Abstract
Morningness/eveningness (M/E) is a stable, quantifiable measure reflecting preferred circadian phase. Two prior studies suggest that bipolar I disorder (BP1) cases are more likely to have lower M/E scores, i.e., be evening types compared with control groups. These studies did not recruit controls systematically and did not evaluate key clinical variables. We sought to replicate the reported associations in a large, well defined sample, while evaluating potential confounding factors. Adults with bipolar disorder (BP) were compared with community controls drawn randomly from the same residential areas (190 cases and 128 controls). M/E was evaluated using the composite scale of morningness (CSM). After accounting for variables correlated with M/E, BP cases had significantly lower CSM scores than controls (i.e., more evening-type or fewer morning-type). There were no significant differences in M/E scores between BP1 or BP2 disorder cases (n = 134 and 56, respectively). CSM scores were stable over approximately 2 years in a subgroup of participants (n = 52). Individuals prescribed anxiolytic drugs, antidepressants, antipsychotic drugs, mood stabilizers or stimulant drugs had significantly lower age-corrected CSM scores compared with persons not taking these drugs. BP cases are more likely to be evening types, suggesting circadian phase delay in BP cases. Individuals with elevated depressive mood scores are more likely to be evening types. Our results suggest a replicable relationship between circadian phase and morbid mood states.
Keywords: Bipolar disorder, Depression, Morningness/eveningness, Chronotypes, Circadian phase, Lifestyle
1. Introduction
The notion of dividing the world into morning ‘larks’ and night ‘owls’ (i.e., into different “chronotypes”) is a familiar one. Morning-type and evening-type individuals differ in the phase position of their endogenous circadian oscillator (Kerkhof and Van Dongen, 1996; Duffy et al., 2001). In addition to predicting the timing of sleep onset and offset, such measures are also associated with inter-individual differences in the phase (timing) of physiological circadian rhythms in plasma cortisol and core body temperature, as well as behavioral rhythms related to eating, exercise and performance (Horne and Ostberg, 1976; Ishihara et al., 1987; Monk et al., 1991; Matsumoto et al., 1996; Nebel et al., 1996; Baehr et al., 2000). M/E variation is normally distributed in the population. M/E measures are stable over 6- to 13-month periods among control individuals (Greenwood, 1994; Caci et al., 2000; Taillard et al., 2004). They can also predict suitability for night shift work, supporting their practical utility (Costa et al., 1989; Matsumoto et al., 1996).
M/E variation can be easily estimated with pen and paper self-report questionnaires (Horne and Ostberg, 1976; Smith et al., 1989). Web-based tools, with precise questionnaires related to weekday and weekend preferences are also available (e.g., http://www.imp-muenchen.de/MCTQ-English) (Zavada et al., 2005; Wittmann et al., 2006). M/E scores may be considered as estimates of preferred circadian phase and thereby utilized for indirect evaluation of circadian phase, which may otherwise require repeated observations over longer periods. Thus, M/E measures can be used to indirectly test the hypothesis that circadian abnormalities mediate the pathogenesis of bipolar disorder (BP) (Healy, 1987; Mitterauer, 2000; Manji et al., 2001). M/E scores are impacted by several factors; most notably aging, with older individuals tending to be morning types (Drennan et al., 1991; Carrier et al., 1997; Caci et al., 2005a,b). Cultural differences have also been noted (Caci et al., 2005a,b). Other studies involving college students and adolescents have also suggested that self-rated depression scores may be correlated with M/E estimates, such that individuals with higher depressive scores are more likely to be evening types (Chelminski et al., 1999; Caci et al., 2005a,b). Patients with major depressive disorder also have been reported to be significantly more evening type than controls (Drennan et al., 1991). Thus, it is necessary to evaluate the impact of demographic and key clinical variables when testing the circadian hypothesis of BP pathogenesis.
Our earlier analyses revealed that M/E scores among BP1 cases were significantly lower (i.e., more evening type) than both community based control individuals and cases with schizophrenia/schizoaffective disorder (SZ/SZA) cases (Mansour et al., 2005). Recently, a study in Korea also showed that adults with BP1 had more ‘evening’ patterns when compared with controls (Ahn et al., 2008). Both studies evaluated the impact of age and gender on M/E variables. Thus, replicated evidence consistent with circadian phase alterations in BP is now available. However, neither study evaluated the impact of other demographic features or clinical variables such as medication, mood state at the time of the M/E evaluation, chronicity, or co-morbid disorders. The number of cases investigated in both studies was relatively small (90 patients or less), and the control individuals were not selected systematically. In the present study, un-duplicated BP cases were compared with carefully screened, randomly selected control individuals and the impact of key measures was evaluated.
2. Methods and materials
2.1. Clinical
All participants were recruited as part of an ongoing high risk BP study (Bipolar Offspring Study, BIOS, #MH 060952-06, Principal Investigator, Boris Birmaher, M.D.). All individuals were assessed using the same procedures by Bachelor's or Master's-level interviewers and staffed by board certified psychiatrists. Most assessments were carried out in the subjects’ homes. All adult participants with available M/E data were included.
2.1.1. Cases
The cases comprised outpatients with BP (DSM IV, Diagnostic and Statistical Manual of Mental Disorders, American Psychiatric Association, 1994). Individuals with moderate to severe mental retardation, as well as those with schizophrenia, organic mental disorders, mood disorder secondary to substance abuse, medications (e.g., corticosteroids), or neurological conditions such as multiple sclerosis and epilepsy were excluded. Only adults living within a 200-mile radius of Pittsburgh and those having offspring between 2 and 18 years of age were eligible to participate. The participants were recruited through advertisement (42.5%), ongoing adult BP studies (48.5%) or outpatient clinics (9.1%). None of the participants were included in our earlier analysis (Mansour et al., 2005). The cases included individuals with BP1 (n=134) and BP2 (n=56, Table 1).
Table 1.
Demographic and clinical characteristics of the participants.
| Bipolar (n=190) | Controls (n=128) | Statistical analysis |
||||
|---|---|---|---|---|---|---|
| Test statistic | df | Effect size | P value | |||
| Age | 39.76 (21.80, 60.59) | 40.51 (24.98, 61.39) | Z=−0.561 | NA | 0.58 | |
| Gender (male/female) | 37/153 (19.5%/80.5%) | 30/98 (23.4%/76.6%) | χ2=0.723 | 1 | NA | 0.40 |
| Ethnicity (C/A–A/O) | 170/16/4 (89.5/8.4/2.1%) | 104/20/4 (81.3/15.6/3.1%) | χ2=4.422 | 2 | NA | 0.11 |
| Socio-economic statusa | 33 (11, 66) | 36 (11, 66) | Z=−1.973 | 0.11 | 0.048 | |
| Global Assessment of Function (current) | 62 (12, 91) | 85 (55, 100) | Z=−11.513 | r=−0.65 | <0.0001 | |
| Beck Depression Inventory score | 18 (0, 45) | 4 (0, 41) | Z=−9.332 | r=−0.52 | <0.0001 | |
| Composite scale of morningness score | 33 (13, 55) | 39 (21, 52) | Z=−5.489 | r=−0.31 | <0.0001 | |
| Co-existing conditions | ||||||
| Substance abuse | 122 (64.2%) | 34 (26.6%) | χ2=43.38 | 1 | r=0.37 | <0.0001 |
| Anxiety (GAD, SAD or SP) | 145 (76.3%) | 21 (16.4%) | χ2=110.01 | 1 | r=0.59 | <0.0001 |
| ADHD | 52 (27.4%) | 5 (3.9%) | χ2=28.62 | 1 | r=0.30 | <0.0001 |
| ODD or CD | 69 (36.3%) | 8 (6.3%) | χ2=37.67 | 1 | r=0.34 | <0.0001 |
| Dysthymia | 5 (2.6%) | 9 (7.0%) | χ2=3.52 | 1 | NA | 0.06 |
| Major depressive disorder | – | 28 (21.9%) | – | – | ||
| Any co-morbid disorder | 171 (90%) | 61 (47.7%) | χ2=69.50 | 1 | r=0.47 | <0.0001 |
Values for continuous variables shown as median (range); df: degrees of freedom. NA: not analyzed.
C: Caucasian; A–A: African–American; O: other ethnic group; GAD: Generalized anxiety disorder; SAD: Separation anxiety disorder; SP: Social Phobia; ADHD: Attention deficit hyperactivity disorder; ODD: Oppositional defiant disorder; CD: conduct disorder.
Derived from the Hollingshead-Redlich index; lower scores indicate lower socio-economic status.
2.1.2. Community controls
Community controls were drawn from the same residential neighborhoods as the cases (n = 128). The neighborhoods were identified using the area code and the first 3 digits of the cases’ telephone numbers, as well as the postal ZIP code. The controls were recruited by the University Center for Social and Urban Research (UCSUR), an independent center at the University of Pittsburgh experienced in performing telephone recruitment and screening services. UCSUR staff employed random digit-dialing sampling to ascertain potential control individuals. The inclusion and exclusion criteria for the controls were identical to the cases, with the additional requirement that individuals with lifetime or current diagnoses of bipolar spectrum disorders were ineligible. Individuals who reported a first-degree relative with BP were also excluded.
2.2. Evaluations
The Structured Clinical Interview-DSM IV (SCID) was administered to all participants (First et al., 1997; Han and Hong, 2000). Based on the Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present and Lifetime Version (K-SADS-PL) (Kaufman et al., 2000), items to assess history of childhood psychiatric disorders, including lifetime attention deficit hyperactive disorder (ADHD), oppositional defiant disorder (ODD), separation anxiety disorder (SAD) and conduct disorder (CD), were added to the SCID. Additional details were obtained from medical records as needed. Current mood status was rated using the Beck Depression Inventory (Beck et al., 1961). Overall severity was estimated using the Global Assessment of Function (GAF). Socio-economic status (SES) was evaluated using the Hollingshead-Redlich index (Hollingshead and Redlich, 1958). Participants also completed the composite scale of morningness, a validated adaptation of the Horne–Ostberg scale that is used to assess morningness/eveningness (M/E) (Horne and Ostberg, 1976; Smith et al., 1989; Greenwood, 1994). This self-report questionnaire gauges diurnal preference for activity and is composed of 13 items. Scores range from 13–55. CSM scores were treated as continuous variables.
Interviewers completed assessments after intensive training for all instruments and after ≥80% agreement with a certified rater. The overall SCID and K-SADS kappa values for psychiatric disorders were ≥0.8. All psychiatric history narratives and ratings were presented to a board of certified psychiatrist for diagnostic confirmation. For cases with BP, two psychiatrists also independently reviewed all narratives, rating scales and depression scores for consensus regarding the diagnosis of BP (DA and BB). When necessary, participants’ medical and psychiatric records were obtained and reviewed. DSM IV criteria were used for all diagnoses.
2.2.1. Co-existing conditions
The following groups of disorders were present or had been present among BP cases, as well as controls: substance abuse; generalized anxiety disorder (GAD), separation anxiety disorder (SAD), or social phobia (SP); attention deficit hyperactivity disorder (ADHD); oppositional defiant disorder (ODD) or conduct disorder (CD), dysthymia and major depressive disorder (MDD). The frequency of each disorder was compared between BP cases and controls. To enable subsequent multivariate analyses with a convenient number of covariates, these disorders were classified into three groups: Group 1 – disruptive behaviors disorders (ADHD, ODD or CD); Group 2 – depression, anxiety and related conditions (GAD, SAD, social phobia, dysthymia or MDD); Group 3 – substance abuse disorders (SUD, alcohol or illicit substances).
2.2.2. Medications
Details of prescribed medications were obtained from the participants and from their medical records. The following groups of medications were prescribed most frequently and were included for analysis: anti-allergic drugs, analgesics, anxiolytic drugs, antidepressants, anti-psychotic drugs, mood stabilizers, stimulants, drugs for asthma, drugs for cardiovascular diseases, contraceptives, anti-diabetic drugs, thyroxine and drugs for peptic ulcer. Other medications prescribed to fifteen or less participants were not included in the analyses, with the exception of stimulants (n = 14 participants among BP cases and controls). The stimulant drugs were nevertheless included in the analyses, because of known impacts on circadian function.
All participants provided written informed consent. The study was approved by the University of Pittsburgh Institutional Review Board.
2.3. Statistical analysis
Group-wise comparisons included parametric or non-parametric tests as appropriate, implemented in the Statistical Package for Social Sciences (SPSS, version 11.0). Age corrections for CSM scores and principle components analyses were conducted using the Statistical Analysis software (SAS). Age correction was conducted using linear regression, with CSM score as the outcome and age as the independent variable. Residual scores were then divided by the standard deviation to generate standardized residual scores. The Shapiro–Wilk test was used to test for deviation from normality. All P values are based on two-tailed tests with α = 0.05. Corrections for multiple comparisons were not applied, as the case–control comparisons of M/E scores tested prior published results.
3. Results
3.1. Demographic and clinical variables
The BP cases did not differ significantly from the controls with regard to age, gender or ethnicity (Table 1). The cases were more likely than the controls to have co-morbid diagnoses, more disability estimated using the Global Assessment of Function (GAF), lower SES scores and higher scores on the Beck Depression Inventory (BDI). A significantly higher lifetime prevalence of the following groups of disorders was noted among BP cases: substance abuse; generalized anxiety disorder (GAD), separation anxiety disorder (SAD), or social phobia; attention deficit hyperactivity disorder (ADHD); oppositional defiant disorder (ODD) or conduct disorder (CD). The prevalence of dysthymia and major depressive disorder (MDD) was higher among the controls, because MDD was an exclusion criterion for the cases (Table 1).
3.2. CSM scores and case–control differences
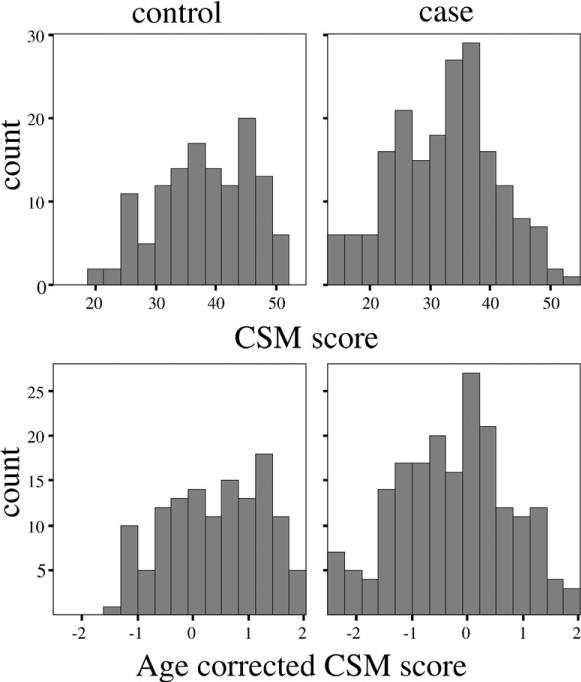
The CSM scores were distributed normally among cases, though deviation was noted among the controls using the Shapiro–Wilk test (Fig. 1, cases: W=0.99, not significant, controls: W=0.97, P=0.01; kurtosis=0.810). There were significant case–control differences with regard to CSM scores (Table 1). Age-corrected CSM scores for BP1 cases were also significantly different from the controls (P<0.0001), as were the scores for BP2 cases (P=0.001). Analysis of variances also revealed that age-corrected CSM scores did not differ significantly between BP1 or BP2 cases (P=0.89). All BP cases were combined for subsequent analyses.
Fig. 1.
Distribution of composite scale (CSM) scores among cases and controls. The top panel shows the uncorrected CSM scores for controls (left) and cases (right). The lower panel shows the CSM scores, adjusted for age. Age correction was conducted using linear regression, with CSM score as the outcome and age as the independent variable. Residual scores were then divided by the standard deviation to generate standardized residual scores.
3.3. Correlations between CSM scores and other demographic/clinical variables
The relationship between CSM scores and key variables was evaluated separately among the BP cases and controls (Table 2).
Table 2.
Correlations between Composite Scale of Morningness scores and clinical variables among BP cases and controls.
| CSM | BDI | GAF | SES | Age at intake | |
|---|---|---|---|---|---|
| CSM | −0.172 | −0.085 | 0.152 | 0.164 | |
| BDI | −0.280 (<0.0001) | −0.386 (<0.0001) | −0.366 (<0.0001) | −0.053 | |
| GAF | 0.218 (0.003) | −0.501 (<0.0001) | 0.371 (<0.0001) | 0.033 | |
| SES | 0.078 | −0.331 (<0.0001) | 0.252 (0.0005) | 0.0813 | |
| Age at intake | 0.180 (0.013) | −0.227 (0.002) | 0.133 | 0.220 (0.002) | |
| Age at onset of BP | 0.021 | −0.033 | 0.040 | 0.118 | 0.235 (0.001) |
Results are presented above the diagonal for controls and below the diagonal for cases. All correlations are reported as Spearman's rho (two-tailed significance value shown in brackets only when P<0.05). Significant correlations are in bold, with P values in parentheses.
CSM: Composite Scale of Morningness score; BDI: Beck Depression Inventory score; GAF: Global Assessment of Function (current); SES: socio-economic status (derived from Hollingshead-Redlich index; lower scores indicate lower SES); BP: Bipolar disorder.
Among the control individuals alone, CSM scores were not significantly correlated with GAF or SES. There were non-significant trends for negative correlation with BDI (P=0.052) and age (P=0.065).
Among the BP cases alone, CSM scores were positively correlated with age and GAF scores, but not with SES or age at onset of BP. There was significant negative correlation between CSM scores and BDI ratings (Table 2). CSM scores were not significantly correlated with rapid cycling patterns or seasonality of illness. They were not significantly different among cases with single mood episodes or those with recurrent episodes (data not shown).
There were no significant associations between CSM scores and gender or ethnicity among the cases or the controls (data not shown). Significant negative correlations were observed among the BP cases as well as controls with regard to other pairs of variables such as GAF and BDI, GAF and SES, as well as SES and BDI (P<0.01 or better, see Table 2).
The cases and controls were analyzed together to evaluate associations between CSM scores and certain co-morbid conditions, because these variables were present among the cases as well as the controls. Comparisons between individuals with or without the groups of co-morbid conditions defined above (groups 1–3) revealed significant differences in age-corrected CSM scores for each of the comparisons (see Table 3; group 1, P<0.0001; group 2, P=0.0001; group 3, P<0.0001). Individuals with each of these conditions had lower CSM scores, i.e., were more likely to be evening types. The results indicated that co-morbidity could be an important contributor for the case–control difference in CSM scores.
Table 3.
Age-corrected Composite Scale of Morningness scores in relation to co-morbid disorders and medications.
| Variable | Absent | Present | Test statistic | Effect size | P value |
|---|---|---|---|---|---|
| Disorders | |||||
| Group 1: Disruptive behaviors disordersa | 0.28, 4.35 (215) | −0.43, 4.37 (103) | Z=−4.783 | r=−0.27 | P<0.0001 |
| Depression, anxiety and related conditions | 0.22, 4.35 (134) | −0.13, 4.37 (184) | Z=−3.811 | r=−0.21 | P=0.0001 |
| Substance abuse disorders | 0.30, 4.35 (162) | −0.18, 4.37 (156) | Z=−4.380 | r=−0.25 | P<0.0001 |
| Medications | |||||
| Anxiolytics | 0.10, 4.55 (262) | −0.004, 4.10 (56) | Z=−2.008 | r=−0.11 | P=0.045 |
| Antidepressants | 0.19, 4.35 (201) | −0.25, 4.37 (117) | Z=−3.407 | r=−0.19 | P=0.001 |
| Antipsychotic drugs | 0.14, 4.54 (239) | −0.29, 4.40 (79) | Z=−2.184 | r=−0.12 | P=0.029 |
| Mood stabilizers | 0.20, 4.54 (185) | −0.17, 4.37 (133) | Z=−3.260 | r=−0.18 | P=0.001 |
| Stimulants | 0.10, 4.55 (304) | −0.58, 2.66 (14) | Z=−2.491 | r=−0.14 | P=0.013 |
Values shown as median, range (n). The analyses included cases as well as controls. Composite Scale of Morningness scores are shown as age-corrected residual scores.
Only statistically significant comparisons using the Mann-Whitney U test are listed (P<0.05).
Disorders.
Group 1: disruptive behaviors disorders (attention deficit hyperactivity disorder, conduct disorder and oppositional defiant disorder; data on history of childhood psychiatric disorders based on additional items from the K-SADS-PL); group 2: depression, anxiety and related conditions (generalized anxiety disorder, social anxiety disorder, social phobia, dysthymia, major depressive disorder); group 3: substance abuse disorder (alcohol and illicit substances).
Significant differences in age-corrected CSM scores were also observed among individuals prescribed, versus those not prescribed anxiolytic drugs, antidepressants, antipsychotic drugs, mood stabilizers or stimulants (Table 3). Individuals receiving these drugs had lower CSM scores, i.e., were more likely to be evening types.
3.4. CSM score differences between BP cases and controls: accounting for correlated variables
Due to the significant inter-correlations between BDI, GAF and SES scores, principal component analysis was first conducted using these variables. Two components were derived with a cumulative eigenvalue of 0.88. BDI and GAF had a major impact on the first component, arbitrarily denoted as ‘BGS’ (eigenvectors: BDI = –0.64, GAF = 0.62, SES = 0.45). SES had a larger impact on the second component, denoted ‘SGB’ (eigenvectors: SES = 0.89, GAF = –0.37, BDI = 0.27). BGS, one of the two derived variables continued to be significantly associated with CSM scores (rho = 0.335, P<0.0001). The derived principal components were used as covariates for linear regression analyses.
Linear regression analyses were conducted, using age corrected CSM score as the dependent variable. Diagnosis (BP or not-BP), gender and ethnicity were also included as covariates in conjunction with the derived principal components from BDI, GAF and SES scores. All three groups of co-morbid disorders, as well as the medications that appeared to impact CSM scores were also included as covariates, though there was no satisfactory way to partial out the individual effects of these variables. After step-wise elimination of non-significant covariates, the following variables were found to be significantly associated with CSM scores: diagnosis of BP, group 1 disorders (ADHD, OD or CD) and the first derived principle component (BGS, Table 4).
Table 4.
Significant predictors of age corrected composite scale of morningness scores.
| Non-standardized coefficients |
Standardized coefficients |
t | Significance | ||
|---|---|---|---|---|---|
| B | Standard error | Beta | |||
| Constant | 0.298 | 0.097 | 3.075 | 0.002 | |
| Group 1: Disruptive behaviors disordersa | −0.248 | 0.129 | −0.116 | −1.918 | 0.056 |
| BGSb | 0.128 | 0.049 | 0.174 | 2.626 | 0.009 |
| Bipolar disorder | −0.350 | 0.130 | −0.172 | −2.687 | 0.008 |
Group 1: disruptive behaviors disorders (attention deficit hyperactivity disorder, conduct disorder and oppositional defiant disorder; data on history of childhood psychiatric disorders based on additional items from the K-SADS-PL).
First principal component derived from BDI, GAF and SES scores.
3.5. Temporal stability of CSM scores
Repeat M/E measures were available for a sub-group of the participants (BP cases, n = 35; controls, n = 17). The initial and repeat measures were highly correlated (Spearman's rho = 0.72, P<0.0001). Pair-wise comparisons of CSM scores for each individual did not differ significantly in either BP cases or controls (mean interval: 2.09±0.13 years, BP cases: time point 1: 34.03±9.61, time point 2: 33.20±8.42; controls: time point 1: 39.41±6.91, time point 2: 42.06±5.33; all values as mean±standard deviation of age-corrected CSM scores, paired t-tests). In addition, general linear models involving repeated measures analysis did not reveal significant difference in either group across the two sets of measures (data not shown).
4. Discussion
We found that BP cases were more likely to be evening types in comparison with screened control individuals. These results replicate our earlier study (Mansour et al., 2005), as well as a subsequent Korean study (Ahn et al., 2008). The consistent results from three independent, non-overlapping samples, which were ascertained in two very different cultural situations thus suggest that the case–control differences cannot be attributed merely to chance. The prior studies did not evaluate the impact of demographic and clinical factors that might affect M/E scores. In the present analyses, the case–control differences were evident even after accounting for several key variables. Our results thus highlight robust case–control differences with regard to M/E, but the mechanism/s are uncertain. Our study was not intended to be a test of the classical phase delay hypothesis of depression, as it involved BP1 cases. Though an individual's preferred circadian phase as measured by the CSM may be taken as a reasonable approximation of his/her actual circadian phase, more direct physiological measures need to be investigated.
We also detected positive correlations between M/E scores and some indices of severity, such as GAF scores and BDI scores. Thus, individuals with more severe depressive mood ratings are more likely to be evening types. Comparable ratings for elevated mood were unavailable in the present dataset and need to be evaluated. The correlation between M/E score and BDI was highly significant among the BP cases in our sample, with similar trends among the control individuals (although the magnitude of the correlation was modest among the cases). Similar correlations have also been reported in an earlier study of college students (Chelminski et al., 1999) In the present analyses, CSM scores were not significantly correlated with rapid cycling patterns or seasonality of illness. They were also similar among cases with single mood episodes or those with recurrent episodes (data not shown).
Several co-morbid disorders were noted among the BP cases at rates that are consistent with the published literature. We detected significant associations between CSM scores and some of these conditions, as well as medications used to treat them. The co-morbid disorders include anxiety, depressive disorders, substance abuse and a history of certain childhood disorders. Individuals with any of these conditions were more likely to be evening types than persons who did not have the co-morbid diagnosis; i.e., in the same direction as the BP case–control differences. The relationship between depression and circadian phase delay has been suggested before (Wehr and Goodwin, 1983; Wirz-Justice, 2006). Since there were more MDD cases among the controls than among the BP cases, it is important to note that this distribution would tend to reduce the BP case–control differences noted here. On the other hand, BDI ratings indicated more severe depressive symptoms among the cases. The relationship between alcohol consumption and abuse of illicit substances on circadian function is also known (Rothenfluh and Heberlein, 2002; Danel et al., 2006; Taylor et al., 2006), but the associations between CSM scores and the other disorders are novel, to our knowledge. It is uncertain whether the associations reflect distinct sub-groups of these disorders.
The associations between CSM scores and prescribed anxiolytic drugs, antidepressant drugs and mood stabilizers are also novel. However, they are difficult to interpret because the associations were also present with some of the disorders for which these medications were presumably prescribed. Since the prescribed doses were not available, it is also not possible to evaluate dose–effect relationships. Separate investigations are necessary to understand these relationships further. Since the analyses reported here were not corrected for multiple comparisons, replicate studies are necessary.
Apart from these associations, our analyses also suggest that the M/E scores can be stable over even long periods as reported previously among healthy individuals (Greenwood, 1994; Caci et al., 2000). We noted significant correlation for pairs of CSM scores rated approximately 2 years apart among BP cases or controls. The temporal stability was present not only among control individuals, but also BP cases. These intriguing findings are consistent with a significant heritability for CSM scores (Klei et al., 2005). They need to be evaluated independently. Our analyses did not detect significant differences in CSM scores between BP1 and BP2 cases. These results, together with the correlations with mood severity suggest that M/E may represent a marker for morbid mood variation rather than for a particular diagnostic group. The prospect for associations between M/E scores and specific symptom domains in BP might also be productively explored.
This study has a number of limitations. It is uncertain whether the M/E differences pre-date the onset of BP, or represent an epiphenomenon. This possibility could be addressed by investigating at risk individuals or patients early in the course of their illness. Alternatively, the relationship between circadian phase and BP genesis could be addressed indirectly by investigating unaffected, adult relatives who are not cohabiting with BP cases. Such individuals share liability for BP, but are not prone to many illness related variables, such as medications. If M/E has a pathogenic role for BP, it would be predicted that the unaffected relatives would also be represented by a preponderance of evening types in comparison with controls. Such studies would, of course, need to take into account the impact of co-habitee lifestyle.
Future studies will also need to explore the impact of lifestyle on the differences noted here. For example, the patients are more likely to be unemployed than the controls. Employment status could conceivably affect CSM scores and thus explain a portion of the observed case–control differences. This possibility was tested indirectly by evaluating socio-economic status as a covariate in our analyses. Since all participants had young offspring, it is also possible that child rearing requirements influenced the CSM scores. However, there is at present no evidence to suggest that presence of young offspring differentially affect the BP patients and the control individuals. The case–control differences reported here were noted also in two earlier studies, even though presence of offspring was not an inclusion criterion in those studies (Mansour et al., 2005; Ahn et al., 2008). The CSM scores among the controls deviated somewhat from a normal distribution. This may reflect selection bias. To take account of the modest skewness among the controls, we utilized non-parametric tests when comparing cases and controls. It should also be noted that the questions in the CSM might be considered to be biased towards estimates of morningness. Our findings would thus be strengthened if they could be replicated using a scale that is biased towards evening chronotypes, e.g., the MCTQ (Wittmann et al., 2006).
In conclusion, CSM score differences between BP cases and controls were replicated even after accounting for a number of confounding variables. The magnitude of the case–control difference is modest, but the presence of several other factors that impact CSM scores makes it difficult to estimate the true effect size accurately. The M/E differences may not be specific to BP, and significant correlations between CSM scores and some indices of illness severity were noted. In a subgroup of the participants, M/E scores were stable over approximately 2 years. If the CSM scores are indeed stable over prolonged periods among BP cases, they may serve as useful indices of selected aspects of illness severity. This possibility merits further evaluation.
Acknowledgements
We thank the participants in our study. The research reported was supported in part by grants from the National Institutes of Health (MH060952-06 to BB; MH63420, MH56242 and MH66263 to VLN; MH57881 to BD; AG13396, and AG020677 to THM, a Mental Health Intervention Research Center grant # MH 30915 to DJK and an Advanced Center for Interventions and Services Research (ACISR) for Early-Onset Mood and Anxiety Disorders #MH066371 to DB).
Footnotes
Financial disclosures
DJK has served on the advisory boards of Eli Lilly & Company, Forest Pharmaceuticals, Inc., Pfizer, Inc., and Solvay/Wyeth Pharmaceuticals and also served as a consultant for Servier Amerique. ST has participated in speakers’ bureaux for the following companies: Astra-Zeneca, Inc., Bristol-Myers-Squibb, Inc., Forest Pharmaceuticals, Inc., GlaxoSmithKline and Wyeth, Inc. He has also served on investigator initiated research funded by Novartis and Bristol-Myers-Squibb, Inc. THM has served as a consultant to Cephalon, Inc., Takeda, Inc., and Sepracor, Inc. VLN received a research grant from Lundeck Research USA after this manuscript was submitted.
References
- Ahn YM, Chang J, Joo YH, Kim SC, Lee KY, Kim YS. Chronotype distribution in bipolar 1 disorder and schizophrenia in a Korean sample. Bipolar Disorders. 2008;10:271–275. doi: 10.1111/j.1399-5618.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- Baehr EK, Revelle W, Eastman CI. Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness–eveningness. Journal of Sleep Research. 2000;9:117–127. doi: 10.1046/j.1365-2869.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Caci H, Nadalet L, Staccini P, Myquel M, Boyer P. The composite scale of morningness: further psychometric properties and temporal stability. European Psychiatry. 2000;15:278–281. doi: 10.1016/s0924-9338(00)00231-5. [DOI] [PubMed] [Google Scholar]
- Caci H, Adan A, Bohle P, Natale V, Pornpitakpan C, Tilley A. Transcultural properties of the composite scale of morningness: the relevance of the “morning affect” factor. Chronobiology International. 2005a;22:523–540. doi: 10.1081/CBI-200062401. [DOI] [PubMed] [Google Scholar]
- Caci H, Robert P, Dossios C, Boyer P. Morningness–Eveningness for Children Scale: psychometric properties and month of birth effect. Encephale. 2005b;31:56–64. doi: 10.1016/s0013-7006(05)82372-3. [DOI] [PubMed] [Google Scholar]
- Carrier J, Monk TH, Buysse DJ, Kupfer DJ. Sleep and morningness–eveningness in the ‘middle’ years of life (20–59 y). Journal of Sleep Research. 1997;6:230–237. doi: 10.1111/j.1365-2869.1997.00230.x. [DOI] [PubMed] [Google Scholar]
- Chelminski I, Ferraro FR, Petros TV, Plaud JJ. An analysis of the “eveningness–morningness” dimension in “depressive” college students. Journal of Affective Disorders. 1999;52:19–29. doi: 10.1016/s0165-0327(98)00051-2. [DOI] [PubMed] [Google Scholar]
- Costa G, Lievore F, Casaletti G, Gaffuri E, Folkard S. Circadian characteristics influencing interindividual differences in tolerance and adjustment to shiftwork. Ergonomics. 1989;32:373–385. doi: 10.1080/00140138908966104. [DOI] [PubMed] [Google Scholar]
- Danel T, Vantyghem MC, Touitou Y. Responses of the steroid circadian system to alcohol in humans: importance of the time and duration of intake. Chronobiology International. 2006;23:1025–1034. doi: 10.1080/07420520600920742. [DOI] [PubMed] [Google Scholar]
- Drennan MD, Klauber MR, Kripke DF, Goyette LM. The effects of depression and age on the Horne–Ostberg morningness–eveningness score. Journal of Affective Disorders. 1991;23:93–98. doi: 10.1016/0165-0327(91)90096-b. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness–eveningness, usual wake time, and circadian phase. Behavioral Neuroscience. 2001;115:895–899. doi: 10.1037//0735-7044.115.4.895. Order. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders — Clinical Version (SCID — I/CV) American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- Greenwood KM. Long-term stability and psychometric properties of the Composite Scale of Morningness. Ergonomics. 1994;37:377–383. doi: 10.1080/00140139408963653. [DOI] [PubMed] [Google Scholar]
- Han OS, Hong JP. SCID-I; Structured Clinical Interview for Axis I Disorder of DSM-IV. Hana Medical Publication; Seoul, Korea: 2000. [Google Scholar]
- Healy D. Rhythm and blues. Neurochemical, neuropharmacological and neuropsychological implications of a hypothesis of circadian rhythm dysfunction in the affective disorders. Psychopharmacology (Berl) 1987;93:271–285. doi: 10.1007/BF00187243. [DOI] [PubMed] [Google Scholar]
- Hollingshead A, Redlich FC. Social Class and Mental Illness. Wiley; New York: 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness–eveningness in human circadian rhythms. International Journal of Chronobiology. 1976;4:97–110. [PubMed] [Google Scholar]
- Ishihara K, Miyasita A, Inugami M, Fukuda K, Miyata Y. Differences in sleep–wake habits and EEG sleep variables between active morning and evening subjects. Sleep. 1987;10:330–342. doi: 10.1093/sleep/10.4.330. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-Sads-Pl. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1208. doi: 10.1097/00004583-200010000-00002. [DOI] [PubMed] [Google Scholar]
- Kerkhof GA, Van Dongen HP. Morning-type and evening-type individuals differ in the phase position of their endogenous circadian oscillator. Neuroscience Letters. 1996;218:153–156. doi: 10.1016/s0304-3940(96)13140-2. [DOI] [PubMed] [Google Scholar]
- Klei L, Reitz P, Miller M, Wood J, Maendel S, Gross D, Waldner T, Eaton J, Monk TH, Nimgaonkar VL. Heritability of morningness–eveningness and self-report sleep measures in a family based sample of 521 Hutterites. Chronobiology International. 2005;22:1041–1054. doi: 10.1080/07420520500397959. [DOI] [PubMed] [Google Scholar]
- Manji HK, Moore GJ, Chen G. Bipolar disorder: leads from the molecular and cellular mechanisms of action of mood stabilizers. British Journal of Psychiatry. 2001;(Supplement 41):s107–s119. [PubMed] [Google Scholar]
- Mansour H, Wood J, Logue T, Chowdari KV, Dayal M, Monk T, Kupfer D, Devlin B, Nimgaonkar V. Circadian phase variation in Bipolar I Disorder. Chronobiology International. 2005;22:571–584. doi: 10.1081/CBI-200062413. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Kamata S, Naoe H, Mutoh F, Chiba S. Investigation of the actual conditions of hospital nurses working on three rotating shifts: questionnaire results of shift work schedules, feelings of sleep and fatigue, and depression. Seishin Shinkeigaku Zasshi. 1996;98:11–26. [PubMed] [Google Scholar]
- Mitterauer B. Clock genes, feedback loops and their possible role in the etiology of bipolar disorders: an integrative model. Medical Hypotheses. 2000;55:155–159. doi: 10.1054/mehy.1999.1039. [DOI] [PubMed] [Google Scholar]
- Monk TH, Reynolds C.F.r., Buysse DJ, Hoch CC, Jarrett DB, Jennings JR, Kupfer DJ. Circadian characteristics of healthy 80-year-olds and their relationship to objectively recorded sleep. Journal of Gerontology. 1991;46:M171–M175. doi: 10.1093/geronj/46.5.m171. [DOI] [PubMed] [Google Scholar]
- Nebel LE, Howell RH, Krantz DS, Falconer JJ, Gottdiener JS, Gabbay FH. The circadian variation of cardiovascular stress levels and reactivity: relationship to individual differences in morningness/eveningness. Psychophysiology. 1996;33:273–281. doi: 10.1111/j.1469-8986.1996.tb00424.x. [DOI] [PubMed] [Google Scholar]
- Rothenfluh A, Heberlein U. Drugs, flies, and videotape: the effects of ethanol and cocaine on Drosophila locomotion. Current Opinion in Neurobiology. 2002;12:639–645. doi: 10.1016/s0959-4388(02)00380-x. [DOI] [PubMed] [Google Scholar]
- Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. Journal of Applied Psychology. 1989;74:728–738. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- Taillard J, Philip P, Chastang JF, Bioulac B. Validation of Horne and Ostberg morningness–eveningness questionnaire in a middle-aged population of French workers. Journal of Biological Rhythms. 2004;19:76–86. doi: 10.1177/0748730403259849. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Tio DL, Bando JK, Romeo HE, Prolo P. Differential effects of alcohol consumption and withdrawal on circadian temperature and activity rhythms in Sprague-Dawley, Lewis, and Fischer male and female rats. Alcoholism: Clinical and Experimental Research. 2006;30:438–447. doi: 10.1111/j.1530-0277.2006.00048.x. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Goodwin FK. Circadian Rhythms in Psychiatry. The Boxwood Press; Pacific Grove CA: 1983. [Google Scholar]
- Wirz-Justice A. Biological rhythm disturbances in mood disorders. International Clinical Psychopharmacology. 2006;21(Suppl 1):S11–S15. doi: 10.1097/01.yic.0000195660.37267.cf. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiology International. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- Zavada A, Gordijn MC, Beersma DG, Daan S, Roenneberg T. Comparison of the Munich Chronotype Questionnaire with the Horne–Ostberg's Morningness–Eveningness Score. Chronobiology International. 2005;22:267–278. doi: 10.1081/cbi-200053536. [DOI] [PubMed] [Google Scholar]