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro E, Esselink RJA, Xie J, Hommel M, Benabid AL, Pollak P. The impact on Parkinson's disease of electical parameter settings in STN stimulation. Neurology. 2002;59:706–713. doi: 10.1212/wnl.59.5.706. [DOI] [PubMed] [Google Scholar]
- Murer MG, Riquelme LA, Tseng KY, Pazo JH. Substantia nigra pars reticulata single unit activity in normal and 6-OHDA-lesioned rats: Effects of intrastriatal apomorphine and subthalamic lesions. Synapse. 1997;27:278–293. doi: 10.1002/(SICI)1098-2396(199712)27:4<278::AID-SYN2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati L-N. Functional anatomy of the basal ganglia.II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Rev. 1995;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- Ryan LJ, Sanders DJ. Subthalamic nucleus lesion regularizes firing patterns in globus pallidus and substantia nigra pars reticulate neurons in rats. Brain Res. 1993;626:327–331. doi: 10.1016/0006-8993(93)90596-f. [DOI] [PubMed] [Google Scholar]
- Shen K-Z, Johnson SW. Presynaptic GABAB and adenosine A1 receptors regulate synaptic transmission to rat substantia nigra reticulata neurones. J Physiol. 1997;505:153–163. doi: 10.1111/j.1469-7793.1997.153bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K-Z, Johnson SW. Subthalamic stimulation evokes complex EPSCs in the rat substantia nigra pars reticulata in vitro. J Physiol. 2006;573:697–709. doi: 10.1113/jphysiol.2006.110031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermann L, Wojtecki L, Gross J, Lehrke R, Voges J, Maarouf M, Treuer H, Sturm V, Schnitzler A. Ten-hertz stimulation of subthalamic nucleus deteriorates motor symptoms in Parkinson’s disease. Move Disord. 2004;19:1328–1333. doi: 10.1002/mds.20198. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Deep brain stimulation for neurologic and neuropsychiatric disorders. Neuron. 2006;52:197–204. doi: 10.1016/j.neuron.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, Starr PA, Subramanian T, Watts RL, DeLong MR. Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Exp Brain Res. 1999;125:397–409. doi: 10.1007/s002210050696. [DOI] [PubMed] [Google Scholar]
- Windels F, Bruet N, Poupard A, Feuerstein C, Bertrand A, Savasta M. Influence of the frequency parameter on extracellular glutamate and γ-aminobutyric acid in substantia nigra and globus pallidus during electrical stimulation of subthalamic nucleus in rats. J Neurosci Res. 2003;72:259–267. doi: 10.1002/jnr.10577. [DOI] [PubMed] [Google Scholar]