Abstract
To understand the trafficking of endocytosed hemoglobin (Hb) in Leishmania, we investigated the characteristics of in vitro fusion between endosomes containing biotinylated Hb (BHb) and avidin–horseradish peroxidase (AHRP). We showed that early endosome fusion in Leishmania is temperature and cytosol dependent and is inhibited by ATP depletion, ATPγS, GTPγS and N-ethylmaleimide treatment. The Rab5 homolog from Leishmania donovani, LdRab5, was cloned and expressed. Our results showed that homotypic fusion between the early endosomes in Leishmania is Rab5 dependent. Early endosomes containing BHb fused efficiently with late endosomes in a process regulated by Rab7, whereas no fusion between early and late endosomes was detected using fluid phase markers. Pre-treatment of early endosomes containing BHb with monoclonal antibody specific for the C-terminus of the Hb receptor (HbR) or the addition of the C-terminal cytoplasmic fragment of the HbR specifically inhibited the fusion with late endosomes, suggesting that signal(s) mediated through the HbR cytoplasmic tail promotes the fusion of early endosomes containing Hb with late endosomes.
Keywords: endocytosis/Hb/Leishmania/Rab/reconstitution
Introduction
Endocytosis is a fundamental process that mediates internalization, sorting and degradation of endocytosed molecules (Wileman et al., 1985). In protozoan parasites, endocytosis is mainly studied in trypanosomatids (Clayton et al., 1995; Liu et al., 2000), and a few receptor systems mediating efficient supply of nutrients have been identified (Voyiatzaki and Soteriadou 1992; Bastin et al., 1996). However, the intracellular route and mechanism of transport of internalized materials in these parasites remain largely unknown.
Recent studies have established that transport of cargo along the endocytic pathway requires a series of coordinated and specific vesicle fusion events regulated by small GTP-binding proteins of the Rab family (Zerial and McBride, 2001). Among the endocytic Rabs, Rab7 is involved in transport from the early to late compartment (Feng et al., 1995; Mukhopadhyay et al., 1997b), whereas Rab5 regulates transport from the plasma membrane to the early compartment as well as homotypic fusion among early endosomes (Gorvel et al., 1991; Mukhopadhyay et al., 1997a). Indications of similar endocytic and secretory pathways in trypanosomatids, reported recently (McConville et al., 2002), remain to be characterized. Homologs of Rab4, Rab5, Rab7 and Rab11 have been identified in Trypanosoma brucei. Rab4 and Rab11 appear to be involved in recycling, while different isoforms of Rab5 regulate distinct steps in endocytosis (Field and Field, 1997; Field et al., 1998; Pal et al., 2002). Rab7, and to a lesser extent Rab4, are associated with the endocytic pathway in Dictyostelium discoideum (Temesvari et al., 1994; Laurent et al., 1998). In Toxoplasma gondii, Rab5 regulates cholesterol acquisition from the host cell (Robibaro et al., 2002) and Rab6 is involved in sorting of post-Golgi secretory granules (Stedman et al., 2003). However, regulation of intracellular trafficking by Rab GTPases in protozoan parasites remains to be elucidated. Recently, we have shown that endocytosis of hemoglobin (Hb) in Leishmania is mediated through receptors located in the flagellar pocket (Sengupta et al., 1999), possibly to generate intracellular heme after degradation of internalized Hb, as Leishmania lack a complete heme biosynthetic pathway (Sah et al., 2002). In order to understand the regulation of Hb trafficking in Leishmania, we have reconstituted endosome fusions using endosomes containing Hb purified from Leishmania promastigotes. Here we report the cloning and expression of Rab5 from L.donovani and show that the early endosome in Leishmania is a very dynamic compartment and promotes fusion with early and late compartments during Hb trafficking.
Results
Cloning and expression of Rab5 homolog from L.donovani
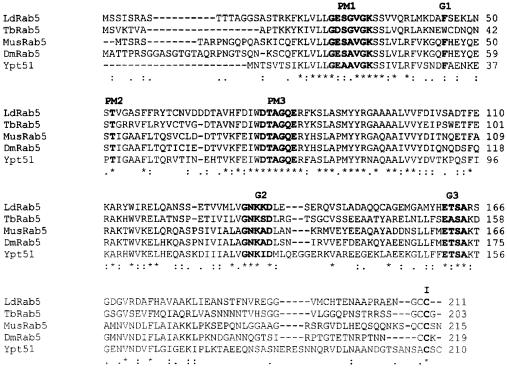
To clone the Rab5 homolog from L.donovani, a BLAST (Karlin and Altschul, 1993) search was carried out using human Rab5 as a query. Genes from divergent genera of protozoa showed significant similarity with human Rab5, e.g. D.discoideum (88%), T.gondii (75%), Plasmodium falciparum (78%), Entamoeba histolytica (78%) and T.brucei (61%). As Trypanosma are closest to Leishmania, a second search was carried out using T.brucei Rab5B sequence as a query, which revealed a putative Rab5-like sequence from L.major with 72% homology. Putative start and stop codons were predicted and appropriate forward and reverse primers were used to amplify a fragment (636 bp) from L.donovani cDNA by PCR. The PCR product was cloned, sequenced and hypothetically translated (211 amino acids). A BLAST search revealed that the cloned protein (LdRab5) has ∼91% similarities with L.major putative Rab5, 65% with T.brucei Rab5, 66% with T.gondii Rab5, 62% with D.melanogaster Rab5 and 59% with human and mouse Rab5. Comparison of LdRab5 sequence with other Rab5 sequences using ClustalW multiple sequence alignment (Version 1.8; Thompson et al., 1994) demonstrated the presence of conserved Rab protein features (Stenmark and Olkkonen, 2001) including the GTP-binding region, effector loop and C-terminal isoprenylation motif (Figure 1).
Fig. 1. Multiple alignment of the amino acid sequence of LdRab5 with Rab5 sequences from different organisms. Our LdRab5sequence data have been submitted to the GenBank database under accession No. AY337265. Residues implicated in guanine phosphate binding (PM1–3), GTP/GDP binding (G1–3) and the isoprenylation motif (I) are marked in bold. Tb, T.brucei; Mm, M.musculus; Dm, D.melanogaster; Ypt, S.cerevisiae; Hs, H.sapiens; Ld, L.donovani; Lm, L.major; Tg, T.gondii; Pf, P.falciparum.
Characterization of Rab5 from L.donovani
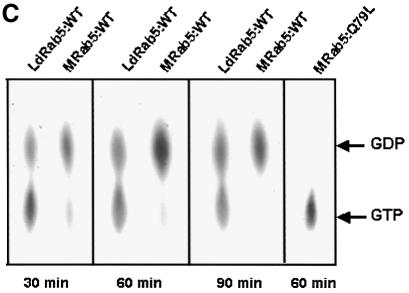
GTP binding and GTPase activities of GST–LdRab5 were determined using free GST and mammalian GST–Rab5 as controls. Figure 2A shows that unlike free GST, GST–LdRab5 bound a significant amount of [α-32P]GTP. The binding of [α-32P]GTP was competed out by unlabeled GTP and GDP but not by ATP, indicating the specificity of guanine nucleotide binding of LdRab5 (Figure 2B). However, kinetic analysis of GTPase activity revealed that LdRab5 GTPase activity is comparatively reduced with respect to mammalian Rab5 (Figure 2C).
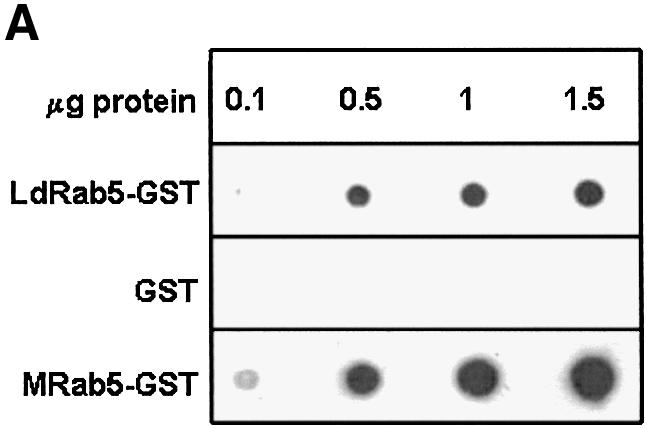
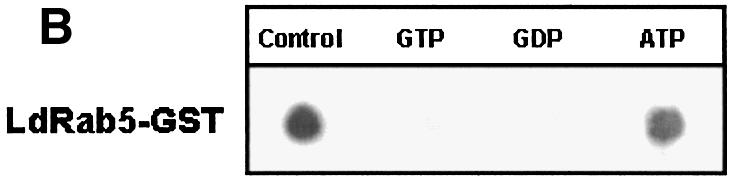
Fig. 2. Characterization of Rab5 from L.donovani. (A) GTP binding of the indicated concentrations of LdRab5 was detected using a [α-32P]GTP overlay assay. Free GST and mammalian Rab5 were used as control. (B) [α-32P]GTP binding was measured in the absence (control) or presence of 1 mM of the indicated nucleotides. (C) The kinetics of GTPase activity of LdRab5 were determined as described in Materials and methods. Mammalian Rab5 wild-type (WT) protein and Rab5:Q79L, a GTP locked mutant, were used as control. Results are representative of three independent preparations.
Specificity of antibodies against LdRab5 and LdRab7
Antibodies against LdRab5 and LdRab7 were raised to characterize Hb trafficking in Leishmania. Western blot analysis showed that antibodies against LdRab5 and LdRab7 specifically recognized an ∼23 kDa and an ∼27 kDa protein, respectively, from Leishmania lysate (Figure 3) and did not cross-react with GST (data not shown).
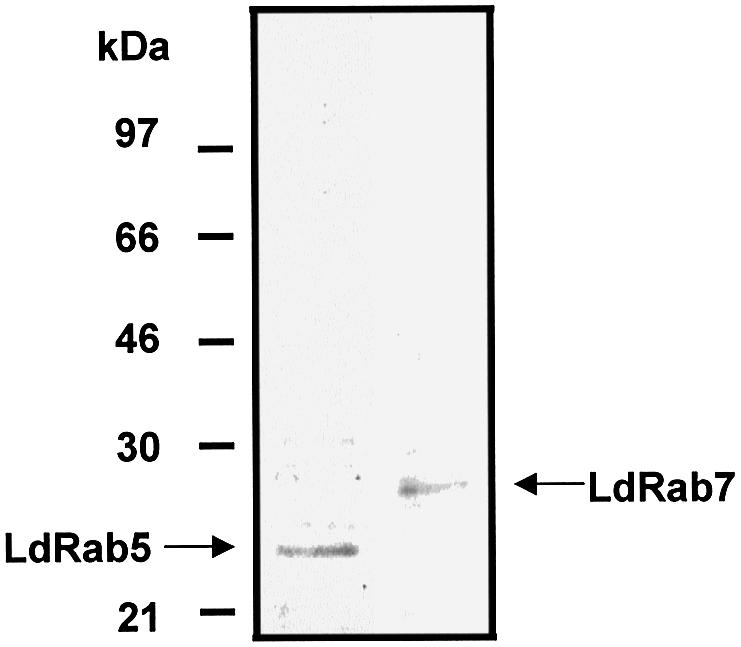
Fig. 3. Specificity of Leishmania Rab5 and Rab7 antibodies. Leishmania cell lysate (40 µg/lane) was analyzed by western blot using specific antibodies against LdRab5 and LdRab7. Results of western blots are representative of three independent preparations.
Subcellular fractionation and preparation of Rab5-enriched early endosomal compartment from Leishmania
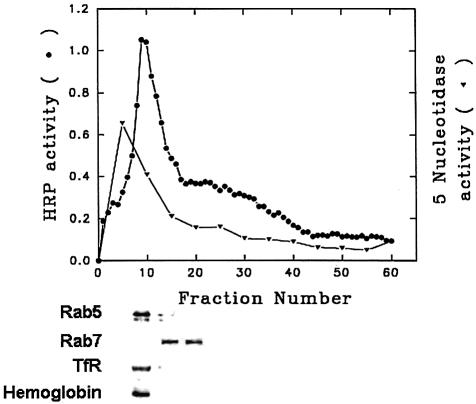
Previously we showed that Hb is internalized efficiently into the endocytic compartment within 5 min in Leishmania (Sengupta et al., 1999). Thus, internalization of biotinylated Hb (BHb) for 5 min was used to label the endocytic compartment. Another set of endosomes was labeled by 5 min internalization of avidin–horseradish peroxidase (AHRP), a fluid phase marker (Gorvel et al., 1991). In order to characterize compartments labeled with AHRP, subcellular fractionation was carried out and HRP activity was estimated from different fractions (50 µl) collected from the top of the gradient. Maximum HRP activity was obtained at the 8–30% interface (Figure 4). No significant HRP activity was detected at the top of the gradient, indicating that most of the endosomes were intact. Five successive fractions were pooled, washed and solubilized in SDS buffer, and western blot analyses were carried out to determine the nature of the compartments. Results presented in Figure 4 show that Rab5 and transferrin receptor were predominantly present in fraction numbers 6–10, which retained maximum HRP activity, whereas a Rab7-positive compartment was found in the later fractions with relatively low HRP activity. The doublet observed for Rab5 might be isoforms or prenylated and non-prenylated protein. Maximum activity of 5′-nucleotidase, a plasma membrane marker, was detected in the lighter fractions having relatively lower HRP activity (Figure 4). Hb was also detected in the fractions enriched in Rab5 and transferrin receptor, when similar fractionation was carried out following 5 min internalization of BHb.
Fig. 4. Subcellular fractionation of the L.donovani endocytic compartment. Promastigotes were disrupted after internalization of AHRP, and PNS was loaded onto a discontinuous sucrose gradient as described in Materials and methods. After centrifugation, 50 µl fractions were collected from the top of the gradient and analyzed for the presence of HRP. Five successive fractions from the top of the gradient were pooled, washed and analyzed by western blot for the presence of the indicated proteins (TfR, transferrin receptor). 5′-Nucleotidase activity was also measured from the pooled fractions. Similar fractionation was carried out using BHb as a probe, and the presence of Hb in the fractions was analyzed by western blot using anti-Hb antibody. Results from western blots are representative of three independent preparations.
Reconstitution and characterization of in vitro endosome–endosome fusion in Leishmania
Reconstitution of endosome fusion has been used successfully to determine the requirements for endocytosis (Gorvel et al., 1991). The results presented in Figure 5A show a typical in vitro fusion experiment in which two sets of endosomes containing BHb or AHRP were incubated for 1 h at 23°C in the presence of an ATP-regenerating system containing different concentrations of gel-filtered cytosol prepared from Leishmania promastigotes. Maximum fusion between the endosomes was observed at 1 mg/ml cytosol concentration. No fusion was detected in the absence of cytosol, suggesting a role for the cytosolic components in endosome fusion. The extent of early endosome fusion in Leishmania was significantly more at 23°C than at 37°C, whereas no fusion was detected at 4°C (data not shown). To determine the energy requirement, endosome fusion was carried out in an ATP-depleting system [250 mM sucrose, 0.5 mM EGTA, 20 mM HEPES-KOH pH 7.2, 1 mM dithiothreitol (DTT), 1.5 mM MgCl2, 100 mM KCl containing 5 mM glucose and 25 U/ml hexokinase, and 0.25 mg/ml avidin as scavenger]. Under these conditions, as well as in the presence of a 4.5 µM ATPγS-containing ATP-regenerating system, significant inhibition of fusion was observed between endosomes, indicating that both ATP and ATP hydrolysis are required for this process (Figure 5B).
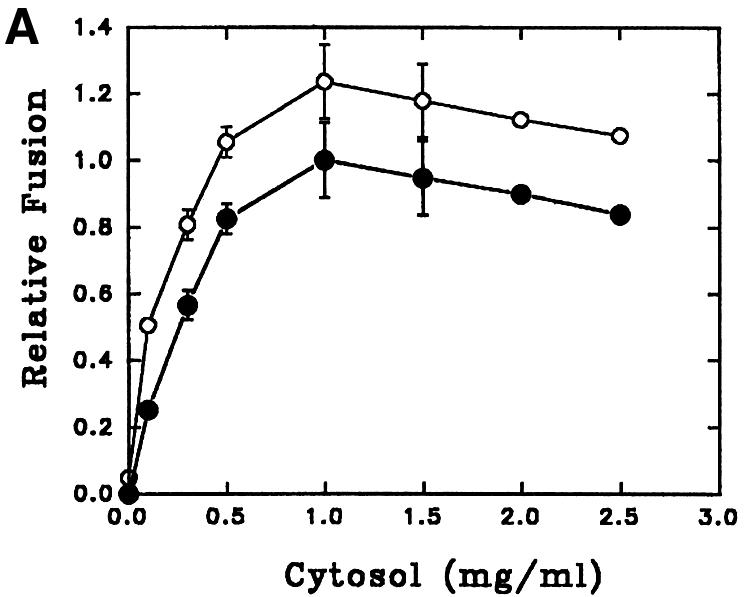
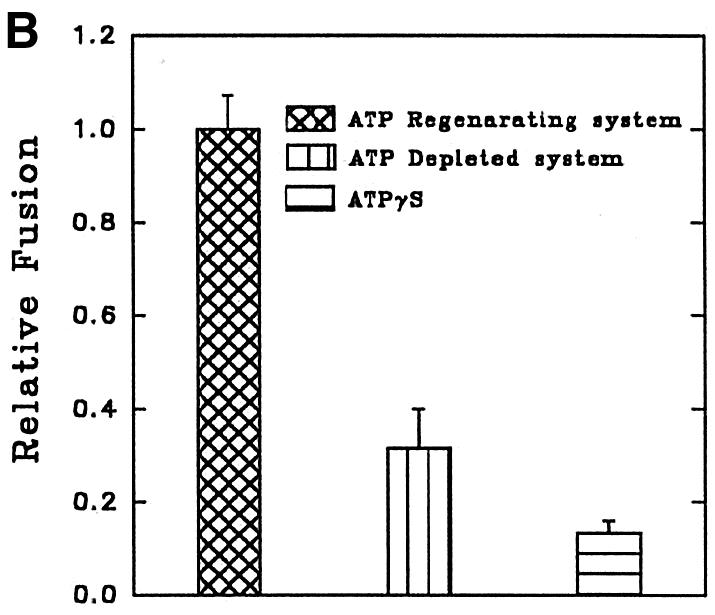
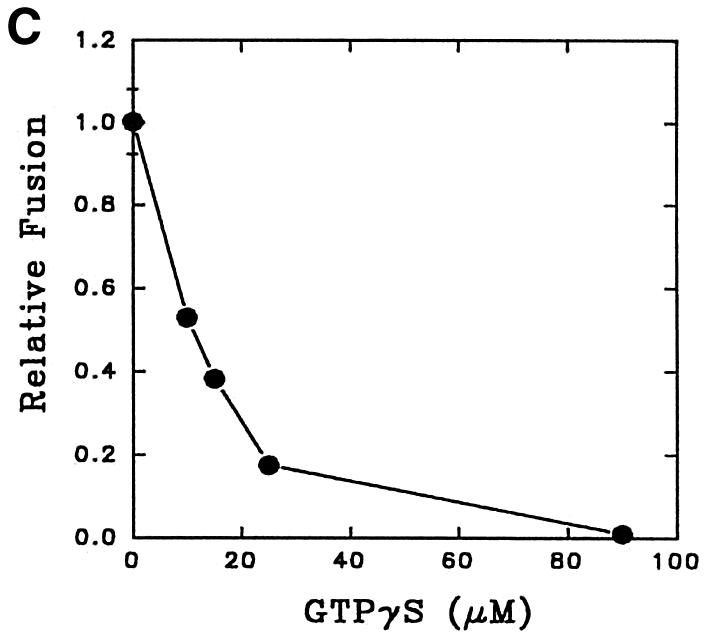
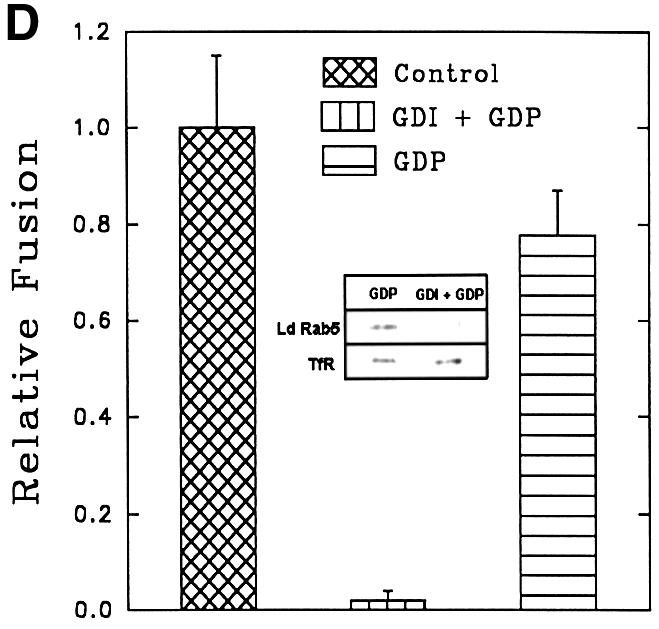
Fig. 5. Characterization of in vitro early endosome fusion in Leishmania. (A) Two sets of early endosomes containing BHb or AHRP were incubated in the presence of an ATP-regenerating fusion buffer supplemented with different concentrations of gel-filtered Leishmania cytosol for 1 h at 23°C. Specific fusion (filled circles) was measured by subtracting the value obtained in the absence of cytosol from the total fusion (open circles) observed in the presence of cytosol as indicated in Materials and methods. Maximum fusion was observed at 1 mg/ml of cytosol, which was normalized to 1 U, and results are expressed as relative fusion of three independent experiments ± SD. One unit corresponds to 12 ng of HRP activity/mg of protein. (B) In vitro fusion between endosomes from Leishmania was carried out in an ATP-regenerating system (Control), ATP-depleted system or in the presence of ATPγS (4.5 µM). Fusion obtained in the control was chosen as 1 U, and the results are expressed as relative fusion of three independent experiments ± SD. One unit corresponds to 13.6 ng of HRP activity/mg of protein. (C) Fusion between endosomes in Leishmania was carried out in an ATP-regenerating system in the presence of different concentrations of GTPγS and 1.5 mg/ml cytosol. Fusion obtained in the absence of GTPγS was chosen as 1 U, and the results are expressed as relative fusion of three independent experiments ± SD. One unit corresponds to 12.6 ng of HRP activity/mg of protein. (D) Endosomes containing BHb treated either with GDP (1 mM) alone or with GDI (6 µg/ ml) as described (Mukherjee et al., 2000) were centrifuged and pellets were analyzed for the presence of Rab protein by western blot using anti-LdRab5. Transferrin receptor (TfR) was used as a control (inset). Treated and untreated (control) endosomes containing BHb were used in the fusion assay. Fusion obtained with untreated endosomes was chosen as 1 U, and results are expressed as relative fusion of three independent experiments ± SD. One unit corresponds to 14.5 ng of HRP activity/mg of protein.
About 80% fusion between early endosomes at a high cytosol concentration (1.5 mg/ml) was inhibited by 25 µM GTPγS, a non-hydrolyzable analog of GTP (Figure 5C). Moreover, treatment of endosomes containing BHb with 6 µg/ml of mammalian GDI (GDP dissociation inhibitor) in the presence of 1 mM GDP specifically removed Leishmania Rab protein, i.e. Rab5, but not transferrin receptor (Figure 5D, inset). Rab-depleted endosomes containing BHb inhibited fusion with endosomes containing AHRP by ∼90%, indicating that Rab GTPases may be regulating this fusion process (Figure 5D).
Role of NSF-like protein in endosome fusion in Leishmania
In vitro fusion between endosomes from mammalian cells is inhibited by N-ethylmaleimide (NEM) treatment and can be restored by the addition of NEM-sensitive factor (NSF) (Diaz et al., 1989). To determine the role of NSF in early endosome fusion in Leishmania, fusion was carried out in the presence of different concentrations of NEM. Figure 6A shows that ∼70% of the fusion was inhibited by 0.3 mM NEM. Endosome fusion was also significantly abrogated in cytosol immunodepleted with antibody against mammalian NSF, which detected an ∼70 kDa protein, presumably the NSF homolog, in Leishmania lysate (Figure 6B, inset).
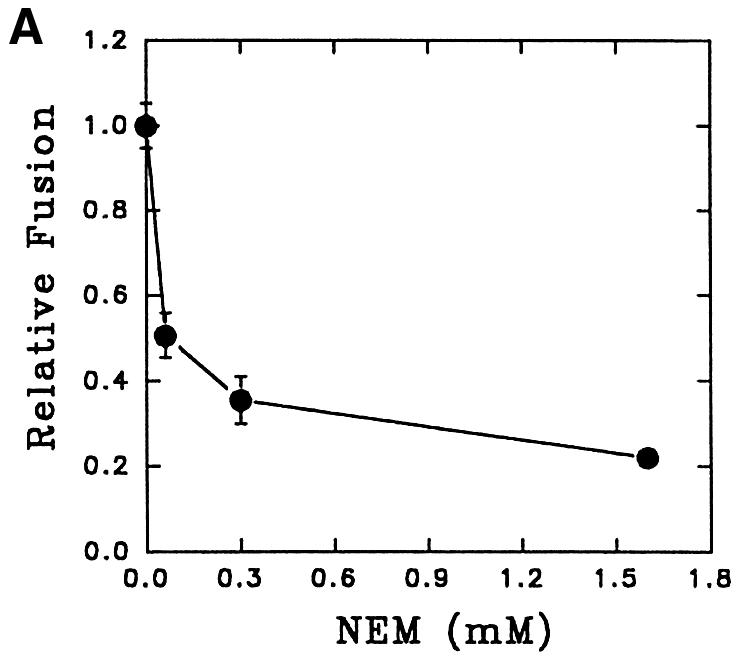
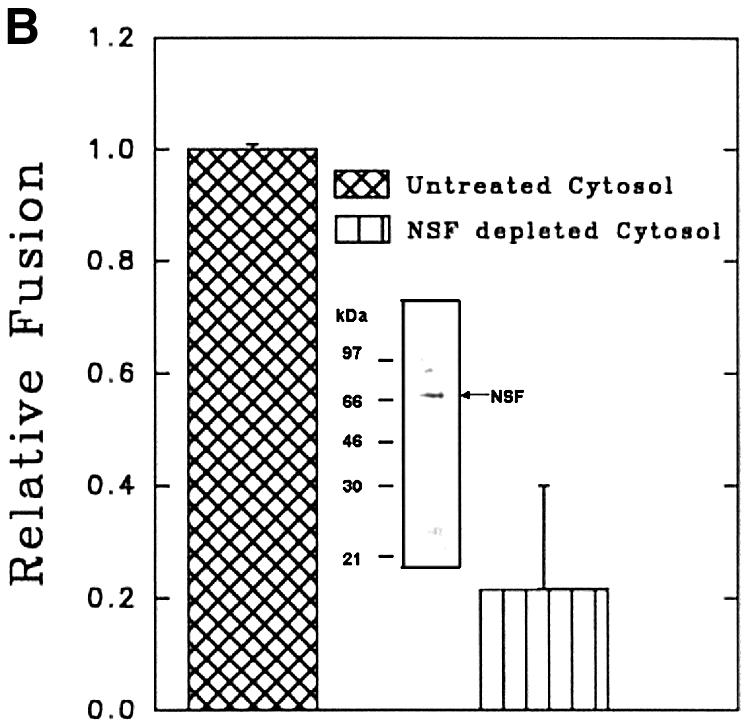
Fig. 6. Role of NSF-like protein in endosome fusion in Leishmania. (A) Cytosol prepared from Leishmania was treated with different concentrations of NEM (30 min at 4°C) and excess NEM was quenched with 3 mM DTT. Fusion was carried out in an ATP-regenerating system containing NEM-treated cytosol. Fusion obtained in the absence of NEM was chosen as 1 U, and the results are expressed as relative fusion of three independent experiments ± SD. One unit corresponds to 10.6 ng of HRP activity/mg of protein. (B) Endosome fusion was carried out in the presence of NSF-immunodepleted cytosol as indicated in Materials and methods. Fusion obtained with untreated cytosol was chosen as 1 U, and the results are expressed as relative fusion of three independent experiments ± SD. One unit corresponds to 11.2 ng of HRP activity/mg of protein. Inset shows western blot analysis of Leishmania cell lysate (40 µg/ lane) with antibodies against mammalian NSF. Results from western blots are representative of three independent preparations.
Role of endocytic Rabs in intracellular trafficking of Hb
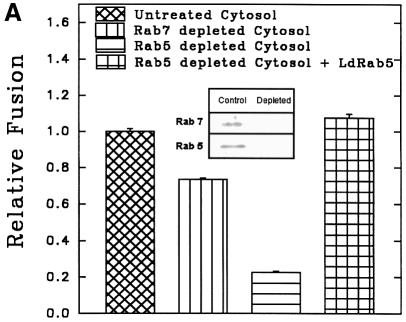
To determine the role of endocytic Rabs in endosome fusion in Leishmania, fusion of BHb-loaded early endosomes with early endosomes containing AHRP was carried out in the presence of Rab5- or Rab7-immunodepleted cytosol. Anti-LdRab5 and anti-LdRab7 specifically depleted the respective proteins from Leishmania cytosol (Figure 7A, inset). Fusion between early endosomes was inhibited by ∼75% in Rab5-depleted cytosol, whereas Rab7 depletion reduced fusion by only ∼25% (Figure 7A). Addition of in vitro prenylated LdRab5 (Lombardi et al., 1993) to the Rab5-depleted system completely restored fusion to the control level (Figure 7A). Previous studies have shown that early endosomes can fuse with early endosomes, whereas in vitro fusion between early and late endosomes does not occur (Gorvel et al., 1991). This prompted us to test heterotypic fusion between early and late endosomes in Leishmania. Accordingly, late endosomal fractions were prepared by 5 min internalization of AHRP followed by 15 min chase at 23°C, as described previously (Gorvel et al., 1991; Laurent et al., 1998), and separated by sucrose gradient. Partial characterization of fractions containing maximum HRP activity revealed that these vesicles predominantly contain Rab7, a late endosomal marker, but no Rab5 or transferrin receptor, whereas 5 min internalized AHRP vesicles possess early endosomal markers such as Rab5 and transferrin receptor (Figure 7B). In contrast to the previous study (Gorvel et al., 1991), ∼80% fusion between BHb-loaded early endosomes and late endosomes containing AHRP was observed in Leishmania (Figure 7C). However, consistent with the earlier report, Figure 7C shows that early endosomes containing fluid phase markers such as avidin and biotinylated HRP (BHRP) fuse efficiently, whereas fusions of early endosomes containing avidin with late endosomes containing BHRP is significantly inhibited. Moreover, Figure 7D shows that the observed heterotypic fusion between BHb-loaded early endosomes and late endosome containing AHRP is significantly inhibited in the presence of Rab7-depleted cytosol but not by Rab5-depleted cytosol. Addition of in vitro prenylated LdRab7 to the Rab7-depleted system significantly restored fusion (Figure 7D). These results indicate that the cytoplasmic tail of the Hb receptor (HbR) from early endosomes containing BHb may possibly promote this heterotypic fusion.
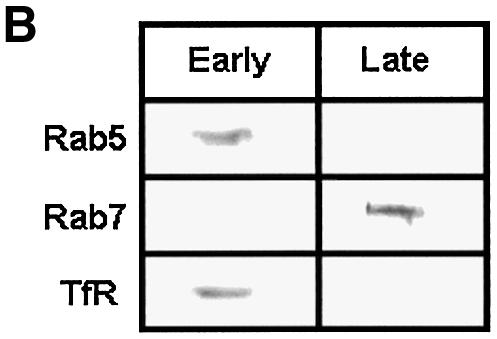
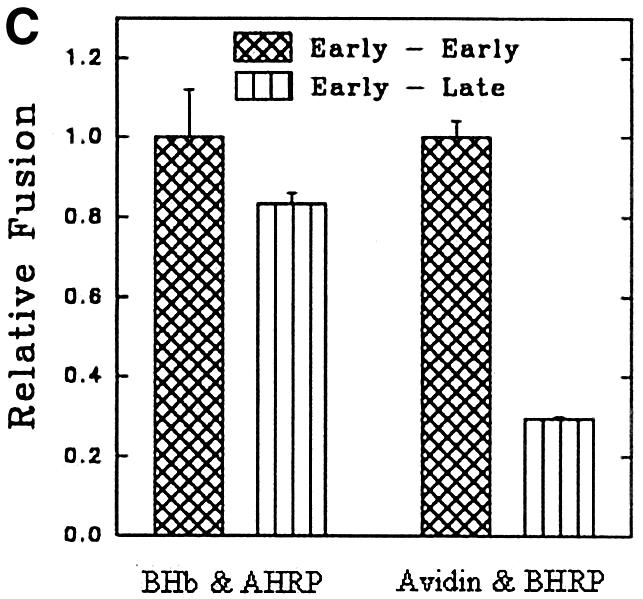
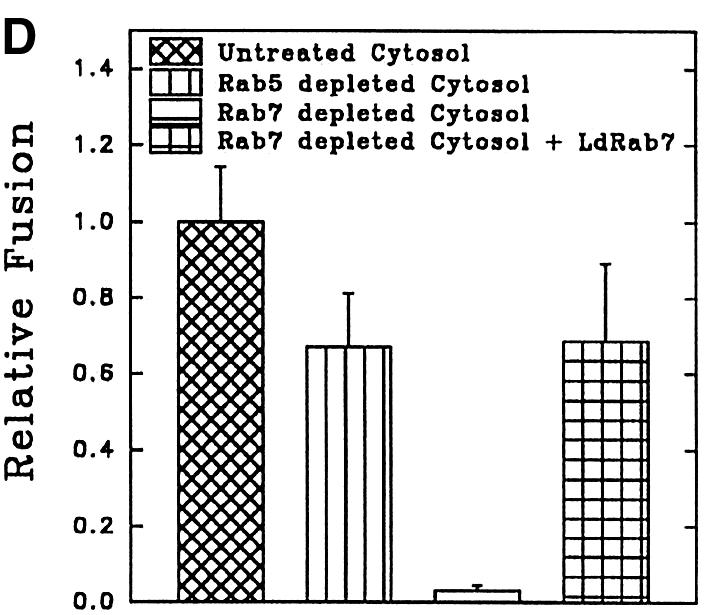
Fig. 7. Role of endocytic Rabs in endosome fusion in Leishmania. (A) Fusion between early endosomes containing BHb or AHRP was carried out in an ATP-regenerating system in the presence of untreated cytosol, LdRab7-depleted cytosol, LdRab5-depleted cytosol or LdRab5-depleted cytosol supplemented with 500 ng of in vitro prenylated LdRab5 as described in Materials and methods. Fusion obtained with untreated cytosol was chosen as 1 U, and the results are expressed as relative fusion of three independent experiments ± SD. One unit corresponds to 13.2 ng of HRP activity/mg of protein. Inset shows immunodepletion of Rab5 or Rab7 from Leishmania cytosol using specific antibodies. (B) AHRP-labeled early and late endosomes purified by sucrose density gradient were analyzed for the presence of early and late compartment-specific markers by western blot using specific antibodies (TfR, transferrin receptor). (C) Fusion of early endosomes containing BHb with early or late endosomes containing AHRP was carried out in an ATP-regenerating system in the presence of cytosol. Similarly, fusion of early endosomes containing fluid phase marker such as avidin was carried out with early or late endosomes containing BHRP. Fusion obtained between the early endosomes in the respective system was chosen as 1 U, and results are expressed as relative fusion of three independent experiments ± SD. One unit corresponds to 15.2 and 12.8 ng of HRP activity/mg of protein in the fusion assay of early endosomes containing BHb and avidin, respectively. (D) Heterotypic fusion between BHb-loaded early endosomes and late endosomes containing AHRP was carried out in an ATP-regenerating system in the presence of untreated cytosol, LdRab5-depleted cytosol, LdRab7-depleted cytosol or LdRab7-depleted cytosol supplemented with 500 ng of in vitro prenylated LdRab7. Fusion obtained with untreated cytosol was chosen as 1 U, and results are expressed as relative fusion of three independent experiments ± SD. One unit corresponds to 12.4 ng of HRP activity/mg of protein.
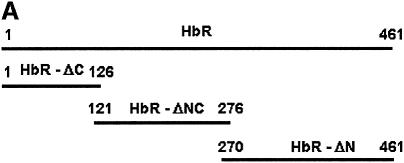
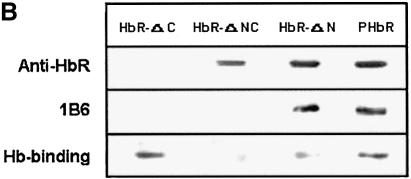
To determine the role of the cytoplasmic tail of the HbR in heterotypic fusion, we have cloned and expressed different deletion mutants of the receptor as GST fusion proteins (Figure 8A): the N-terminus (HbR-ΔC), middle region (HbR-ΔNC) and C-terminus (HbR-ΔN). Figure 8B shows that polyclonal antibody against HbR purified from Leishmania (PHbR) recognized HbR-ΔNC and HbR-ΔN, while a monoclonal antibody (1B6) specifically recognized HbR-ΔN. In addition, HbR-ΔC predominantly bound to Hb, in comparison with other fragments. PHbR was used as a positive control. No binding was observed with GST (data not shown). When BHb-loaded early endosomes were pre-treated with 1B6, heterotypic fusion with late endosomes containing AHRP was significantly inhibited. Moreover, addition of HbR-ΔN in the fusion assay inhibited ∼80% of fusion, whereas no significant inhibition was observed with HbR-ΔC or HbR-ΔNC (Figure 8C).
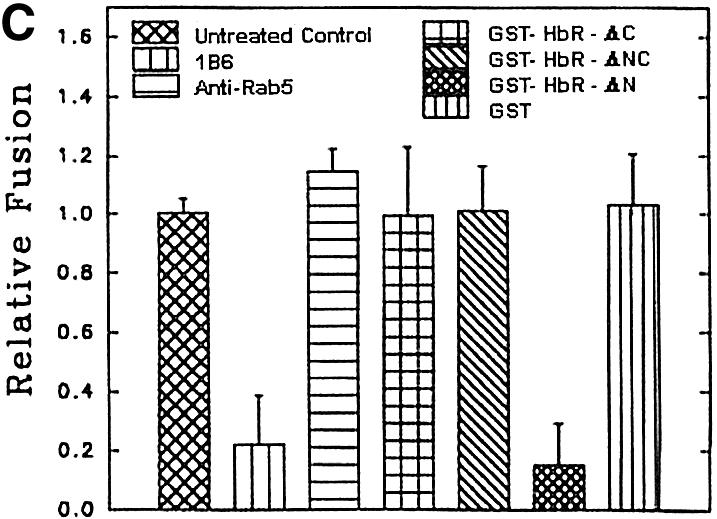
Fig. 8. Role of HbR cytoplasmic tail in heterotypic fusion in Leishmania. (A) Deletion mutants HbR-ΔC, HbR-ΔNC and HbR-ΔN, corresponding to the N-terminus, middle region and C-terminus of HbR, respectively, were expressed and purified as GST fusion proteins. (B) Purified HbR and deletion mutants (1 µg/lane) were analyzed by western blot using anti-HbR polyclonal antibody (top), monoclonal antibody, 1B6 (middle) and hemoglobin binding (bottom) as described in Materials and methods. (C) To determine the role of the HbR cytoplasmic domain, BHb-containing early endosomes were incubated with 1B6, anti-LdRab5 antibody or the indicated deletion mutants of HbR (280 ng) for 30 min at 4°C. Subsequently, late endosomes containing AHRP were added to treated early endosomes along with cytosol containing an ATP-regenerating system, and fusion was carried out as described in Materials and methods. Fusion obtained with untreated endosomes was chosen as 1 U, and the results are expressed as relative fusion of three independent experiments ± SD. One unit corresponds to 12.6 ng of HRP activity/mg of protein.
Localization of Rab5 in Leishmania
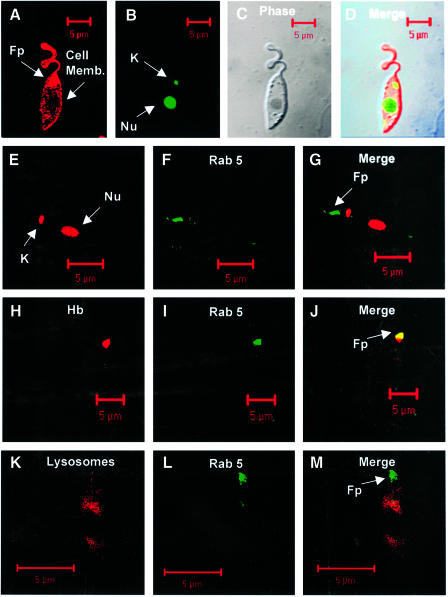
Immunolocalization showed that L.donovani Rab5 is localized in the anterior end near the flagellar reservoir (Figure 9G), which is indicated by the presence of nucleus and kinetoplast (Figure 9D). Also, Rab5 co-localized with 5 min internalized Hb (Figure 9J), which is clearly separated from the Lysotracker Red-labeled perinuclear lysosomal compartment (Figure 9M).
Fig. 9. Confocal images showing the localization of Rab5 in L.donovani promastigotes. Cells were fixed in formaldehyde and Rab5 was visualized by anti-LdRab5 antibody and subsequently probed with goat anti-mouse Alexa Fluor 488-labeled second antibody (F, G, I, J, L and M) as described in Materials and methods. Early endosomes were visualized by 5 min uptake of BHb, probed with avidin–Texas red (H and J). Yellow indicates co-localization of Rab5 with Hb in early endosomes (J). Nucleus (Nu) and kinetoplast (K) were labeled with propidium iodide (E and G), and lysosome-like compartments were visualized by pre-incubating the promastigotes with Lysotracker Red (K and M) for 30 min at 23°C. The upper panel shows the phase image (C) of plasma membrane labeled with FM4-64 (A) and kinetoplast and nucleus stained with Syto green (B and D). Fp indicates the flagellar pocket.
Discussion
To understand the intracellular trafficking of Hb in L.donovani promastigotes and its sorting in the early endocytic compartment, we have used an in vitro reconstitution assay (Gruenberg et al., 1989) of endosomes isolated from Leishmania promastigotes using appropriate receptor-mediated or fluid phase endocytic probes. The cell fractionation data and partial characterization of purified vesicles revealed that 5 min internalization of an appropriate endocytic probe specifically labeled early endosomes enriched in Rab5 and transferrin receptor, whereas 5 min internalization followed by 15 min chase specifically labeled late endosomes containing Rab7, as observed in previous studies (Gorvel et al., 1991; Laurent et al., 1998). These results are in agreement with the findings that Rab5-positive early endosomes regulate early events of endocytosis (Gorvel et al., 1991; Mukhopadhyay et al., 1997a), whereas Rab7, localized in late endosomes, serves as a targeting signal to the late compartment (Feng et al., 1995).
Our results show that several features of homotypic early endosome fusion in Leishmania are similar to fusion events described previously in mammalian cells (Gruenberg et al., 1989; Gorvel et al., 1991). Thus, early endosome fusion in Leishmania requires cytosol, ATP and its hydrolysis. A significantly higher level of fusion between endosomes from Leishmania was observed at its optimal growth temperature of 23°C than at 37°C (data not shown). This is not surprising since Dictyostelium, which grows optimally at 21–28°C, exhibits significant fusion at a similar temperature (Lenhard et al., 1992). It has been shown in several systems that Rab-GDI in the presence of GDP specifically depletes Rab proteins from the membrane (Funato et al., 1997) and exhibits broad substrate specificity across species (Attal and Langsley, 1996; Chaturvedi et al., 1999). In agreement with this, mammalian GDI along with GDP selectively stripped off Leishmania Rab protein from the endosomes, rendering them fusion incompetent, demonstrating the role of Rab proteins in this fusion.
Another ubiquitous factor required for vesicle fusion in mammalian cells is NSF, a homohexamer having both ATP-binding and hydrolyzing activities (May et al., 2001). The current model suggests that NSF in its ATP-bound state binds to the membrane through soluble NSF attachment protein (SNAP), and ATP hydrolysis of NSF triggers rearrangement of v-SNARE and t-SNARE (SNAP receptor), which actually mediate membrane fusion (Chen and Scheller, 2001). As NSF is an ATPase and because our findings that ATPγS and NEM treatment inhibit fusion of Leishmania endosomes, we explored the role of NSF-like protein in this fusion. NSF is reported to be well conserved among different organisms, and antibodies against NSF from one organism cross-react with others (Weidenhaupt et al., 1998). Accordingly, in our study, anti-mammalian NSF antibody specifically recognizes an ∼70 kDa protein in Leishmania, and fusion carried out in the presence of cytosol immunodepleted using this antibody is significantly inhibited, demonstrating the role of NSF-like protein in endosome fusion in Leishmania. These results along with others suggest that, as in higher eukaryotic cells, an NSF-mediated SNARE complex is likely to regulate endocytosis in unicellular protozoa (Chaturvedi et al., 1999; Bogdanovic et al., 2000).
Previous studies have shown that Rab5 regulates homotypic fusion between early endosomes. To determine the role of Rab5 in endosome fusion in Leishmania, we have cloned and expressed LdRab5, which specifically binds GTP. However, GTPase activity of LdRab5 is lower than mammalian Rab5. It has been shown that consensus sequences of Rabs in switch I (IGVDF) and switch II (KLQIW) regions are crucial for GTP hydrolysis and GDP/GTP exchange and this sequence is sensitive to alteration. The switch I and switch II regions of LdRab5 consist of VGASF and HFDIW, respectively, which may possibly explain the relatively low GTPase activity of LdRab5 as compared with its mammalian equivalent. However, LdRab5 contains RYKS and YYRGA, the signature motifs of the Rab subfamily (Pereira-Leal and Seabra, 2000; Stenmark and Olkkonen, 2001). Immunolocalization shows that Rab5 co-localizes with the 5 min internalized Hb-containing compartment, indicating that Rab5 in Leishmania is localized in an early endocytic compartment. Recently, it has been shown that LmRab7 localized in the perinuclear late endosome/lysosome compartment in L.mexicana (Denny et al., 2002). Subsequently, LdRab5, LdRab7 and specific antibodies were used to characterize endosome fusion in Leishmania. We observe that LdRab5 regulates fusion of BHb-loaded early endosomes with early endosomes containing AHRP from Leishmania, resembling the results of an earlier report (Gorvel et al., 1991). However, early endosome fusion in Dictyostelium appears to be regulated by Rab7, suggesting that Rab7 may couple both ends of the endocytic pathway in D.discoideum (Laurent et al., 1998).
It has been shown in different reconstitution systems that early endosomes are capable of homotypic fusion in vitro, whereas heterotypic fusion between early endosomes and late endosomes does not occur in vitro (Gorvel et al., 1991; Laurent et al., 1998). Similarly, early and late endosomes prepared from Leishmania promastigotes after fluid phase uptake of avidin and BHRP, respectively, do not fuse as observed in other systems. In contrast, early endosomes containing a receptor-mediated endocytic probe, BHb, drive the fusion with both early and late compartments in Leishmania, and Rab7 regulates this heterotypic fusion.
Several studies indicate that the signals for endocytosis and intracellular trafficking often reside in the cytoplasmic domain of the receptor. For example, deletion of core kinase sequences from the distal region of the cytoplasmic domain of the epidermal growth factor (EGF) receptor impaired proper trafficking of the receptor to the late/lysosomal compartment (Kornilova et al., 1996). Similarly, a sequence distal to the endocytic motif of cation-independent mannose 6-phosphate receptor in the cytoplasmic tail is required for efficient transport to late endosomes (Juuti-Uusitalo et al., 2000). In addition, dendritic cells express DEC-205, an endocytic receptor-like macrophage mannose receptor (MMR). However, unlike MMR, DEC-205 receptor recycles from the late compartment and the targeting signal is localized in the distal region of the cytoplasmic tail (Mahnke et al., 2000). Thus, it is tempting to speculate that the cytoplasmic tail of HbR projecting from the early endosome may transduce some signal(s) to mediate fusion with late endosomes in Leishmania.
In order to prove unequivocally that the cytoplasmic domain of the HbR regulates heterotypic fusion, we have cloned and expressed different deletion mutants of HbR, which is a transmembrane protein having kinase activity (data not shown). Topology prediction (TMPred; Hofmann and Stoffel, 1993) of HbR sequence and the observed maximum binding of Hb with HbR-ΔC suggest that possibly the N-terminus is the extracellular domain of HbR. Our results show that BHb-loaded early endosomes pre-treated with monoclonal antibody (1B6), specific to the C-terminus of HbR, significantly inhibit heterotypic fusion with late endosomes containing AHRP. Similarly, addition of HbR-ΔN in the fusion assay inhibited ∼80% of fusion, whereas no significant inhibition was observed with HbR-ΔC or HbR-ΔNC. These results demonstrate that signal transduced from the HbR tail projecting from the early endosomal compartment is blocked by a C-terminus-specific antibody or competed by HbR-ΔN, indicating that the signal mediated through the C-terminal cytoplasmic tail of HbR may promote the fusion with late endosomes.
In conclusion, our results represent the first documentation that endocytosis in unicellular parasitic protozoa such as Leishmania is regulated by small GTP-binding proteins of the Rab family through vesicle fusion. Interestingly, our results have shown that early endosomes containing Hb in Leishmania fuse efficiently with both early and late compartments. We suggest that Hb in Leishmania first moves to an early endosomal compartment where Rab5-dependent rapid exchange between the endosomes occurs. Subsequently, Hb is targeted to the late/lysosomal compartment through signals mediated by the cytoplasmic tail of the receptor, which is Rab7 dependent. It will be interesting to determine the nature of signal(s) mediated through the receptor tail, which promotes heterotypic fusion between early endosomes and late endosomes in Leishmania.
Materials and methods
Materials
Avidin, AHRP, N-hydroxy succicinimidobiotin (NHS-Bio) and bicinchoninic acid (BCA) were purchased from Pierce Biochemicals (Rockford, IL). Recombinant mammalian GDI and Rab5 constructs were kindly provided by Dr Philip Stahl (Washington University School of Medicine, St Louis, MO). A mouse monoclonal anti-Rab5 antibody was received as a gift from Dr A.Wandinger-Ness (Northwestern University, Evanston, IL). Alexa Fluor 488, goat anti-mouse IgG and Lysotracker Red were purchased from Molecular Probes (Eugene, OR). All HRP-labeled secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Enhanced chemiluminescence (ECL) reagents were from Amersham Biosciences (Amersham, UK). Unless otherwise stated, all other reagents were obtained from Sigma Chemical Co (St. Louis, MO). Anti-Hb, anti-HbR and anti-NSF polyclonal or monoclonal antibodies were raised in mice by standard techniques (Overkamp et al., 1988; Celis et al., 1994). Hb was biotinylated using NHS-Bio as described previously (Gruenberg et al., 1989). Cytosol was prepared from Leishmania using a procedure similar to that described previously (Mukherjee et al., 2000).
Cells
Leishmania donovani promastigotes (UR 6) were obtained from the Indian Institute of Chemical Biology, Kolkata, India. Cells were routinely maintained on blood agar slants containing glucose, peptone, sodium chloride, beef heart extract, rabbit blood and gentamycin (Roy, 1932). For experimental use, cells were harvested in phosphate-buffered (10 mM pH 7.2) saline (0.15 M) from 3-day-old slants.
Cloning and expression of Rab5 homolog from L.donovani
A putative Rab5-like sequence with substantial homology to T.brucei Rab5B was identified from the L.major genome using BLAST. Accordingly, forward (5′-GGATCCATGTCATCCATCAGTCGC-3′) and reverse (5′-GAATTCCTAGCAGCATCCGTTCTCT-3′) primers were designed against the start and stop codons, respectively, of the putative Rab5 sequence of L.major. RT–PCR was performed using these primers to amplify the open reading frame of the putative Rab5 sequence using L.donovani cDNA. mRNA isolated from Leishmania promastigotes using an Oligotex mRNA kit (Qiagen, Germany) was used for cDNA synthesis using a Thermo Script RT–PCR kit (Gibco-BRL) as per the manufacturer’s instructions. Subsequently, PCR was performed using the above primers in a Perkin-Elmer thermocycler for 30 cycles: denaturation at 94°C for 3 min; annealing at 55°C for 30 s; and extension at 72°C for 1 min. The PCR product was cloned into pGEMT-easy vector (Promega) and sequenced using m13 universal primers in an automated sequencer. Subsequently, the PCR product was cloned into BamHI–EcoRI sites of the pGEX-4T-2 vector (Amersham Biosciences) and transformed into Escherichia coli.
Expression and purification of LdRab5
Transformed E.coli were grown in LB and induced with 0.2 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h at 37°C for expression of GST–LdRab5. Cells were harvested, lysed by sonication and lysate was treated with Triton X-100 (1%). Cellular debris was removed by centrifugation at 13 000 g for 10 min at 4°C. The fusion protein, GST–LdRab5, was purified from the supernatant using reduced glutathione beads by standard procedures.
Generation of antibodies against LdRab5 and LdRab7
GST–LdRab5 immobilized on glutathione beads was cleaved with 10 U of thrombin (Pharmacia) in cleavage buffer (50 mM Tris–HCl pH 7.5 containing 150 mM NaCl and 2.5 mM CaCl2) for 3 h at 24°C, and beads were removed by centrifugation (Smith and Corcoran, 1993). After ensuring the purity of the released LdRab5 by SDS–PAGE, mice were immunized with this protein to raise antibodies by standard techniques (Overkamp et al., 1988). We have also cloned and expressed Rab7 from L.donovani (data not shown) and raised antibody against LdRab7 using a similar procedure. The specificity of the antibodies against the respective proteins was determined by western blot analysis using Leishmania cell lysate.
GTP overlay assay
GTP binding of LdRab5 was detected in an overlay assay (Via et al., 1997). Briefly, different amounts of GST–LdRab5 and mammalian GST–Rab5 proteins blotted onto nitrocellulose membranes were incubated with 1 µCi/ml of [α-32P]GTP (3000 Ci/mM, NEN) in 50 mM phosphate buffer pH 7.5 containing 5 mM MgCl2, 1 mM EGTA and 0.3% Tween-20, washed and visualized by autoradiography.
GTPase assay
The GTPase activity of LdRab5 was determined as described previously (Stenmark et al., 1994). Briefly, 2 pmol of immobilized Rab5 was incubated with buffer A (20 mM Tris–HCl pH 7.8, 100 mM NaCl, 5 mM MgCl2, 1 mM Na-phosphate and 10 mM 2-mercaptoethanol) for 20 min at 25°C and bound nucleotides were eluted with 1 M guanidine-HCl. Immobilized proteins were then incubated with 2 pmol [α-32P]GTP (800 Ci/mmol, Perkin Elmer Life Sciences, MA) in 20 µl of buffer A for 10 min at 0°C and incubated for various times at 23°C. Samples were washed and incubated in 8 µl of buffer B (0.2% SDS, 2 mM EDTA, 10 mM GDP, 10 mM GTP pH 7.5) and heated for 2 min at 70°C. An aliquot was analyzed using thin-layer chromatography and visualized by autoradiography.
Preparation of early endosomes from Leishmania
Leishmania promastigotes were incubated with BHb (2 mg/ml) in internalization medium (MEM containing 10 mM HEPES and 5 mM glucose pH 7.4) for 5 min at 23°C, to label the early endosomal compartment. Internalization of BHb was stopped by adding cold medium, and cells were washed three times with cold homogenization buffer (HB; 20 mM HEPES, 250 mM sucrose and 2 mM EGTA, pH 7.2 containing protease inhibitors). Uninternalized BHb bound to the cells was quenched by incubating the cells with excess avidin (200 µg/ml) at 4°C for 30 min. After washing with HB, 5 × 109 cells were resuspended in 12 ml of HB, equilibrated in a pre-cooled nitrogen cavitation bomb (Parr Instrument company, IL) with 750 p.s.i. N2 for 25 min and disrupted by release of N2 from the bomb (Shapiro et al., 1989). The unbroken cells, nuclei and other cell debris were removed by low speed centrifugation at 500 g for 10 min at 4°C. The post-nuclear supernatant (PNS) was snap-frozen in liquid nitrogen. The enriched endosomal fraction from the PNS was prepared as described previously (Mukherjee et al., 2000). Briefly, thawed PNS was diluted with HB (1:3) and centrifuged at 20 000 g for 1 min at 4°C. The resultant supernatant was again centrifuged at 100 000 g for 5 min at 4°C. The pellet enriched in early endosomal vesicles was used for in vitro fusion assays. Another set of endosomes containing AHRP was prepared similarly.
Likewise, late endosomes were labeled by 5 min internalization followed by 15 min chase of AHRP or BHRP at 23°C. HRP-labeled enriched late endosomal vesicles were purified from the sucrose gradient as described below.
Subcellular fractionation of L.donovani endocytic compartment
To characterize the compartments labeled by 5 min internalization of AHRP by L.donovani promastigotes at 23°C, subcellular fractionation was carried out as described previously (Laurent et al., 1998). Briefly, cells were disrupted after internalization of AHRP and 0.5 ml of PNS was loaded onto a discontinuous sucrose density gradient formed by layering 0.35 ml of 54%, 1.45 ml of 40% and 1.45 ml of 30% sucrose in HB. After centrifugation in an MLS 50 rotor (Beckman TL100) at 100 000 g for 1 h at 4°C, 50 µl fractions were collected from the top of the gradient and samples were analyzed for the presence of HRP, 5′-nucleotidase activity (Graham, 1993) and various compartment-specific markers. A similar fractionation was carried out using BHb as probe, and the presence of Hb in fractions was detected by western blot analysis using anti-Hb antibody.
In vitro reconstitution of endosome fusion in Leishmania
Reconstitution of fusion between endosomes prepared from Leishmania was carried out as described earlier (Gorvel et al., 1991). Briefly, two sets of endosomes containing BHb or AHRP were mixed in fusion buffer (250 mM sucrose, 0.5 mM EGTA, 20 mM HEPES-KOH pH 7.2, 1 mM DTT, 1.5 mM MgCl2, 100 mM KCl, including an ATP-regenerating system, 1 mM ATP, 8 mM creatine phosphate, 31 U/ml creatine phosphokinase and 0.25 mg/ml avidin as scavenger) supplemented with gel-filtered (G-25 Sephadex) cytosol prepared from Leishmania. Fusion was carried out for 1 h at 23°C and the reaction was stopped by chilling on ice. The membrane was solubilized in solubilization buffer (SB; PBS containing 1% Triton X-100 and 0.2% methylbenzethonium chloride with 0.25 mg/ml avidin as scavenger), and BHb–AHRP complexes were immunoprecipitated using anti-Hb antibody. The HRP activity associated with the BHb–AHRP complex was measured as fusion units using O-phenylenediamine as chromogenic substrate (Gruenberg et al., 1989). Fusion carried out in the absence of cytosol was low and was subtracted from the corresponding values to determine specific fusion. Maximum fusion obtained at 1 mg/ml cytosol concentration was expressed as 1 U of relative fusion. HRP activity corresponding to 1 U is specified in the figure legends.
Immunodepletion of Leishmania cytosol using specific antibodies
To deplete Rab5 from Leishmania cytosol, 100 µl of protein A/G plus–agarose (Santa Cruz Biotechnology) was incubated with 10 µl of anti-LdRab5 antibody in PBS overnight at 4°C. The antibody–protein A/G–agarose complex was washed and blocked with 1 mg/ml bovine serum albumin (BSA) for 1 h at 4°C. Subsequently, 600 µg of cytosol containing protease inhibitors was added to the protein A/G–agarose–anti-LdRab5 complex and incubated for 1 h at 4°C as described previously (Mukherjee et al., 2001). Rab5-depleted Leishmania cytosol was separated from the agarose beads by centrifugation. NSF- or Rab7-depleted Leishmania cytosol was prepared similarly using the respective antibodies.
Generation of HbR deletion mutants
Recently we have cloned and expressed HbR from L.donovani (data not shown). Different deletion mutants corresponding to the 5′ end (1–378 bp), middle region (363–828 bp) and 3′ end (810–1383 bp) of the gene were amplified by PCR using appropriate forward and reserve primers designed against the HbR sequence. These fragments were cloned into pGEMT-easy vector, sequenced and subcloned into pGEX-4T-2 vector. The GST fusion proteins, HbR-ΔC, HbR-ΔNC and HbR-ΔN, corresponding to the N-terminus, middle region and C-terminus of HbR, respectively, were expressed in E.coli and purified by standard procedure.
Hemoglobin binding activity of HbR
Purified HbR and different deletion mutants were subjected to SDS–PAGE (1 µg of each protein) and transferred to a nitrocellulose membrane. After blocking with 5% BSA and incubating with 1 µg/ml Hb, the membrane was incubated with anti-Hb antibody and subsequently probed with HRP-labeled secondary antibodies. Hb bound to various proteins was visualized by ECL.
Immunolocalization of Rab5 in L.donovani
In order to localize Rab5 in L.donovani promastigotes, cells were washed three times with PBS and fixed in 4% formaldehyde in PBS for 20 min at 4°C. Following the washes, thin smears of cells were prepared on glass slides and permeabilized with 0.4% saponin for 20 min at 24°C. Subsequently, cells were incubated with 10% fetal calf serum (FCS) to block non-specific binding, washed and probed with anti-LdRab5 antibody in PBS containing 0.4% saponin for 30 min at 24°C. Cells were washed three times with PBS and incubated with Alexa Fluor 488-labeled goat anti-mouse secondary antibody in the same buffer for 30 min at 24°C. Finally, the cells were washed and incubated for 15 min at 24°C with 5 µg/ml of propidium iodide to label the nucleus and kinetoplast. Similarly, early endosomes were labeled by 2 h binding of BHb at 4°C, washed, followed by 5 min uptake at 23°C and visualized by avidin–Texas red. Promastigotes were pre-incubated with Lysostracker Red (10 µM) for 30 min at 23°C in PBS to label the lysosome-like compartment, before fixing the cells. Slides were mounted with antifade reagents (Molecular probes) and viewed in an LSM 510 confocal microscope using an oil immersion objective.
Acknowledgments
Acknowledgements
We are grateful to Philip Stahl, Washington University School of Medicine, St Louis, MO, for critically reviewing the manuscript. These studies are supported by grants from the Department of Science and Technology, Government of India to A.M. and Department of Biotechnology to the National Institute of Immunology.
References
- Attal G. and Langsley,G. (1996) A Plasmodium falciparum homologue of a rab specific GDP dissociation inhibitor. Mol. Biochem. Parasitol., 79, 91–95. [DOI] [PubMed] [Google Scholar]
- Bastin P., Stephan,A., Raper,J., Saint-Remy,J.M., Opperdoes,F.R. and Courtoy,P.J. (1996) An M(r) 145,000 low-density lipoprotein (LDL)-binding protein is conserved throughout the kinetoplastida order. Mol. Biochem. Parasitol., 76, 43–56. [DOI] [PubMed] [Google Scholar]
- Bogdanovic A., Bruckert,F., Morio,T. and Satre,M. (2000) A syntaxin 7 homologue is present in Dictyostelium discoideum endosomes and controls their homotypic fusion. J. Biol. Chem., 275, 36691–36697. [DOI] [PubMed] [Google Scholar]
- Celis A., Dejgaard,K. and Celis,J.E. (1994) Production of mouse monoclonal antibodies. In Celis,J.E. (ed.), Cell Biology, a Laboratory Handbook. Academic Press, New York, pp. 269–275. [Google Scholar]
- Chaturvedi S., Qi,H., Coleman,D., Rodriguez,A., Hanson,P.I., Striepen,B., Ross,D.S. and Joiner,K. (1999) Constitutive calcium-independent release of Toxoplasma gondii dense granules occurs through the NSF/SNAP/SNARE/Rab machinery. J. Biol. Chem., 274, 2424–2431. [DOI] [PubMed] [Google Scholar]
- Chen Y.A. and Scheller,R.H. (2001) SNARE-mediated membrane fusion. Nature Rev. Mol. Cell Biol., 2, 98–106. [DOI] [PubMed] [Google Scholar]
- Clayton C., Hausler,T. and Blattner,J. (1995) Protein trafficking in kinetoplast protozoa. Microbiol. Rev. 59, 325–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny P.W., Lewis,S., Tempero,J.E., Goudling.D., Ivens,A.C., Field,M.C. and Smith,D.F. (2002) Leishmania RAB7: characterization of terminal endocytic stages in an intracellular parasites. Mol. Biochem. Parasitol., 123, 105–113. [DOI] [PubMed] [Google Scholar]
- Diaz R., Mayorga,L.S., Weidman,P.J., Rothman,J.E. and Stahl,P.D. (1989) Vesicle fusion following receptor-mediated endocytosis requires a protein active in Golgi transport. Nature, 339, 398–400. [DOI] [PubMed] [Google Scholar]
- Feng Y, Press,B. and Wandinger-Ness,A. (1995) Rab7: an important regulator of late endocytic membrane traffic. J. Cell Biol., 131, 1435–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. and Field,M.C. (1997) Tandem duplication of rab genes followed by sequence divergence and acquisition of distinct functions in Trypanosoma brucei. J. Biol. Chem., 272, 10498–10505. [DOI] [PubMed] [Google Scholar]
- Field H., Farjah,M., Pal,A., Gull,K. and Field,M.C. (1998) Complexity of trypanosomatid endocytosis pathways revealed by Rab4 and Rab5 isoforms in Trypanosoma brucei. J. Biol. Chem., 273, 32102–32110. [DOI] [PubMed] [Google Scholar]
- Funato F., Beron,W., Yang,C.Z., Mukhopadhyay,A. and Stahl,P.D. (1997) Reconstitution of phagosome–lysosome fusion in streptolysin-O-permeabilized cells. J. Biol. Chem., 272, 16147–16151. [DOI] [PubMed] [Google Scholar]
- Gorvel J.P., Chavrier,P., Zerial,M. and Gruenberg,J. (1991) rab5 controls early endosome fusion in vitro. Cell, 64, 915–925. [DOI] [PubMed] [Google Scholar]
- Graham J.M. (1993) Identification of subcellular organelles. In Graham,J.M and Higgins,J. (eds), Biomembrane Protocols. Humana Press, New Jersey, Vol. 19, pp. 1–18. [Google Scholar]
- Gruenberg J., Griffths,G. and Howell,K.E. (1989) Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J. Cell Biol., 108, 1301–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann K. and Stoffel,W. (1993) TMbase—a database of membrane spanning proteins segments. Biol. Chem. Hoppe-Seyler, 347, 166–166. [Google Scholar]
- Juuti-Uusitalo K., Airenne,K.J., Laukkanen,A., Punnonen,E., Olkkonen,V.M., Gruenberg,J., Kulomaa,M. and Marjomaki,V. (2000) Selective targeting of avidin/mannose 6-phosphate receptor chimeras to early to late endosomes. Eur. J. Cell Biol., 79, 458–468. [DOI] [PubMed] [Google Scholar]
- Karlin S. and Altschul,S.F. (1993) Application and statistics for multiple high-scoring segments in molecular sequences. Proc. Natl Acad. Sci. USA, 90, 5873–5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornilova E., Sorkina,T., Beguinot,L. and Sorkin,A. (1996) Lysosomal targeting of epidermal growth factor receptors via a kinase-dependent pathway is mediated by the receptor carboxyl-terminal residues 1022–1023. J. Biol. Chem., 271, 30340–30346. [DOI] [PubMed] [Google Scholar]
- Laurent O., Bruckert,F., Adessi,C. and Satre,M. (1998) In vitro reconstituted Dictyostelium discoideum early endosome fusion is regulated by Rab7 but proceeds in the absence of ATP-Mg2+ from the bulk solution. J. Biol. Chem., 273, 793–799. [DOI] [PubMed] [Google Scholar]
- Lenhard J.M., Mayorga,L. and Stahl,P.D. (1992) Characterization of endosome–endosome fusion in a cell-free system using Dictyostelium discoideum.J. Biol. Chem., 267, 1896–1903. [PubMed] [Google Scholar]
- Liu J., Qiao,X., Du,D. and Lee,M.G. (2000) Receptor-mediated endocytosis in the procyclic form of Trypanosoma brucei.J. Biol. Chem., 275, 12032–12040. [DOI] [PubMed] [Google Scholar]
- Lombardi D., Soldati,T., Reiderer,M.A., Goda,Y., Zerial,M. and Pfeffer,S.R. (1993) Rab9 functions in transport between late endosome to trans Golgi network. EMBO J., 12, 677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahnke K., Guo,M., Lee,S., Sepulveda,H., Swain,S.L. and Nussenzweig,M. (2000) The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J. Cell Biol., 151, 673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A.P., Whiteheart,S.W. and Weiss,I.W. (2001) Unraveling the mechanism of the vesicle transport ATPase NSF, the N-ethylmaleimide-sensitive factor. J. Biol. Chem., 276, 21991–21994. [DOI] [PubMed] [Google Scholar]
- McConville M.J., Mullin,K.A., Ilgoutz,S.C. and Teasdale,R.D. (2002) Secretory pathway of trypanosomatid parasites. Microbiol. Mol. Biol. Rev., 66, 122–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee K., Siddiqi,S.A., Hashim,S., Raje,M., Basu,S.K. and Mukhopadhyay,A. (2000) Live Salmonella recruits N-ethylmaleimide-sensitive fusion protein on phagosomal membrane and promotes fusion with early endosome. J. Cell Biol., 148, 741–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee K., Parashuraman,S., Raje,M. and Mukhopadhyay,A. (2001) SopE acts as a Rab5-specific nucleotide exchange factor and recruits non-prenylated Rab5 on Salmonella-containing phagosomes to promote fusion with early endosomes. J. Biol. Chem., 276, 23607–23615. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A., Barbieri,A.M., Funato,K., Roberts,R. and Stahl,P.D. (1997a) Sequential actions of rab5 and rab7 regulate endocytosis in the Xenopus oocytes. J. Cell Biol., 136, 1227–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay A., Funato,F. and Stahl,P.D. (1997b) Rab7 regulates transport from early to late endocytic compartments in Xenopus oocytes. J. Biol. Chem., 272, 13055–13059. [DOI] [PubMed] [Google Scholar]
- Overkamp D., Mohammed,A.S., Cartledge,C. and London,J. (1988) Production of polyclonal antibodies in ascitic fluid of mice: techniques and applications. J. Immunol., 9, 51–68. [DOI] [PubMed] [Google Scholar]
- Pal A., Hall,B.S., Nesbeth,D.N., Field,H.I. and Field,M.C. (2002) Differential endocytic functions of Trypanosoma brucei Rab5 isoforms reveal a glycophosphatidylinositol-specific endosomal pathway. J. Biol. Chem., 277, 9529–9539. [DOI] [PubMed] [Google Scholar]
- Pereira-Leal J.B. and Seabra,M.C. (2000) The mammalian Rab family of small GTPases: definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily. J. Mol. Biol., 301, 1077–1087. [DOI] [PubMed] [Google Scholar]
- Robibaro B., Stedman,T.T., Coppens,I., Ngo,H.M., Pypaert,M., Bivona,T., Nam,H.W. and Joiner,K.A. (2002) Toxoplasma gondii Rab5 enhances cholesterol acquisition from host cells. Cell. Microbiol., 4, 139–152. [DOI] [PubMed] [Google Scholar]
- Roy J.C. (1932) Cultivation of various Leishmania parasites in solid medium. Ind. J. Med. Res., 20, 355–367. [Google Scholar]
- Sah J.F., Ito,H., Kolli,B.K., Peterson,D.A., Sassa,S. and Chang,K.P. (2002) Genetic rescue of Leishmania deficiency in porphyrin synthesis creates mutants suitable for analysis of cellular events in uroporphyria and for photodynamic therapy. J. Biol. Chem., 277, 14902–14909. [DOI] [PubMed] [Google Scholar]
- Sengupta S., Tripathi,J., Tandon,R., Raje,M., Roy,R.P., Basu,S.K. and Mukhopadhyay,A. (1999) Hb endocytosis in Leishmania is mediated through a 46-kDa protein located in the flagellar pocket. J. Biol. Chem., 274, 2758–2765. [DOI] [PubMed] [Google Scholar]
- Shapiro S.Z. and Webster,P. (1989) Coated vesicles from the protozoan parasite Trypanosoma brucei: purification and characterization. J. Protozool., 36, 344–349. [DOI] [PubMed] [Google Scholar]
- Smith D.B. and Corcoran,L.M. (1993) Expression and purification of glutathione-S-transferase fusion protein. In Janssen,K. (ed.), Current Protocols in Molecular Biology. John Wiley, New York, 16.7.1–16.7.81. [DOI] [PubMed] [Google Scholar]
- Stedman T.T., Sussmann,A.R. and Joiner,K.A. (2003) Toxopalsma gondii Rab6 mediates a retrograde pathway for sorting of constitutively secreted proteins to the Golgi complex. J. Biol. Chem., 278, 5433–5443. [DOI] [PubMed] [Google Scholar]
- Stenmark H. and Olkkonen,V.M. (2001) The Rab GTPase family. Genome Biol., 2, 3007.1–3007.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H., Parton,R.G., Steele-Mortimer,O., Lutcke,A., Gruenberg,J. and Zerial,M. (1994) Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J., 13, 1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temesvari L., Rodriguez-Paris,J., Bush,J., Steck,T.L and Cardelli,J. (1994) Characterization of lysosomal membrane proteins of Dictyostelium discioideum: a complex population of acidic integral membrane glycoprotein, rab GTP binding proteins and vacuolar ATPase subunits. J. Biol. Chem., 269, 25719–25727. [PubMed] [Google Scholar]
- Thompson J.D., Higgins,D.G. and Gibson,T.J. (1994) CLUSTAL 2: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res., 22, 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via L.E., Deretic,D., Ulmer,R.J., Hibler,N.S., Huber,L.A. and Deretic,V. (1997) Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J. Biol. Chem., 272, 13326–13331. [DOI] [PubMed] [Google Scholar]
- Voyiatzaki C.S. and Soteriadou,K.P. (1992) Identification and isolation of the Leishmania transferrin receptor. J. Biol. Chem., 267, 9112–9117. [PubMed] [Google Scholar]
- Weidenhaupt M., Bruckert,F. and Satre,M. (1998) Identification of the Dictyostelium discoideum homolog of the N-ethylmaleimide-sensitive protein. Gene, 207, 53–60. [DOI] [PubMed] [Google Scholar]
- Wileman T., Harding,C. and Stahl,P.D. (1985) Receptor-mediated endocytosis. Biochem. J., 232, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M. and McBride,H. (2001) Rab proteins as membrane organizers. Nature Rev. Mol. Cell Biol., 2, 107–117. [DOI] [PubMed] [Google Scholar]