Abstract
In acute promyelocytic leukemia (APL), the translocation t(15;17) induces a block at the promyelocytic stage of differentiation in an all-trans-retinoic acid (ATRA)-responsive manner. Here we report that upon treatment with ATRA, t(15;17) cells (NB4) reveal a very rapid increase in protein level and binding activity of C/EBPβ, a C/EBP family member, which was not observed in an ATRA-resistant NB4 cell line. We further provide evidence that ATRA mediates a direct increase of C/EBPβ, only in PML–RARA (promyelocytic leukemia–retinoic acid receptor α)-expressing cells. In addition, transactivation experiments indicate that the PML–RARA fusion protein, but not PML–RARA mutants defective in transactivation, strongly transactivates the C/EBPβ promoter. These results suggest that PML–RARA mediates ATRA-induced C/EBPβ expression. Finally, we demonstrate the importance of C/EBPβ in granulocytic differentiation. We show that not only does C/EBPβ induce granulocytic differentiation of non-APL myeloid cell lines independent of addition of ATRA or other cytokines, but also that C/EBPβ induction is required during ATRA-induced differentiation of APL cells. Taken together, C/EBPβ is an ATRA-dependent PML–RARA target gene involved in ATRA-induced differentiation of APL cells.
Keywords: APL/C/EBP/granulopoiesis/PML–RARA/retinoic acid
Introduction
Acute myeloid leukemias are often associated with specific chromosomal translocations that rearrange genes important for the commitment and the differentiation of hematopoietic cells. The t(15;17) translocation found in acute promyelocytic leukemia (APL) juxtaposes the promyelocytic (PML) gene to the retinoic acid receptor α (RARA) gene (Borrow et al., 1990; de The et al., 1990). It is believed that the aberrant PML–RARA fusion protein resulting from the translocation plays a role in the accumulation of the blasts arrested at the promyelocytic stage. This hypothesis is supported by studies of the expression of PML–RARA in transgenic mice, resulting in the development of leukemia with striking APL features (Brown et al., 1997; Grisolano et al., 1997; He et al., 1997). The interesting feature of the PML–RARA-bearing blasts is that they are acutely sensitive to therapeutic doses of all-trans-retinoic acid (ATRA) which is used effectively for the treatment of t(15;17) patients (Castaigne et al., 1990; for a review see Mistry et al., 2003). However, the precise mechanism by which the PML–RARA fusion protein blocks the cells at the promyelocytic stage, and how retinoic acid (RA) reverses this block is still unclear.
It is believed that PML–RARA acts as a dominant-negative factor on both the RARA and PML pathways (de The et al., 1991; Kakizuka et al., 1991). It has been shown that PML–RARA retains most functional domains of the RARA protein and behaves as an abnormal RA receptor with altered transactivation properties, suggesting a defect in the RA pathway to be responsible for the promyelocytic block. This has been strongly supported by experiments showing that PML–RARA recruits co-repressors and histone deacetylase that cannot be dissociated with physiological doses of RA, thus inhibiting RA-induced transcriptional activation (Grignani et al., 1998; He et al., 1998; Lin et al., 1998). However, recently it has become evident that disruption in the RA signaling pathway does not afford an explanation as to how the cells become specifically blocked at the promyelocytic stage since granulocyte lineage commitment is not impaired in RARA and retinoic acid receptor γ (RARG)-deficient mice (Kastner et al., 2001).
The sensitivity of promyelocytes in APL to ATRA remains an enigma. One explanation is that PML–RARA not only participates in the block of cell differentiation, but is also involved in the response of cells to ATRA. PML–RARA has been shown to homodimerize and act as a potent transcriptional repressor, acquiring an oncogenic potential which is reversible upon ATRA treatment (Lin and Evans, 2000; Minucci et al., 2000). Moreover, PML–RARA has been involved in the regulation of genes during APL differentiation (Park et al., 1999; Moog-Lutz et al., 2001). In addition, ATRA resistance, a major problem in ATRA treatment, is mainly associated with defects in the RARA E-domain of PML–RARA (Imaizumi et al., 1998; Duprez et al., 2000). These findings highlight the role of the fusion protein in mediating the sensitivity of APL cells to ATRA.
In this work, we tested the hypothesis that ATRA could target members of the leucine zipper transcription factor family, the CCAAT/enhancer-binding protein (C/EBP) family, during APL differentiation. These transcription factors are implicated in the growth or the differentiation of several tissues or cell lineages including the myeloid lineage (for a review see Lekstrom-Himes and Xanthopoulos, 1998). In myeloid development, the effect of the C/EBP family on cell proliferation and differentiation is mediated by their ability to bind DNA (often containing the sequence CCAAT) and control gene expression (reviewed in Tenen et al., 1997), although recent studies provide evidence that these factors also regulate cell fate by protein–protein interaction, independent of their transcriptional activity (reviewed in McKnight, 2001). Three members of this family, C/EBPα, C/EBPβ and C/EBPε have been shown to be involved at different stages of myeloid development. C/EBPα plays a critical role for the engagement of the cells to the granulocytic lineage (Zhang et al., 1997; Radomska et al., 1998), while C/EBPε is important late in the granulocytic differentiation process (Yamanaka et al., 1997). However, the role of C/EBPβ is less clear. Although C/EBPβ knockout mice do not show any defects in myeloid differentiation, recent reports show that C/EBPβ can drive immature cells into granulocytes (Popernack et al., 2001; Iwama et al., 2002). The apparent lack of any myeloid defects in C/EBPβ mice may suggest that overlapping expression of C/EBP members provides functional redundancy among the different members (Hu et al., 1998).
Here, we report that ATRA targets C/EBPβ during APL differentiation. We show that ATRA increases C/EBPβ activity and we demonstrate that this activation is due to an increase in C/EBPβ RNA and protein levels and is one of the earliest events following ATRA treatment. We show that PML–RARA mediates ATRA-induced upregulation of C/EBPβ through the C/EBPβ proximal promoter and that ATRA resistance of APL cells correlates with the loss of C/EBPβ induction. ATRA-independent expression of C/EBPβ can drive multipotent hematopoietic cells to granulocytic differentiation. Inhibition of C/EBPβ, obtained after using a dominant-negative C/EBP or using small interfering RNA (siRNA) against C/EBPβ, reduces ATRA-induced maturation of APL cells. Therefore, we propose that C/EBPβ is a major target of PML–RARA during APL differentiation.
Results
An increase in C/EBP binding activity is an early event in APL cells in response to ATRA
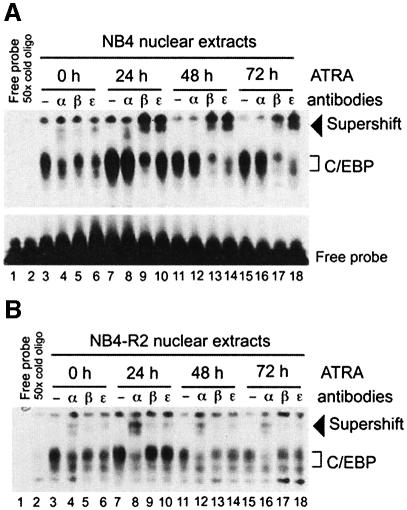
Because of the known role of C/EBP proteins in mediating granulocytic differentiation, we investigated C/EBP activity during ATRA-induced APL differentiation. Electrophoretic mobility shift assays (EMSAs) were performed using a C/EBP-binding site probe from the human granulocyte colony-stimulating factor (G-CSF) receptor promoter (Smith et al., 1996) with nuclear extract from APL ATRA-sensitive NB4 cells. Consistent with previous findings (Lutz et al., 2001), C/EBP binding was detected in NB4 cells (Figure 1A, lane 3). Addition of specific antibody against either C/EBPα, C/EBPβ or C/EBPε resulted for each family member tested in a partial supershift of the protein–oligonucleotide complex (Figure 1A, lanes 4–6), indicating that these three CEBP proteins were part of the DNA–protein complex initially observed. EMSA of nuclear extract from NB4 cells after ATRA stimulation revealed substantial modification in the DNA binding profile of C/EBP proteins. Levels of C/EBPα binding did not change within 24 h of ATRA treatment (Figure 1A, lane 8), and disappeared after 48 h (Figure 1A, lanes 12 and 16). In contrast, we observed an increase in C/EBPβ binding after 24 h of ATRA treatment (Figure 1A, lanes 9 and 13) as well as an increase of C/EBPε binding (Figure 1A, lanes 10, 14 and 18). The C/EBP DNA binding profile was not modified upon ATRA treatment of NB4-R2 cells, an NB4 subclone which is resistant to ATRA treatment (Duprez et al., 2000) (Figure 1B, lanes 3–18). These data demonstrate that the activity of different C/EBP proteins is modulated upon ATRA treatment and suggest that there is a correlation between ATRA-induced C/EBP binding modification and ATRA-induced APL differentiation.
Fig. 1. ATRA induces changes in the C/EBP DNA binding profile during promyelocytic differentiation. EMSA analysis of nuclear extracts from NB4 (A) and NB4-R2 cells (B) treated with ATRA. A double-stranded oligonucleotide including the C/EBP-binding site from the G-CSF receptor was radiolabeled with 32P and incubated in the absence (free probe; lane 1) or presence of 10 µg of nuclear extracts from cells which were untreated (lanes 3–6), or treated for 24 (lanes 7–10), 48 (lanes 11–14) and 72 h (lanes 15–18) with ATRA. For competition analysis, a 50-fold molar excess of unlabeled oligonucleotide was used (lane 2). Antisera against C/EBPα (α), C/EBPβ (β) or C/EBPε (ε) were added to the binding reaction. Supershifted complexes are indicated with an arrow. Free probe was present in excess in each lane (shown for A only, but similar results apply to the EMSA shown in B and Figure 2).
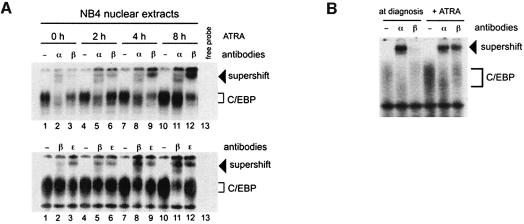
C/EBPε has been described as a target gene responsible for ATRA-induced differentiation (Park et al., 1999). We found an increase in the binding activity of both C/EBPβ and C/EBPε upon ATRA-mediated differentiation. Thus, we next sought to determine the temporal sequence of the two events by studying the binding activity of C/EBPs following a short exposure of NB4 cells to ATRA. We found that C/EBPα binding activity was not modified during 8 h of ATRA treatment, whereas C/EBPβ activity was stimulated as early as 2 h following ATRA treatment (supershifted bands, Figure 2A) and C/EBPε required at least 8 h to display an increase in binding activity (Figure 2A). Thus, C/EBPβ binding activity preceded that of C/EBPε. Bone marrow cells from an APL patient were subjected to EMSA, and we demonstrated that in vitro treatment with ATRA for 15 h increased C/EBPβ binding in the APL sample, resembling that seen in the NB4 cell line (Figure 2B). Our results thus implicate C/EBPβ as a new RA target in ATRA-induced APL differentiation.
Fig. 2. C/EBPβ DNA binding activity is rapidly enhanced during ATRA-mediated differentiation of APL cells. (A) NB4 cells were treated with 1 µM ATRA for 2 (lanes 4–6), 4 (lanes 7–9) and 8 h (lanes 10–12). EMSAs were performed using the same conditions as in Figure 1. DNA–C/EBP complexes were characterized by supershift experiments using antisera against C/EBPα (α), C/EBPβ (β) or C/EBPε (ε). (B) C/EBPα and C/EBPβ binding profile in primary APL cells. Untreated cells and cells treated for 15 h with 1 µM ATRA were subjected to EMSA analysis using the same conditions as in (A).
ATRA regulates C/EBPβ expression through PML–RARA
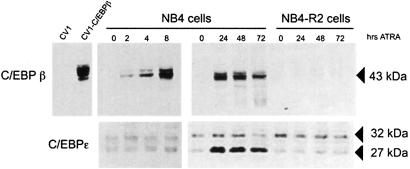
Post-transcriptional modification of C/EBPβ can enhance C/EBPβ transcriptional activity by enhanced DNA binding (Poli et al., 1990). Thus, we were interested in investigating the mechanism by which ATRA enhances C/EBPβ binding. We used a C/EBPβ-specific antibody to analyze the relative protein level in nuclear extracts from cells treated for varying lengths of time with ATRA. In untreated NB4 cells, we were unable to detect the C/EBPβ protein by immunoblotting (Figure 3, upper panel), although the more sensitive EMSA revealed the presence of the protein (Figure 1). Nonetheless, expression of C/EBPβ protein was markedly increased following ATRA treatment. The augmentation in relative abundance of C/EBPβ protein following ATRA treatment (Figure 3, upper panel) correlated with the kinetics observed for the increase in DNA binding activity (Figure 1). Moreover, no change in C/EBPβ protein level was observed in the resistant cell line NB4-R2 (Figure 3, upper panel). However, analyses of the same extracts using a C/EBPε-specific antibody failed to detect any significant changes in expression level of C/EBPε isoforms (32 and 27 kDa) after a short exposure of the cells to ATRA. In agreement with previously published data (Chih et al., 1997), the smaller, 27 kDa isoform increased in abundance after longer exposure of ATRA (Figure 3, lower panel). Therefore, we conclude that ATRA activates C/EBPβ activity by increasing the nuclear level of the protein and that this effect precedes C/EBPε upregulation.
Fig. 3. The increase in C/EBPβ protein after ATRA treatment reflects the increase in DNA binding activity. Nuclear extracts from ATRA-sensitive (NB4) and ATRA-resistant (NB4-R2) cells were tested for C/EBPβ and C/EBPε expression by western blotting following ATRA treatment. The membranes were incubated with antiserum against C/EBPβ (upper panel) and then stripped and incubated with an antiserum against C/EBPε (lower panel). The arrows designate the bands corresponding to the 43 kDa C/EBPβ isoform, as well as the 32 and 27 kDa C/EBPε isoforms. On the left of the figure, specificity of the C/EBPβ antibody is shown using extracts from untransfected CV1cells and from CV1 cells transfected with a human C/EBPβ cDNA.
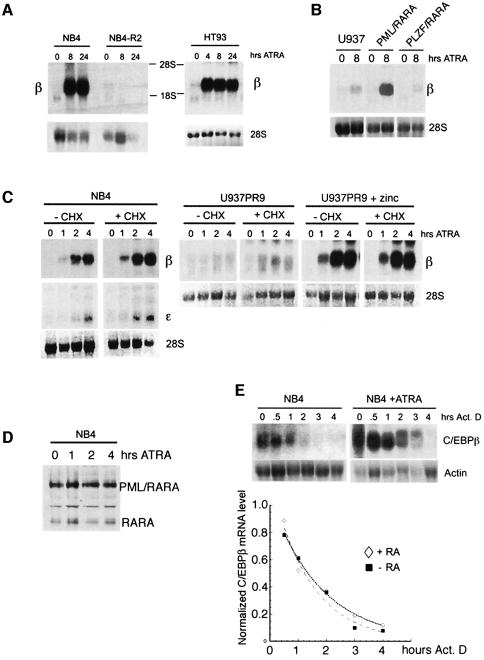
To analyze further the mechanisms involved in ATRA-induced C/EBPβ upregulation, we studied the expression of C/EBPβ RNA. In agreement with the increase in protein levels, C/EBPβ RNA expression was increased following exposure of the NB4 cells to ATRA, while no induction was observed in the NB4-R2 resistant cells (Figure 4A). In addition, we also observed the same pattern of ATRA-induced C/EBPβ induction in another RA-sensitive APL cell line, HT93 (Figure 4A), indicating that this effect is not an artifact of the NB4 cell system. To study the APL-specific nature of C/EBPβ induction by ATRA, we took advantage of the U937-derived cell lines, U937-PR9 and U937-B412 which contain respectively a zinc-inducible PML–RARA and PLZF–RARA (Ruthardt et al., 1997). Using this system, we demonstrated that the level of C/EBPβ mRNA expression was strongly enhanced with ATRA in PML–RARA-expressing cells (Figure 4B). This enhanced expression required the presence of the PML–RARA fusion protein, since C/EBPβ remained only a little responsive to ATRA in U937-PR9 cells that were not pre-treated with zinc. More interestingly, induction of the fusion protein PLZF–RARA that is associated with an ATRA-resistant form of APL has no effect on C/EBPβ expression after ATRA treatment (Figure 4B). Treatment of NB4 cells for short periods of time revealed that the increase in C/EBPβ RNA occurred after only 1 h. The expression profile of C/EBPβ RNA following ATRA treatment was not perturbed by pre-treating the cells with cycloheximide, indicating that de novo protein synthesis is not required for ATRA to exert its effect (Figure 4C, left panel). The same short ATRA treatment did not modify C/EBPβ RNA in myeloid U937-PR9 cells in which PML–RARA was not expressed (Figure 4C, middle panel) but strongly enhanced C/EBPβ RNA in the same cells pre-treated with zinc, similar to that observed in NB4 cells (Figure 4C, right panel). These observations indicate that the direct induction of C/EBPβ mRNA by ATRA is PML–RARA specific. Given the literature showing that prolonged ATRA treatment results in PML–RARA degradation, the presence of PML–RARA was checked in the nuclear extract of NB4 cells after a short time of ATRA stimulation (Figure 4D). For the 4 h treatment time tested, we observe no degradation of the PML–RARA fusion protein. Taken together, our data demonstrate that ATRA directly regulates C/EBPβ expression and that this regulation requires the presence of the PML–RARA fusion protein.
Fig. 4. The presence of PML–RARA is required to rapidly induce C/EBPβ RNA. (A) Northern blot analysis of total RNA isolated from NB4, NB4-R2 and HT93 cells untreated or treated with 1 µM ATRA for the indicated time. The 28S rRNA level is shown as a loading control. (B) Comparison by northern blot of C/EBPβ induction after ATRA treatment between PML–RARA- and PLZF–RARA-expressing cells. PML–RARA and PLZF–RARA were induced respectively by pre-treating the U937-PR9 cells or the U937-B412 cells with zinc for 15 h. (C) Northern blot analysis of total RNA from NB4, U937-PR9 or zinc-pre-treated U937-PR9 cells untreated or treated with 1 µM ATRA for 1, 2 or 4 h in the absence (–) or presence (+) of cycloheximide (CHX). The 28S rRNA level is shown as a loading control. (D) Level of PML–RARA protein after a short period of ATRA treatment is revealed by western blot analysis of NB4 nuclear extracts using an anti-RARA antibody. (E) Northern blot analysis of the half-life of C/EBPβ mRNA in uninduced and ATRA-induced NB4 cells. NB4 cells were pre-treated with 1 µM ATRA for 4 h and then actinomycin D (10 µg/ml) was added for the indicated times to the cells prior to extraction of RNA. Results are presented as RNA level normalized against actin with respect to time.
In order to investigate whether ATRA induced post-transcriptional stabilization of C/EBPβ mRNA, the mRNA half-life was measured in uninduced and ATRA-induced NB4 cells by exposing the cells to actinomycin D for the times indicated in Figure 4E. The average half-life of the mRNA was 2 h in both uninduced and ATRA-induced NB4 cells (Figure 4E), suggesting a transcriptional effect of ATRA rather than post-transcriptional mRNA stabilization accounting for the upregulation of steady-state level of C/EBPβ mRNA.
ATRA treatment activates the C/EBPβ promoter in a PML–RARA-dependent manner
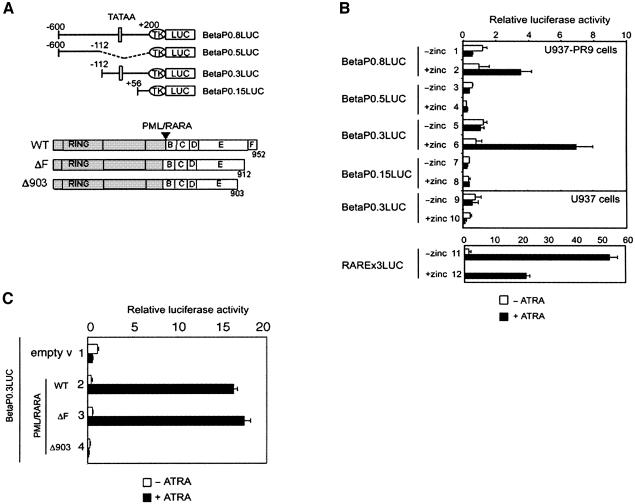
ATRA is known to exert its effects by transcriptional activation via the heterodimer RAR/RXR (Perez et al., 1993), and transcriptional activation by ATRA has been shown to be mediated through an RA response element (RARE) motif composed of direct repeats of (A/G)G(G/T)TCA (Naar et al., 1991; Umesono et al., 1991). As our results suggested that ATRA was mediating PML–RARA activation of C/EBPβ transcription, we assessed the efficiency of PML–RARA in activating the C/EBPβ promoter in the absence or presence of ATRA. We subcloned 0.8 kb of the human C/EBPβ promoter region (bp –600 to +200) which includes the putative TATA-binding protein-binding site and transcriptional initiation site, into a luciferase (LUC) reporter vector (Figure 5A). Transfection of the resulting BetaP0.8LUC reporter construct into U937-PR9 cells demonstrated that the C/EBPβ promoter did not respond like a RA-responsive promoter. Treatment of the non-zinc-stimulated cells with ATRA failed to significantly transactivate the promoter, compared with the control RARE-LUC reporter plasmid (Figure 5B, compare lane 1 with lane 11). In contrast, the relative luciferase activity was enhanced in the presence of PML–RARA after ATRA treatment (Figure 5B, lane 2), showing that the C/EBPβ promoter region was activated only in the presence of the fusion protein after ATRA treatment (Figure 5B). This correlated well with our northern blot results (Figure 4), which demonstrated elevation of C/EBPβ expression exclusively in PML–RARA-expressing cells. Deletion analyses of the C/EBPβ promoter implicated the region between bp –112 and +56 as being important for this activation (Figure 5B).
Fig. 5. C/EBPβ promoter is specifically activated by PML–RARA in the presence of ATRA. (A) Schematic representation of promoter constructs used in transient transfections shown in (B) and (C). The white rectangle labeled ‘TATAA’ represents the putative TATA box of the C/EBPβ promoter. (B) U937-PR9 cells were transiently transfected with the different deletions of the C/EBPβ reporter constructs. PML–RARA induction was obtained by pre-treating U937-PR9 cells with zinc for 15 h. Relative luciferase activity in the absence (white bar) or presence (black bar) of 2 µM ATRA is presented. U937 cells (without PML–RARA) were treated with zinc for 15 h to control for the effect of zinc treatment. The RAREx3LUC reporter vector was used as a control for ATRA treatment. (C) C-terminal PML–RARA deletion mutants were tested for their ability to activate the C/EBPβ promoter in U937-PR9 cells. The BetaP0.3LUC reporter was co-transfected in non-zinc-stimulated U937-PR9 cells with expression vector for PML–RARA wild-type and mutants (lanes 3–5). Relative luciferase activity in the absence (white bar) or presence (black bar) of 2 µM ATRA is presented.
To investigate whether the integrity of the fusion protein is necessary for the transactivation, C-terminal deletion mutants of PML–RARA were tested (Figure 5C). PML–RARAΔ903, a mutant which lacks half of α-helix 12 of the ligand-binding domain, could repress basal transcriptional activity but, importantly, this repression could not be overcome with ATRA treatment (Figure 5C, lane 4). Deletion of the F domain did not affect the transcription activity of PML–RARA (Figure 5C). These results demonstrate that a functional ligand-binding domain is required for the transactivation by PML–RARA. It is noteworthy that this Δ903 mutant has been shown to be expressed in the ATRA-resistant NB4 subclone NB4-R2 (Duprez et al., 2000).
C/EBPβ can induce granulocytic differentiation of a multipotential hematopoietic cell line
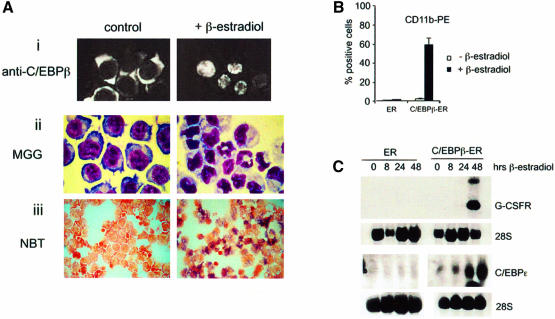
Our data strongly implicate an important role for C/EBPβ in ATRA-induced promyelocytic differentiation of APL cells and suggested that C/EBPβ directed differentiation. To determine whether C/EBPβ can induce hematopoietic cells to undergo differentiation to granulocytes, we used the multipotent K562 cell line as a model (Sutherland et al., 1986) that does not express C/EBPα, C/EBPβ or C/EBPε. Therefore, K562 cells were transduced with a pBabePuro retrovirus encoding either an estrogen receptor hormone-binding domain fused to C/EBPβ (C/EBPβ-ER) or the estrogen receptor hormone-binding domain alone (ER). When infected with the C/EBPβ-ER or ER viruses, the expressed exogenous proteins were located in the cytoplasm, thus partitioning any nuclear activities they might have (Figure 6A). However, upon treatment of infected cells with β-estradiol for 48 h, the exogenous protein translocated to the nucleus (Figure 6A, i), thus allowing the direct study of any associated nuclear C/EBPβ activity. Following β-estradiol treatment, the C/EBPβ-ER-infected cells developed morphological changes characteristic of granulocytic differentiation (Figure 6A, ii), the cells stained positive for the nitroblue tetrazolium (NBT) reduction assay (Figure 6A, iii) and showed an increase in CD11b expression (Figure 6B). In order to characterize further granulocytic differentiation following the induction of C/EBPβ, the expression of granulocytic-specific genes was analyzed by northern blot RNA prepared before and after varying times of β-estradiol treatment. C/EBPβ nuclear translocation led to the specific induction of G-CSF receptor and C/EBPε RNA (Figure 6C). Thus, in conclusion, the translocation of C/EBPβ into the nucleus of K562 cells drives the cells to differentiate into mature granulocytes as assessed by morphological changes, NBT reduction assay, expression of a cell surface differentiation marker and granulocyte-specific gene expression.
Fig. 6. C/EBPβ directs differentiation of a multipotent hematopoietic cell line. (A) β-Estradiol induces nuclear localization of the C/EBPβ-ER fusion protein followed by granulocytic differentiation. (i) Stably transfected K562 cells were subjected to immunofluorescence using an anti-C/EBPβ antibody after treatment for 24 h with vehicle only (left panel) or β-estradiol (right panel). Cell maturation was evaluated by morphology (ii) and by NBT reduction assay (iii) following 48 h of treatment with vehicle only (left panel) or β-estradiol (right panel). (B) Cell surface expression of CD11b was determined by FACS. Cells transfected with empty ER vector or with C/EBPβ-ER were treated with vehicle only or with β-estradiol for 72 h. (C) RNA expression of the G-CSF receptor (upper panel) or C/EBPε (lower panel) was assessed using northern blot analysis. As indicated in the figure, cells infected with empty vector (ER) or C/EBPβ-ER were treated for different times with β-estradiol.
C/EBPβ activity is required for ATRA induction of APL cell differentiation
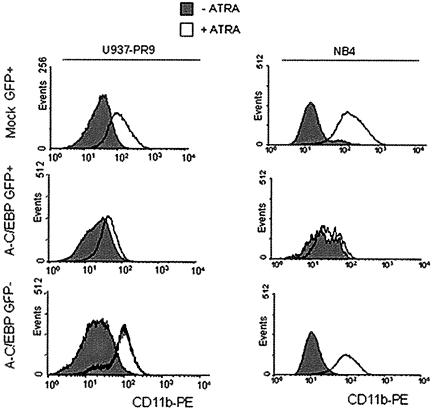
To investigate further the role of C/EBPβ in APL cell differentiation, the effect of overexpression of a dominant-negative form of C/EBP was examined in U937-PR9 and NB4 cells. The dominant-negative C/EBP (A-C/EBP) contains an acid amphipathic helix juxtaposed to a 49 amino acid leucine zipper domain of C/EBP. This combination allows formation of stable heterodimer with other C/EBP proteins and inhibits C/EBP gene activation (Olive et al., 1996). By using a retrovirus-mediated gene transfer system (Iwama et al., 2002), we expressed A-C/EBP in PML–RARA-expressing cells. Following infection, cells were treated with ATRA and expression of the cell surface differentiation marker CD11b was evaluated (Figure 7). Cells transfected with the control empty vector or the green fluorescent protein (GFP)-negative population of A-C/EBP-infected cells exhibited an increase in expression of the CD11b marker upon ATRA treatment. The GFP-positive population from the A-C/EBP-infected cells did not show an increase of CD11b expression after ATRA treatment in either zinc-pre-treated U937-PR9 or NB4 cells (Figure 7). These data suggest that disruption of C/EBP function leads to a block in ATRA-induced APL cell differentiation. To look specifically for the involvement of C/EBPβ in the ATRA-regulated maturation process, we used DNA vector-based RNAi technology to suppress C/EBPβ expression in NB4 cells. NB4 cells were transfected with the empty vector MSCV-U6 or the MCV-U6-beta vector, and transfected cells were selected in media containing puromycin. One month after selection, cells were treated with ATRA. As shown as Figure 8A, cells that had been transfected with MSCV-U6-beta had a significantly reduced level of C/EBPβ expression in response to ATRA. The maturation state of the cells was assessed by morphology and we demonstrated a correlation between the low level of C/EBPβ expression (Figure 8A) and an immature shape of the cells (Figure 8B) in response to ATRA. Quantification of maturation was obtained by NBT reduction assay, and a poor response of the low C/EBPβ-expressing cells to ATRA treatment could be confirmed (Figure 8C).
Fig. 7. Inhibition of ATRA-induced granulocytic differentiation by a dominant-negative C/EBP. U937-PR9 (left panel) and NB4 (right panel) cells were infected with a virus expressing the dominant- negative form of C/EBP, A-C/EBP (bottom two panels) or mock virus (top panels). The infected cells were treated with ATRA to induce differentiation. CD11b expression in the GFP-negative cell population (bottom panels) or GFP-positive cell population (top two panels) was examined by flow cytometry 72 h after induction of differentiation.
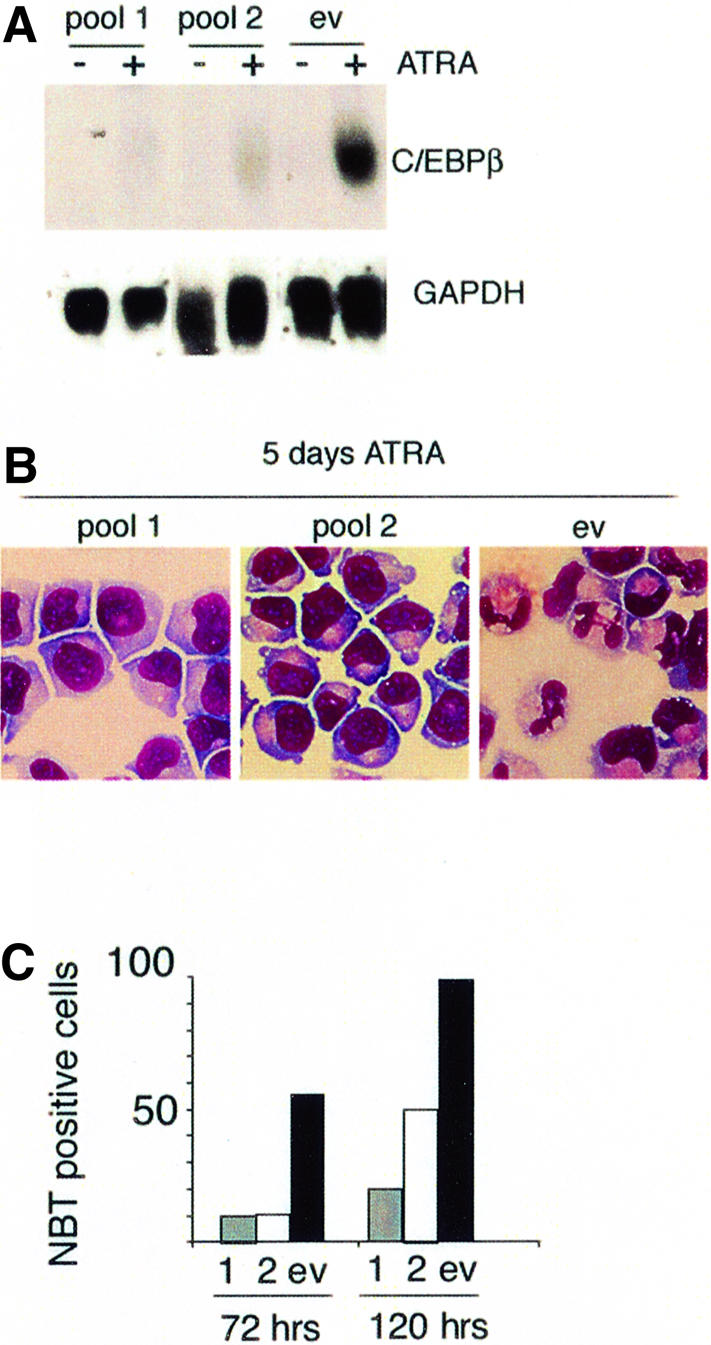
Fig. 8. Reduction of C/EBPβ expression induces reduction of ATRA-induced differentiation of NB4 cells. (A) C/EBPβ expression was evaluated by northern blot after 4 h of ATRA treatment in two different pools of cells transfected with the RNAi vector MSCV-U6-beta (pool1 and pool2) in comparison with cells transfected with an empty vector MSCV-U6 (ev). Cell maturation after 5 days of ATRA treatment was evaluated by morphology using MGG staining (B) and/or by NBT reduction assay (C). Results are presented as the percentage of NBT-positive cells and correspond to a count of 500 cells.
In summary, C/EBPβ is the first C/EBP activity induced following ATRA treatment of APL cells (Figures 2 and 3), and induction of C/EBPε by C/EBPβ is not dependent on ATRA (Figure 6). These results support a primary role for C/EBPβ in the differentiation of APL cells.
Discussion
Is C/EBPβ activation necessary for normal granulopoiesis?
In this study, we have shown the importance of the role of C/EBPβ in inducing granulocytic differentiation. The role of C/EBPβ in myeloid cells was first determined by the study of C/ebpβ–/– mice which present a defect in macrophage activation (Screpanti et al., 1995; Tanaka et al., 1995). However, the exact role of C/EBPβ in myeloid differentiation is unclear. Depending upon which system is used, gain-of-function experiments have shown a role for C/EBPβ in either monocytic differentiation, eosinophil maturation (Nerlov et al., 1998) or, recently, in granulocytic differentiation (Popernack et al., 2001; Iwama et al., 2002; this study). We show that expression of C/EBPβ in the multipotent cell line K562 leads to its preferential differentiation towards granulocytes, as indicated by cell morphology and gene expression induction. Interestingly, in this cell line without any ATRA treatment, C/EBPβ induces C/EBPε, an important mediator of granulocytic differentiation (Park et al., 1999). One possibility would be that C/EBPε is a target of C/EBPβ and participates in the differentiation of the cells. In addition, we present evidence that C/EBPβ is enhanced and required in ATRA-induced differentiation of the APL cells. We show that inhibiting C/EBP activity and more specifically C/EBPβ expression dramatically reduces the APL response to ATRA. In addition, during ATRA-induced APL cell differentiation, C/EBPβ modulates granulocytic-specific genes and binds to the G-CSF receptor promoter in vivo (data not shown). However, it remains to be determined whether C/EBPβ is indispensable for normal granulopoiesis, or whether what we observed in APL cells is specific to this cell pathology. C/EBP activity is known to be necessary for granulocytic differentiation, since our previous work showed that the loss of C/EBPα impairs granulopoiesis (Zhang et al., 1997). The role of C/EBPα versus C/EBPβ in granulopoiesis has not yet been reported. However, a gene replacement strategy has shown that when C/EBPβ is expressed from the C/ebpα gene locus, C/EBPβ can functionally replace C/EBPα to restore granulopoiesis (Jones et al., 2002). Moreover, immortalized fetal liver C/ebpα–/– cells can give rise to granulocytes under interleukin-3 (IL-3) and granulocyte–macrophage colony-stimulating factor (GM-CSF) stimulation, and this granulocytic differentiation is characterized by an increase in C/EBPβ expression (Zhang et al., 2002).
C/EBPβ is targeted by PML–RARA
Downstream events leading to ATRA-induced differentiation of APL cells are still poorly understood. A list of potential ATRA target genes has been drawn from multiple studies (reviewed in Melnick and Licht, 1999), which have been complemented by reports using systematic analyses, leading to the identification of new potential ATRA target genes (Liu et al., 2000). In spite of these efforts, the important target genes have yet to be identified. In this work, we provide evidence that a specific myeloid transcription factor, C/EBPβ, is targeted by ATRA during APL differentiation. We provide evidence that C/EBPβ is a very early response gene, directly regulated after 1 h of ATRA treatment. This induction is not dependent on a RARE in the C/EBPβ promoter and does not require a normal RA transduction pathway. This probably explains why C/EBPβ has not yet been described as an RA-responsive gene in normal cells, and that direct repeats of the RARE motif, (A/G)G(G/T)TCA, are not found in the C/EBPβ proximal promoter. Our data reveal that ATRA induction of C/EBPβ occurs only in the presence of the PML–RARA fusion protein, placing PML–RARA as the mediator of this effect. Thus, C/EBPβ is a gene that is not regulated by a normal retinoic pathway but specifically targeted by the fusion protein PML–RARA. This novel finding supports the hypothesis that PML–RARA is an active member of the response of APL cells to ATRA and fits with the model stipulating that PML–RARA expression confers sensitivity to ATRA in myeloid cell lines (Ruthardt et al., 1997).
One enigma posed by our data is how the PML–RARA fusion protein, which is involved in blocking cell differentiation by repressing transcription, could then become the stimulator of the differentiation by becoming a transcriptional activator. This process could be difficult to picture in the light of the extensive literature showing that PML–RARA is degraded upon ATRA treatment (Duprez et al., 1996; Zhu et al., 1999). However, we show that C/EBPβ activation occurs as early as 1 h after ATRA stimulation and that within the first few hours of treatment, PML–RARA is still present in the cell and is not degraded. This confirms previous data showing that the degradation process is neither complete nor immediate after the addition of ATRA (Duprez et al., 1996; Zhu et al., 1999). PML–RARA has been shown to activate target promoters in vitro in response to ATRA, and this activation involves the formation of a stable multimeric complex involving the coiled-coil region of PML (Lin and Evans, 2000; Minucci et al., 2000). More recently, evidence that PML–RARA binds directly to in vivo target promoters was reported (Di Croce et al., 2002). These models support the hypothesis that PML–RARA can directly bind DNA sites via the RARA DNA-binding domain and repress transcription in an ATRA-reversible way. Our work illustrates the fact that PML–RARA activates a promoter in response to ATRA, and we were able to determine the region in the C/EBPβ promoter that mediates PML–RARA activation. This activation is probably the result of an indirect effect of the fusion protein on this promoter as we could not demonstrate any RARE and any direct binding of PML–RARA to the DNA element (data not shown). We hypothesize that this activation is achieved by PML–RARA-mediated modification of the activity of a transcription factor, which is important for the regulation of C/EBPβ. This model is supported by previous observations showing that PML–RARA can modify transcription factor activity through direct interaction (e.g. Gata-2; Tsuzuki et al., 2000) or by recruiting co-repressors and HDAC1 (e.g. Mad; Khan et al., 2001). Additional studies aimed at identification of proteins binding to the C/EBPβ promoter will be required in order to understand the mechanism of activation.
In summary, our data support a model in which C/EBPβ can play an important role in granulocytic differentiation and, more specifically, at the promyelocytic stage of myeloid maturation. Our model suggests that in ATRA-stimulated APL cells, PML–RARA activates C/EBPβ, providing a novel additional mechanism to explain the unique sensitivity of APL cells to ATRA. Identifying other genes activated by ATRA in association with PML–RARA and understanding how these genes are regulated may be especially useful for the treatment of relapsed APL patients.
Materials and methods
Constructs
An 800 bp DNA fragment from the C/EBPβ promoter region encompassing bp –600 to +200 was amplified by PCR using genomic DNA from U937 cells as template with the following oligos: sense, 5′-TCCCGTCTGTGAAAATGGCAG-3′; antisense, 5′-ACCAGGCG TTGCATGACG-3′. The cDNA product was cloned into a thymidine kinase minimal promoter luciferase reporter plasmid, PT81-LUC (Nordeen, 1988). BetaP0.3 and BetaP0.5 were generated by digesting the 800 bp promoter fragment using the internal restriction site SacII. P0.15 was generated by PCR using the oligos 5′-TTCACTAAT AGCGGCCACC-3′ and 5′-ACCAGGCGTTGCATGACG-3′. The unmodified (‘empty’) PT81-LUC was used as a negative control. A LUC reporter construct containing three copies of a RARE upstream of the tk promoter was used as a control for a RARE. PSG5-RARA, PSG5-PML–RARA and PML–RARAΔ907 expression vectors have been described previously (Duprez et al., 2000). PML–RARΔF was obtained by digesting full-length PSG5-PML–RARA with KpnI and BamHI (KpnI cuts in the PML part) and by replacing the digested fragment with a PCR-amplified PML–RARA which lacks the F domain. For retrovirus construction, a human C/EBPβ cDNA fragment encoding the full-length protein was inserted in-frame with the murine estrogen receptor-α vector (Zhang et al., 2002).
Cell culture and evaluation of cell differentiation
Human acute promyelocytic cell lines NB4, NB4-R2 and HT93 and human myelomonoblastic cell lines U937, U937-PR9 or U937-B412 were maintained at exponential growth in RPMI supplemented with 10% fetal calf serum (BioWhittaker, Walkersville, MD). U937-PR9 and U937-B412 contain respectively PML–RARA cDNA and PLZF–RARA cDNA under the control of the zinc-inducible human metallothionein promoter (Ruthardt et al., 1997). ATRA (Sigma Chemical Co., St Louis, MO) was used at final concentration of 1 µM or as indicated in the figure legends, and cycloheximide (Sigma Chemical Co.) at a final concentration of 10 µg/ml. When using ATRA and cycloheximide together, cycloheximide was added 30 min before ATRA treatment. For mRNA stability studies, actinomycin D was used at 10 µg/ml and added for the times indicated in the figures. For the generation of the cell lines with conditional C/EBPβ expression, K562 cells were electroporated with pBabePuro vector or pBabePuroC/EBPβ-ER. The presence of the fusion protein C/EBPβ-ER was screened in the different puromycin-resistant clones by western blot analysis using a C/EBPβ-specific antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Three independent clones were analyzed. For virus infection, we used a producer cell line of the dominant-negative C/EBP virus, A-C/EBP (Iwama et al., 2002). Between 2 and 4% of the cells were GFP positive 48 h after infection. Differentiation was evaluated by morphology using light microscopy of May–Grunwald–Giemsa staining and NBT staining or by flow cytometry analysis. We assessed the expression of the differentiation-related surface marker CD11b with a specific phycoerythrin (PE)-conjugated mouse monoclonal antibody (Pharmingen, San Diego, CA). Results were analyzed on a FACscan flow cytometer (Beckton Dickinson, Mountain View, CA) using Cellquest software.
Protein extracts, western blotting and EMSA
Nuclear proteins were extracted by an NP-40 detergent lysis procedure. For western blotting, nuclear extracts (20 µg) were separated on 10% SDS–polyacrylamide gels and electrophoretically transferred to nitrocellulose membranes (Bio-Rad). Proteins of interest were revealed with a C/EBPβ monoclonal antibody or C/EBPε polyclonal antibody (Santa Cruz Biotechnology) or with an antibody raised against the RARA F-region (Ab9α-F, a gift from P.Chambon). To assess C/EBP binding activity, a double-stranded G-CSF receptor promoter oligonucleotide extending from bp –57 to –38 was used as a probe (Smith et al., 1996). EMSA was performed by incubating 10 µg of nuclear extract with 1 ng of the radiolabeled probe in binding buffer [10 mM HEPES pH 7.9, 50 mM KCl, 2.5 mM MgCl2, 1 mM dithiothreitol (DTT)] supplemented with 1 mg/ml bovine serum albumin (BSA), 10% glycerol and 0.5 µg of poly(dI–dC) (Amersham, Piscataway, NJ) for 30 min at room temperature. For competition experiments, a 50-fold excess of unlabeled competitor oligonucleotide was added before addition of labeled oligonucleotide. For supershift experiments, 1.5 µl of specific C/EBPα, C/EBPβ or C/EBPε antisera (Santa Cruz Biotechnology) were added before addition of the radiolabeled probe. Complexes were resolved in a 4% non-denaturing polyacrylamide gels in 1× TBE.
Northern blot analysis
Northern blot analysis was performed as described previously (Duprez et al., 1996). A fragment corresponding to bp 1427–1771 of the human C/EBPβ cDNA was amplified by PCR and used as a probe. The C/EBPε probe was a 0.5 kb PstI fragment of the pJurkat1 clone (Yamanaka et al., 1997). The G-CSF receptor probe was a 0.7 kb SacII–NdeI fragment of the human G-CSF receptor cDNA.
Transient transfection and luciferase assays
Transactivation studies of the C/EBPβ promoter were performed in U937 or U937-PR9 cells. Transfections were carried out using Effecten reagent (Qiagen, Valencia, CA). Total cDNA transfected per assay was 0.5 µg: 0.4 µg of the reporter plasmid and 0.1 µg of a reference plasmid (CMV β-gal). PML–RARA was induced by adding 100 µM ZnSO4 to the culture medium 15 h before transfection. When expression vectors were added, total cDNA transfected per assay was 1 µg: 0.5 µg of the reporter plasmid, 0.25 µg of an expression plasmid and 0.25 µg of a reference plasmid (CMV β-gal). After the addition of DNA, cells were incubated in the presence or absence of ATRA at 2 µM final concentration for an additional 15 h. Cell lysates were assayed for luciferase activity (Promega, Madison, WI) and β-gal activity (Tropix, Bedfort, MA). Luciferase activity values are standardized against β-galactosidase activity obtained with the reference plasmid.
siRNA
For knocking-down C/EBPβ expression in the NB4 cell line, we used DNA-based vector-delivered siRNA technology. The targeted sequence of C/EBPβ was empirically determined in the C/EBPβ coding sequence but was verified by BLAST searches to ensure the non-homology with other C/EBP genes. Based on the strategy described in Devroe et al. (2002), two complementary oligos were designed corresponding to nucleotides 1280–1300 of the C/EBPβ mRNA sequence (accession number AY193834): oligo1, GGCCAACTTCTACTACGAGGaagcttCC TCTCGTAGTAGAAGTTGGCCCTTTTTG; and oligo 2, ATTCAAAA AGGGCCAACTTCTACTACGAGGaagcttCCTCGTAGTAGAAGTT GGCC. The oligos were annealed and inserted in a modified MSCV-U6 siRNA retroviral expression vector digested with ApaI and EcoRI (Devroe et al., 2002). The inserted sequence was verified by sequencing. NB4 cells were electroporated with MSCV-U6-beta vector or MSCV-U6 empty vector and selected with puromycin (1 µg/ml). Pools of cells were treated with ATRA and subjected to northern blot analysis or differentiation assay.
Acknowledgments
Acknowledgements
We thank A.Iwama and C.Vinson for the A-C/EBP producer cell line, P.G.Pelicci for the gift of U937-PR9 and U937-B412 cells, M.Lanotte for the gift of the NB4-R2 cells, R.Stone for providing the APL patient sample, P.Chambon for the gift of the anti-RARA antibody, M.Ewen for providing suggestions for the C/EBPβ siRNA, P.Silver for the gift of the MSCV-U6, H.Radomska for valuable suggestions, K.Geary for technical assistance and A.Saurin for critically reading the manuscript. We are also grateful to M.Sieweke for allowing experiments to be performed in his laboratory. This work was supported by the CNRS to E.D, DFG WA 1584/1-1 to K.W., and NIH grants (CA66996 and HL56745) to D.G.T.
References
- Borrow J., Goddard,A.D., Sheer,D. and Solomon,E. (1990) Molecular analysis of acute promyelocytic leukemia breakpoint cluster region on chromosome 17. Science, 249, 1577–1580. [DOI] [PubMed] [Google Scholar]
- Brown D., Kogan,S., Lagasse,E., Weissman,I., Alcalay,M., Pelicci,P.G., Atwater,S. and Bishop,J.M. (1997) A PMLRARα transgene initiates murine acute promyelocytic leukemia. Proc. Natl Acad. Sci. USA, 94, 2551–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaigne S., Chomienne,C., Daniel,M.T., Berger,R., Miclea,J.M., Ballerini,P. and Degos,L. (1990) Retinoic acids in treatment of acute promyelocytic leukemia. Nouv. Rev. Fr. Hematol., 32, 36–38. [PubMed] [Google Scholar]
- Chih D.Y., Chumakov,A.M., Park,D.J., Silla,A.G. and Koeffler,H.P. (1997) Modulation of mRNA expression of a novel human myeloid-selective CCAAT/enhancer binding protein gene (C/EBPε). Blood, 90, 2987–2994. [PubMed] [Google Scholar]
- de The H., Chomienne,C., Lanotte,M., Degos,L. and Dejean,A. (1990) The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor α gene to a novel transcribed locus. Nature, 347, 558–561. [DOI] [PubMed] [Google Scholar]
- de The H., Lavau,C., Marchio,A., Chomienne,C., Degos,L. and Dejean,A. (1991) The PML–RARα fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell, 66, 675–684. [DOI] [PubMed] [Google Scholar]
- Devroe E. and Silver,P. (2002) Retrovirus-delivered siRNA. BMC Biotechnol., 2, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Croce L. et al. (2002) Methyltransferase recruitment and DNA hypermethylation of target promoters by an oncogenic transcription factor. Science, 295, 1079–1082. [DOI] [PubMed] [Google Scholar]
- Duprez E., Lillehaug,J.R., Gaub,M.P. and Lanotte,M. (1996) Differential changes of retinoid-X-receptor (RXRα) and its RARα and PML–RARα partners induced by retinoic acid and cAMP distinguish maturation sensitive and resistant t(15;17) promyelocytic leukemia NB4 cells. Oncogene, 12, 2443–2450. [PubMed] [Google Scholar]
- Duprez E., Benoit,G., Flexor,M., Lillehaug,J.R. and Lanotte,M. (2000) A mutated PML/RARA found in the retinoid maturation resistant NB4 subclone, NB4-R2, blocks RARA and wild-type PML/RARA transcriptional activities. Leukemia, 14, 255–261. [DOI] [PubMed] [Google Scholar]
- Grignani F. et al. (1998) Fusion proteins of the retinoic acid receptor-α recruit histone deacetylase in promyelocytic leukaemia. Nature, 391, 815–818. [DOI] [PubMed] [Google Scholar]
- Grisolano J.L., Wesselschmidt,R.L., Pelicci,P.G. and Ley,T.J. (1997) Altered myeloid development and acute leukemia in transgenic mice expressing PML–RARα under control of cathepsin G regulatory sequences. Blood, 89, 376–387. [PubMed] [Google Scholar]
- He L.Z., Tribioli,C., Rivi,R., Peruzzi,D., Pelicci,P.G., Soares,V., Cattoretti,G. and Pandolfi,P.P. (1997) Acute leukemia with promyelocytic features in PML/RARα transgenic mice. Proc. Natl Acad. Sci. USA, 94, 5302–5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L.Z., Guidez,F., Tribioli,C.,D., Ruthardt,M., Zelent,A. and Pandolfi,P.P. (1998) Distinct interactions of PML–RARα and PLZF–RARα with co-repressors determine differential responses to RA in APL. Nat. Genet., 18, 126–135. [DOI] [PubMed] [Google Scholar]
- Hu H.M., Baer,M., Williams,S.C., Johnson,P.F. and Schwartz,R.C. (1998) Redundancy of C/EBPα, -β and -δ in supporting the lipopolysaccharide-induced transcription of IL-6 and monocyte chemoattractant protein-1. J. Immunol., 160, 2334–2342. [PubMed] [Google Scholar]
- Imaizumi M. et al. (1998) Mutations in the E-domain of RAR portion of the PML/RAR chimeric gene may confer clinical resistance to all-trans retinoic acid in acute promyelocytic leukemia. Blood, 92, 374–382. [PubMed] [Google Scholar]
- Iwama A. et al. (2002) Reciprocal roles for CCAAT/enhancer binding protein (C/EBP) and PU.1 transcription factors in Langerhans cell commitment. J. Exp. Med., 195, 547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L.C., Lin,M.L., Chen,S.S., Krug,U., Hofmann,W.K., Lee,S., Lee,Y.H. and Koeffler,H.P. (2002) Expression of C/EBPβ from the C/ebα gene locus is sufficient for normal hematopoiesis in vivo. Blood, 99, 2032–2036. [DOI] [PubMed] [Google Scholar]
- Kakizuka A., Miller,W.H.,Jr, Umesono,K., Warrell,R.P.,Jr, Frankel,S.R., Murty,V.V., Dmitrovsky,E. and Evans,R.M. (1991) Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RARα with a novel putative transcription factor, PML. Cell, 66, 663–674. [DOI] [PubMed] [Google Scholar]
- Kastner P., Lawrence,H.J., Waltzinger,C., Ghyselinck,N.B., Chambon,P. and Chan,S. (2001) Positive and negative regulation of granulopoiesis by endogenous RARα. Blood, 97, 1314–1320. [DOI] [PubMed] [Google Scholar]
- Khan M.M. et al. (2001) Role of PML and PML-RARα in Mad-mediated transcriptional repression. Mol. Cell, 7, 1233–1243. [DOI] [PubMed] [Google Scholar]
- Lekstrom-Himes J. and Xanthopoulos,K.G. (1998) Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J. Biol. Chem., 273, 28545–28548. [DOI] [PubMed] [Google Scholar]
- Lin R.J. and Evans,R.M. (2000) Acquisition of oncogenic potential by RAR chimeras in acute promyelocytic leukemia through formation of homodimers. Mol. Cell, 5, 821–830. [DOI] [PubMed] [Google Scholar]
- Lin R.J., Nagy,L., Inoue,S., Shao,W., Miller,W.H.,Jr and Evans,R.M. (1998) Role of the histone deacetylase complex in acute promyelocytic leukaemia. Nature, 391, 811–814. [DOI] [PubMed] [Google Scholar]
- Liu T.X. et al. (2000) Gene expression networks underlying retinoic acid-induced differentiation of acute promyelocytic leukemia cells. Blood, 96, 1496–14504. [PubMed] [Google Scholar]
- Lutz P.G., Houzel-Charavel,A., Moog-Lutz,C. and Cayre,Y.E. (2001) Myeloblastin is an Myb target gene: mechanisms of regulation in myeloid leukemia cells growth-arrested by retinoic acid. Blood, 97, 2449–2456. [DOI] [PubMed] [Google Scholar]
- McKnight S. (2001) McBindall: a better name for CCAAT/enhancer binding proteins. Cell, 107, 259–261. [DOI] [PubMed] [Google Scholar]
- Melnick A. and Licht,J.D. (1999) Deconstructing a disease: RARα, its fusion partners and their roles in the pathogenesis of acute promyelocytic leukemia. Blood, 93, 3167–3215. [PubMed] [Google Scholar]
- Minucci S. et al. (2000) Oligomerization of RAR and AML1 transcription factors as a novel mechanism of oncogenic activation. Mol. Cell, 5, 811–820. [DOI] [PubMed] [Google Scholar]
- Mistry R.A., Pedersen,E.W., Solomon,E. and Grimwade,D. (2003) The molecular pathogenesis of acute promyelocytic leukemia: implications for the clinical management of the disease. Blood Rev., 17, 71–79. [DOI] [PubMed] [Google Scholar]
- Moog-Lutz C. et al. (2001) PRAM-1 is a novel adaptor protein regulated by retinoic acid (RA) and promyelocytic leukemia (PML)–RA receptor α in acute promyelocytic leukemia cells. J. Biol. Chem., 276, 22375–22381. [DOI] [PubMed] [Google Scholar]
- Naar A.M., Boutin,J.M., Lipkin,S.M., Yu,V.C., Holloway,J.M., Glass,C.K. and Rosenfeld,M.G. (1991) The orientation and spacing of core DNA-binding motifs dictate selective transcriptional responses to three nuclear receptors. Cell, 65, 1267–1279. [DOI] [PubMed] [Google Scholar]
- Nerlov C., McNagny,K.M., Doderlein,G., Kowenz-Leutz,E. and Graf,T. (1998) Distinct C/EBP functions are required for eosinophil lineage commitment and maturation. Genes Dev., 12, 2413–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen S.K. (1988) Luciferase reporter gene vectors for analysis of promoters and enhancers. Biotechniques, 6, 454–458. [PubMed] [Google Scholar]
- Olive M., Williams,S.C., Dezan,C., Johnson,P.F. and Vinson,C. (1996) Design of a C/EBP-specific, dominant-negative bZIP protein with both inhibitory and gain-of-function properties. J. Biol. Chem., 271, 2040–2047. [DOI] [PubMed] [Google Scholar]
- Park D.J., Chumakov,A.M., Vuong,P.T., Chih,D.Y., Gombart,A.F., Miller,W.H.,Jr and Koeffler,H.P. (1999) CCAAT/enhancer binding protein ε is a potential retinoid target gene in acute promyelocytic leukemia treatment. J. Clin. Invest., 103, 1399–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez A., Kastner,P., Sethi,S., Lutz,Y., Reibel,C. and Chambon,P. (1993) PMLRAR homodimers: distinct DNA binding properties and heteromeric interactions with RXR. EMBO J., 12, 3171–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli V., Mancini,F.P. and Cortese,R. (1990) IL-6DBP, a nuclear protein involved in interleukin-6 signal transduction, defines a new family of leucine zipper proteins related to C/EBP. Cell, 63, 643–653. [DOI] [PubMed] [Google Scholar]
- Popernack P.M., Truong,L.T., Kamphuis,M. and Henderson,A.J. (2001) Ectopic expression of CCAAT/enhancer binding protein β (C/EBPβ) in long-term bone marrow cultures induces granulopoiesis and alters stromal cell function. J. Hematother. Stem Cell Res., 10, 631–642. [DOI] [PubMed] [Google Scholar]
- Radomska H.S., Huettner,C.S., Zhang,P., Cheng,T., Scadden,D.T. and Tenen,D.G. (1998) CCAAT/enhancer binding protein α is a regulatory switch sufficient for induction of granulocytic development from bipotential myeloid progenitors. Mol. Cell. Biol., 18, 4301–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthardt M., Testa,U., Nervi,C., Ferrucci,P.F., Grignani,F., Puccetti,E., Peschle,C. and Pelicci,P.G. (1997) Opposite effects of the acute promyelocytic leukemia PML–retinoic acid receptor α (RARα) and PLZF–RARα fusion proteins on retinoic acid signalling. Mol. Cell. Biol., 17, 4859–4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screpanti I. et al. (1995) Lymphoproliferative disorder and imbalanced T-helper response in C/EBPβ-deficient mice. EMBO J., 14, 1932–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L.T., Hohaus,S., Gonzalez,D.A., Dziennis,S.E. and Tenen,D.G. (1996) PU.1 (Spi-1) and C/EBPα regulate the granulocyte colony-stimulating factor receptor promoter in myeloid cells. Blood, 88, 1234–1247. [PubMed] [Google Scholar]
- Sutherland J.A., Turner,A.R., Mannoni,P., McGann,L.E. and Turc,J.M. (1986) Differentiation of K562 leukemia cells along erythroid, macrophage and megakaryocyte lineages. J. Biol. Response Mod., 5, 250–262. [PubMed] [Google Scholar]
- Tanaka T. et al. (1995) Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell, 80, 353–361. [DOI] [PubMed] [Google Scholar]
- Tenen D.G., Hromas,R., Licht,J.D. and Zhang,D.E. (1997) Transcription factors, normal myeloid development and leukemia. Blood, 90, 489–519. [PubMed] [Google Scholar]
- Tsuzuki S., Towatari,M., Saito,H. and Enver,T. (2000) Potentiation of GATA-2 activity through interactions with the promyelocytic leukemia protein (PML) and the t(15;17)-generated PML–retinoic acid receptor α oncoprotein. Mol. Cell. Biol., 20, 6276–6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesono K., Murakami,K.K., Thompson,C.C. and Evans,R.M. (1991) Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell, 65, 1255–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka R., Kim,G.D., Radomska,H.S., Lekstrom-Himes,J., Smith,L.T., Antonson,P., Tenen,D.G. and Xanthopoulos,K.G. (1997) CCAAT/enhancer binding protein ε is preferentially up-regulated during granulocytic differentiation and its functional versatility is determined by alternative use of promoters and differential splicing. Proc. Natl Acad. Sci. USA, 94, 6462–6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.E., Zhang,P., Wang,N.D., Hetherington,C.J., Darlington,G.J. and Tenen,D.G. (1997) Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein α-deficient mice. Proc. Natl Acad. Sci. USA, 94, 569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P.., Nelson,E.A., Radomska,H.S., Iwasaki-Arai,J, Akashi,K, Friedman,A.D. and Tenen,D.G. (2002) Induction of granulocytic differentiation by two pathways. Blood, 99, 4406–4412. [DOI] [PubMed] [Google Scholar]
- Zhu J., Gianni,M., Kopf,E., Honore,N., Chelbi-Alix,M., Koken,M., Quignon,F., Rochette-Egly,C. and de The,H. (1999) Retinoic acid induces proteasome-dependent degradation of retinoic acid receptorα (RARα) and oncogenic RARα fusion proteins. Proc. Natl Acad. Sci. USA, 96, 14807–14812. [DOI] [PMC free article] [PubMed] [Google Scholar]