Abstract
Feed forward control and estimates of the future state of the motor system are critical for fast and coordinated movements. One framework for generating these predictive signals is based on the central nervous system implementing internal models. Internal models provide for representations of the input–output properties of the motor apparatus or their inverses. It has been widely hypothesized that the cerebellum acquires and stores internal models of the motor apparatus. The results of psychophysical, functional imaging and transcranial magnetic stimulation studies in normal subjects support this hypothesis. Also, the deficits in patients with cerebellar dysfunction can be attributed to a failure of predictive feed forward control and/or to accurately estimate the consequences of motor commands. Furthermore, the computation performed by the cerebellar-like electrosensory lobes in several groups of fishes is to predict the sensory consequences of motor commands. However, only a few electrophysiological investigations have directly tested whether neurons in the cerebellar cortex have the requisite signals compatible with either an inverse or forward internal model. Our studies in the monkey performing manual pursuit tracking demonstrate that the simple spike discharge of Purkinje cells does not have the dynamics-related signals required to be the output of an inverse dynamics model. However, Purkinje cell firing has several of the characteristics of a forward internal model of the arm. A synthesis of the evidence suggests that the cerebellum is involved in integrating the current state of the motor system with internally generated motor commands to predict the future state.
Keywords: Primate, Cerebellum, Purkinje cell, Simple spike, Internal models
Review
Internal models are widely discussed as a mechanism by which the central nervous system (CNS) generates feed forward commands and/or makes predictions about the upcoming states. Forward internal models predict the state of the system, either the motor variables or the sensory output, as a consequence of the current state of the system and the motor commands. Inverse dynamics models, in response to the desired trajectory, generate the signals required to produce the joint torques/forces used to control a movement. Numerous psychophysical findings support the hypothesis that the CNS uses internal models. Prominent examples include the anticipatory changes in grip forces during predictable manipulations of object load [11, 17] and our ability to adapt and generalize reaching movements in novel dynamic environments [38, 42]. Furthermore, sensory feedback loops have delays that are too long and gains too low to control fast and coordinated movements.
Many investigators postulate that the cerebellum acquires and stores internal models of the motor system [16, 18, 26, 37, 44]. Support for these hypotheses is largely based on deficits in patients with cerebellar dysfunction or on functional imaging studies. Consistent with the inverse dynamics hypothesis, cerebellar patients have difficulty adapting to external force fields [23, 40]. Changes in the activation of the cerebellum following motor learning in normal subjects also implicate this structure in the acquisition and storage of inverse models [8, 10, 16, 37].
A few electrophysiological investigations have tested whether Purkinje cells are the output of an inverse dynamics model. Direct predictions of this hypothesis are that these neurons must provide the signals needed to specify the dynamics of a movement and should be closely coupled to muscle activity [13, 36, 42]. The first studies involved Purkinje cell recordings in the ventral paraflocculus/flocculus during the ocular following response in monkeys [13, 20, 39]. Simple spike and complex spike firing were reconstructed using a combination of eye position, velocity, and acceleration. These kinematic signals were interpreted in terms of their dynamic counterparts, that is, the elastic, viscous, and inertial forces needed to control the eye. However, the kinematic signals are not equivalent to forces [30]. In fact, it has been well documented that Purkinje cell firing signals eye movement kinematics [22, 24].
The transient changes in Purkinje cell firing that occurs when an external force load is switched from resistive to assistive during elbow movements have been taken as evidence for an inverse dynamics model in the cerebellum [45]. However, that study did not explicitly control limb kinematics or systematically vary the magnitude of the external loads. There was no measure of effect size. Most importantly, the study did not demonstrate that the changes in simple spike firing in response to the switch in force loads specified joint torques and/or limb EMG activity as required for the output of an inverse dynamics model.
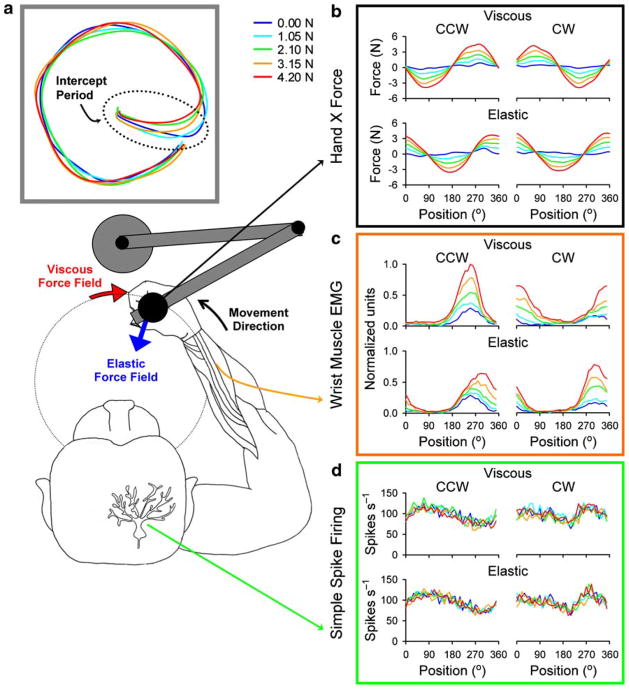
To rigorously test whether Purkinje cell firing is the output of an inverse dynamics model requires systematically varying forces while keeping kinematics constant. To test for arm movements, we recorded Purkinje cells in the intermediate and lateral cerebellum while monkeys accurately tracked a small target as viscous and elastic force fields of varying loads were imposed (Fig. 1a) [32]. Hand kinematics, including the path and speed, were not significantly altered by either the type of force field or the magnitude of the load (Fig. 1a). Hand forces and arm EMG activity increased linearly with the load and their spatial tuning (i.e., position of maximal response during clockwise (cw) and counterclockwise (ccw) tracking) differed markedly between viscous and elastic force fields (Fig. 1b and c). Under viscous loads, the hand forces and arm EMG activity were highly dependent on the direction as evidenced by the nearly 180° out-of-phase modulation for ccw and cw tracking. Under elastic loads, arm dynamics were highly dependent on position as shown by the nearly in-phase modulation of the forces and EMG activity independent of the direction of movement.
Fig. 1.
a Schematic of monkey performing the circular tracking task and the hand paths under varying viscous loads and the null force field. b Viscous (left column) and elastic (right column) force fields have distinct hand force profiles with the former being a function of tracking direction. c Muscle EMG activities follow the external forces. d Purkinje cell simple spike discharge, while modulated by the task kinematics, is not altered by the task dynamics (i.e., type and strength of the external force fields); from [32], with permission
The simple spike discharge of Purkinje cells was essentially not affected, with 91% of the cells (151/166) not significantly modulated by force type or load (Fig. 1d). Furthermore, the spatial tuning pattern was not altered, a finding that further uncouples Purkinje cell firing from limb EMG activity. These results are inconsistent with the hypothesis that Purkinje cell discharge in this region of the cerebellum is the output of an inverse dynamics model of the arm.
Evidence implicating the cerebellum as a forward internal model is also primarily based on deficits in patients with cerebellar disorders [1, 27, 43] and on functional imaging studies in normal subjects [10, 16, 19, 34, 37]. In patients with cerebellar pathology, predictive, feed forward control is defective during a wide variety of motor behaviors [1, 15, 21, 27–29, 40, 43]. In contrast, on-line or reactive control is relatively intact. The failure of patients with cerebellar degeneration to adapt to visuomotor perturbations may be due to mismatch between the predicted and actual sensory outcome of motor commands [43]. Furthermore, the increase in reach errors that occurs with transcranial magnetic stimulation of the lateral cerebellum in healthy subjects may reflect the disruption of an accurate estimate of hand position [25]. These observations suggest that the cerebellum generates feed-forward or predictive signals as envisioned by forward internal models.
The cerebellar cortical circuitry appears designed to make predictions about the upcoming state. Cerebellum-like structures, such as the electrosensory lobe in elasmobranches, have an architecture similar to the mammalian cerebellum [2–4]. Common features include a molecular layer with densely packed parallel fibers that synapse on the apical dendrites of the principal cells (i.e., similar to Purkinje cells). The parallel fibers originate in a granule cell layer that receives inputs from higher motor and sensory areas. Principal cells also receive a second class of afferents onto their basilar dendrites and this input relays sensory information from the periphery (i.e., similar to the climbing fiber projection to Purkinje cells). In several teleosts, these cerebellum-like structures generate predictions of the sensory consequences of the fish’s behavior on its own electrosensory system [2, 4, 5, 7, 14]. In turn, these signals are used to remove predictable features from the sensory input. Therefore, cerebellar-like circuitry has the capacity to function as a forward internal model that predicts the upcoming state.
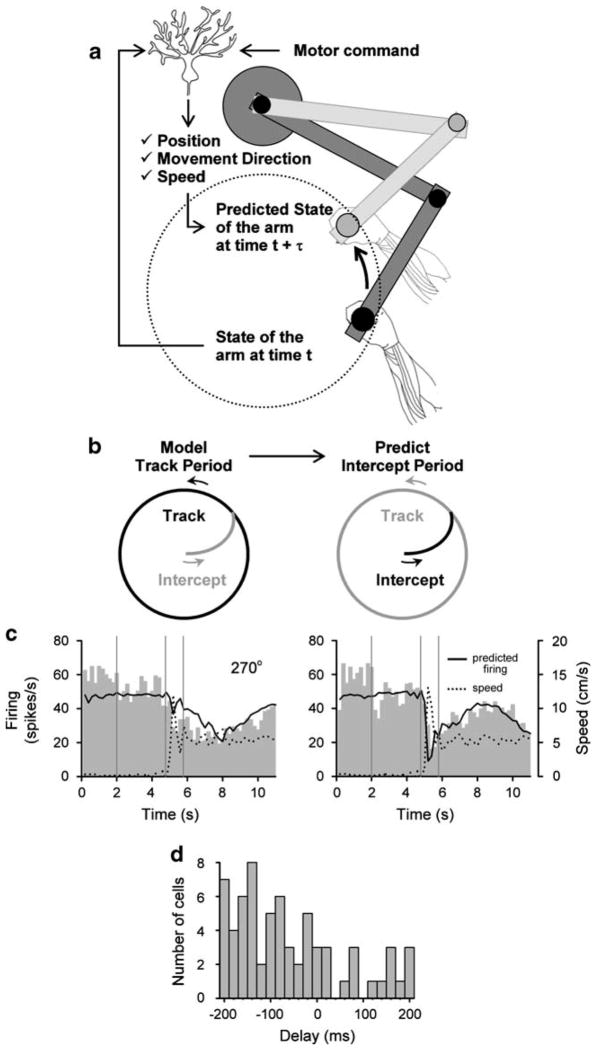
A forward internal model could either produce an estimate of the new motor state (e.g., the position and velocity of the arm) or predict the sensory reafferent signals (e.g., the signals from muscles or joints). While the cerebellum receives the inputs required for a forward internal model, that is the motor command and information on the current state of the system (Fig. 2a) [6, 41], we are not aware of single unit studies explicitly testing the forward internal model hypothesis. Three properties of Purkinje cell firing during arm movements are consistent with these neurons providing a prediction of the upcoming motor state. First, Purkinje cells in lobules IV–VI of the intermediate and lateral cerebellum encode the position, direction of movement and speed of arm movements [9, 12, 31, 35].
Fig. 2.
a Schematic of the forward model prediction in Purkinje cells. State of the arm at time t+τ is predicted based of the state of the arm at time t and the motor command. b Simple spike firing during the Track period of the task is modeled and the same parameters are used to predict the firing during the entire task, including the Intercept period. The τ was estimated from the best regression fit achieved by shifting the firing and kinematic parameters relative to each other over −200 to +200 ms. c Example of the predicted simple spike discharge for medium target speed (5.7 cm/s), for both cw and ccw tracking at a start angle of 270°. d Distribution of delays between simple spike discharge and kinematics. The majority of cells have a negative delay indicating the change in firing preceded the change in kinematics; from [35], with permission
Second, Purkinje cell firing precedes movement kinematics. We demonstrated this in a circular pursuit-tracking paradigm [35] that included both a Track period when the animal tracked the target and an Intercept period when the monkey made a fast-reaching movement from a central location to intercept the target (Fig. 2b). This reaching movement is fundamentally different from pursuit tracking. We took advantage of this difference and the asymmetric properties of the Intercept period to determine the lead/lag between the firing and movement kinematics as well as test the robustness of the kinematic signals carried by Purkinje cells. Using the parameters from a regression model that describes the relation between Purkinje cell firing and hand kinematics during the Track period, we could accurately predict the firing during the Intercept period (Fig. 2b and c). For the majority of recorded Purkinje cells the simple spike discharge preceded the movement kinematics, consistent with a prediction of the upcoming motor state (Fig. 2d).
Third, a forward internal model should provide an estimate of the expected consequences of the motor command under differing movement conditions. Our force field experiment showed that in spite of the large changes in arm dynamics and limb EMG activity, Purkinje cell discharge was unaffected [31]. One interpretation is that the Purkinje cell firing predicts the consequences of arm movements, specifically the position and velocity of the limb [4].
In conclusion, there is converging evidence from human and patient studies, investigations into cerebellum-like structures, and single cell recordings in monkeys that the cerebellum generates predictions of the future state of the system as the kinematic consequences of motor commands. In the monkey, these state estimates can directly inform the CNS about where the hand is, where it is heading, and at what speed, without the inherent delays in sensory feedback. While our focus has been on estimating the upcoming motor state, the cerebellum may provide similar calculations for non-motor systems [4, 33].
Acknowledgments
We wish to thank Michael McPhee for graphics, and Kris Bettin for preparation of the manuscript. Supported in part by NIH grant R01-NS18338.
References
- 1.Bastian AJ. Learning to predict the future: the cerebellum adapts feedforward movement control. Curr Opin Neurobiol. 2006;16 (6):645–649. doi: 10.1016/j.conb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Bell CC. An efference copy which is modified by reafferent input. Science. 1981;214(4519):450–453. doi: 10.1126/science.7291985. [DOI] [PubMed] [Google Scholar]
- 3.Bell CC. Sensory coding and corollary discharge effects in mormyrid electric fish. J Exp Biol. 1989;146:229–253. doi: 10.1242/jeb.146.1.229. [DOI] [PubMed] [Google Scholar]
- 4.Bell CC, Han V, Sawtell NB. Cerebellum-like structures and their implications for cerebellar function. Annu Rev Neurosci. 2008;31:1–24. doi: 10.1146/annurev.neuro.30.051606.094225. [DOI] [PubMed] [Google Scholar]
- 5.Bell CC, Han VZ, Sugawara Y, Grant K. Synaptic plasticity in a cerebellum-like structure depends on temporal order. Nature. 1997;387(6630):278–281. doi: 10.1038/387278a0. [DOI] [PubMed] [Google Scholar]
- 6.Bloedel JR, Courville J. A review of cerebellar afferent systems. In: Brooks VB, editor. Handbook of Physiology, Sect. 1, The Nervous System, Vol. II. Motor Control, Part 2. Williams and Wilkins; Baltimore: 1981. pp. 735–830. [Google Scholar]
- 7.Bodznick D, Montgomery JC, Carey M. Adaptive mechanisms in the elasmobranch hindbrain. J Exp Biol. 1999;202(Pt 10):1357–1364. doi: 10.1242/jeb.202.10.1357. [DOI] [PubMed] [Google Scholar]
- 8.Bursztyn LLCD, Ganesh G, Imamizu H, Kawato M, Flanagan JR. Neural correlates of internal-model loading. Current Biology. 2006;16(24):2440–2445. doi: 10.1016/j.cub.2006.10.051. [DOI] [PubMed] [Google Scholar]
- 9.Coltz JD, Johnson MT, Ebner TJ. Cerebellar Purkinje cell simple spike discharge encodes movement velocity in primates during visuomotor arm tracking. J Neurosci. 1999;19(5):1782–1803. doi: 10.1523/JNEUROSCI.19-05-01782.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diedrichsen J, Hashambhoy Y, Rane T, Shadmehr R. Neural correlates of reach errors. J Neurosci. 2005;25(43):9919–9931. doi: 10.1523/JNEUROSCI.1874-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flanagan J, Wing AM. The role of internal models in motion planning and control: evidence from grip force adjustments during movements of hand-held loads. J Neurosci. 1997;17(4):1519–1528. doi: 10.1523/JNEUROSCI.17-04-01519.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu QG, Flament D, Coltz JD, Ebner TJ. Relationship of cerebellar Purkinje cell simple spike discharge to movement kinematics in the monkey. J Neurophysiol. 1997;78(1):478–491. doi: 10.1152/jn.1997.78.1.478. [DOI] [PubMed] [Google Scholar]
- 13.Gomi H, Shidara M, Takemura A, Inoue Y, Kawano K, Kawato M. Temporal firing patterns of Purkinje cells in the cerebellar ventral paraflocculus during ocular following responses in monkeys I. Simple spikes. J Neurophysiol. 1998;80(2):818–831. doi: 10.1152/jn.1998.80.2.818. [DOI] [PubMed] [Google Scholar]
- 14.Han VZ, Grant K, Bell CC. Reversible associative depression and nonassociative potentiation at a parallel fiber synapse. Neuron. 2000;27(3):611–622. doi: 10.1016/s0896-6273(00)00070-2. [DOI] [PubMed] [Google Scholar]
- 15.Horak FB, Diener HC. Cerebellar control of postural scaling and central set in stance. J Neurophysiol. 1994;72(2):479–493. doi: 10.1152/jn.1994.72.2.479. [DOI] [PubMed] [Google Scholar]
- 16.Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B, Yoshioka T, Kawato M. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature. 2000;403(6766):192–195. doi: 10.1038/35003194. [DOI] [PubMed] [Google Scholar]
- 17.Johansson RS, Cole KJ. Sensory-motor coordination during grasping and manipulative actions. Curr Opin Neurobiol. 1992;2 (6):815–823. doi: 10.1016/0959-4388(92)90139-c. [DOI] [PubMed] [Google Scholar]
- 18.Kawato M. Internal models for motor control and trajectory planning. Curr Opin Neurobiol. 1999;9(6):718–727. doi: 10.1016/s0959-4388(99)00028-8. [DOI] [PubMed] [Google Scholar]
- 19.Kawato M, Kuroda T, Imamizu H, Nakano E, Miyauchi S, Yoshioka T. Internal forward models in the cerebellum: fMRI study on grip force and load force coupling. Prog Brain Res. 2003;142:171–188. doi: 10.1016/S0079-6123(03)42013-X. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi Y, Kawano K, Takemura A, Inoue Y, Kitama T, Gomi H, Kawato M. Temporal firing patterns of Purkinje cells in the cerebellar ventral paraflocculus during ocular following responses in monkeys II. Complex spikes. J Neurophysiol. 1998;80 (2):832–848. doi: 10.1152/jn.1998.80.2.832. [DOI] [PubMed] [Google Scholar]
- 21.Lang CE, Bastian AJ. Cerebellar subjects show impaired adaptation of anticipatory EMG during catching. J Neurophysiol. 1999;82(5):2108–2119. doi: 10.1152/jn.1999.82.5.2108. [DOI] [PubMed] [Google Scholar]
- 22.Leung HC, Suh M, Kettner RE. Cerebellar flocculus and paraflocculus Purkinje cell activity during circular pursuit in monkey. J Neurophysiol. 2000;83(1):13–30. doi: 10.1152/jn.2000.83.1.13. [DOI] [PubMed] [Google Scholar]
- 23.Maschke M, Gomez CM, Ebner TJ, Konczak J. Hereditary cerebellar ataxia progressively impairs force adaptation during goal-directed arm movements. J Neurophysiol. 2004;91(1):230–238. doi: 10.1152/jn.00557.2003. [DOI] [PubMed] [Google Scholar]
- 24.Medina JF, Lisberger SG. Variation, signal, and noise in cerebellar sensory-motor processing for smooth-pursuit eye movements. J Neurosci. 2007;27(25):6832–6842. doi: 10.1523/JNEUROSCI.1323-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miall RC, Christensen LO, Cain O, Stanley J. Disruption of state estimation in the human lateral cerebellum. PLoS Biol. 2007;5(11):e316. doi: 10.1371/journal.pbio.0050316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miall RC, Weir DJ, Wolpert DM, Stein JF. Is the cerebellum a Smith predictor? J Mot Behav. 1993;25(3):203–216. doi: 10.1080/00222895.1993.9942050. [DOI] [PubMed] [Google Scholar]
- 27.Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci. 2006;26(36):9107–9116. doi: 10.1523/JNEUROSCI.2622-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nixon PD, Passingham RE. Predicting sensory events. The role of the cerebellum in motor learning. Exp Brain Res. 2001;138 (2):251–257. doi: 10.1007/s002210100702. [DOI] [PubMed] [Google Scholar]
- 29.Nowak DA, Hermsdorfer J, Rost K, Timmann D, Topka H. Predictive and reactive finger force control during catching in cerebellar degeneration. Cerebellum. 2004;3(4):227–235. doi: 10.1080/14734220410019057. [DOI] [PubMed] [Google Scholar]
- 30.Ostry DJ, Feldman AG. A critical evaluation of the force control hypothesis in motor control. Exp Brain Res. 2003;153(3):275–288. doi: 10.1007/s00221-003-1624-0. [DOI] [PubMed] [Google Scholar]
- 31.Pasalar S, Ebner TJ. Invariant prediction of movement kinematics by Purkinje cell simple spike discharge [Abstract] Soc Neurosci Abstr 2007 [Google Scholar]
- 32.Pasalar S, Roitman AV, Durfee WK, Ebner TJ. Force field effects on cerebellar Purkinje cell discharge with implications for internal models. Nat Neurosci. 2006;9:1404–1411. doi: 10.1038/nn1783. [DOI] [PubMed] [Google Scholar]
- 33.Paulin MG. Evolution of the cerebellumas a neuronal machine for Bayesian state estimation. J Neural Eng. 2005;2(3):S219–S234. doi: 10.1088/1741-2560/2/3/S06. [DOI] [PubMed] [Google Scholar]
- 34.Pollok B, Gross J, Kamp D, Schnitzler A. Evidence for anticipatory motor control within a cerebello-diencephalic-parietal network. J Cogn Neurosci. 2008;20(5):828–840. doi: 10.1162/jocn.2008.20506. [DOI] [PubMed] [Google Scholar]
- 35.Roitman AV, Pasalar S, Johnson MT, Ebner TJ. Position, direction of movement, and speed tuning of cerebellar Purkinje cells during circular manual tracking in monkey. J Neurosci. 2005;25 (40):9244–9257. doi: 10.1523/JNEUROSCI.1886-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schweighofer N, Arbib MA, Kawato M. Role of the cerebellum in reaching movements in humans. I. Distributed inverse dynamics control. Eur J Neurosci. 1998;10(1):86–94. doi: 10.1046/j.1460-9568.1998.00006.x. [DOI] [PubMed] [Google Scholar]
- 37.Shadmehr R, Holcomb HH. Neural correlates of motor memory consolidation. Science. 1997;277(5327):821–825. doi: 10.1126/science.277.5327.821. [DOI] [PubMed] [Google Scholar]
- 38.Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci. 1994;14:3208–3224. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shidara M, Kawano K, Gomi H, Kawato M. Inverse-dynamics model eye movement control by Purkinje cells in the cerebellum. Nature. 1993;365(6441):50–52. doi: 10.1038/365050a0. [DOI] [PubMed] [Google Scholar]
- 40.Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. J Neurophysiol. 2005;93(5):2809–2821. doi: 10.1152/jn.00943.2004. [DOI] [PubMed] [Google Scholar]
- 41.Stein JF, Glickstein M. Role of the cerebellum in visual guidance of movement. Physiol Rev. 1992;72(4):967–1017. doi: 10.1152/physrev.1992.72.4.967. [DOI] [PubMed] [Google Scholar]
- 42.Thoroughman KA, Shadmehr R. Electromyographic correlates of learning an internal model of reaching movements. J Neurosci. 1999;19(19):8573–8588. doi: 10.1523/JNEUROSCI.19-19-08573.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol. 2007;98:54–62. doi: 10.1152/jn.00266.2007. [DOI] [PubMed] [Google Scholar]
- 44.Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998;2:338–347. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto K, Kawato M, Kotosaka S, Kitazawa S. Encoding of movement dynamics by Purkinje cell simple spike activity during fast arm movements under resistive and assistive force fields. J Neurophysiol. 2006;97:1588–1599. doi: 10.1152/jn.00206.2006. [DOI] [PubMed] [Google Scholar]