Abstract
Purpose:
KRAS mutations are found in ~ 25% of lung adenocarcinomas in Western countries and, as a group, have been strongly associated with cigarette smoking. These mutations are predictive of poor prognosis in resected disease as well as resistance to treatment with erlotinib or gefitinib.
Experimental Design:
We determined the frequency and type of KRAS codon 12 and 13 mutations and characterized their association with cigarette smoking history in patients with lung adenocarcinomas.
Results:
KRAS mutational analysis was performed on 482 lung adenocarcinomas, 81 (17%) of which were obtained from patients who had never smoked cigarettes. KRAS mutations were found in 15% (12/81; 95% CI 8%-24%) of tumors from never smokers. Similarly, 22% (69/316; 95% CI 17%-27%) of tumors from former smokers, and 25% (21/85; 95% CI 16%-35%) of tumors from current smokers had KRAS mutations. The frequency of KRAS mutation was not associated with age, gender, or smoking history. The number of pack years of cigarette smoking did not predict an increased likelihood of KRAS mutations. Never smokers were significantly more likely than former or current smokers to have a transition mutation (G→A) rather than the transversion mutations known to be smoking related (G→T or G→C; p<0.0001).
Conclusions:
Based upon our data, KRAS mutations are not rare among never smokers with lung adenocarcinoma and such patients have a distinct KRAS mutation profile. The etiologic and biological heterogeneity of KRAS mutant lung adenocarcinomas is worthy of further study.
Introduction
Since the identification of somatic epidermal growth factor receptor (EGFR) mutations, there has been heightened interest in the molecular basis of lung cancer in patients who never smoked cigarettes (1-3). Somatic mutations in EGFR have been identified in approximately 15% of all patients with lung adenocarcinoma, with the proportion increasing to 50% in patients who never smoked cigarettes. There is an inverse relationship between cigarette smoking history and frequency of EGFR mutations, with the frequency of EGFR mutations decreasing significantly among patients who smoked more than 15 pack years (4). Such refined understanding of the relationship between smoking history and presence of EGFR mutations has allowed the design of clinical trials which use smoking history to enrich the number of patients with somatic EGFR mutations (5-7).
In contrast to EGFR mutations, KRAS mutations were initially identified in patients with lung adenocarcinoma who had a history of heavy cigarette smoking and were thought to be uncommon in patients without a history of smoking cigarettes (8). These mutations are found in ~ 25% of lung adenocarcinomas in western countries but are less common in Asian populations (9, 10). KRAS mutations have been associated with poor prognosis in resected non-small cell lung cancer (NSCLC) (11-13), lack of survival benefit from adjuvant chemotherapy (14), and resistance to erlotinib or gefitinib (15). More than 95% of KRAS mutations in lung cancer occur in codons 12 and 13. In both KRAS and TP53, transversions (substituting a pyrimidine for a purine or purine for a pyrimidine) are more common than transitions (substituting purine for purine or pyrimidine for pyrimidine) identifying a molecular signature for the carcinogenic effects of cigarette smoke (16, 17). A detailed analysis of KRAS mutations in relation to smoking history has not been performed. Using a cohort of patients with lung adenocarcinoma, we sought to determine the frequency and type of KRAS mutations in a large series of patients with known smoking histories.
Materials and Methods
Tumor specimens were obtained from an institutional tumor bank of patients who had undergone non-small cell lung cancer resections between 2002 and 2007 as well as patients with metastatic non-small cell lung cancer who had KRAS mutation testing performed as part of clinical trials or during routine clinical practice. Since KRAS mutations are rare in squamous tumors, only specimens with a histologic diagnosis of lung adenocarcinoma were included. All tumor specimens used for KRAS sequencing had >50% tumor. Specimens were not routinely microdissected. This retrospective review was performed under a waiver of authorization approved by the Memorial Sloan-Kettering Cancer Center Institutional Review Board. Standard direct sequencing was used to identify KRAS codon 12 and 13 mutations in tumors (15).
Patient smoking history was obtained by review of a patient-completed smoking questionnaire and the medical record. The prospectively administered questionnaire contained the following questions: Have you smoked more than 100 cigarettes in your life?; Are you currently smoking?; How many years have you been a regular smoker?; and On average, how many cigarettes did you smoke per day? The smoking questionnaire was administered at the time of the first evaluation by a thoracic surgeon or medical oncologist at this institution. Never smokers had smoked <100 cigarettes. Former smokers had previously smoked cigarettes but quit smoking more than one year prior to diagnosis of lung cancer. Pack years of smoking was defined as [(average number of cigarettes per day/20) × years smoking].
Results
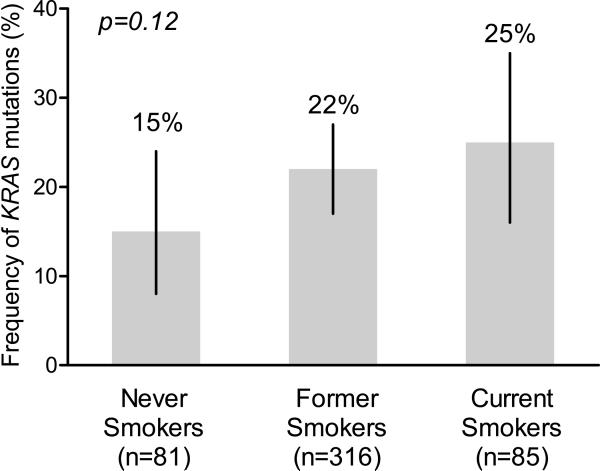
In 482 lung adenocarcinomas, KRAS mutations in codons 12 or 13 were found in 21% (102/482; 95% CI 18%-25%). Patients whose tumors harbored KRAS mutations were not significantly different from patients with KRAS wild type tumors with regard to gender, age or prior smoking history (Table 1). KRAS mutations were identified in 15% (12/81; 95% CI 8%-24%) of never smokers, 22% (69/316; 95% CI 17%-27%) of former smokers, and 25% (21/85; 95% CI 16%-35%) of current smokers (Figure). No tumor with a KRAS mutation had a mutation in EGFR. There were no significant differences in frequency of KRAS mutations by category of smoking history (Mantel-Haenszel Chi-Square p=0.12). We next examined the frequency of KRAS mutation by pack years of cigarette smoking (Table 2). There was no trend in frequency of KRAS mutation by pack years of cigarette smoking (Mantel-Haenszel Chi-Square p=0.19). To determine whether there was a cut point for pack years of cigarette smoking above which KRAS mutations were more frequent, a receiver operating characteristic (ROC) curve was generated. The area under the ROC curve was 0.56 (data not shown) suggesting no value in using pack years of cigarette smoking to predict KRAS mutational status.
Table 1.
KRAS codon 12 and 13 mutations and clinical characteristics
| All | mutant KRAS |
wild type KRAS |
p | |
|---|---|---|---|---|
| Total | 482 | 102 (21%) | 380 (79%) | |
| Men | 197 (41%) | 40 (39%) | 157 (41%) | 0.73* |
| Women | 285 (59%) | 62 (61%) | 223 (59%) | |
| Never smokers | 81 (17%) | 12 (12%) | 69 (18%) | 0.14* |
| Former/current smokers | 401 (83%) | 90 (88%) | 311 (82%) | |
| Age, median (range) | 68 (30-89) | 68 (33-85) | 67 (30-89) | 0.98** |
Fisher's exact test
Wilcoxon rank sum test
Figure.
Frequency of KRAS mutation by smoking history. Mantel-Haenszel Chi-Square test for trend was used to calculate p-value.
Table 2.
Frequency of KRAS mutation by smoking history in pack years (py)
| Mut | wt | Total | Frequency | 95% CI | |
|---|---|---|---|---|---|
| Never Smokers | 12 | 69 | 81 | 15% | 8%-23% |
| <5 py | 3 | 25 | 28 | 11% | 2%-28% |
| 6-10 py | 3 | 25 | 28 | 11% | 2%-28% |
| 11-15 py | 6 | 13 | 19 | 32% | 13%-57% |
| 16-25 py | 10 | 45 | 55 | 18% | 9%-31% |
| 26-50 py | 40 | 106 | 146 | 27% | 20%-35% |
| 51-75 py | 16 | 51 | 67 | 24% | 14%-36% |
| >75 py | 12 | 46 | 58 | 21% | 11%-33% |
| total | 102 | 380 | 482 | 21% | |
To determine whether the type of KRAS mutation identified in never smokers correlated with the previously described dominance of transversions in smoking associated cancers, we compared the type of KRAS mutation found in never smokers to those found in former or current smokers (Table 3). Never smokers were significantly more likely (Fisher's Exact p<0.0001) to have a transition mutation. The ratio of transition:transversion for never smokers was 11:1 as compared with 17:73 for former or current smokers.
Table 3.
KRAS mutation type as a function of smoking history
| KRAS | ||||
|---|---|---|---|---|
| Mutation | Nucleotide | Former/current | Never | Total |
| G12A | GGT➔GCT | 13 | 0 | 13 |
| G12C | GGT➔TGT | 38 | 0 | 38 |
| G12V | GGT➔GTT | 20 | 1 | 21 |
| G13C | GGC➔TGC | 2 | 0 | 2 |
| G13D | GGC➔GAC | 1 | 0 | 1 |
| G12D | GGT➔GAT | 15 | 10 | 25 |
| G12S | GGT➔AGT | 1 | 1 | 2 |
| Total | 90 | 12 | ||
Discussion
In these patients with lung adenocarcinoma, we have demonstrated that KRAS mutations are not rare in never smokers. This is a striking finding given the widespread perception that cigarette smoking and KRAS mutations are invariably linked (reviewed in (16)). The association between cigarette smoking and KRAS mutations has been inferred from a number of series that included a relatively small numbers of patients who never smoked cigarettes. For example, Nelson et al examined tumors from 365 patients with non-small cell lung cancer, of which only 22 were never smokers (18). Among the patients in that series in which KRAS mutational analysis was performed, there were only 16 never smokers, none of whom had KRAS mutations. However, another series which included some never smokers did identify KRAS mutation in 14% (3/21) of never smokers (19). A difference between our series and previous series is the method of collection of smoking history. We determined smoking history using prospectively collected smoking questionnaires completed by patients with a diagnosis of lung cancer. These patients completed a detailed questionnaire which included the age of onset of smoking, the average number of cigarettes per day, the number of years in which they smoked cigarettes, and the time that the patient quit smoking cigarettes. The characteristics of patients included in this analysis are similar to the patient population seen at our institution with regard to age, gender, and smoking history.
The KRAS mutations observed in these never smokers, in addition to being more frequent than previously reported, are more likely to be transitions, unlike the transversions more common in patients with a history of cigarette smoking. In both KRAS and TP53, transversions (substituting a pyrimidine for a purine or purine for a pyrimidine) are more common than transitions (substituting purine for purine or pyrimidine for pyrimidine) (16, 17). The etiology of G→T transversions in tumors from patients with lung cancer is thought to be related to exposure to polycyclic aromatic hydrocarbons found in cigarette smoke (20). In the case of TP53, investigators have recently noted that TP53 G→T transversions were distinctly uncommon in lung adenocarcinomas with EGFR mutations, a mutation more commonly seen in never smokers (21).
Since patients without smoking history represent ~15% of patients with lung cancer, it is critical that any analysis seeking to examine the biology of these tumors examine a relatively large number of patients with NSCLC (22, 23). Relatively little is understood about the biology and epidemiology of lung cancer in never smokers. A number of possible causative factors have been suggested including exposure to environmental tobacco smoke or radon, as well as genetic and hormonal abnormalities (reviewed in(24, 25)). The distinct profile of KRAS mutations observed here in never smokers further suggests that such cancers may not be caused by environmental tobacco exposure. Whether this etiologic heterogeneity within KRAS mutant lung adenocarcinomas is associated with differences in cooperating genetic lesions and overall biologic behavior warrants further investigation. Finally, since tumors from never smokers may have KRAS mutations, and such mutations have been associated with resistance to erlotinib and gefitinib (15), molecular analysis of NSCLC specimens for KRAS mutations may improve clinician's ability to predict response and resistance to therapy with erlotinib or gefitinib.
Footnotes
Statement of Clinical Relevance:
Mutations in the KRAS oncogene are found in about 25% of lung adenocarcinomas in Western countries. Studies have linked KRAS mutations with poor prognosis in non-small cell lung cancer as well as resistance to treatment with erlotinib or gefitinib. These mutations have been reported to be strongly associated with cigarette smoking. However, previous studies which explored association of smoking with KRAS mutation did not include large numbers of patients who never smoked cigarettes. We report that KRAS mutations are found in 15% of lung adenocarcinomas from patients who never smoked cigarettes compared with 22% in patients with a history of smoking cigarettes, a statistically insignificant difference. Furthermore, the frequency of KRAS mutation was not associated with age, gender, or smoking history making it difficult to predict which tumors have KRAS mutations by any clinical characteristics. Based on these data, we believe that molecular testing for KRAS mutations is necessary to identify this subgroup of patients with a different response to some treatments.
References
- 1.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 2.Paez JG, Janne PA, Lee JC, et al. EGFR Mutations in Lung Cancer: Correlation with Clinical Response to Gefitinib Therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 3.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101:13306–11. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pham D, Kris MG, Riely GJ, et al. Use of cigarette-smoking history to estimate the likelihood of mutations in epidermal growth factor receptor gene exons 19 and 21 in lung adenocarcinomas. J Clin Oncol. 2006;24:1700–4. doi: 10.1200/JCO.2005.04.3224. [DOI] [PubMed] [Google Scholar]
- 5.Cappuzzo F, Ligorio C, Janne PA, et al. Prospective study of gefitinib in epidermal growth factor receptor fluorescence in situ hybridization-positive/phospho-Akt-positive or never smoker patients with advanced non-small-cell lung cancer: the ONCOBELL trial. J Clin Oncol. 2007;25:2248–55. doi: 10.1200/JCO.2006.09.4300. [DOI] [PubMed] [Google Scholar]
- 6.Kris MG, Pao W, Zakowski M, et al. Prospective trial with preoperative gefitinib to correlate lung cancer response with EGFR exon 19 and 21 mutations and to select patients for adjuvant therapy. In: Grunberg SM, editor. Am Soc Clin Oncol; 2006 ASCO Annual Meeting; Atlanta, GA. 2006.2006. p. 369s. [Google Scholar]
- 7.Sequist LV, D. M, D. S, et al. iTARGET: A phase II trial to assess the response to gefitinib in epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer (NSCLC) tumors. Journal of Clinical Oncology; 2007 ASCO Annual Meeting; 2007.2007. [Google Scholar]
- 8.Rodenhuis S vdWM, Mooi WJ, et al. Mutational activation of the K-ras oncogene: A possible pathogenetic factor in adenocarcinoma of the lung. N Engl J Med. 1987;317:929–35. doi: 10.1056/NEJM198710083171504. [DOI] [PubMed] [Google Scholar]
- 9.Buttitta F, Barassi F, Fresu G, et al. Mutational analysis of the HER2 gene in lung tumors from Caucasian patients: mutations are mainly present in adenocarcinomas with bronchioloalveolar features. International journal of cancer. 2006;119:2586–91. doi: 10.1002/ijc.22143. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki M, Shigematsu H, Iizasa T, et al. Exclusive mutation in epidermal growth factor receptor gene, HER-2, and KRAS, and synchronous methylation of nonsmall cell lung cancer. Cancer. 2006;106:2200–7. doi: 10.1002/cncr.21853. [DOI] [PubMed] [Google Scholar]
- 11.Graziano SL, Gamble GP, Newman NB, et al. Prognostic significance of K-ras codon 12 mutations in patients with resected stage I and II non-small-cell lung cancer. J Clin Oncol. 1999;17:668–75. doi: 10.1200/JCO.1999.17.2.668. [DOI] [PubMed] [Google Scholar]
- 12.Slebos R, Kibbelaar R, Dalesio O. K-RAS oncogene activation as a prognostic marker in adenocarcinoma of the lung. N Engl J Med. 1990;323:561–5. doi: 10.1056/NEJM199008303230902. [DOI] [PubMed] [Google Scholar]
- 13.Rosell R, Molina F, Moreno I, et al. Mutated K-ras gene analysis in a randomized trial of preoperative chemotherapy plus surgery versus surgery in stage IIIA non-small cell lung cancer. Lung Cancer. 1995;12(Suppl 1):S59–70. doi: 10.1016/0169-5002(95)00421-v. [DOI] [PubMed] [Google Scholar]
- 14.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–97. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 15.Pao W, Wang TY, Riely GJ, et al. KRAS Mutations and Primary Resistance of Lung Adenocarcinomas to Gefitinib or Erlotinib. PLoS Med. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahrendt SA, Decker PA, Alawi EA, et al. Cigarette smoking is strongly associated with mutation of the K-ras gene in patients with primary adenocarcinoma of the lung. Cancer. 2001;92:1525–30. doi: 10.1002/1097-0142(20010915)92:6<1525::aid-cncr1478>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 17.Hainaut P, Pfeifer GP. Patterns of p53 G toT transversions in lung cancer reflect the primary mutagenic signature of DNA-damage by tobacco smoke. Carcinogenesis. 2001;22:367–74. doi: 10.1093/carcin/22.3.367. [DOI] [PubMed] [Google Scholar]
- 18.Nelson HH, Christiani DC, Mark EJ, Wiencke JK, Wain JC, Kelsey KT. Implications and prognostic value of K-ras mutation for early-stage lung cancer in women. J Natl Cancer Inst. 1999;91:2032–8. doi: 10.1093/jnci/91.23.2032. [DOI] [PubMed] [Google Scholar]
- 19.Tsao MS, Aviel-Ronen S, Ding K, et al. Prognostic and predictive importance of p53 and RAS for adjuvant chemotherapy in non small-cell lung cancer. J Clin Oncol. 2007;25:5240–7. doi: 10.1200/JCO.2007.12.6953. [DOI] [PubMed] [Google Scholar]
- 20.Hecht SS. Tobacco smoke carcinogens and lung cancer. Journal of the National Cancer Institute. 1999;91:1194–210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 21.Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64:8919–23. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- 22.Parkin DM, Pisani P, Lopez AD, Masuyer E. At least one in seven cases of cancer is caused by smoking. Global estimates for 1985. International journal of cancer. 1994;59:494–504. doi: 10.1002/ijc.2910590411. [DOI] [PubMed] [Google Scholar]
- 23.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 24.Subramanian J, Govindan R. Lung cancer in never smokers: a review. J Clin Oncol. 2007;25:561–70. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- 25.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nat Rev Cancer. 2007;7:778–90. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]