Abstract
Although some individuals who abuse methamphetamine have considerable cognitive deficits, no prior studies have examined whether neurocognitive functioning is associated with outcome of treatment for methamphetamine dependence. In an outpatient clinical trial of bupropion combined with cognitive behavioral therapy and contingency management (Shoptaw et al., 2008), 60 methamphetamine-dependent adults completed three tests of reaction time and working memory at baseline. Other variables that were collected at baseline included measures of drug use, mood/psychiatric functioning, employment, social context, legal status, and medical status. We evaluated the relative predictive value of all baseline measures for treatment outcome using Classification and Regression Trees (CART; Breiman, 1984), a nonparametric statistical technique that produces easily interpretable decision rules for classifying subjects that are particularly useful in clinical settings. Outcome measures were whether or not a participant completed the trial and whether or not most urine tests showed abstinence from methamphetamine abuse. Urine-verified methamphetamine abuse at the beginning of the study was the strongest predictor of treatment outcome; two psychosocial measures (e.g., nicotine dependence and Global Assessment of Functioning) also offered some predictive value. A few reaction time and working memory variables were related to treatment outcome, but these cognitive measures did not significantly aid prediction after adjusting for methamphetamine usage at the beginning of the study. On the basis of these findings, we recommend that research groups seeking to identify new predictors of treatment outcome compare the predictors to methamphetamine usage variables to assure that unique predictive power is attained.
Keywords: methamphetamine, treatment, relapse, neuropsychology, cognitive, abstinence, retention, predictors, prediction, outcome
1. Introduction
Research on variables that can predict success in treatment for methamphetamine (MA) dependence has a limited but evolving literature. Consistent evidence suggests that poorer treatment outcome is associated with a greater frequency of pre-treatment MA abuse (Maglione et al., 2000b; Brecht et al., 2005; Brecht et al., 2006; Hillhouse et al., 2007; Maglione et al., 2000a; Shoptaw et al., 2008). A more extensive history of previous treatment (Brecht et al., 2000; Brecht et al., 2006; Hillhouse et al., 2007) and lower educational attainment (Brecht et al., 2005; Brecht et al., 2006; Hillhouse et al., 2007) have also been associated with poorer MA treatment outcome, but conflicting findings have been reported (Maglione et al., 2000b; Maglione et al., 2000a). Other variables with limited and/or inconsistent evidence in predicting poorer outcome include greater MA craving (Hartz et al., 2001), legal coercion of treatment (Brecht et al., 2005), outpatient (versus residential) treatment (Brecht et al., 2006), shorter treatment duration (Brecht et al., 2000), selling MA (Brecht et al., 2000), presence of a disability (Brecht et al., 2005), and intravenous injection as the typical route of MA administration (Brecht et al., 2005; Hillhouse et al., 2007). Age, gender, and ethnicity have also been differentially associated with outcomes, but the findings have varied by study (Brecht et al., 2000; Maglione et al., 2000b; Hillhouse et al., 2007; Maglione et al., 2000a; Hser et al., 2005).
The association of lower levels of education with poorer treatment outcomes in some studies suggests that cognitive function may play a role in a participant’s ability to persist in treatment and to resist relapse. At face value, poor cognitive function would seem to be associated with a reduced effectiveness of cognitive treatment strategies (e.g., cognitive behavioral therapy), poor insight, and possibly reduced control over drug craving and early drop-out. Several studies have documented cognitive deficits in at least a subset of MA-abusing participants (Simon et al., 2000; Kalechstein et al., 2003; Salo et al., 2002; Gonzalez et al., 2004; Monterosso et al., 2005; Salo et al., 2007; Woods et al., 2005; Hoffman et al., 2006; Simon, In review; see review and meta-analysis byScott et al., 2007); these individuals may be at particular risk for relapse and poor treatment outcome. However, to our knowledge, no published studies have tested whether cognitive performance can predict success in treatment for MA dependence. We thus sought to examine the relationship between cognition and treatment outcome in MA dependence. Given the noted relationships between the frequency of MA abuse and other drug use variables with treatment outcome, we also sought to determine whether cognitive performance is more or less predictive of treatment success than these variables with established value.
In an outpatient treatment study (Shoptaw et al., 2008), we conducted a randomized, placebo-controlled clinical trial of bupropion, in conjunction with cognitive behavioral therapy (CBT) and contingency management, for MA dependence (for more information regarding these treatments see (Rawson et al., 1999; Lee and Rawson, 2008). Compared to placebo, bupropion failed to significantly impact overall MA use, MA craving, and study retention (Shoptaw et al., 2008). During the baseline testing period in this study, participants completed two measures of processing speed/reaction time and one measure of working memory. These measures have been found to be sensitive to cognitive and brain dysfunction in MA and other substance-abusing populations (Mendrek et al., 2005; Newton et al., 2004; Verdejo-Garcia et al., 2006; Xu et al., 2005), as well as other medical conditions (e.g., AIDS; Hardy and Hinkin, 2002). We tested their value in predicting treatment outcome for MA dependence, as measured by the ability to abstain from MA abuse and to complete treatment. We also evaluated the relationship between treatment outcome and several drug use and psychosocial variables to determine their relative predictive strength. Psychosocial variables included demographic information, psychiatric symptomology, functional status, social context, legal status, and medical status.
2. Methods
2.1 Participants
Sixty MA-dependent adults (38 males, 22 females, age 35.08 ± 10.70 years), who were seeking treatment, participated in the study. Twelve of the 73 participants in the original study were excluded because they did not have cognitive data; another participant was excluded because, upon further review, it was determined that he/she met criteria for MA abuse but not dependence (excluded participants did not differ from the remaining participants in abstinence during the trial or study completion, p > .5). The participants were of diverse ethnic heritage (Caucasian N = 38, Hispanic N = 18, African-American N = 1, Asian-American N =1, and Other N = 2) and educational background (less than a high school degree N = 10; high school diploma or GED N = 21; some college N = 23; college degree or higher = 6). Participants met none of the following exclusion criteria: (1) medical condition that would interfere with safe study participation (e.g., active tuberculosis, unstable diabetes, uncontrolled hypertension, symptomatic AIDS [HIV+ was permitted], or elevated liver enzymes [> 3 times the normal upper limit]), (2) current neurological disorder or major psychiatric disorder (e.g., schizophrenia or bipolar disorder; see below for specific psychiatric diagnoses included), (3) current suicidal intention or plan, (4) taking a prescription medication that is known to interact with bupropion, (5) current dependence on cocaine, opiates, alcohol, or benzodiazepines, (6) history of alcohol dependence within the last 3 years, (7) history of seizures, (8) history of anorexia or bulimia, and (9) history of sensitivity to bupropion. Female participants were not pregnant or lactating, and they were willing to use an acceptable method of birth control.
All participants met criteria for amphetamine (methamphetamine) dependence as verified by the Structured Clinical interview for the DSM-IV-TR (SCID). They reported using MA an average of 16.50 days within the 30 days (± 10.50 days) prior to enrollment. Mean duration of regular use was 10.32 years (± 8.18 years). Primary route of administration included smoking (63%), snorting (22%), intravenous injection (13%), or the oral route (2%). Most participants regularly smoked cigarettes (67%), with those smoking demonstrating a mean score of 3.38 (± 2.23) on the Fagerström Test for Nicotine Dependence. Five participants were positive for HIV infection (without an AIDS-defining condition), and three had medically stable hepatitis C infection. In addition to amphetamine dependence, current Axis I diagnoses included substance-induced mood disorder (N = 8), marijuana dependence (N = 7), substance-induced anxiety disorder (N = 6), substance-induced psychotic disorder (N = 4), marijuana abuse (N = 2), and several diagnoses with N = 1 (psychotic disorder not otherwise specified [NOS], alcohol abuse, PTSD, body dysmorphic disorder, sedative abuse). Because of the limited sample sizes available for most diagnoses, only two collapsed diagnostic variables were included in the following analyses: substance-induced psychiatric condition (N = 13) and marijuana dependence or abuse (N = 9).
2.3. Procedures
Participants were recruited from the greater Los Angeles area, and were seen at one of three clinical research sites (Rancho Cucamonga, Hollywood, LA). Advertisements in newspapers, radio, and the internet offered treatment for individuals looking to quit using MA, in a research-based counseling and medication trial. Interested individuals contacted the researchers on a toll-free research line. After receiving a detailed description of the protocol, they were provided written informed consent, following the guidelines of the UCLA Office for Protection of Research Subjects. Potential participants underwent a two-week baseline/screening period to determine eligibility for enrollment in the medication trial. All measures used to predict treatment outcome were administered during this baseline period, which included a physical exam by a study physician with blood tests and EKG. Following baseline, eligible participants were randomly assigned to receive either bupropion (300mg) or placebo daily, in a double-blind fashion. Contingency management treatment began on the second day of the baseline period, while CBT did not begin until the medication trial. Excluding the baseline period and follow-up visits, the study lasted twelve weeks. Participants visited the study clinic three times per week (Monday, Wednesday, and Friday), with urine drug tests (Phamatech, Inc., San Diego, CA) completed on each visit. In addition to voucher monies earned in contingency management, participants received $20 for completing baseline assessments and termination assessments ($40 if they completed both).
2.3. Assessments
2.3.1. Neuropsychological Measures
Simple Reaction Time (SRT; (Newton et al., 2004; Hardy and Hinkin, 2002))
a computerized test of simple processing speed and reaction time in which letters are pseudo-randomly presented from the set A, a, G, g, T, t, H, h, one after the other, and participants are instructed to press a button on the response box with their dominant forefinger as quickly as possible when they see a letter. Each letter is presented for 500 ms, with a 2500 ms interstimulus interval. A total of 31 trials are presented and mean reaction time is calculated.
Choice Reaction Time. (CRT; (Newton et al., 2004; Hardy and Hinkin, 2002))
a computerized test of selective attention and reaction time. As with the SRT, the letters A, a, G, g, T, t, H, and h are pseudo-randomly presented one after the other. However, in the CRT, participants must quickly press a blue response button to the presentation of G, g, H, or h; and a red response button to the presentation of A, a, T, or t. Thirty trials are administered and the screen presentation is identical to the SRT test. Mean reaction time and the number of errors are calculated. Because the CRT differs from the SRT only by the added demand to make a decision and discriminate between stimuli, mean reaction time on the CRT minus mean reaction time on the SRT can provide an index of decision making speed (Hardy and Hinkin, 2002).
N-back Task, (variation based on Smith et al., 1996)
a computerized test of working memory and selective attention. As with the above tasks, the letters A, a, G, g, T, t, H, h are pseudo-randomly presented on the computer screen in a similar format (with a different order of letter presentation). In the 1-back condition, the participant must press a blue button on a response box as quickly as possible if the verbal identity of the presented letter matches the verbal identity of the letter that immediately preceded it. If the currently presented letter does NOT match the preceding letter, the participant must quickly press a red button. Note that the case of the letter is not relevant to matching verbal identity. In the 2-back condition, a blue button press is required if the verbal identity of the current letter matches the verbal identity of the letter that was presented two trials back (if not, a red button press is required). A total of 64 trials are completed, 32 in each condition, and reaction time and errors are calculated.
2.3.2. Drug Use and Psychosocial Measures
All of the following measures were administered during the baseline period, except the Substance Use Inventory which was administered every study day.
Addiction Severity Index—Lite (ASI—Lite; (McLellan et al., 1992))
a clinical interviewing instrument used to quantify problems associated with substance abuse in seven domains: alcohol use, drug use, employment, family and social relationships, legal, psychological, and medical. Index scores can be calculated to indicate problem severity in each domain.
Brief Symptom Inventory (BSI; (Derogatis and Melisaratos, 1983))
a 53-item questionnaire that measures nine domains of psychological and emotional distress experienced during the last 30 days (e.g., anxiety, paranoid ideation). A Global Severity Index also provides a measure of the mean item endorsement across all domains.
Quality of Well-Being Scale—Self Administered Version (QWB-SA; (Kaplan et al., 1997))
a self-report measure of the general health-related quality of life. In our analyses we only used a subset of items tapping perceptions of current health status and functional limitations.
Substance Use Inventory (SUI; (Sobell et al., 1986))
a self-report measure of frequencies and quantities of drug (MA, cigarettes, other drugs) and alcohol use.
Fagerström Test of Nicotine Dependence (Heatherton et al., 1991)
a 6-item self-report measure of nicotine dependence that was developed and revised from the earlier Fagerström Tolerance Questionnaire (Fagerstrom, 1978).
Craving Visual Analog Scale (VAS)
a single item in which severity of MA craving is rated on a visual analog scale.
Clinical Global Impression (CGI)
a one-item visual analog scale rating completed by the experimenters and the participant to evaluate global severity of MA dependence.
Beck Depression Inventory-II (BDI-II; (Beck et al., 1996))
a 21-item self-report measure of depressive symptoms.
2.5. Statistical Methods
Our principal goal was to identify factors predictive of treatment outcome and their relative importance. We considered two dichotomous primary outcome measures, mostly abstinent and completer status. Participants were considered abstinent in a given week if they had at least one MA-free urine specimen and no urines positive for MA. Thus the subject had to both actively participate during a week and be negative on all urine tests taken that week. Subjects were considered mostly abstinent over the course of the study if they were abstinent for more than half of the 12 treatment weeks (7 or more weeks). This cut-point was chosen because the distribution of weeks abstinent was bimodal with a natural break at 7 weeks, and because this cut-point assured reasonable reduction of MA use while also maintaining acceptable sample size in the mostly abstinent group (n = 19). Self-report data confirmed that the mostly abstinent variable was associated with reduction in MA use (16 of the 19 mostly abstinent participants reported a reduction in MA use during the study of at least 50% based on the average days used before the study compared to average days used during the study). A participant was considered a completer if he/she attended at least one session in each of the 12 weeks of treatment. This assured consistent study attendance as well as acceptable completer sample size (n = 18).
Univariate analyses (t-tests, chi-squared tests, correlations) were used descriptively to identify individual variables that were associated with outcome (predictors considered are listed in the Supplementary Materials.) In order to investigate potential linear relationships between predictors and outcome, we included the continuously-measured number of abstinent weeks (abstinent defined as above) in these univariate analyses. However, our primary analytic approach was to develop a sequence of multivariate predictive models using Classification and Regression Trees (CART; Breiman, 1984) with the dichotomous outcome variables described above.
CART is a non-parametric technique that recursively partitions the space of predictor variables so that subjects in the same region are as similar as possible with regard to outcome. In our study, CART identified variable values to classify subjects according to dichotomous treatment outcomes (e.g., completer status). CART searches through the list of possible predictors to identify the single variable and cut-point that divides the data into the two sub-regions or “nodes” that best separate the outcome classes (e.g., completers vs. non-completers). The splitting process is repeated for each sub-region until no significant improvements can be made. The result is a tree or nested sequence of subgroups which describes a simple set of rules for assigning a person to a predicted outcome. Trees are typically grown until the terminal nodes are relatively small, at which point they are pruned back to minimize the predictive error rate for new subjects (i.e. to avoid overfitting). CART also calculates the relative importance of all predictor variables in producing the classification rule, which, in addition to obvious clinical implications, provides a useful tool for identifying predictors whose significance may have been masked in the tree building process due to multicollinearity or other factors. (At each split CART evaluates the usefulness of other predictors as proxies, so variables that track well with multiple splits may have high importance ratings even if they are not included in the primary tree.) CART has several strengths compared to model-based methods like logistic regression, including: a) the ability to handle large numbers of predictor variables even when the sample size is small, without overfitting; b) excellent ability to capture interactions; c) automatic handling of missing data through the use of proxy variables; and d) production of simple classification rules that can be applied clinically through a set of yes or no questions.
We constructed two sequences of classification trees, one with mostly abstinent as the outcome, and one with completer status as the outcome. The initial trees included all the predictor variables listed in the Supplementary Materials. Note, however, that multiple urine tests for MA were completed during the baseline screening period from which several different baseline usage variables could be constructed. In our primary models we included only frequency of initial MA use, with infrequent use defined as less than or equal to two positive urine tests for MA during the baseline period, and frequent use as three or more positive tests during baseline. We originally chose this construct based on its use in prior studies (Shoptaw et al., 2008), but CART also confirmed that the optimal division of the initial urine data was whether the person had three or more positive tests.
In addition to the primary CART trees described above, two additional trees were built for each outcome. First, we replaced the frequency of initial MA use variable with whether the subject had a urine screen positive for MA metabolites on their first baseline urine. Many studies have only a single urine test to assess baseline severity of use and the objective was to see how much this would reduce their ability to predict treatment outcome. Finally, we removed all baseline urinalysis measures to see whether cognitive and other supplementary measures could achieve comparable predictive ability. Trees were evaluated on prediction accuracy (measured using cross-validation, a statistical procedure which refits the model leaving out each of the subjects in turn and then generates predictions for the omitted cases, thus creating accurate estimates of how well the tree would classify new subjects), and the set of splits and variable importance weights.
3. Results
3.1. Predictive Models
About one-third of the participants were able to stay mostly abstinent or to complete treatment (mostly abstinent n = 19; complete n = 18). Fifteen of the 19 mostly abstinent participants were also completers (79%). N-back data from six outlier participants was excluded because they failed to respond to half or more (≥ 16) of the items on the 1-back condition, 2-back condition, or both, suggesting that they did not exert appropriate effort on the task and/or did not fully understand the instructions (in contrast, the majority of participants had 1 or fewer non-responses).
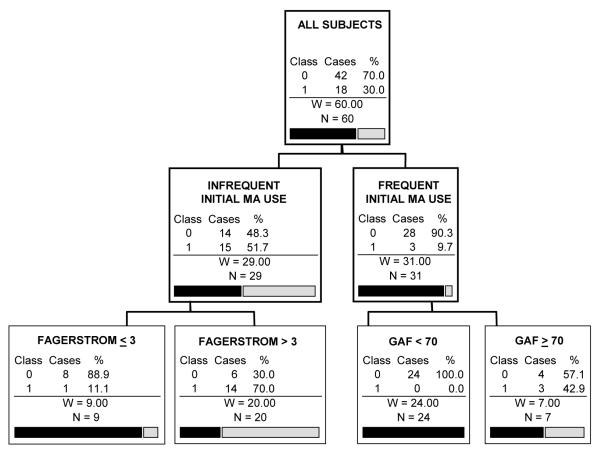
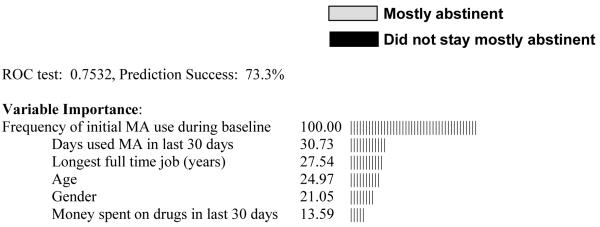
The CART analysis with mostly abstinent as the outcome and all possible predictors included is shown in Figure 1. The only split that remains significant after pruning is based on whether or not the subject was a frequent initial user of MA (> 2 baseline urine tests positive for MA). Among subjects who were frequent users, 90.3% failed to stay mostly abstinent over the course of the study, while among subjects who were infrequent users, 55.2% stayed mostly abstinent. Other variables with high importance ratings were self-reported MA use over the last 30 days, years of full-time employment, age, gender, and money spent on drugs in the last 30 days. The cross-validated prediction success rate for this tree is 73.3%. Although this prediction rate is only modestly better overall than predicting that no one remains abstinent (68% success rate), the CART tree successfully adds to prediction by identifying sub-groups of participants with considerably different rates of abstinence. While guessing that no one remains abstinent would be a reasonable estimation for frequent initial users of MA (∼10% error), infrequent initial users of MA would regularly be misclassified by this rule (∼55% error).
Figure 1.
CART prediction tree of the ability of participants to stay mostly abstinent in treatment.
(the caption should go above the figure, the gray-scaled legend [abstinent or not] should be placed to the right of the top figure box, and the remaining information below should go underneath the figure)
Note: INFREQUENT INITIAL MA USE: ≤ 2 positive urine tests during first two study weeks (i.e., baseline); FREQUENT INITIAL MA USE: > 2 positive urine tests during first two study weeks. Class variables: 0 = Did not stay mostly abstinent; 1 = Mostly abstinent.
CART analysis specifications: Gini splitting criterion with 5-fold cross-validation; minimum terminal node size of n=5, with cost complexity pruning with larger trees penalized similar to the AIC criterion.
When a single baseline urine was substituted for the multi-test frequency of usage variable in the prediction of mostly abstinent, no splits remained significant after pruning. Although drug usage variables other than urine tests were again included in this model (money spent on drugs recently, recent problems due to drugs), the prediction success was 46.7% and did not improve prediction over chance guessing. Similarly, an optimal tree was not produced when all baseline urine testing data were excluded from the model.
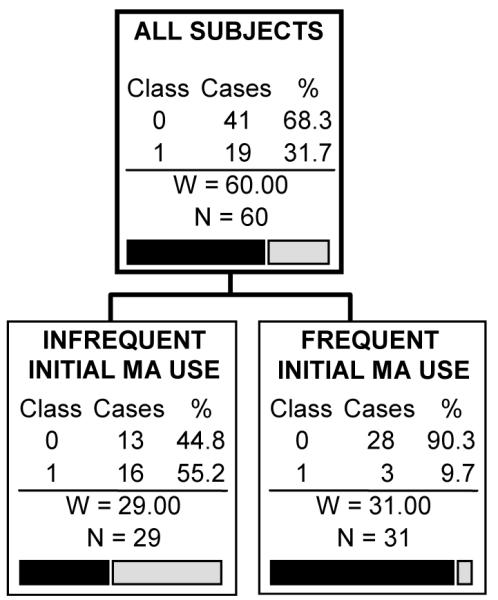
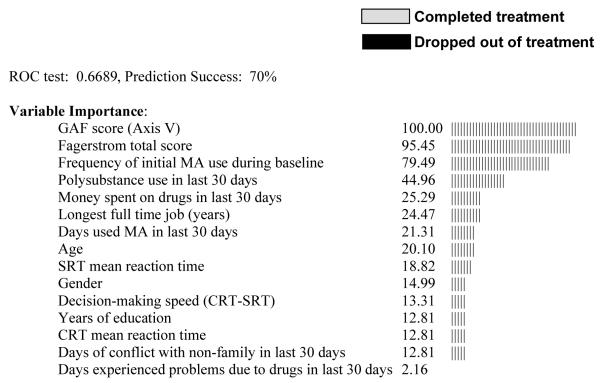
Analyses with completer status as the dependent variable showed a similar premium on measures of initial drug usage. The tree for which all predictors were included is shown in Figure 2. It split first on frequency of initial MA use. Among participants with infrequent initial MA use, there was a subsequent split based on the Fagerström test score, while for participants with frequent initial MA use, there was a subsequent split on the Axis 5 Global Assessment of Functioning (GAF) score (see the Discussion section for further details). Other variables with high importance ratings were self-reported drug problems (polysubstance abuse, money spent on drugs, MA abuse in the 30 days before enrollment in the study, recent drug problems), demographics (age, gender, education), employment history, recent social conflict and some of the cognitive tests (mean SRT, mean CRT and their difference). The overall prediction success rate for this tree was 70%. When the multi-test baseline urinalysis variable was replaced by only the first baseline urine test, an optimal tree was not produced, nor was an optimal tree produced if all baseline urine testing was excluded.
Figure 2.
CART prediction tree of the ability of participants to complete treatment.
(the caption should go above the figure, the gray-scaled legend [completed or dropped out of treatment] should be placed to the right of the top figure box, and the remaining information below should go underneath the figure)
Note: INFREQUENT INITIAL MA USE: ≤ 2 positive urine tests in first two study weeks (i.e., baseline); FREQUENT INITIAL MA USE: > 2 positive urine tests in first two study weeks; FAGERSTROM = Fagerstrom Test of Nicotine Dependence; GAF = Global Assessment of Functioning score (Axis 5); SRT = Simple Reaction Time; CRT = Choice Reaction Time. Class variables: 0 = Dropped out of treatment; 1 = Completed treatment.
CART analysis specifications: Gini splitting criterion with 5-fold cross-validation; minimum terminal node size of n=5, with cost complexity pruning with larger trees penalized similar to the AIC criterion.
3.2. Univariate Associations
To further investigate the relationship between predictor variables and the continuous and dichotomous outcome measures outside of the CART analyses, we conducted univariate analyses between the 104 potential predictor variables and the outcome measures previously described. The statistically significant (p < .05, uncorrected for multiple comparisons) results of these analyses are shown in Table 1. However, it should be noted that because of the number of associations tested, a high probability of Type I error exists for these data. A full listing of univariate associations for all variables tested can be found in Supplementary Materials1. We see that current or recent drug usage variables at baseline are the best predictors of outcome, showing strong relationships with both weeks abstinent and completing treatment. Other variables positively related to a favorable outcome included having a higher number of lifetime treatments for drugs and alcohol, recent treatment for drugs/alcohol (prior to study entry), and higher nicotine dependence score. Variables negatively associated with a favorable outcome included living with someone with an alcohol problem, current or past problems with depression, recent social conflict, and greater involvement with the legal system. Table 2 displays the cognitive performance of the dichotomous outcome groups. Only the number of errors on the 1-back test was significantly associated with outcome in univariate analyses (p < .05, uncorrected), in which participants who completed treatment made fewer errors than those who dropped out (all other p values > .05).
Table 1.
Significant univariate associations (uncorrected) between baseline variables and the number of methamphetamine (MA) abstinent weeks and study completion.
| Variable Type | Measure | Weeks Abstinent | Study Completion |
|---|---|---|---|
| Cognitive Performance | |||
| 1-back total errors | N-Back | NS | t(52) = 2.10* |
| Drug Usage | |||
| Number of MA-free urine screens during baseline | Urine | r = .58*** | t(58) = -3.00** |
| Frequency of initial MA use (freq/infreq) a, b | Urine | t(58) = 4.61*** | χ2 = 12.61*** |
| Drug screen on first baseline day (pos/neg) a | Urine | t(58) = 3.29** | χ2 = 4.78* |
| Days of MA use in the 30 days before baseline | ASI | r = -.27* | NS |
| Self-rated severity of MA dependence | CGI | r = -.25* | NS |
| Number of lifetime drug treatments | ASI | r = .29* | NS |
| Number of lifetime alcohol treatments | ASI | r = .37** | NS |
| Days treated for drugs/alcohol in last 30 days | ASI | r = .38** | NS |
| Nicotine dependence total score | FG | NS | t(37) = -2.18* |
| Mood/Psychiatric Function | |||
| Depressed in last 30 days (yes/no) a | ASI | NS | χ2 = 6.57* |
| Significant depression in lifetime (yes/no) a | ASI | NS | χ2 = 8.93** |
| Legal Status | |||
| Legal index score | ASI | NS | t(58) = 2.37* |
| Social Context | |||
| Live with someone with alcohol problem (yes/no) a | ASI | t(57) = 3.25* | NS |
| Days of conflicts with non-family in last 30 days | ASI | NS | t(58) = 2.40* |
Note: Urine = urinalysis for methamphetamine; ASI = Addiction Severity Index—Lite; CGI = Clinical Global Impression; FG = Fagerstrom Test of Nicotine Dependence.
Denotes a categorical scale of measurement. All other variables were on a continuous scale.
Frequency of initial MA use: Infrequent: ≤ 2 positive MA urines during first two study weeks (i.e., baseline). Frequent: > 2 positive tests.
NS = Nonsignificant p > .05
r = Pearson correlation
t = independent samples t-test
χ2 = chi-square test
p < .05 uncorrected for multiple comparisons
p < .01 uncorrected for multiple comparisons
p < .001 uncorrected for multiple comparisons
Table 2.
Means and standard deviations of baseline cognitive variables for participants with different treatment outcomes.
| Cognitive Variable | Mostly Abstinent (N = 19) |
Not Abstinent (N = 40) |
Completer (N = 18) |
Non-completer (N = 41) |
|---|---|---|---|---|
| SRT mean reaction time | 363.31 ± 79.92 | 363.69 ± 56.53 | 255.32 ± 63.95 | 267.19 ± 64.87 |
| CRT mean reaction time | 553.98 ± 78.84 | 574.96 ± 131.43 | 565.29 ± 87.75 | 569.49 ± 128.74 |
| CRT total errors | 1.79 ± 2.01 | 2.90 ± 2.75 | 2.00 ± 1.97 | 2.78 ± 2.79 |
| Decision speed (CRT — SRT) | 290.67 ± 89.82 | 311.27 ± 116.37 | 309.96 ± 94.98 | 302.30 ± 114.53 |
| 1-back mean reaction time | 663.63 ± 183.36 | 674.42 ± 189.07 | 668.84 ± 180.44 | 672.50 ± 189.84 |
| 1-back total errors | 1.50 ± 2.59 | 1.75 ± 3.15 | 0.77 ± .83 | 1.98 ± 3.37* |
| 1-back total trials correct | 30.29 ± 2.59 | 30.15 ± 3.41 | 31.08 ± .86 | 29.90 ± 3.60 |
| 2-back mean reaction time | 1017.25 ± 334.04 | 963.81 ± 361.89 | 1072.49 ± 343.57 | 947.60 ± 354.17 |
| 2-back total errors | 2.07 ± 3.10 | 2.20 ± 3.07 | 1.62 ± 2.26 | 2.34 ± 3.27 |
| 2-back total trials correct | 26.14 ± 5.80 | 27.40 ± 4.87 | 28.23 ± 4.27 | 26.71 ± 5.34 |
Note: Mostly Abstinent: attended at least 7 treatment weeks with a negative urine for MA and no positive urines; Not Abstinent: attended less than 7 weeks with a negative urine for MA and no positive urines; Completer: attended at least one treatment session in each of the 12 treatment weeks; Non-completer: did not attend at least one treatment session in each of the 12 treatment weeks; SRT = Simple Reaction Time; CRT = Choice Reaction Time.
p < .05 uncorrected, Completer vs. Non-completer
Because cognitive function may be moderated by current drug use, we compared the cognitive performance of those who tested positive for MA on the cognitive testing day to those who tested negative. No differences were found in these analyses for any cognitive variable (all p values > .05).
Since contingency management treatment was provided during the two week baseline period as a means of supporting participants while the baseline assessments were completed, MA use during the baseline period may reflect the effectiveness of this early contingency management treatment. However, it also may reflect the typical frequency of MA use independent of treatment. To explore this possibility, we examined the difference between those with infrequent initial MA use (≤ 2 positive urine tests at baseline) to those with frequent initial MA use (> 2 positive urines at baseline) on the number of days they reported using MA in the month prior to treatment entry. Those with frequent initial use endorsed using significantly more days in the last month (M = 21.8 days, S.D. = 8.1) than those with infrequent initial use (M = 10.8 days, S.D. = 9.8; p < .00), confirming that initial use during the beginning of the study was associated with use levels prior to treatment initiation.
4. Discussion
Although a few neurocognitive and psychiatric variables were associated with treatment outcome, the frequency of MA abuse at study outset was a much stronger predictor of outcome. Specifically, participants with two or fewer urine tests positive for MA metabolite during the first two weeks of the study (i.e., baseline period) were much more likely to complete treatment and achieve abstinence from MA in the majority of the treatment weeks. In contrast, participants with three or more MA-metabolite positive urines during the baseline period were more likely to drop out and continue to abuse MA regularly. These findings are consistent with several other studies finding an association between baseline frequency of MA abuse and treatment success (Peterson et al., 2006; Maglione et al., 2000b; Brecht et al., 2005; Brecht et al., 2006; Hillhouse et al., 2007; Maglione et al., 2000a; Shoptaw et al., 2008). Generally speaking, the best predictor of future MA abuse is the frequency of current MA abuse.
Because contingency management treatment was provided during the two week baseline period (in contrast with CBT and study medication which began after the two baseline weeks), MA use during the baseline period may reflect response to the initial contingency management treatment, as well as the baseline frequency of MA use prior to treatment (indeed, MA use in the 30 days prior to enrollment and during the two week baseline period were strongly related in our study). Still, the relationship we found between MA abuse at baseline and treatment outcome may be partly accounted for by the relationship between early and later responses to contingency management. While this complicates the interpretation of initial MA use, our results are nonetheless apt to be generalizable to the situation in the majority of substance abuse treatment programs in which baseline urinalysis is not conducted prior to treatment initiation. Further, although the relationship between baseline MA abuse and the mostly abstinent outcome may have been spuriously inflated due to the similarity in metric (both are measured by urinalysis), such measurement issues would not explain the strong predictive power of baseline MA abuse for the study completion outcome variable, which relied on session attendance rather than urinalysis. As anticipated, those participants who were mostly abstinent also tended to be completers (79%), but differences in predictors between these two outcomes do indicate somewhat different correlates. In sum, use patterns during the initial stages of treatment are likely to be quite predictive of subsequent outcome, both in terms of continued MA abuse and treatment attendance.
While self-reported MA use was not as predictive of treatment outcome as was urine-verified use, several self-report measures were identified in the CART importance ratings as associated with outcome. The importance ratings indicate the degree to which the variables could be used as proxies for the (best) predictors identified in the CART trees. Self-report measures with high importance ratings included days of MA used in the month preceding the study, longest full-time job (years), recent money spent on MA, and frequency of recent polysubstance abuse. However, when baseline urinalysis in the models was restricted to only the first urine obtained or was removed altogether, the subsequent prediction trees did not exceed chance guessing. Thus when considered individually, no variable other than the one derived from multiple initial urine tests was exceptionally predictive of treatment outcome. This observation suggests that treatment programs wishing to identify participants at risk for treatment failure should use multiple urine tests if possible.
Variables other than urinalysis may become most useful in predicting subsets of MA users who are successful or unsuccessful after initial MA usage is known. The elegance of the CART analysis lies in the ability to easily visualize interactions between multiple variables. For example, in the analysis of treatment completion (see Figure 2), the Global Assessment of Functioning (GAF) score from the DSM-IV diagnosis (i.e., Axis V) specifically aided prediction for frequent users of MA at baseline. For frequent users, a low GAF score (<70) was associated with an exceptionally high probability of treatment drop-out (100%). In contrast, for those who showed infrequent initial MA abuse, the most discriminating variable after urinalysis was the Fagerström Test of Nicotine Dependence. Infrequent MA abusers with low Fagerström scores were quite likely to drop out of treatment (88% drop out), while infrequent users with higher Nicotine Dependence scores more likely to remain in treatment (30% drop out). In this way, CART prediction trees provide straightforward expectations regarding the treatment success of samples and subsamples of MA participants, and may be particularly useful in clinical settings in which an individual’s risk for treatment noncompliance can be quickly estimated from a few baseline measures. However, because the sample sizes of some of the CART nodes were small (i.e., GAF and Fagerstrom nodes), additional research in different treatment settings is needed to determine the generalizability of the interactions.
A GAF score above 70 is associated with transient psychiatric symptoms and no more than slight functional impairment (Association, 2000). In contrast, scores below 70 suggest at least mild symptoms, with increasingly significant functional impairment as the GAF score lowers. In the GAF results in our study, this indicates that frequent baseline users of MA judged to have almost any functional impairment were exceptionally likely to drop out of treatment (100%). Frequent users with limited functional impairment were more likely to stay in treatment (43%), although even these individuals still dropped out of treatment more than half of the time. This finding reiterates the fact that frequent baseline MA abuse was associated with poor treatment outcome in the majority of cases.
It is not clear why a low level of nicotine dependence was associated with treatment drop out in the infrequent MA abusers. These individuals had lower levels of dependence on MA and nicotine and may not have been sufficiently motivated to complete treatment due to a perception that a brief period of treatment was sufficient for their severity of disease. It is also worth noting that the medication examined in the clinical trial, bupropion, is approved for smoking cessation treatment. While this might raise the possibility that infrequent MA users with higher levels of nicotine dependence might have been retained in the trial due to a benefit of bupropion on their cigarette smoking behavior, we found no evidence of an interaction between medication administration, nicotine dependence, and treatment completion in our analyses.
A few neurocognitive measures were associated with treatment outcome (1-back errors in univariate analyses; SRT and CRT in the importance ratings for study completion), but these variables were not the best predictors of outcome when compared to other indices. Cognitive performance was not related to a gross measure of MA abuse at the time of cognitive testing (testing positive or negative for MA on the testing day), so differences in use at the time of testing did not appear to explain the weakness of predictability demonstrated by cognitive measures. However, the day on which participants received cognitive testing during the baseline period was variable, and the time elapsed since last use of MA at the time of cognitive testing was not known. Thus, we cannot be certain that recent MA usage factors did not add variability into the measurement of cognitive function. Also, because the cognitive measures were only measured once, it is possible that cognitive changes occurred during treatment (possibly associated with frequency of MA use) which were not captured by the single-point assessment.
Our findings on the predictive value of cognitive measures are less robust than those from studies of individuals who abuse cocaine, in which treatment completion has been associated with cognitive performance in a number of domains, including attention, memory, spatial processing, and global cognitive functioning (Aharonovich et al., 2003; Aharonovich et al., 2006). However, in contrast with study completion, cognitive variables from these studies have been inconsistently linked with the actual use of cocaine during treatment. Similarly, these studies did not compare cognitive variables to those of initial cocaine use during the beginning of treatment in predicting outcome (only prior self-reported use). It is therefore unclear whether markers of initial drug use would supersede cognitive variables in prediction utility, as demonstrated by our current results. Because our study and those on cocaine treatment have differed in the cognitive tests used and the treatments implemented, it is unclear whether cognition is more or less predictive of outcome for treatment of dependence on the two drugs. Additional studies with comparable methods are needed to further explore these issues, and we recommend that they also include urine-verified measures of initial use to ensure that unique predictive power is attained. Future studies may also benefit from using other neurocognitive measures than those currently implemented. For instance, measures tapping specific domains of function believed to be central to drug use behavior (e.g., inhibitory control, risk taking) may provide better predictive ability than the measures of reaction time and working memory currently implemented.
In the current results, the greatest accuracy in prediction was achieved for participants who were unsuccessful in treatment (dropped out or continued to use), rather than those who were successful. For example, in the CART analysis of those staying mostly abstinent (figure 1), frequent initial MA abuse strongly predicted continued use of MA during treatment (28 of 31 frequent users were unable to stay mostly abstinent). However, infrequent initial abuse of MA was not as accurate in predicting the converse—the ability of participants to remain abstinent (only 13 of 29 infrequent users were able to stay mostly abstinent). This difference in predicting treatment failure versus treatment success was replicated in other analyses. This suggests that, in general, it is easier to identify individuals who will do poorly in treatment, rather than those who will do well. Because the CART procedure automatically attempts to maximize the prediction of both treatment success and treatment failure, it can be concluded that none of the 100+ variables we analyzed were extremely accurate in predicting treatment success. Finding variables that accurately predict treatment success for MA-dependent individuals is a challenge that warrants additional investigation.
Supplementary Material
Footnotes
Additional data analyses for this report are available as supplementary material with the online version of this paper at doi:xxx/j.drugalcdep.xxx …
These materials are available with the online version of the paper at doi:xxx/j.drugalcdep.xxx …
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81:313–322. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Aharonovich E, Nunes E, Hasin D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug Alcohol Depend. 2003;71:207–211. doi: 10.1016/s0376-8716(03)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association AP. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. American Psychiatric Association; Washington, DC: 2000. Text Revision. [Google Scholar]
- Beck AT, Steer RA, Brown GK. BDI-II manual. The Psychological Corporation; San Antonio: 1996. [Google Scholar]
- Brecht ML, Greenwell L, Anglin MD. Methamphetamine treatment: trends and predictors of retention and completion in a large state treatment system (1992-2002) J Subst Abuse Treat. 2005;29:295–306. doi: 10.1016/j.jsat.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Brecht ML, Greenwell L, von Mayrhauser C, Anglin MD. Two-year outcomes of treatment for methamphetamine use. J Psychoactive Drugs Suppl. 2006;3:415–426. doi: 10.1080/02791072.2006.10400605. [DOI] [PubMed] [Google Scholar]
- Brecht ML, von Mayrhauser C, Anglin MD. Predictors of relapse after treatment for methamphetamine use. J Psychoactive Drugs. 2000;32:211–220. doi: 10.1080/02791072.2000.10400231. [DOI] [PubMed] [Google Scholar]
- Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and regression trees. Wadsworth; Belmont, CA: 1984. [Google Scholar]
- Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychological Medicine. 1983;13:595–605. [PubMed] [Google Scholar]
- Fagerstrom KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Rippeth JD, Carey CL, Heaton RK, Moore DJ, Schweinsburg BC, Cherner M, Grant I. Neurocognitive performance of methamphetamine users discordant for history of marijuana exposure. Drug and Alcohol Dependence. 2004;76:181–190. doi: 10.1016/j.drugalcdep.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Hardy DJ, Hinkin CH. Reaction time performance in adults with HIV/AIDS. J Clin Exp Neuropsychol. 2002;24:912–929. doi: 10.1076/jcen.24.7.912.8391. [DOI] [PubMed] [Google Scholar]
- Hartz DT, Frederick-Osborne SL, Galloway GP. Craving predicts use during treatment for methamphetamine dependence: a prospective, repeated-measures, within-subject analysis. Drug and Alcohol Dependence. 2001;63:269–276. doi: 10.1016/s0376-8716(00)00217-9. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hillhouse MP, Marinelli-Casey P, Gonzales R, Ang A, Rawson RA. Predicting intreatment performance and post-treatment outcomes in methamphetamine users. Addiction. 2007;102(Suppl 1):84–95. doi: 10.1111/j.1360-0443.2007.01768.x. [DOI] [PubMed] [Google Scholar]
- Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology (Berl) 2006;188:162–170. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- Hser YI, Evans E, Huang YC. Treatment outcomes among women and men methamphetamine abusers in California. J Subst Abuse Treat. 2005;28:77–85. doi: 10.1016/j.jsat.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Newton TF, Green M. Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. Neurophysiology Clin. 2003;15:215–220. doi: 10.1176/jnp.15.2.215. [DOI] [PubMed] [Google Scholar]
- Kaplan RM, Sieber WJ, Ganiats TG. The quality of well-being scale: Comparison of the interviewer-administered version with a self-administered questionnaire. Psychological Health. 1997;12:783–791. [Google Scholar]
- Lee NK, Rawson RA. A systematic review of cognitive and behavioural therapies for methamphetamine dependence. Drug Alcohol Rev. 2008;27:309–317. doi: 10.1080/09595230801919494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglione M, Chao B, Anglin D. Residential treatment of methamphetamine users: Correlates of drop-out from the California Alcohol and Drug Data System (CADDS) Addiction Research. 2000a;8:65–79. [Google Scholar]
- Maglione M, Chao B, Anglin MD. Correlates of outpatient drug treatment drop-out among methamphetamine users. J Psychoactive Drugs. 2000b;32:221–228. doi: 10.1080/02791072.2000.10400232. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The fifth edition of the Addiction Severity Index. J SubstAbuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Monterosso JR, Simon SL, Jarvik M, Brody AL, Olmstead R, Domier C, Cohen MS, Ernst M, London ED. Working memory in cigarette smokers: Comparison to non-smokers and effects of abstinence. 2005. [DOI] [PMC free article] [PubMed]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug and Alcohol Dependence. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Newton TF, Kalechstein AD, Hardy DJ, Cook IA, Nestor L, Ling W, Leuchter AF. Association between quantitative EEG and neurocognition in methamphetamine-dependent volunteers. ClinNeurophysiol. 2004;115:194–198. doi: 10.1016/s1388-2457(03)00314-6. [DOI] [PubMed] [Google Scholar]
- Peterson JD, Wolf ME, White FJ. Repeated amphetamine administration decreases D1 dopamine receptor-mediated inhibition of voltage-gated sodium currents in the prefrontal cortex. Journal of Neuroscience. 2006;26:3164–3168. doi: 10.1523/JNEUROSCI.2375-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson R, McCann M, Huber A, Shoptaw S, Higgins ST, Silverman K. Motivating behavior change among illicit-drug abusers: Research on contingency management interventions., Contingency management and relapse prevention as stimulant abuse treatment interventions. American Psychological Association; Washington, DC, US: 1999. pp. 57–74. [Google Scholar]
- Salo R, Nordahl TE, Natsuaki Y, Leamon MH, Galloway GP, Waters C, Moore CD, Buonocore MH. Attentional control and brain metabolite levels in methamphetamine abusers. Biol Psychiatry. 2007;61:1272–1280. doi: 10.1016/j.biopsych.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Possin K, Leamon M, Gibson DR, Galloway GP, Flynn NM, Henik A, Pfefferbaum A, Sullivan EV. Preliminary evidence of reduced cognitive inhibition in methamphetamine-dependent individuals. Psychiatry Research. 2002;111:65–74. doi: 10.1016/s0165-1781(02)00111-7. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Steward T, Wang J, Swanson AN, De La Garza R, Newton T, Ling W. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96:222–232. doi: 10.1016/j.drugalcdep.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL, Dean AC, Cordova X, Monterosso JR, London ED. Methamphetamine dependence and neuropsychological functioning: Evaluating change during early abstinence. Psychology of Addictive Behavior. doi: 10.15288/jsad.2010.71.335. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL, Domier C, Sim T, Richardson K, Rawson RA, Huber A, Ling W. Cognitive correlates of chronic methamphetamine use. Drug and Alcohol Dependence. 2000;60:511. [Google Scholar]
- Smith EE, Jonides J, Koeppe RA. Dissociating verbal and spatial working memory using PET. Cerebral Cortex. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students’ recent drinking history: utility for alcohol research. Addict Behav. 1986;11:149–161. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A, Recknor EC, Perez-Garcia M. Executive dysfunction in substance dependent individuals during drug use and abstinence: an examination of the behavioral, cognitive and emotional correlates of addiction. J Int Neuropsychol Soc. 2006;12:405–415. doi: 10.1017/s1355617706060486. [DOI] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Conover E, Gongvatana A, Gonzalez R, Carey CL, Cherner M, Heaton RK, Grant I. Deficient strategic control of verbal encoding and retrieval in individuals with methamphetamine dependence. Neuropsychology. 2005;19:35–43. doi: 10.1037/0894-4105.19.1.35. [DOI] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Rodriguez P, Simon SL, Brody A, Jarvik M, Domier CP, Olmstead R, Ernst M, London ED. Brain activity in cigarette smokers performing a working memory task: effect of smoking abstinence. Biol Psychiatry. 2005;58:143–150. doi: 10.1016/j.biopsych.2005.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.