Abstract
Angiopoietin (Angpt)-1 and angpt-2 are circulating proteins first ascribed opposing roles in embryonic angiogenesis. Both bind the Tie-2 receptor on endothelial cells, but angpt-1 is a Tie-2 agonist, whereas angpt-2 antagonizes Tie-2 signaling. In the developed vasculature, angpt-1 protects against vascular leak, while angpt-2 promotes increased vascular permeability. Since alterations in vascular permeability are common in septic shock, we obtained plasma from critically ill children within 24 h of diagnosis of the systemic inflammatory response syndrome (SIRS, n=20), sepsis (n=20), or septic shock (n=61), as well as 15 healthy controls. Plasma levels of angpt-1 and angpt-2 were measured via a commercially available ELISA. Plasma angpt-2 levels were significantly elevated in children with septic shock when compared to healthy children, as well as critically ill children with either SIRS or sepsis, and circulating angpt-2 levels appeared to correlate with disease severity and outcome. In addition, plasma angpt-1 levels were significantly decreased in critically ill children with septic shock compared to critically ill children with either SIRS or sepsis. Given the contrasting effects of angpt-2 and angpt-1 on the vascular endothelium, these two factors may play an important role in the pathophysiology of septic shock in children, and further studies are warranted.
Introduction
Sepsis and its related syndromes account for significant morbidity and mortality in critically ill patients. Sepsis is the 10th leading cause of death overall and accounts for nearly $17 billion in annual health care expenditures in the United States alone (1–3). Sepsis remains a significant health problem in children as well, accounting for nearly 4,500 deaths and close to $2 billion per year in healthcare expenditures nationwide (4). Shock and subsequent multiple organ dysfunction remain significant risk factors for mortality in these patients (5). For example, multiple organ dysfunction syndrome (MODS) is associated with a mortality rate between 30 to 50% in children with septic shock (6–8).
Septic shock is characterized by a systemic inflammatory response which is accompanied by fever, hypotension, and impaired tissue perfusion and oxygen delivery. The classic cardiovascular response to septic shock is a profound decrease in systemic vascular resistance accompanied by resistance to both endogenous and exogenous vasoactive agents (i.e., vasoplegia). Loss of the normal capillary integrity leading to a net extravasation of fluid out of the vascular space and into the tissues is an important feature as well. Septic shock is therefore characterized by fundamental alterations in the intrinsic function of the microcirculation, which ultimately play an important role in the pathogenesis of MODS and subsequent morbidity and mortality (9–11).
Capillary integrity is tightly regulated and appears to reflect a balance between (i) contractile forces between endothelial cells (EC) that create intercellular gaps and (ii) the adhesive forces between these EC that restrict the formation of these intercellular gaps. An increase in contractile force within EC via phosphorylation of the myosin light chain (MLC) is associated with increased capillary permeability (12, 13). Recently, the circulating vascular growth factor, angiopoetin-2 (angpt-2) was shown to increase capillary permeability via MLC phosphorylation in cultured human microvascular endothelial cells (HMVEC) (14). Collectively, the angiopoietins are a family of endogenously derived vascular growth factors which are necessary for embryonic and postnatal blood vessel formation and stabilization. Two members of the angiopoietin family have been well characterized in humans, namely angiopoietin-1 (angpt-1) and angpt-2, both of which appear to act on the Tie2 (tyrosine kinase with immunoglobulin-like loop and epidermal growth factor homology domains) receptor tyrosine kinase found primarily on EC. Despite both binding the same receptor with equal affinity, angpt-1 and angpt-2 appear to act as an agonist/antagonist pair under most conditions. For example, angpt-1 activates the Tie-2 receptor complex on vascular endothelial cells and appears to promote vessel stability, decrease adhesion molecule expression, and promote endothelial cell survival (15–18), while angpt-2 initially blocks the Tie-2 receptor and destabilizes blood vessels, increases adhesion molecule expression, and potentiates inflammation (15, 19, 20). However, angpt-2 has also been shown to act as a partial Tie-2 agonist under certain conditions (21).
Elevated plasma levels of angpt-2 and soluble Tie-2 receptor have been noted in adults with congestive heart failure (22). Parikh et al (14) recently showed that plasma angpt-2 levels were significantly elevated in adults with sepsis-induced acute lung injury, and this elevation contributed to endothelial injury in human microvascular endothelial cells (HMVEC) ex vivo. Plasma angpt-2 levels also correlated with worsening indices of lung function. Importantly, recombinant angpt-1 reversed these effects in HMVEC ex vivo (14). Collectively, these data suggest that the angpt-2/Tie-2 receptor complex may be an important target in patients with septic shock and MODS. Pre-formed angpt-2 can be released from ECs stimulated by inflammatory ligands, such as TNF-α (23). As such, angpt-2 may serve as an important biomarker of endothelial injury in this population. Accordingly, we hypothesized that critically ill children with septic shock would express high levels of circulating angpt-2 when compared to healthy controls.
Methods
Patient selection
We conducted a prospective analysis of plasma samples obtained following informed consent with approval from the Institutional Review Boards at Children’s Hospital of Pittsburgh and Cincinnati Children’s Hospital Medical Center. Samples were collected within the first 24 hours of admission to the pediatric intensive care unit (PICU). Plasma samples were analyzed prospectively such that the investigators were blinded to all demographic information and annotated clinical data at the time of sample selection. SIRS, sepsis, and septic shock were diagnosed according to the definitions of the American College of Chest Physicians/Society of Critical Care Medicine (24) modified specifically for pediatrics (25).
Each patient was assigned an Organ Failure Index (OFI) score upon admission to the PICU as previously described by Doughty et al (26). Organ failure was scored according to the following criteria: cardiovascular failure, mean arterial blood pressure < 5th percentile for age or requirement for vasopressors or inotropes after appropriate volume resuscitation (excluding dopamine ≤ 5 μg/kg/min); pulmonary failure, PaO2/FiO2 < 300 and requirement for mechanical ventilation; renal failure, urine output < 1 mL/kg/hr for ≥ 8 hrs in children < 30 kg, or plasma creatinine > 1.0 mg/dL (> 88 μmol/L); hematologic failure, prothrombin time and partial thromboplastin time > 1.5 × normal for age, and platelet count < 100,000 × 103 thrombocytes/mm3; hepatic failure, alanine aminotransferase and aspartate aminotransferase > 100 units/L and total plasma bilirubin > 1.0 mg/dL (> 17 μmol/L); central nervous system failure, Glasgow Coma Scale < 12 in the absence of sedation. Organ failure was defined as meeting all criteria for organ dysfunction using the most abnormal values for the first 24 hours of admission to the PICU. One point was given for each failed organ, for a total possible score of 6.
As a control, plasma samples from otherwise healthy children undergoing elective surgical procedures were analyzed prospectively with approval from the Cincinnati Children’s Hospital Medical Center IRB. Blood samples from children in the control group were obtained during the pre-operative visit or immediately prior to surgery. For each group, samples were collected in tubes containing sodium citrate and were centrifuged immediately at 4,000 rpm × 10 minutes in order to separate plasma from the cellular components. Samples were stored in 50 μL aliquots in order to avoid multiple freeze-thaw cycles at −70 °C until later analysis.
Enzyme-linked immunosorbent assay (ELISA)
Angpt-1 and angpt-2 levels were measured using commercially available ELISA assay kits (Quantikine Human Angiopoietin-1 Immunoassay and Quantikine Human Angiopoietin-2 Immunoassay, respectively; R&D Systems, Inc., Minneapolis, MN) using the manufacturer’s protocol.
Statistical analysis
Plasma angpt-1 and angpt-2 levels are expressed as median, interquartile range due the nonparametric nature of the data. Nonparametric tests (Mann-Whitney, Wilcoxon signed rank, Spearman) were used for comparison of groups and correlation analyses as indicated with Bonferroni correction for multiple analyses. A p value less than or equal to 0.05 was considered statistically significant.
Results
Plasma samples from critically ill children with SIRS (n=20), sepsis (n=20), or septic shock (n=61) were analyzed (Table 1). In addition, control samples were obtained from fifteen otherwise healthy, non-critically ill children undergoing elective surgical procedures (median age, 32 months). Blood cultures were positive in 34 children in the septic shock group (55%) (n=17 gram-positive bacteria, n=15 gram-negative bacteria, and 3 fungal), eight children had positive viral serologies, and infection was clinically suspected in the remaining children with negative blood cultures (n=19). Blood cultures were positive in 15 children in the sepsis group (75%) (n=10 gram-positive bacteria, n=4 gram-negative bacteria), 2 children had positive viral serologies, and infection was clinically suspected in the remaining children with negative blood cultures (n=4). The majority of the children in the septic shock group (n=33 or 54%) had chronic illnesses as predisposing factors. For example, 17 children were solid organ transplant recipients, 5 children had undergone hematopoietic stem cell transplant, 7 children had underlying malignancies, and 20 children were receiving corticosteroids at the time of enrollment. Twelve children had neutropenia (defined as an absolute neutrophil count <1,000 cells/mm3). Thirty-two children were thrombocytopenic (platelet count < 100,000 cells/mm3). Ten children did not survive (mortality rate 16%). Our cohort was therefore representative of previously published series involving children with septic shock and multiple organ dysfunction syndrome (MODS) (4, 7, 27).
Table 1.
| HEALTHY CONTROL | SIRS | SEPSIS | SEPTIC SHOCK | |
|---|---|---|---|---|
| Age, months (median, IQR) |
32 (10–100) |
67 (24–94) |
24 (12–83) |
39 (11–112) |
| Gender (M:F) | 9:6 | 13:7 | 9:11 | 30:31 |
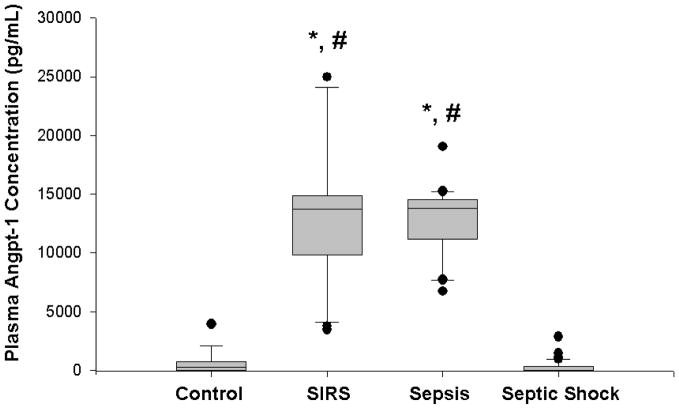
Plasma angpt-1 levels upon admission to the PICU were not significantly different in children with septic shock (median, 15 pg/ml, IQR 15–349 pg/ml) compared to healthy children (median, 285 pg/ml, IQR 15–705 pg/ml; p=NS). However, there was a significant difference in angpt-1 levels in children with SIRS (median, 13,758 pg/mL, IQR 10,198–14,843 pg/mL) compared to both healthy controls and children with septic shock. Similarly, angpt-1 levels were significantly increased in children with sepsis (median, 13,798 pg/mL, IQR 11,197–14,504 pg/mL) compared to both healthy controls and children with septic shock (Figure 1). The difference between children with SIRS and children with sepsis, however, was not significant. Nor were the plasma angpt-1 levels different between those children with septic shock who survived (median, 15 pg/mL, IQR 15–338 pg/mL) compared to those children who did not survive (median, 60 pg/mL, IQR 15–420 pg/mL, p=NS). Finally, because the presence of chronic illness may have adverse effects on the vascular endothelium, we compared plasma angpt-1 levels in critically ill children with septic shock with chronic illness as a predisposing factor. Plasma anpt-1 levels were not significantly different between these two groups (chronic illness: median 15 pg/mL, IQR 15–259 pg/mL; no chronic illness: median 15 pg/mL, IQR 15–383 pg/mL; p=0.8).
Figure 1. Box and whisker plot of plasma angpt-1 levels in healthy children (n=15) versus critically ill children with SIRS (n=20), sepsis (n=20), and septic shock (n=61).
Plasma was obtained within 24 h of diagnosis of SIRS, sepsis, or septic shock. Plasma from the healthy children was obtained during the pre-operative evaluation. * p<0.05 compared to healthy controls; # p<0.05 compared to septic shock.
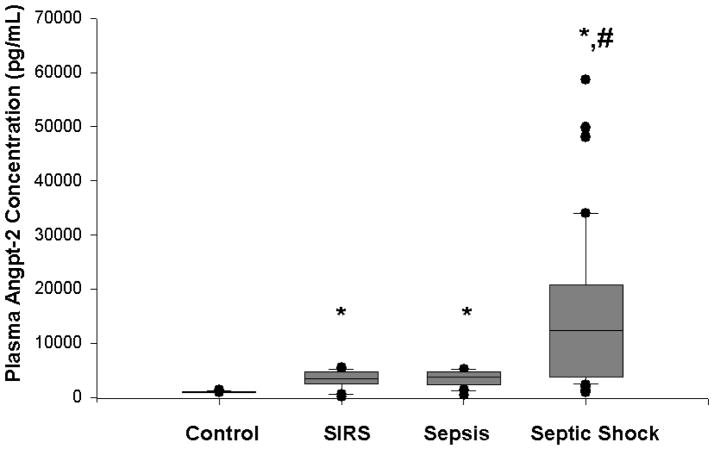
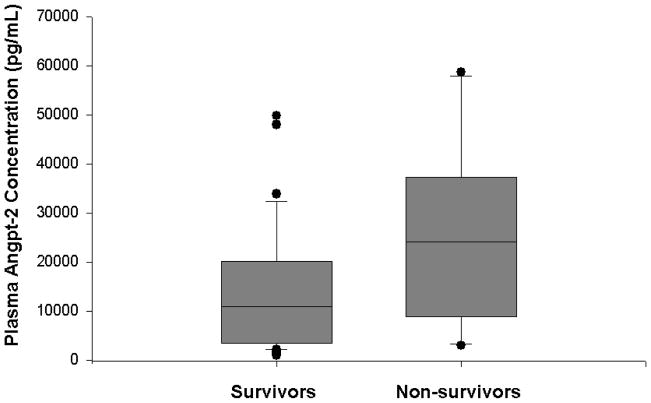
In contrast, plasma angpt-2 levels upon admission to the PICU were significantly increased in children with septic shock (median, 12,400 pg/mL, IQR 3742–20,843 pg/ml) compared to healthy children (median, 1,010 pg/ml, IQR 958–1,092 pg/mL; p<0.001), children with SIRS (median, 3411 pg/mL, IQR 2536–4626 pg/mL; p<0.05), and children with sepsis (median, 3814 pg/mL, IQR 2688–4693 pg/mL; p<0.05) (Figure 2). Plasma angpt-2 levels were significantly increased in children with SIRS or sepsis compared to healthy children, though the difference between children with SIRS and children with sepsis was not significant. There was no significant difference in plasma angpt-2 levels in males compared to females, (10,825 pg/mL vs 6170 pg/mL, p=NS), and there was no correlation between plasma angpt-2 levels and age (r= −0.052, p=NS). Plasma angpt-2 correlated positively with OFI on day 1 (r=0.42, p<0.001). While there was a trend in the difference between plasma angpt-2 levels upon admission to the PICU in non-survivors (median, 24,123 pg/mL, IQR 9565–33,175 pg/mL) compared to survivors (median, 10,850 pg/mL, IQR 3490–19,720 pg/mL), the difference was not statistically significant (p=0.06) (Figure 3). Furthermore, while the plasma angpt-2 levels tended to be higher in critically ill children with septic shock who had chronic illness as a predisposing factor (median 13,175 pg/mL, IQR 7194–33156 pg/mL) compared to those without chronic illness as a predisposing factor (median 9425 pg/mL, IQR 3380–17238 pg/mL), the difference was not significant (p=0.06).
Figure 2. Box and whisker plot of plasma angpt-2 levels in healthy children (n=15) versus critically ill children with SIRS (n=20), sepsis (n=20), and septic shock (n=61).
Plasma was obtained within 24 h of diagnosis of SIRS, sepsis, or septic shock. Plasma from the healthy children was obtained during the pre-operative evaluation. * p<0.05 compared to healthy controls; # p<0.05 compared to SIRS and sepsis.
Figure 3. Box and whisker plot of plasma angpt-2 levels upon admission to the PICU in survivors vs non-survivors with septic shock.
Angpt-2 levels were higher in non-survivors (n=10) compared to survivors, though the difference was not significant (24122 pg/mL vs. 10850 pg/mL, p=0.058).
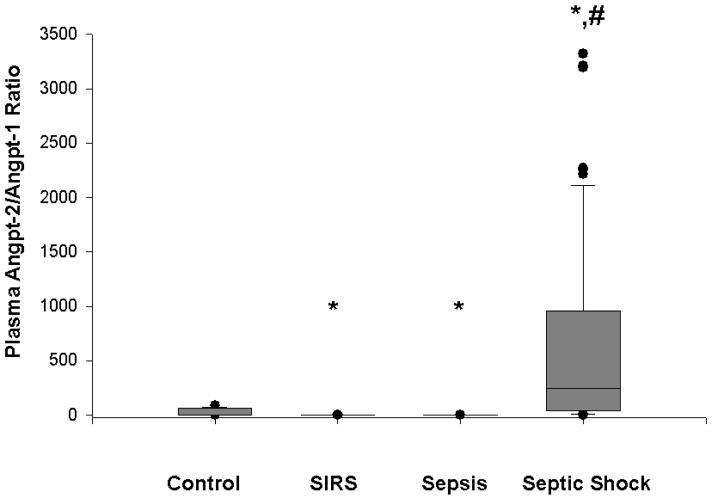
Because of opposing effects at the Tie-2 receptor, it is possible that the ratio of angpt-2/angpt-1 is more important in the regulation of capillary permeability. We determined that the ratio between angpt-2 and angpt-1 ratio was significantly greater in children with septic shock (median, 243, IQR 44–957; p<0.001) compared to healthy children (median 4, IQR 2–63), critically ill children with SIRS (median 0.3, IQR 0.1–0.3; p<0.05), and critically ill children with sepsis (median 0.3, IQR 0.2–0.3; p<0.05) (Figure 4). The angpt-2/angpt-1 ratio was significantly decreased in critically ill children with SIRS or sepsis compared to healthy controls. However, there was no significant difference in the ratio between survivors with septic shock (median, 229, IQR 28–1034) and non-survivors with septic shock (median, 348, IQR 50–553; p=NS). Finally, there were no significant differences in the angpt-2/angpt-1 ration in those critically ill children with septic shock with chronic illness as a predisposing factor (median 261, IQR 44–1175) compared to those without chronic illness (median 221, IQR 41–795).
Figure 4. Box and whisker plot of plasma angpt-2/angpt-1 ratios in healthy children (n=15) versus critically ill children with SIRS (n=20), sepsis (n=20), and septic shock (n=61).
Plasma was obtained within 24 h of diagnosis of SIRS, sepsis, or septic shock. Plasma from the healthy children was obtained during the pre-operative evaluation. * p<0.05 compared to healthy controls. # p<0.05 compared to SIRS and sepsis.
Discussion
Pediatric septic shock and related syndromes continue to account for substantial morbidity and mortality in the United States each year with an estimated 0.56 cases per 1,000 population per year (4). Approximately 10% of children diagnosed with septic shock will die (4). Multiple organ failure remains a significant risk factor for morbidity and mortality in these children (5–8). Recent attention has focused on the alterations in the intrinsic function of the microcirculation in sepsis and MODS (9–11). For example, several studies have suggested that vascular endothelial growth factor (VEGF) plays an important role in the pathophysiology of capillary leak and associated hypotension that is characteristic of septic shock and MODS (28–32).
The angiopoietin family of endogenously derived angiogenic factors may play a similar role in the pathophysiology of capillary leak. For example, plasma angpt-2 levels are significantly elevated in adults with sepsis-induced acute lung injury and appear to correlate with worsening indices of lung function (14). Bhandari et al recently showed that angpt-2 mediates pulmonary capillary leak associated with hyperoxic lung injury (19). Angpt-2 may further exacerbate inflammation and capillary leak through its pro-inflammatory and chemotactic effects (20, 33, 34). In contrast, angpt-1 may have anti-inflammatory and other stabilizing effects on the microcirculation (14, 18, 35–37).
Herein we show that plasma angpt-2 levels are significantly elevated in children with septic shock compared to healthy children, critically ill children with SIRS, and critically ill children with sepsis. Plasma angpt-2 levels correlated with OFI on the first day of admission to the PICU, with a trend towards increasing mortality. Recently, Orfanos et al (38) measured serum angpt-2 levels in adults with severe sepsis and noted a positive linear relationship between angpt-2 and other proinflammatory cytokines. In this study, serum angpt-2 levels correlated with increasing disease severity, as measured by APACHE and SOFA scores, though no correlation between angpt-2 and mortality was observed.
In our study, there were no significant differences in plasma angpt-1 levels in children with septic shock compared to healthy controls. It is interesting to note, however, that plasma angpt-1 levels were significantly increased in critically ill children with both SIRS and sepsis. In the two aforementioned studies (14, 38), plasma angpt-1 was not measured. However, Chong et al. (22) recently noted elevated plasma angpt-2 levels in adults with congestive heart failure (CHF). In these patients, plasma angpt-1 levels were not significantly different compared to healthy controls. These investigators further surmised that a “relative deficiency” of angpt-1 levels was at least partially responsible for the degree of vascular endothelial injury in these patients (22). It is tempting to speculate that septic shock results in a more severe degree of injury to the vascular endothelium, leading to a relative deficiency of the protective angiogenesis factor, angpt-1. The ratio between these two contrasting angiogenesis factors may therefore be more important in determining the fate of vascular endothelial cells rather than the absolute angpt-1 and angpt-2 levels (37). The significant increase in angpt-2/angpt-1 ratios in critically ill children with septic shock compared to critically ill children with SIRS or sepsis supports this line of reasoning. It is interesting to note further that a recent report suggest that cell-based angpt-1 therapy counteracts the vascular inflammation and pulmonary vascular leak in a rat model of experimental acute lung injury (39). Collectively, these data suggest a potential role for angpt-1 therapy in critically ill patients with septic shock.
Several limitations to our experimental approach deserve mention. Given the preliminary nature of our study, we chose to examine a relatively small sample of children with SIRS, sepsis, or septic shock with a relatively limited clinical data set. As such, we only measured plasma angpt-1 and angpt-2 concentrations on the first day of hospitalization in the PICU. Further study will be required to assess whether angpt-1 and angpt-2 levels fluctuate over time and to examine associations with other inflammatory mediators and vascular growth factors during septic shock. In addition, the relatively small number of non-survivors in the current cohort may have prohibited us from being able to discern differences in these angiogenesis factors between survivors and non-survivors. All of these questions remain an active focus in our laboratory, and we are currently planning a larger clinical trial to address these questions.
In conclusion, we show that plasma angpt-2 levels are significantly elevated in children with septic shock when compared to healthy children, as well as critically ill children with either SIRS or sepsis. In addition, plasma angpt-1 levels are significantly decreased in critically ill children with septic shock compared to critically ill children with either SIRS or sepsis. With the previously described role angpt-2 plays in the vascular remodeling process, elevated levels seen in septic children may account for some of the capillary leak phenomena seen in these patients. Further studies are necessary to better define the role of these angiopoietins in the pathophysiology of sepsis.
Acknowledgments
Supported by the National Institute of General Medical Sciences, KO8 GM077432 (DSW), RO1 GM67202 (BZ), and RO1 GM064619 (HRW)
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Anderson RN, Smith BL. Deaths: Leading causes for 2001. Natl Vital Stat Rep. 2003;52:1–85. [PubMed] [Google Scholar]
- 3.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 4.Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167:695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- 5.Han YY, Carcillo JA, Dragootta MA, Bills DM, Watson RS, Westerman ME, Orr RA. Early reversal of pediatric-neonatal septic shock by community physicians is associated with improved outcome. Pediatrics. 2003;112:793–799. doi: 10.1542/peds.112.4.793. [DOI] [PubMed] [Google Scholar]
- 6.Proulx F, Gauthier M, Nadeau D, Lacroix J, Farrell CA. Timing and predictors of death in pediatric patients with multiple organ system failure. Crit Care Med. 1994;22:1025–1031. doi: 10.1097/00003246-199406000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Proulx F, Fayon M, Farrell CA, Lacroix J, Gauthier M. Epidemiology of sepsis and multiple organ dysfunction syndrome in children. Chest. 1996;109:1033–1037. doi: 10.1378/chest.109.4.1033. [DOI] [PubMed] [Google Scholar]
- 8.Leclerc F, Leteurtre S, Duhamel A, Grandbastien B, Proulx F, Martinot A, Gauvin F, Hubert P, Lacroix J. Cumulative influence of organ dysfunctions and septic state on mortality of critically ill children. Am J Respir Crit Care Med. 2005;171:348–353. doi: 10.1164/rccm.200405-630OC. [DOI] [PubMed] [Google Scholar]
- 9.Hinshaw LB. Sepsis/septic shock: Participation of the microcirculation: An abbreviated review. Crit Care Med. 1996;24:1072–1078. doi: 10.1097/00003246-199606000-00031. [DOI] [PubMed] [Google Scholar]
- 10.Lush CW, Kvietys PR. Microvascular dysfunction in sepsis. Microcirculation. 2000;7:83–101. doi: 10.1038/sj.mn.7300096. [DOI] [PubMed] [Google Scholar]
- 11.Spronk PE, Zandstra DF, Ince C. Bench-to-bedside review: Sepsis is a disease of the microcirculation. Crit Care. 2004;8:462–468. doi: 10.1186/cc2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia JG, Davis HW, Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: Role of myosin light chain phosphorylation. J Cell Physiol. 1995;163:510–522. doi: 10.1002/jcp.1041630311. [DOI] [PubMed] [Google Scholar]
- 13.Verin AD, Patterson CE, Day MA, Garcia JG. Regulation of endothelial cell gap formation and barrier function by myosin-associated phosphatase activities. Am J Physiol. 1995;269:L99–L108. doi: 10.1152/ajplung.1995.269.1.L99. [DOI] [PubMed] [Google Scholar]
- 14.Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, Sukhatme VP. Excess Circulating Angiopoietin-2 May Contribute to Pulmonary Vascular Leak in Sepsis in Humans. PLoS Med. 2006;3:e46. doi: 10.1371/journal.pmed.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 16.Papapetropoulos A, Garcia-Cardena G, Dengler TJ, Maisonpierre PC, Yancopoulos GD, Sessa WC. Direct actions of angiopoietin-1 on human endothelium: Evidence for network stabilization, cell survival, and interaction with other angiogenic factors. Lab Invest. 1999;79:213–223. [PubMed] [Google Scholar]
- 17.Kim I, Moon SO, Park SK, Chae SW, Koh GY. Angiopoietin-1 reduces VEGF-stimulated leukocyte adhesion to endothelial cells by reducing ICAM-1, VCAM-1, and E-selection expression. Circ Res. 2001;89:477–479. doi: 10.1161/hh1801.097034. [DOI] [PubMed] [Google Scholar]
- 18.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–3. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 19.Bhandari V, Choo-Wing R, Lee CG, Zhu Z, Nedrelow JH, Chupp GL, Zhang X, Matthay MA, Ware LB, Homer RJ, Lee PJ, Geick A, de Fougerolles AR, Elias JA. Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nat Med. 2006;12:1286–1293. doi: 10.1038/nm1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, Sobke A, Herrmann M, Preissner K, Vajkoczy P, Augustin HG. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12:235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 21.Kim I, Kim JH, Moon SO, Kwak HJ, Kim NG, Koh GY. Angiopoietin-2 at high concentration can enhance endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Oncogene. 2000;19:4549–4552. doi: 10.1038/sj.onc.1203800. [DOI] [PubMed] [Google Scholar]
- 22.Chong AY, Caine GJ, Freestone B, Blann AD, Lip GY. Plasma angiopoietin-1, angiopoietin-2, and angiopoietin receptor tie-2 levels in congestive heart failure. J Am Coll Cardiol. 2004;43:423–428. doi: 10.1016/j.jacc.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 23.Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103:4150–4156. doi: 10.1182/blood-2003-10-3685. [DOI] [PubMed] [Google Scholar]
- 24.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 25.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 26.Doughty LA, Kaplan SS, Carcillo JA. Inflammatory cytokine and nitric oxide responses in pediatric sepsis and organ failure. Crit Care Med. 1996;24:1137–1143. doi: 10.1097/00003246-199607000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson JD, Pollack MM, Ruttimann UE, Glass NL, Yeh TS. Outcome of pediatric patients with multiple organ system failure. Crit Care Med. 1986;14:271–274. doi: 10.1097/00003246-198604000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Mura M, dos Santos CC, Stewart D, Liu M. Vascular endothelial growth factor and related molecules in acute lung injury. J Appl Physiol. 2004;97:1605–1617. doi: 10.1152/japplphysiol.00202.2004. [DOI] [PubMed] [Google Scholar]
- 29.Nolan A, Weiden MD, Thurston G, Gold JA. Vascular endothelial growth factor blockade reduces plasma cytokines in a murine model of polymicrobial sepsis. Inflammation. 2004;28:271–277. doi: 10.1007/s10753-004-6050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Flier M, van Leeuwen HJ, van Kessel KP, Kimpen JL, Hoepelman AI, Geelen SP. Plasma vascular endothelial growth factor in severe sepsis. Shock. 2005;23:35–38. doi: 10.1097/01.shk.0000150728.91155.41. [DOI] [PubMed] [Google Scholar]
- 31.Pickkers P, Sprong T, van Eijk L, van der Hoeven H, Smits P, van Deuren M. Vascular endothelial growth factor is increased during the first 48 hours of human septic shock and correlates with vascular permeability. Shock. 2005;24:508–512. doi: 10.1097/01.shk.0000190827.36406.6e. [DOI] [PubMed] [Google Scholar]
- 32.Yano K, Liaw PC, Mullington JM, Shih S-C, Okada H, Bodyak N, Kang PM, Toltl L, Belikoff B, Buras J, Simms BT, Mizgerd JP, Carmeliet P, Karumanchi SA, Aird WA. Vascular endothelial growth factor is an important determinant of sepsis morbididty and mortality. J Exp Med. 2006;203:1447–1458. doi: 10.1084/jem.20060375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roviezzo F, Tsigkos S, Kotanidou A, Bucci M, Brancaleone V, Cirino G, Papapetropoulos A. Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Therap. 2005;314:738–744. doi: 10.1124/jpet.105.086553. [DOI] [PubMed] [Google Scholar]
- 34.Sturn DH, Feistritzer C, Mosheimer BA, Djanani A, Bijuklic K, Patsch JR, Wiedermann CJ. Angiopoietin affects neutrophil migration. Microcirculation. 2005;12:393–403. doi: 10.1080/10739680590960296. [DOI] [PubMed] [Google Scholar]
- 35.Witzenbichler B, Westermann D, Knueppel S, Schultheiss HP, Tschope C. Protective role of angiopoietin-1 in endotoxic shock. Circulation. 2005;111:97–105. doi: 10.1161/01.CIR.0000151287.08202.8E. [DOI] [PubMed] [Google Scholar]
- 36.Hall E, Brookes ZL. Angiopoietin-1 increases arteriolar vasoconstriction to phenylephrine during sepsis. Regul Pept. 2005;131:34–7. doi: 10.1016/j.regpep.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Brindle NPJ, Saharinen P, Alitalo K. Signaling and functions of angiopoietin-1 in vascular protection. Circ Res. 2006;98:1014–1023. doi: 10.1161/01.RES.0000218275.54089.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orfanos SE, Kotanidou A, Glynos C, Athanasiou C, Tsigkos S, Dimopoulou I, Sotiropoulou C, Zakynthinos S, Armaganidis A, Papapetropoulos A, Roussos C. Angiopoietin-2 is increased in severe sepsis: correlation with inflammatory mediators. Crit Care Med. 2007;35:199–206. doi: 10.1097/01.CCM.0000251640.77679.D7. [DOI] [PubMed] [Google Scholar]
- 39.MCCarter SD, Mei SH, Lai PF, Zhang Q, Parker CH, Suen RS, Hood RD, Zhao YD, Deng Y, Han RN, Dumont DJ, Stewart DJ. Cell-based angiopoietin-1 gene therapy for acute lung injury. [Accessed April 30, 2007];Am J Respir Crit Care Med. doi: 10.1164/rccm.200609-1370OC. Epub ahead of print: http://ajrccm.atsjournals.org/cgi/reprint/200609-1370OCv1. [DOI] [PubMed]