Abstract
Serotonin-1A receptors may play a role in the pathophysiology of depression and suicide. In postmortem brain tissue, agonist binding to serotonin-1A receptors is reportedly increased or unchanged in depression or suicide, while neuroimaging studies report a decrease in antagonist binding to these receptors in subjects with depression. In this study, both agonist and antagonist radioligand binding to serotonin-1A receptors were examined in postmortem orbitofrontal cortex from subjects with major depressive disorder (MDD). Brain tissue was collected at autopsy from 11 subjects with MDD and 11 age- and gender-matched normal control subjects. Two depressed subjects had a recent psychoactive substance use disorder. Six subjects with MDD had a prescription for an antidepressant drug in the last month of life, and, of these six, postmortem bloods from only two subjects tested positive for an antidepressant drug. There was no significant difference between cohorts for age, postmortem interval or tissue pH. The receptor agonist [3H]8-OH-DPAT or the antagonist [3H]MPPF were used to autoradiographically label serotonin-1A receptors in frozen sections from cytoarchitectonically-defined left rostral orbitofrontal cortex (area 47). There was no significant difference between depressed and control subjects in agonist binding to serotonin-1A receptors. However, antagonist binding was significantly decreased in outer layers of orbitofrontal cortex in MDD. This observation in postmortem tissue confirms reports using an antagonist radioligand in living subjects with depression. Decreased antagonist binding to serotonin-1A receptors in outer layers of orbitofrontal cortex suggests diminished receptor signaling and may be linked to corresponding neuronal changes detected previously in these depressed subjects.
Keywords: Serotonin-1A receptor, Depression, Postmortem, Orbitofrontal cortex
1. Introduction
Various approaches in clinical research implicate serotonin in the pathophysiology of depression. Evidence for the involvement of serotonin in depression and its treatment comes from reports of the efficacy of chronic antidepressant treatments that enhance serotonin neurotransmission, of tryptophan depletion studies in remitted subjects with depression, of diminished neuroendocrine responses to serotonergic stimuli in depression, of changes in the serotonin transporter and serotonin receptors in brain imaging or studies of postmortem brain in depression, and of a polymorphism of the promoter gene for the serotonin-1A receptor associating with depression (Blier and de Montigny, 1999; Mann, 1999; Meyer et al., 2003; Stockmeier, 2003; Lemonde et al., 2003; Drevets et al., 2007).
Probing serotonin receptor binding characteristics in vivo using positron emission tomography (PET) or in postmortem brain tissue is a useful although somewhat indirect means of assessing the serotonergic system in depression. Using the serotonin-1A receptor antagonist [11C]WAY-100635, binding potential is significantly decreased in the orbitofrontal, anterior cingulate, occipital, or parietal cortex in medicated or unmedicated subjects experiencing a major depressive episode and is decreased in subjects whose MDD is in remission and are free of antidepressant medication for six or more months (Drevets et al., 2000, 2007; Sargent et al., 2000; Bhagwagar et al., 2004). However, other neuroimaging studies report either no change in binding potential to serotonin-1A receptors in these regions in depressed subjects exposed to antide-pressants or an increase in binding in antidepressant-naïve subjects with MDD (Meltzer et al., 2004; Parsey et al., 2006).
Serotonin-1A receptors have been examined in postmortem cerebral cortex in suicide victims and subjects with a history of a mood disorder using the selective serotonin-1A receptor agonist [3H]8-hydroxy-2-(di-n-propyl)aminotetralin (8-OH-DPAT). These studies using a radiolabeled agonist have yielded varied results. Matsubara et al. (1991) detected an increase in the number of serotonin-1A receptors in dorsolateral prefrontal cortex (dlPFC, Brodmann areas 8+9) of suicide victims dying of so-called non-violent means. Arango et al. (1995) reported increases in radioligand binding to serotonin-1A receptors in ventrolateral (area 45 and area 46) but not in other areas of prefrontal cortex of suicide victims. In contrast, other studies reported no significant changes in agonist-labeled serotonin-1A receptors in prefrontal cortex, even when suicide victims were classified as either depressed or not depressed (Dillon et al., 1991, dlPFC areas 8+9; Arranz et al., 1994, anterior dorsolateral and orbitofrontal cortex, areas 9+10+11; Lowther et al., 1997, anterior prefrontal area 10, and occipital areas 17+18; Stockmeier et al., 1997, area 10).
One reason for the differing results between studies of living subjects with depression and postmortem tissue from subjects with depression could be in the choice of radioligand. The serotonin-1A receptor can be either coupled to or uncoupled from a GTP binding protein. The serotonin-1A receptor agonist, 8-OH-DPAT, used in all postmortem studies, binds only to the coupled receptor, whereas the receptor antagonist WAY-100635, used in neuroimaging studies, binds to both the coupled and uncoupled receptor (Gozlan et al., 1995; Palego et al., 1997; Lanfumey and Hamon, 2004). Thus, neuroimaging studies in cerebral cortex using a receptor antagonist may be detecting a decrease in the total population of serotonin-1A receptors (coupled plus uncoupled) in depression; whereas agonist binding only to G-protein coupled receptors may not be affected in depression. Independent of the issue of radioligands used for receptor labeling, the expression of mRNA for the serotonin-1A receptor was significantly decreased in superficial layers of dorsolateral prefrontal cortex of subjects with MDD (Lopez-Figueroa et al., 2004).
A newer radioligand antagonist for the serotonin-1A receptor, [3H]4-(2’-methoxy-)-phenyl-1-[2’-(N-2”-pyridal)-p-fluorobenzamido]ethyl-piperazine (MPPF), has been developed (Kung et al. 1996). MPPF has lower affinity than WAY-100635 for the serotonin-1A receptor and, as such, the binding of MPPF may be more responsive to changes in endogenous levels of serotonin (Passchier et al., 2000). However, the binding potential of [18F]MPPF to serotonin-1A receptors is not significantly affected by short-term tryptophan depletion or by physiological increases in synaptic serotonin levels or by chronic treatment with fluoxetine, an antidepressant medication that selectively blocks the reuptake of serotonin (Praschak-Rieder et al., 2004; Udo de Haes et al., 2006; Aznavour et al., 2006).
The objective of this study was to examine agonist vs. antagonist radioligand binding to serotonin-1A receptors in adjacent sections of postmortem orbitofrontal cortex from subjects with MDD as compared to normal control subjects. It was hypothesized that antagonist but not agonist binding to serotonin-1A receptors would be significantly decreased in depression. It was also of interest to determine whether any putative lamina-specific changes in serotonin-1A receptors corresponded to neuroanatomical changes detected in immediately adjacent orbitofrontal cortex of the same depressed subjects; changes such as reduced thickness of gray matter and altered neuronal and glial density and neuronal soma size (Rajkowska et al., 1999).
2. Method
2.1. Human subjects and tissue selection
Brain tissue was obtained at autopsy at the coroner’s office of Cuyahoga County, Cleveland, OH. The study was performed according to the declaration of Helsinki and with a protocol approved by the University Institutional Review Board. Informed written consent was obtained from the next-of-kin for all subjects. Characteristics of the control and depressed subjects are recorded in Table 1 and Table 2. A coronal block of left orbitofrontal cortex (rostral part of Brodmann area 47) was collected, frozen in isopentane cooled by dry ice and stored at −80 °C. The frozen piece of orbitofrontal cortex was located immediately caudal to fixed coronal sections defined by cytoarchitectonic criteria and taken from all of the same depressed and many of the same control subjects for cell counting examination by Rajkowska et al. (1999).
Table 1.
Characteristics of the normal control subjects.
| Age/gender | Cause of death | PMI (hrs) /pH | Toxicology | Medicationsa | Axis I diagnosis |
|---|---|---|---|---|---|
| 23/f | Motor vehicle accident | 11/6.84 | Nothing detected | None | none |
| 27/f | CVD | 15/7.01 | Nothing detected | None | None |
| 30/m | CVD | 19/6.98 | Nothing detected | None | None |
| 35/m | CVD | 25/6.74 | Nothing detected | None | None |
| 46/m | CVD | 11/6.95 | Nothing detected | None | None |
| 46/f | Homicide | 24/6.32 | Nothing detected | None | None |
| 49/f | CVD | 29/6.57 | Nothing detected | None | None |
| 50/f | CVD | 27/6.74 | Nothing detected | None | None |
| 50/ m | CVD | 12/6.54 | Nothing detected | None | None |
| 58/m | CVD | 22/6.78 | Nothing detected | None | None |
| 71/m | Cardiac rupture | 24/6.82 | Nothing detected | None | None |
Abbreviations: CVD, cardiovascular disease; f, female; m, male; pH, tissue pH; PMI, postmortem interval.
Psychoactive medications prescribed in the last month of life. Only psychoactive compounds are listed under toxicology.
Table 2.
Characteristics of the subjects with major depressive disorder.
| Age/ gender |
Cause of death | PMI (hrs) / pH |
Toxicology | Medicationsa | Axis I diagnosis | Duration of illness (years) |
|---|---|---|---|---|---|---|
| 30/m | Sigsw – chest, suicide | 18/6.91 | Ethanol 0.07% | None | MDD, chronic, moderate | 3 |
| 34/f | Carbon monoxide, suicide | 24/6.27 | Ethanol 0.12%, CO, alprazolam | Alprazolama, valproatea, trazodone, risperidone | MDD, recurrent, severe; panic attacks with agoraphobia | 20 |
| 36/m | Undetermined | 11/6.96 | Diphenhydramine | None | MDD, recurrent, moderate | 3 |
| 40/f | CVD | 25/6.32 | Morphine, codeine, hydrocodone | Fluoxetinea, temazepama, hydrocodonea | MDD, recurrent, in full remission | 5 |
| 42/f | Suicide overdose – propoxyphene and acetaminophen | 24/6.62 | Propoxyphene, acetaminophen, | Propoxyphenea, fluoxetinea, amitriptylinea, paroxetinea, diazepama, | MDD, chronic, moderate; polysubstance dependence; bulemia nervosa | 26 |
| 42/m | Drowning, suicide | 20/6.64 | Sertraline, ethanol 0.02% | Sertralinea | MDD, single episode, severe | 0.25 |
| 46/m | Gunshot, homicide | 17/6.26 | Nothing detected | None | MDD, single episode, mild | 1 |
| 50/f | Hanging, suicide | 23/6.83 | Nothing detected | Clomipramine, fluoxetine, thiothixene | MDD, recurrent, moderate, with psychosis, mood congruent | 5 |
| 54/m | Accidental CO poisoning | 23/6.24 | CO, phenobarbital, phenytoin | Sertralinea | MDD, single episode, moderate, chronic | 3 |
| 63/f | Pulmonary thromboembolism | 24/6.32 | Amitriptyline, chlorpromazine, amantadine, lidocaine | Amitriptylinea, chlorpromazinea, clonazepama, amantidinea | MDD, recurrent, moderate; Polysubstance abuse | 30 |
| 86/m | Stab to chest and throat, suicide | 21/6.23 | Nothing detected | Fluoxetinea, atenolola, leuprolide | MDD, recurrent, severe, with melancholia | 20 |
Abbreviations: CO, carbon monoxide; CVD, cardiovascular disease; f, female; MDD, major depressive disorder; m, male; pH, tissue pH; PMI, postmortem interval; sigsw, self-inflicted gunshot wound.
Psychoactive medications prescribed in the last month of life. Only psychoactive compounds are listed under toxicology.
Psychiatric assessments of all subjects were performed via informant-based retrospective interviews as previously described (Stockmeier et al., 1998). A trained interviewer administered the schedule for affective disorders and schizophrenia: lifetime version (SADS-L; Spitzer and Endicott, 1978) to next-of-kin about three months after the death to determine current and lifetime axis I psychopathology. Diagnoses for axis I major mental disorders were independently assessed by a clinical psychologist and a psychiatrist, and consensus diagnosis was reached in conference using information from the knowledgeable informants, the coroner’s office, and available inpatient and outpatient records. Eleven subjects met the DSM-IV criteria for MDD (American Psychiatric Association, 1994). Ten of these subjects were experiencing a depressive episode within the last two weeks of their lives, and one subject with MDD was in remission. Six subjects with MDD had an antidepressant drug prescription in the last month of life, while postmortem blood from only two subjects tested positive for an antidepressant drug. Medication histories for the depressed subjects are included in Table 2. One subject with MDD also met criteria for polysubstance dependence and another subject for polysubstance abuse.
There was no significant difference between subject groups in age, postmortem interval or tissue pH. The average age (years, mean ± SEM) of the two groups was – Control: 44.1 ± 4.3, MDD: 47.5 ± 4.8. The average postmortem interval (hours, mean ± SEM) of the two groups was – Control: 19.9 ± 2.0, MDD: 20.9 ± 1.3. The average tissue pH (mean ± SEM) was – Control: 6.75 ± 0.06, MDD: 6.51 ± 0.09.
2.2. Receptor autoradiography
Autoradiographic measurement of serotonin-1A receptor binding was performed using the receptor agonist [3H]8-hydroxy-2-(di-n-propyl)aminotetralin (8-OH-DPAT) (Pazos et al., 1987; Stockmeier et al., 1998) or the antagonist [3H]4-(2’-methoxy-)-phenyl-1-[2’-(N-2”-pyridal)-p-fluorobenzamido]ethyl-piperazine (MPPF) (Kung et al., 1996). Triplicate (total binding) and duplicate (nonspecific binding) frozen sections (20 μ) of rostral orbitofrontal cortex were thawed and air-dried. After a 30 min pre-incubation at room temperature in buffer containing 170 mM Tris-HCl, 4 mM CaCl2, and 0.01% ascorbic acid (pH 7.7, 24 °C), three sections were incubated in fresh buffer for one hour at 24 °C with [3H]8-OH-DPAT (2 nM, 162.9 Ci/mmol, New England Nuclear, Boston, MA). Nonspecific binding was measured in duplicate serial sections co-incubated with 10 µM serotonin (serotonin–creatinine sulfate complex, Sigma Chemical, St. Louis, MO). Citalopram–hydrobromide (1 µM, Lundbeck, Copenhagen, Denmark) was included in the incubation with [3H]8-OH-DPAT to prevent radioligand binding to the serotonin transporter. Adjacent sections were similarly processed for antagonist radioligand binding. For antagonist binding, after a 30 min pre-incubation at room temperature in buffer containing 50 mM Tris-HCl and 2 mM MgCl2 (pH 7.4, 24 °C), three sections were incubated in fresh buffer for 90 min at 24 °C with [3H]MPPF (1.9 nM, 70.5 Ci/mmol, New England Nuclear). Nonspecific binding was measured in duplicate serial sections co-incubated with 10 µM serotonin (serotonin–HCl, Sigma, St. Louis, MO). Prazosin-HCl (100 nM, RBI, Natick, NJ) and S(–)-sulpiride (1 µM, RBI) were included in the incubation with [3H]MPPF to prevent radioligand binding to alpha-1 adrenergic and dopamine-2 receptors, respectively.
After the incubation, the [3H]8-OH-DPAT-labeled sections were washed twice at 4 °C for five minutes in non-radioactive assay buffer (pH 7.7 at 4 °C), and the [3H]MPPF-labeled sections were washed twice at 4 °C for ten minutes in non-radioactive assay buffer (pH 7.4 at 4 °C). Sections were then dipped in ice-cold water, air-dried, and stored for 24–48 h at 4 °C in sealed slide boxes with capsules containing desiccant. The sections and tritiated plastic standards (American Radiolabeled Chemicals, St. Louis, MO) were apposed to Hyperfilm-3H (Amersham, Arlington Heights, IL) in X-ray cassettes for 8 weeks ([3H]8-OH-DPAT) or 8.5 weeks ([3H]MPPF). Films were processed with Kodak D-19 developer and fixed with Kodak Rapid Fix (Eastman Kodak, Rochester, NY).
Autoradiographic images were digitized and quantified using the microcomputer controlled imaging device (MCID-M5, Imaging Research, Inc., St. Catherines, Ontario). Sections used for radioligand binding were later stained for Nissl substance with cresyl echt violet to facilitate the cytoarchitectonic identification of individual cortical laminae. Only cortical laminae with autoradiographic images registering a relative optical density between 0.2 and 0.8 were quantified. At the optimized exposure times listed above, only antagonist binding in layers I–III and agonist binding in layers I–II met this criterion. Calibrated images of total and nonspecific radioligand binding were digitized and sampled, and nonspecific binding was subtracted from total binding to yield specific binding. Specific binding was between 90 and 96 percent of total binding. The results are expressed in terms of calibrated standards. Both the tissues and the resultant autoradiograms from the matched pairs of subjects were processed and analyzed in parallel, and laboratory personnel were blind to the clinical diagnoses of the subjects.
2.3. Statistical analysis
The main statistical analysis of all 22 subjects was performed with a mixed model ANOVA (SAS, Cary, NC) with one of five dependent measures (antagonist labeling in layers I, II, and III, and agonist labeling in layers I and II). Diagnosis was modeled as a fixed effect and between cohorts pairing of subjects (by age) as a random effect. In addition to these three ANOVA models, 15 ANCOVA models were also evaluated, using the three covariates (age, postmortem interval, and tissue pH) one at a time, along with the five dependent measures listed above. When examining the potential effect of gender, suicide or clinical variability, Student’s t-test was used because of a relatively small number of subjects. Six male depressed subjects were compared with six male controls, and five female depressed subjects were compared with five female controls. Because of the clinical variability of four depressed subjects, the 11 controls were also compared with seven depressed subjects with the following depressed subjects omitted in this statistical test: one subject with MDD that was in full remission, one subject with polysubstance dependence, one subject with sertraline present in plasma, and one subject with polysubstance abuse and amitriptyline present in plasma. The main effect of depression persisted when controlling for the potential confounds of gender or clinical variability. Spearman’s rank correlation coefficients were calculated to evaluate potential interactions between [3H]MPPF binding and age of onset or duration of depression.
3. Results
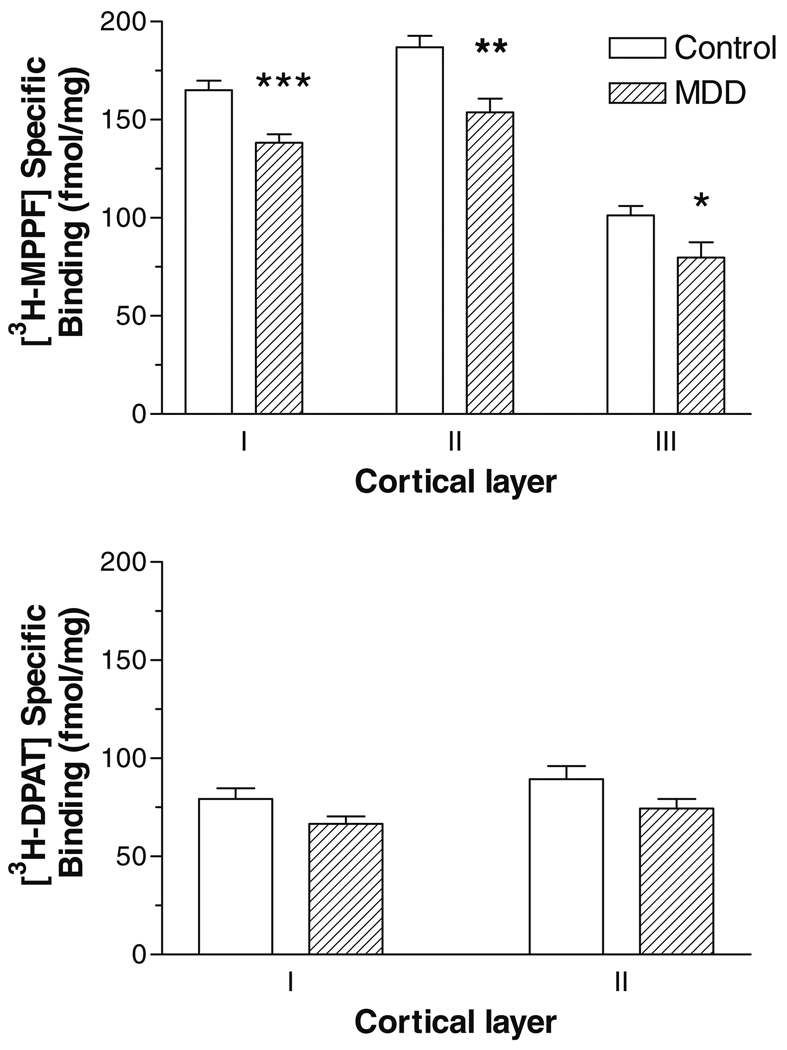
Subjects with MDD showed a statistically significant decrease in radioligand binding of [3H]MPPF, the serotonin-1A receptor antagonist, in the rostral orbitofrontal cortex (Fig. 1 and Fig. 2). There was a significant MDD-related decrease in antagonist binding in layer I (16 percent decrease, F = 16.88, df = 20, p = 0.0005), layer II (18 percent decrease, F = 13.11, df = 20, p = 0.0017), and layer III (21 percent decrease, F = 5.43, df = 20, p = 0.0304). In contrast, there was no statistically significant difference between controls and depressives in the binding of [3H]DPAT, the serotonin-1A receptor agonist, in layer I (F = 3.81, df = 19, p = 0.0658) or in layer II (F = 3.42, df = 19, p = 0.0799) (Fig. 1 and Fig. 2). In the mixed model covariate analysis, none of the three covariates (age, postmortem interval, or tissue pH) made a statistically significant contribution to the model, and the statistical differences noted above with the ANOVA were essentially replicated (data not shown). Specifically, subjects with MDD showed a significant decrease in antagonist but not agonist binding to serotonin-1A receptors when co-varying for age, postmortem interval, or tissue pH.
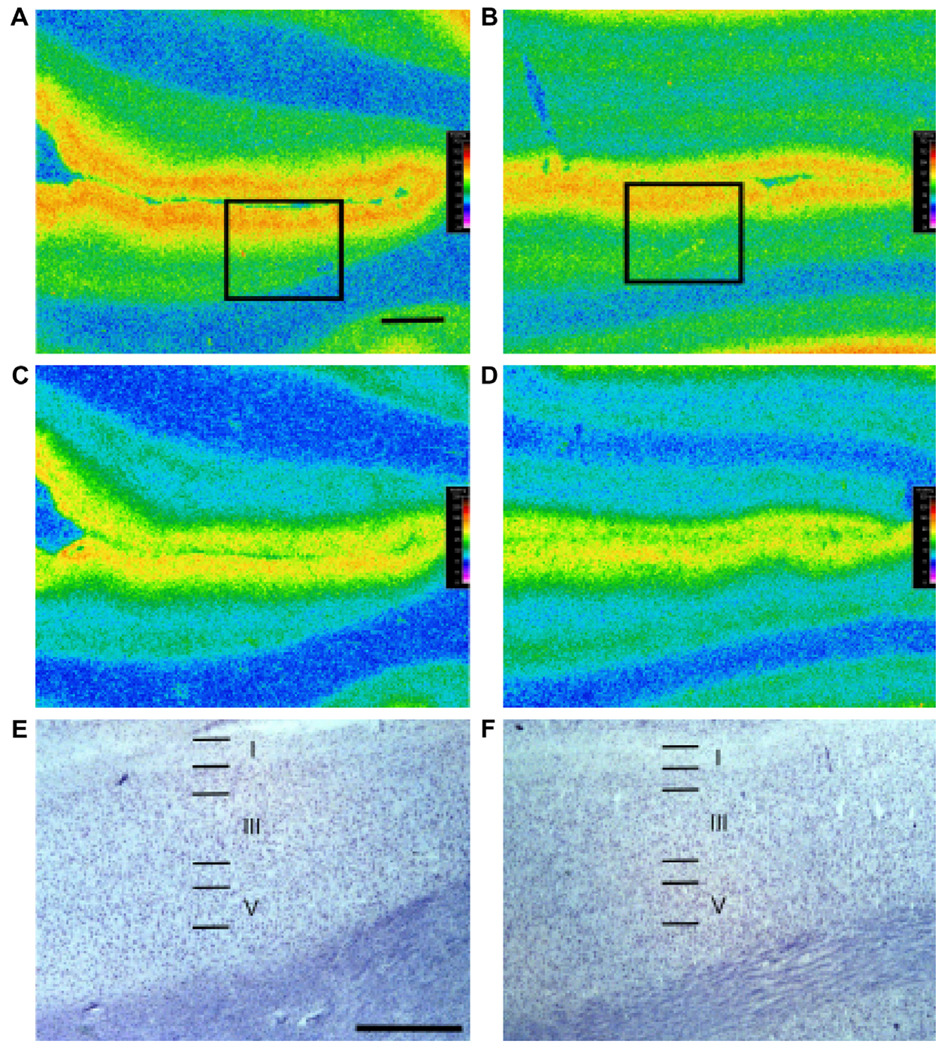
Fig. 1.
Autoradiograms of [3H]MPPF (A, B) and [3H]8-OH-DPAT (C, D) binding to serotonin-1A receptors in adjacent sections of orbitofrontal cortex. Calibrated serotonin-1A autoradiograms from a control subject (A, C) and the age-matched subject with MDD (B, D) are presented. Note the decrease in autoradiographic density in outer cortical layers in the depressed subject compared to the control subject as measured with [3H]MPPF but not with [3H]8-OH-DPAT. The boxes in (A) and (B) are placed over cytoarchitectonically-identified rostral orbitofrontal cortex (Brodmann area 47). Radiolabeled sections used to generate the autoradiograms were subsequently stained for Nissl substance to identify cortical laminae. Laminae (I–VI) for cresyl-violet-stained cortex corresponding to the boxed regions in (A) and (B) are presented in (E) and (F), respectively. The scale bars in (C) and (E) are 2 and 1 mm in length, respectively.
Fig. 2.
Radioligand binding to serotonin-1A receptors in orbitofrontal cortex in subjects with major depressive disorder (MDD) compared with psychiatrically-normal control subjects. [3H]MPPF (top) and [3H]8-OH-DPAT (bottom) binding in the outer cortical laminae is represented on the y-axis as fmol/mg (mean ± SEM). Sections used for receptor autoradiography were subsequently stained for Nissl substance and used to identify the cortical laminae. *** p = 0.0005, **p = 0.0017, * p = 0.03 vs. control subjects.
The potential influence of gender, clinical variability, suicide or allele or genotype frequency was also considered. The observation of a statistically significant decrease in [3H]MPPF but not [3H]DPAT binding was noted in both male and female depressed subjects (mean ± SEM, fmol/mg; layer I, control males: 163.6 ±04.6, MDD males: 147.2 ± 3.4, p = 0.0157; layer II, control males: 189.1 ± 4.6, MDD males: 161.4 ±10.0, p = 0.0304; layer I, control females: 166.6 ± 9.9, MDD females: 127.3 ±5.8, p = 0.009; layer II, control females: 183.1 ± 12.4, MDD females: 144.4 ± 9.2, p = 0.0329. A statistically significant decrease in [3H]MPPF but not [3H]DPAT binding was also noted when the following four depressed subjects were omitted: one subject with MDD that was in full remission, one subject with comorbid polysubstance dependence, one subject with sertraline present in plasma, and one subject with comorbid polysubstance abuse and amitriptyline present in plasma (mean±SEM, fmol/mg; layer I, control: 165.0 ± 4.9, MDD: 139.9 ± 4.8, p = 0.0032; layer II, control: 186.8 ± 5.8, MDD: 156.6 ± 9.2, p = 0.0101). The binding value for the depressed subject in remission was less than the mean of the depression cohort. There were no significant correlations between the age of onset (layer I, r = 0.0137, p = 0.967; layer II, r = −0.137, p = 0.967) or the duration (layer I, r = -0.3503, p = 0.286; layer II, r = 0.0599, p = 0.860) of depression and binding values for [3H]MPPF. Regardless of whether the depressed subject committed suicide, the binding to antagonist-labeled serotonin-1A receptors was also decreased in depression, compared to control subjects (mean ± -SEM, fmol/mg; layer I, control: 165.0 ± 4.9, suicide: 139.4 ± 5.3, p = 0.0047; control: 165.0 ± 4.9, non-suicide: 136.6 ± 7.8, p = 0.0067; layer II, control: 186.8 ± 5.8, suicide: 159.9 ± 9.9, p = 0.0243; control: 186.8 ± 5.8, non-suicide: 146.0 ± 10.0, p = 0.0023). Based on χ2 analysis of genotype frequencies and comparing allele frequencies with Fisher’s exact test, no statistically significant difference in the allele or genotype frequency between control and depressed subjects for the novel C(-1019)G polymorphism was observed (A. Burns and P. Albert, unpublished observation).
4. Discussion
Serotonin-1A receptors were examined autoradiographically in the rostral orbitofrontal cortex of subjects with MDD and age- and gender-matched control subjects. There was a statistically significant effect of diagnosis, in which subjects with MDD had decreased antagonist but not agonist binding to serotonin-1A receptors in superficial layers of orbitofrontal cortex. There was a trend for a reduction in agonist binding to serotonin-1A receptors but it did not reach statistical significance. Covariate statistical analysis revealed that age, postmortem interval or tissue pH did not significantly contribute to the variance among subjects of either cohort. Although the sample size was small for considering a potential influence of gender, antagonist binding to serotonin-1A receptors was significantly decreased in both male and female subjects with depression. A reduction in autoradiographic image intensity in MDD could correspond to either a decrease in serotonin-1A receptor number or affinity, and the current data set does not distinguish between these two possibilities. A reduction in receptor number in depression might be supported by the observation of Lopez-Figueroa et al. (2004) that the expression of mRNA for serotonin-1A receptors was significantly decreased in superficial layers of dorsolateral prefrontal cortex (areas 9 and 46) in MDD. A reduced number of receptors in depression may limit the ability of the brain to compensate under stressful situations where glucocorticoids are known to downregulate serotonin-1A receptor expression (Chalmers et al., 1993; Ou et al., 2001).
A decrease in function in serotonin-1A receptors in orbitofrontal cortex in depression may have a significant effect on prefrontal and subcortical circuitry involved in depression. PET studies record abnormal blood flow or glucose metabolism in the orbital and medial prefrontal cortex, dorsolateral prefrontal cortex, subgenual cingulate cortex and amygdala in depression (Mayberg et al., 1999; Drevets, 2000). Studies in non-human primates reveal that layer III of the orbitofrontal cortex is the main connection between this region and associational and limbic cortical regions (Carmichael and Price, 1995; Rempel-Clower and Barbas, 2000). Layers III and V of the orbitofrontal cortex send axons to the amygdala and layers II and VI receive projections from the amygdala (Aggleton et al., 1980; Porrino et al., 1981). Hence, altered serotonin-1A receptor binding in outer layers of orbitofrontal cortex in depression may significantly contribute to disrupted forebrain circuitry involved in depression.
All of the subjects with MDD and several of the control subjects in the current study were examined morphometrically in an adjacent, formalin-fixed portion of orbitofrontal cortex (Rajkowska et al., 1999). In that study, subjects with depression exhibited a reduction in the thickness of cortical gray matter, smaller sizes of neuronal cell bodies and a lower density of large neurons accompanied by an increase in the density of smaller neurons. This neuronal pathology was most prominent in outer cortical layers II and III where antagonist binding to serotonin-1A receptors is down-regulated. In contrast, changes in glial density were noted in mid to deeper cortical layers in the same depressed subjects (Rajkowska et al., 1999).
The neuropathological and serotonin-1A receptor changes observed postmortem in superficial prefrontal cortical layers may be linked with changes in GABA and glutamate detected in the cerebral cortex of depressed subjects (Sanacora et al., 2004; Hasler et al., 2007; Maciag et al., 2007; Rajkowska et al., 2007). The level of GABA is reduced in depressed subjects not taking antidepressant drugs but these levels normalized with treatment by selective serotonin reuptake inhibitors (SSRI)(Sanacora et al., 2002). The therapeutic effect of SSRI’s may involve interactions between serotonin-1A receptors, GABA and glutamate in outer layers of prefrontal cortex.
The serotonin-1A receptor gene contains a novel C(-1019)G polymorphism and the homozygous G(-1019) allele is enriched two-fold in depressed subjects and enriched four-fold in suicide victims relative to control subjects (Lemonde et al., 2003). However, in our small number of samples, no statistically significant difference in the allele or genotype frequency was observed between control or depressed subjects (A. Burns and P. Albert, unpublished observation). Although the genotype frequency analysis is significantly underpowered, these data suggest that the decrease in antagonist binding to serotonin-1A receptors is likely not due to a mismatch in allele or genotype frequency between the control and depressed subjects.
Serotonin-1A receptor protein was recently measured in prefrontal cortex (area 10, right hemisphere) by Western blotting in depressed and control subjects not included in this study. Receptor protein levels were significantly decreased in females but not males with depression (Szewczyk et al., 2008), whereas the present study revealed a decrease in serotonin-1A binding sites in both females and males with MDD. A possible reason for the difference in results between Szewczyk et al. (2008) and the current study is that two different cortical regions (orbitofrontal vs. frontal pole) were examined in different hemispheres.
There are limitations in this report of decreased antagonist binding to serotonin-1A receptors in MDD. The number of subjects in the study is relatively small, and the results are presented for only one cerebral cortical region. However, our finding of diminished antagonist binding to serotonin-1A receptors is strengthened by the 3-dimensional cell counting study in the same brain region in these depressed subjects (Rajkowska et al., 1999), and are consistent with the PET studies of Drevets et al. (2000; 2007) and Sargent et al. (2000).
Another limitation of this study is the clinical variability in the depressed subjects. While suicide was ruled the cause of death for six of the eleven depressed subjects, it did not alter the main effect of depression. In addition, MDD was in full remission in one subject, and two of the subjects with MDD had comorbid diagnoses of polysubstance dependence or polysubstance abuse. The statistically significant finding of a decrease in antagonist radioligand binding to serotonin-1A receptors in all eleven depressed subjects persisted when data were reanalyzed after excluding four subjects (with a comorbid psychoactive substance use disorder, with an antidepressant medication present in blood, or with MDD in full remission). It does not appear likely that such a prescription alone would precipitate a decrease in antagonist binding to serotonin-1A receptors in MDD as there was no difference in binding between depressed subjects with a history of antidepressant treatment and those without. Furthermore, serotonin-1A binding potential is significantly decreased in orbitofrontal and other prefrontal cortical regions in 1) depressed subjects taking fluoxetine or sertraline, 2) depressed subjects that were medication-free for over one year, and 3) depressed subjects never medicated (Sargent et al., 2000). However, Parsey et al. (2006) reported no change in serotonin-1A binding potential in subjects with MDD recently treated with antidepressant medications and an increase in this binding potential in never medicated subjects with MDD. These varying neuroimaging results may be related to a methodological distinction in that Sargent et al. (2000) used cerebellar gray matter and Parsey et al. (2006) used cerebellar white matter as a reference tissue for determining free ligand concentration and nonspecific binding.
The question arises as to the significance of a decrease in antagonist but not agonist radioligand binding in cerebral cortex in MDD. The serotonin-1A receptor exists in two states: either coupled or uncoupled to a GTP-binding protein such as Gαi or Gαo (Gozlan et al., 1995; Lanfumey and Hamon, 2004). The agonist 8-OH-DPAT binds only to the coupled receptor, while antagonists such as WAY-100635 or MPPF bind to both the coupled and free receptor. Consequently, the density of antagonist-labeled sites is 60–70% higher than agonist-labeled sites in human brain (Burnet et al., 1997). Thus, the current data reporting a decrease in antagonist but not agonist binding in depression suggest a decrease in the number or affinity of serotonin-1A receptors not coupled to Gαi/o protein. A decrease in the number or affinity of the total receptors, as assessed with the receptor antagonist, may restrict the reserve of receptors available to couple to Gαi/o proteins, and thus diminish receptor signaling when additional reserve is needed. There was a trend for a reduction in agonist binding to serotonin-1A receptors, suggesting that a small reduction in receptors coupled to a GTP-binding protein may have contributed to the decrease in total serotonin-1A receptors as measured with the antagonist.
The cellular and laminar location of serotonin-1A receptors is a critical issue regarding the functional significance of a decrease in serotonin-1A receptor binding in depression. In response to stimulation of the dorsal and median raphe nuclei and the release of serotonin in cerebral cortex, serotonin-1A receptors mediate the inhibitory effect of serotonin on cortical pyramidal neurons (Amargos-Bosch et al., 2004). In monkey and human temporal cortex, DeFelipe et al. (2001) found serotonin-1A receptor immunoreactivity on axons of pyramidal neurons proximal to input from GABA-containing chandelier interneurons. These authors also report that soma and proximal portions of dendrites of other neurons were also immunostained but with lower intensity than labeling of the initial segments of pyramidal axons. The neurons immunostained with lower intensity may correspond to the GABA interneurons in rat cerebral cortex that express mRNA or immunoreactivity for the serotonin-1A receptor (Aznar et al., 2003; Santana et al., 2004). Thus, the decrease observed here in antagonist binding to serotonin-1A receptors may reflect a decrease in serotonin-1A receptors localized to GABA interneurons or to the axon initial segments of pyramidal neurons. Consistent with this suggestion, Rajkowska et al. (2007) detected a significant decrease in presumptively-immunolabeled GABA neurons in dorsolateral prefrontal cortex and a strong trend for a decrease in these neurons in orbitofrontal cortex in depression.
In conclusion, subjects with MDD had decreased antagonist but not agonist binding to serotonin-1A receptors in superficial layers of orbitofrontal cortex. The decrease in antagonist binding to serotonin-1A receptors appears to be related to depression and not to suicide or antidepressant therapy. The observation in postmortem tissue confirms reports using an antagonist radioligand in living subjects with depression and suggests diminished receptor signaling.
Acknowledgements
The authors deeply appreciate the assistance of the next-of-kin of the deceased and gratefully acknowledge the support of Elizabeth K. Balraj, M.D., and the staff of the Cuyahoga County Coroner’s Office, Cleveland, Ohio. The authors also gratefully acknowledge Lisa Konick and Drs. James C. Overholser, George Jurjus, Bryan Roth and Herbert Y. Meltzer for psychiatric assessment of the subjects. The authors thank Paul R. Albert, Ph.D., and Ariel M. Burns, M.Sc., of the Ottawa Health Research Institute (Neuroscience), University of Ottawa, Canada, for the genotype frequency analysis. The authors also acknowledge Dr. J. Miguel-Hidalgo, Ph.D., for sectioning the tissues and Drs. Mark Austin and Paul Albert for helpful discussions.
Contributors
C.A. Stockmeier and G. Rajkowska designed the study, wrote the protocol, analyzed the data and wrote the first draft of the manuscript. L. Friedman statistically analyzed the data and assisted in writing the first draft of the manuscript. X. Shi performed the radioreceptor binding assays. E. Howley, G. Clarke and A. Sobanska analyzed the autoradiograms. All authors contributed to and have approved the final manuscript.
Role of funding source
The authors declare that this work was funded by support from The National Institute of Mental Health (MH60451, MH61578, MH63187, MH67996, and RR17701), and an Intramural Research Support grant from The University of Mississippi Medical Center. These funding sources had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of Interest
Grazyna Rajkowska, Ph.D., is a consultant for Eli Lilly and Company. Eimear Howley, M.S., is employed by GlaxoSmithKline, Harlow, Essex, UK, a manufacturer of antidepressant medications. All other authors declare that there are no financial interests or conflicts of interest to disclose.
References
- Aggleton JP, Burton MJ, Passingham RE. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta) Brain Research. 1980;190:347–368. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- Amargos-Bosch M, Bortolozzi A, Puig MV, Serrats J, Adell A, Celada P, et al. Co-expression and in vivo interaction of serotonin-1A and serotonin-2A receptors in pyramidal neurons of prefrontal cortex. Cerebral Cortex. 2004;14:281–299. doi: 10.1093/cercor/bhg128. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre-and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Research. 1995;688:121–133. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- Arranz B, Eriksson A, Mellerup E, Plenge P, Marcusson J. Brain 5-HT1A, 5-HT1D, and 5-HT2 receptors in suicide victims. Biological Psychiatry. 1994;35:457–463. doi: 10.1016/0006-3223(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Aznar S, Qian Z, Shah R, Rahbek B, Knudsen GM. The 5-HT1A serotonin receptor is located on calbindin-and parvalbumin-containing neurons in the rat brain. Brain Research. 2003;959:58–67. doi: 10.1016/s0006-8993(02)03727-7. [DOI] [PubMed] [Google Scholar]
- Aznavour N, Rbah L, Riad M, Reilhac A, Costes N, Descarries L, et al. A PET imaging study of 5-HT(1A) receptors in cat brain after acute and chronic fluoxetine treatment. Neuroimage. 2006;33:834–842. doi: 10.1016/j.neuroimage.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Rabiner EA, Sargent PA, Grasby PM, Cowen PJ. Persistent reduction in brain serotonin-1A receptor binding in recovered depressed men measured by positron emission tomography with [11C]WAY-100635. Molecular Psychiatry. 2004;9:386–392. doi: 10.1038/sj.mp.4001401. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Serotonin and drug-induced therapeutic responses in major depression, obsessive-compulsive and panic disorders. Neuropsycho-pharmacology. 1999;21 Suppl 2:91S–98S. doi: 10.1016/S0893-133X(99)00036-6. [DOI] [PubMed] [Google Scholar]
- Burnet PW, Eastwood SL, Harrison PJ. [3H]WAY-100635 for 5-HT1A receptor autoradiography in human brain: a comparison with [3H]8-OH-DPAT and demonstration of increased binding in the frontal cortex in schizophrenia. Neurochemistry International. 1997;30:565–574. doi: 10.1016/s0197-0186(96)00124-6. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. Journal of Comparative Neurology. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Kwak SP, Mansour A, Akil H, Watson SJ. Corticosteroids regulate brain hippocampal 5-HT1A receptor mRNA expression. Journal of Neuroscience. 1993;13:914–923. doi: 10.1523/JNEUROSCI.13-03-00914.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, Arellano JI, Gomez A, Azmitia EC, Munoz A. Pyramidal cell axons show a local specialization for GABA and 5-HT inputs in monkey and human cerebral cortex. Journal of Comparative Neurology. 2001;433:148–155. doi: 10.1002/cne.1132. [DOI] [PubMed] [Google Scholar]
- Dillon KA, Gross-Isseroff R, Israeli M, Biegon A. Autoradiographic analysis of serotonin 5-HT1A receptor binding in the human brain postmortem: effects of age and alcohol. Brain Research. 1991;554:56–64. doi: 10.1016/0006-8993(91)90171-q. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Greer PJ, Mathis C. Serotonin type-1A receptor imaging in depression. Nuclear Medicine and Biology. 2000;27:499–507. doi: 10.1016/s0969-8051(00)00119-0. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biological Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, et al. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nuclear Medicine and Biology. 2007;34:865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozlan H, Thibault S, Laporte AM, Lima L, Hamon M. The selective 5-HT1A antagonist radioligand [3H]WAY-100635 labels both G-protein-coupled and free 5-HT1A receptors in rat brain membranes. European Journal of Pharmacology. 1995;288:173–186. doi: 10.1016/0922-4106(95)90192-2. [DOI] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Archive of General Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- Kung HF, Stevenson DA, Zhuang ZP, Kung MP, Frederick D, Hurt SD. New 5-HT1A receptor antagonist: [3H]p-MPPF. Synapse. 1996;23:344–346. doi: 10.1002/(SICI)1098-2396(199608)23:4<344::AID-SYN13>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Lanfumey L, Hamon M. 5-HT1 receptors. Current Drug Targets of CNS Neurological Disorders. 2004;3:1–10. doi: 10.2174/1568007043482570. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, et al. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. Journal of Neuroscience. 2003;23:8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Figueroa AL, Norton CS, Lopez-Figueroa MO, Armellini-Dodel D, Burke S, Akil H, et al. Serotonin 5-HT1A, 5-HT1B, and 5-HT2A receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biological Psychiatry. 2004;55:225–233. doi: 10.1016/j.biopsych.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Lowther S, De Paermentier F, Cheetham SC, Crompton MR, Katona CL, Horton RW. 5-HT1A receptor binding sites in postmortem brain samples from depressed suicides and controls. Journal of Affective Disorders. 1997;42:199–207. doi: 10.1016/s0165-0327(96)01413-9. [DOI] [PubMed] [Google Scholar]
- Maciag D, Karolewicz B, O’Dwyer G, Srivastava K, Overholser J, Jurjus G, Dieter L, Herbst N, Stockmeier CA, Rajkowska G. Program No. 707.1.2007 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience; 2007. Decreased level of glutamic acid decarboxylase 67 kDa in the prefrontal cortex in major depression. Online. [Google Scholar]
- Mann JJ. Role of the serotonergic system in the pathogenesis of major depression and suicide. Neuropsychopharmacology. 1999;21:99S–105S. doi: 10.1016/S0893-133X(99)00040-8. [DOI] [PubMed] [Google Scholar]
- Matsubara S, Arora RC, Meltzer HY. Serotonergic measures in suicide brain: 5-HT-1Abinding sites in frontal cortex of suicide victims. Journal of Neural Transmission. 1991;85:181–194. doi: 10.1007/BF01244944. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. American Journal of Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Price JC, Mathis CA, Butters MA, Ziolko SK, Moses-Kolko E, et al. Serotonin-1A receptor binding and treatment response in late-life depression. Neuropsychopharmacology. 2004;29:2258–2265. doi: 10.1038/sj.npp.1300556. [DOI] [PubMed] [Google Scholar]
- Meyer JH, McMain S, Kennedy SH, Korman L, Brown GM, DaSilva JN, et al. Dysfunctional attitudes and 5-HT2 receptors during depression and self-harm. American Journal of Psychiatry. 2003;160:90–99. doi: 10.1176/appi.ajp.160.1.90. [DOI] [PubMed] [Google Scholar]
- Ou XM, Storring JM, Kushwaha N, Albert PR. Heterodimerization of mineralocorticoid and glucocorticoid receptors at a novel negative response element of the 5-HT1A receptor gene. Journal of Biological Chemistry. 2001;276:14299–14307. doi: 10.1074/jbc.M005363200. [DOI] [PubMed] [Google Scholar]
- Palego L, Marazziti D, Rotondo A, Batistini A, Lucacchini A, Naccarato AG, et al. Further characterization of [3H]8-hydroxy-2-(di-n-propyl)aminotetralin binding sites in human brain postmortem. Neurochemistry International. 1997;30:149–157. doi: 10.1016/s0197-0186(96)00050-2. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang YY, et al. Altered serotonin-1A binding in major depression: a [carbonyl-C-11]WAY-100635 positron emission tomography study. Biological Psychiatry. 2006;59:106–113. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Passchier J, van Waarde A, Pieterman RM, Elsinga PH, Pruim J, Hendrikse HN, et al. In vivo delineation of 5-HT1A receptors in human brain with [18F]MPPF. Journal of Nuclear Medicine. 2000;41:1830–1835. [PubMed] [Google Scholar]
- Pazos A, Probst A, Palacios JM. Serotonin receptors in the human brain-III. Autoradiographic mapping of serotonin-1 receptors. Neuroscience. 1987;21:97–122. doi: 10.1016/0306-4522(87)90326-5. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Crane AM, Goldman-Rakic PS. Direct and indirect pathways from the amygdala to the frontal lobe in rhesus monkeys. Journal of Comparative Neurology. 1981;198:121–136. doi: 10.1002/cne.901980111. [DOI] [PubMed] [Google Scholar]
- Praschak-Rieder N, Hussey D, Wilson AA, Carella A, Lee M, Dunn E, et al. Tryptophan depletion and serotonin loss in selective serotonin reuptake inhibitor-treated depression: an [(18)F] MPPF positron emission tomography study. Biological Psychiatry. 2004;56:587–591. doi: 10.1016/j.biopsych.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biological Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology. 2007;32:471–482. doi: 10.1038/sj.npp.1301234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel-Clower NL, Barbas H. The laminar pattern of connections between prefrontal and anterior temporal cortices in the rhesus monkey is related to cortical structure and function. Cerebral Cortex. 2000;10:851–865. doi: 10.1093/cercor/10.9.851. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. American Journal of Psychiatry. 2002;159:663–665. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, et al. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Archives of General Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. Expression of serotonin-1A and serotonin-2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cerebral Cortex. 2004;14:1100–1109. doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, et al. Brain serotonin-1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Archives of General Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Endicott J. Schedule for affective disorders and schizophrenia (SADS) 3rd ed. New York: New York State Psychiatric Institute; 1978. [Google Scholar]
- Stockmeier CA, Dilley GE, Shapiro LA, Overholser JC, Thompson PA, Meltzer HY. Serotonin receptors in suicide victims with major depression. Neuropsychopharmacology. 1997;16:162–173. doi: 10.1016/S0893-133X(96)00170-4. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. Journal of Neuroscience. 1998;18:7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmeier CA. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. Journal of Psychiatry Research. 2003;37:357–373. doi: 10.1016/s0022-3956(03)00050-5. [DOI] [PubMed] [Google Scholar]
- Szewczyk B, Albert PR, Burns AM, Czesak M, Overholser JC, Jurjus GJ, et al. Gender-specific decrease in NUDR and 5-HT1A receptor proteins in the prefrontal cortex of subjects with major depressive disorder. International Journal of Neuropsychopharmacology. 2008;19:1–14. doi: 10.1017/S1461145708009012. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udo de Haes JI, Harada N, Elsinga PH, Maguire RP, Tsukada H. Effect of fenfluramine-induced increases in serotonin release on [18F]MPPF binding: a continuous infusion PET study in conscious monkeys. Synapse. 2006;59:18–26. doi: 10.1002/syn.20209. [DOI] [PubMed] [Google Scholar]