Abstract
Sepsis is currently the 10th leading cause of death overall and accounts for significant healthcare expenditures in the developed world. There are now more deaths attributable to sepsis than coronary artery disease, stroke, or cancer, and it is widely believed that the incidence of sepsis and sepsis-related mortality will continue to rise. Based on these sobering statistics, there is great interest in identifying novel treatments for managing critically ill children and adults with sepsis. Unfortunately, to date, there have been very few successful therapeutic agents employed in the clinical setting. Despite these disappointing results, new therapeutic agents continue to be identified, and there is reason for optimism and hope for the future. Herein, we will briefly review several novel therapeutic adjuncts for the management of critically ill patients with sepsis. We will largely focus on those therapies that directly target the host inflammatory response, specifically those that result in activation of the transcription factor, nuclear factor (NF)-κB. We will also reference some of the patents recently filed that pertain to the host innate immune response and sepsis.
Keywords: Sepsis, Critical illness, Septic shock, Genomics, Gene polymorphisms, LPS, NF-κB, Toll-like receptors, danger signals, heat shock proteins, inflammation, Pediatric, Adult
Introduction
The early recognition, diagnosis, and management of sepsis remains one of the greatest challenges in the field of critical care medicine today. Part of this challenge historically has been the lack of an adequate, standardized definition of sepsis, prompting an international panel of experts from the Society of Critical Care Medicine (SCCM) and the American College of Chest Physicians (ACCP) to propose the now familiar consensus definitions for the systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, and septic shock (Table 1) [1]. These definitions have been subsequently modified for use in critically ill children with sepsis (Table 2). However, these definitions are far from perfect [2–4], and as a result, several experts from SCCM and ACCP re-convened in December, 2001 and proposed a new staging system for sepsis [5]. The “PIRO” staging system for sepsis is modeled after the TNM (Tumor, Nodes, Metastasis) system [6] for staging malignancies and stratifies patients on the basis of their Predisposing conditions, the nature and extent of the insult (Infection), the nature and magnitude of the host Response, and the degree of concomitant Organ dysfunction. The PIRO staging system has many favorable attributes, but will require thorough validation and testing before it is widely adopted and applied in clinical practice [7].
Table 1.
American College of Physicians (ACCP)/Society of Critical Care Medicine (SCCM) Consensus Definitions for SIRS, Infection, Sepsis, Severe Sepsis, and Septic Shock
| Systemic Inflammatory Response Syndrome (SIRS) |
The presence of at least two of the following four criteria, one of which must be abnormal temperature or leukocyte count:
|
| Infection |
| A suspected or proven (i.e. by positive culture, tissue stain, polymerase chain reaction, etc) infection caused by any pathogen OR a clinical syndrome associated with a high probability of infection (e.g. presence of white blood cells in a normally sterile body fluid, chest radiograph consistent with pneumonia, petechial or purpuric rash, etc) |
| Sepsis |
| SIRS + Infection |
| Severe Sepsis (Sepsis with Organ Dysfunction) |
| Sepsis plus either cardiovascular dysfunction or acute respiratory distress syndrome (ARDS) OR two or more other organ dysfunctions |
| Septic Shock |
| Severe Sepsis with arterial hypotension defined by a systolic blood pressure below 90 mm Hg, mean arterial pressure (MAP) < 60 mm Hg, or a reduction in systolic blood pressure >40 mm Hg from baseline, despite adequate volume resuscitation and in the absence of other causes for hypotension. |
Adapted from [1]
Table 2.
Consensus Definitions for Pediatric SIRS, Infection, Sepsis, Severe Sepsis, and Septic Shock
| Systemic Inflammatory Response Syndrome (SIRS) |
The presence of at least two of the following four criteria, one of which must be abnormal temperature or leukocyte count:
|
| Infection |
| A suspected or proven (i.e. by positive culture, tissue stain, polymerase chain reaction, etc) infection caused by any pathogen OR a clinical syndrome associated with a high probability of infection (e.g. presence of white blood cells in a normally sterile body fluid, chest radiograph consistent with pneumonia, petechial or purpuric rash, etc) |
| Sepsis |
| SIRS + Infection |
| Severe Sepsis |
| Sepsis plus either cardiovascular dysfunction or acute respiratory distress syndrome (ARDS) OR two or more other organ dysfunctions (see Table 2) |
| Septic Shock |
| Sepsis and cardiovascular organ dysfunction |
Adapted from Goldstein B, Giroir B, Randolph A, and the Members of the International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005; 6:2–8.
According to the National Center for Health Statistics and the Centers for Disease Control and Prevention, sepsis was the 10th leading cause of death overall in 2004 [8]. Recent estimates suggest that there are between 77 to 240 new cases of sepsis per 100,000 population each year [9, 10]. The incidence of sepsis is expected to further increase by 1.5% every year, resulting in an additional 1 million cases per year by 2020 [9, 11, 12]. Similarly, the incidence of sepsis and its complications continues to increase worldwide [10, 13–18]. Sepsis accounts for nearly $17 billion and €5.8–7.6 billion in annual health care expenditures in the U.S. and Europe, respectively [9, 11, 19]. Sepsis remains a significant health problem in children as well, accounting for nearly 4,500 deaths and close to $2 billion per year in healthcare expenditures nationwide [20]. These statistics do not take into account the additional hidden costs attributed to the loss of productivity related to years of life lost for both critically ill children and adults who succumb to sepsis. Based on these sobering statistics, there is great interest in identifying novel treatments for managing critically ill children and adults with sepsis [21–23].
Unfortunately, promising therapies in pre-clinical models of sepsis have universally failed to live up to initial expectations in subsequent clinical trials in both critically ill adults [23–62] and children [24–26] (Table 3). In fact, to date there have been only three positive clinical trials in critically ill adults with sepsis – early goal-directed therapy (EGDT) [27], activated protein C (drotrecogin alfa, Xigris®, Eli Lilly and Co, Indianapolis, IN) [28], and afelimomab, a monoclonal antibody F(ab′)2 fragment directed against tumor necrosis factor (TNF)-α. [29]. EGDT, which emphasizes aggressive, early reversal of shock via titration of intravenous fluid therapy, optimization of cardiac output with inotropic support, and blood transfusion to predefined resuscitation endpoints (e.g., central venous pressure, blood pressure, and central venous oxygen saturation) was associated with a significant reduction in in-hospital mortality (46.5% vs. 30.5%, standard therapy vs. EGDT, respectively; p=0.009). Recent and controversial questions on the clinical design, research vigor, and conduct of this study have raised several concerns regarding the validity of these findings [30–34], and a five-year, multi-center, randomized trial of early, protocolized management of septic shock – Protocolized Care for Early Septic Shock (ProCESS) (ClinicalTrials.gov NCT00510835) with a planned enrollment of 1,935 patients recently started to further address these concerns. However, preliminary studies suggest that this therapy may be beneficial in selected groups of critically ill children with sepsis as well and provide further support for this type of management strategy in critically ill patients with sepsis [35, 36].
Table 3.
The “dustpile of failed sepsis trials”
| anti-TNF monoclonal antibody (MAb) clinical trials [203, 204] |
| NORAEPT I |
| NORASEPT II |
| INTERSEPT |
| p55 tumor necrosis factor receptor fusion protein [102, 205–207] |
| Fc portion of IgG1 (TNFR:Fc) [205] |
| anti-TNF antibody fragment [208] |
| MAK 195F |
| anti-endotoxin MAb clinical trials [24, 101, 209–217] |
| HA-1A |
| E5 |
| recombinant human interleukin-1 receptor antagonist [103, 218] |
| NOS inhibitor 546C88 [219] |
| pentoxifylline [220] |
| Anti-thrombin III [221–228] |
| Tissue factor pathway inhibitor (TFPI) [229, 230] |
| platelet-activating factor acetylhydrolase (PAF-AH) [231, 232] |
| group IIA secretory phospholipase A [233] |
| Bactericidal/permeability-increasing protein (BPI) [25, 26] |
| Ibuprofen [234, 235] |
The PROWESS study, a prospective, multi-center, randomized, placebo-controlled trial of activated protein C in 1,690 critically ill adults with severe sepsis was able to demonstrate an absolute reduction in mortality of 6.1% (p=0.005) [28]. However, a recent trial of aPC in critically ill children with sepsis was terminated prematurely following an interim analysis showing lack of therapeutic benefit and a trend toward increased complications in the group receiving aPC [37]. Moreover, due to persistent questions regarding the risk:benefit ratio of aPC in less critically ill patients, European pharmaceutical regulatory agencies have mandated an additional phase III clinical trial prior to approval of the drug for clinical use, which is currently in the final planning stages [23, 38].
Finally, in the MONARCS study [29], 2, 634 critically ill patients with severe sepsis were stratified by interleukin (IL)-6 levels into low- and high-risk groups. While the overall results of the trial were negative (35.9% versus 32.2% 28-day mortality in the placebo and afelimomab groups, respectively), there was a significant reduction in mortality in high-risk patients treated with afelimomab (47.6% versus 43.6%, placebo vs. afelimomab, respectively; p=0.041). However, anti-TNF strategies in septic shock have not been adopted because of the inability to replicate the relatively small treatment effect demonstrated in the MONARCS study. Two additional therapies – adrenal corticosteroids [39, 40] and intravenous immunoglobulin (IVIG) [41] have shown promise, though the results on mortality reduction have been inconsistent at best. The use of corticosteroids is discussed in greater detail below.
Herein, we will briefly review several novel therapeutic adjuncts for the management of critically ill patients with sepsis. We will largely focus on those therapies that directly target the host inflammatory response, specifically those that target the transcription factor, nuclear factor (NF)-κB. The interested reader is also directed to several additional reviews of novel therapies directed at the coagulation cascade [42–44], apoptosis [45–49], oxidative stress [50–54], and nitric oxide [22, 55–57]. Recent advances in antibiotic therapy, fluid resuscitation, and inotropic support will not be reviewed due to space limitations.
Pathophysiology of Sepsis
The pathophysiology of sepsis is inherently complex and will only be reviewed briefly here [58, 59]. Traditionally, research efforts in the field of sepsis have largely focused on the innate immune system and conceptually viewed sepsis as a syndrome of hyperinflammation. Under this paradigm, overzealous activation of the host inflammatory response, ostensibly intended for pathogen eradication, becomes dysregulated and consequently causes auto-injury to the host, leading to multiple organ dysfunction syndrome (MODS) and death [60, 61]. Many of the aforementioned studies (Table 3) were directed at this host inflammatory response, particularly the innate immune system, and were largely based upon pre-clinical data showing:
Increased circulating levels of pro-inflammatory mediators in animals following infectious challenge with either LPS or live bacteria
Increased circulating levels of these same pro-inflammatory mediators in critically ill subjects with the sepsis syndrome
Recapitulation of the sepsis syndrome in animals or healthy humans following administration of these same pro-inflammatory mediators (e.g. TNF-α, IL-1β, etc)
Improved survival following neutralization of these pro-inflammatory mediators in animal models of sepsis.
The astute reader will note that these are essentially Koch’s postulates for determining causality for disease [62]. As an example of this kind of approach, several studies have shown that TNF-α levels are increased in both animals and humans with sepsis, TNF-α recapitulates the sepsis syndrome in both animals and healthy humans, and neutralization of TNF-α results in improved survival in several pre-clinical models of sepsis [63, 64]. Based on these data, several so-called mediator-specific agents targeting TNF-α or other similar pro-inflammatory cytokines (e.g. IL-1β) have been proposed and studied in critically ill patients with sepsis. For example, McKenna et al [65] recently filed a patent on the the use of specific compounds that inhibit TNF-α. Unfortunately, as mentioned earlier, few, if any of these mediator-specific agents have been shown to improve outcome in subsequent prospective clinical trials [23, 66], leading to speculation that the sepsis phenotype was more complex than originally believed.
A global view of the inherent complexity of the host inflammatory response has been provided by several genome-level expression studies using cDNA microarrays in various models of sepsis. For example, lipopolysaccharide (LPS) treatment results in the variable expression of 226 genes at 2 h in rats, many of which are involved in the acute phase response, inflammation, cell adhesion, and oxidative respiration [67]. Between 500–1,400 genes were differentially expressed at the 2 h time-point following either burn injury, trauma/hemorrhage, or LPS administration in mice compared to sham animals. More importantly, thirteen of the differentially expressed genes were up- or down-regulated in common among all three models of injury, suggesting an early, highly conserved transcriptional response to injury and inflammation [68]. Low-dose LPS administration to healthy human volunteers resulted in the differential expression of over 1,500 genes, many of which are crucial to the host inflammatory response [69]. Based on these data, it would appear that the sepsis phenotype is inherently more complex than previously believed and provides further rationale to the reasons why clinical trials of therapeutic agents targeted against a single pathway or cytokine have been largely unsuccessful.
Our group recently reported the first genome-level expression data involving children with septic shock during the first 24 h after admission to the pediatric intensive care unit (PICU) [70]. A global view of the data reaffirmed, at a genomic level, that widespread regulation of inflammation- and innate immunity-related genes are fundamental components of septic shock. Perhaps the most intriguing finding generated from this initial study involves the large number of genes that were downregulated in this cohort of children with septic shock. Of interest, approximately 12% of the >1,000 gene probes that were significantly repressed in children with septic shock corresponded to functional annotations and gene ontology terms related to zinc and metal binding [70]. These data suggest that pediatric septic shock is characterized by large scale repression of genes that either depend on zinc homeostasis for normal function or directly participate in zinc homeostasis. In keeping with this assertion, functional validation data demonstrated that children with septic shock who died had significantly lower serum zinc levels compared to children with septic shock who survived [70].
A follow-up study focused on longitudinal expression profiles (i.e. over the initial 3 days of illness) [71]. This study again highlighted the ubiquitous regulation of inflammation- and innate immunity-related genes, and documented expression of genes corresponding to canonical signaling pathways and gene networks in a time-dependent manner. In addition, these longitudinal studies validated the previous observations regarding zinc-related biology and documented the persistence of this particular gene repression pattern during the initial three days of illness. The most intriguing data derived from these longitudinal studies involves the coordinated repression of genes corresponding to lymphocyte function [71]. A large number of genes that were repressed throughout the initial three days of illness corresponded to canonical signaling pathways and gene networks involving T cell receptor signaling, the antigen presentation pathway, and natural killer cell function. These data are consistent with persistent dysfunction of the adaptive immune system in children with septic shock and fit well with evolving paradigms in the field [61, 72].
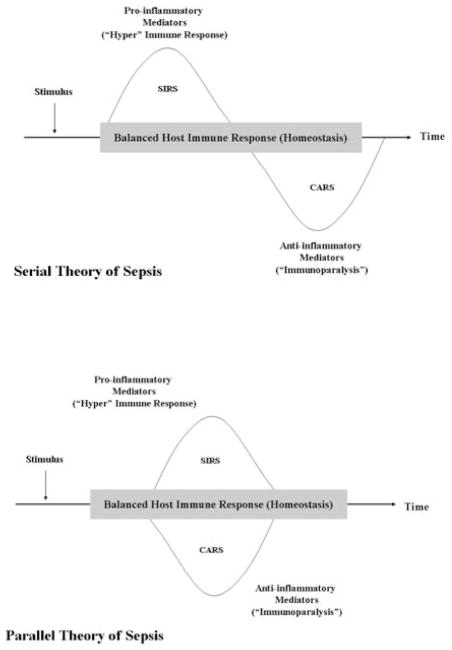
Consistent with these data, the currently available evidence supports the concept that sepsis results from a dysregulated host response, such that the balance between the pro-inflammatory mechanisms that are largely responsible for sepsis and the compensatory, anti-inflammatory mechanisms that counteract, “fine-tune”, and regulate these mechanisms largely determines the nature of the host response. There are two prevailing theories to explain this dysregulated response – namely, the serial theory and the parallel theory of sepsis [73] Fig (1). While it is recognized that these two therapies may be overly simplistic, they adequately convey the concept that the sepsis phenotype is quite heterogeneous and that a “one-size fits all” approach to treatment is not likely to be successful. For example, a shift in the homeostatic balance to a predominantly anti-inflammatory phenotype leads to a state of relative immune suppression or immunoparalysis, resulting in an inability to clear the pathogen and hence, an increased risk of nosocomial infection [74, 75]. These patients would benefit from therapies that are designed to augment or stimulate the host immune response, e.g. interferon-γ [76–78] and/or granulocyte-macrophage colony-stimulating factor (GM-CSF) [79–83]. Conversely, a shift in balance toward a predominantly pro-inflammatory phenotype results in further cellular injury, multiple organ failure, and death. These patients, on the other hand, would benefit from therapies that are designed to suppress or attenuate the host immune response. It is the nature of the host response, then, that largely determines the type of therapy required – either stimulation or attenuation of the host response to infection [84, 85].
Figure 1. Current prevailing theories of the pathophysiology of sepsis. The serial theory of sepsis.
The initial response to an infectious (e.g. bacteria, virus, fungus) or non-infectious (e.g. acute pancreatitis, burns, trauma, etc) insult is characterized by a predominantly pro-inflammatory response (SIRS), which if severe enough leads to further cellular injury and subsequent organ dysfunction. This pro-inflammatory response is soon followed by a period of relative immune suppression or immunoparalysis (Compensatory Anti-inflammatory response syndrome, or CARS), characterized by the release of anti-inflammatory mediators. If severe, immunoparalysis leads to an increased risk of nosocomial infection (a “second hit”) and multiple organ dysfunction syndrome. The parallel theory of sepsis. The initial response to an infectious or non-infectious insult depends upon a multitude of factors and may lead to either a balanced immune response, an overwhelmingly pro-inflammatory response (SIRS), or an overwhelmingly anti-inflammatory response (CARS). The nature of the host response is largely determined by the host’s genetic background, gender, and the specific pathogen involved. Adapted from Ronco C, Tetta C, Mariano F, et al. Interpreting the mechanisms of continuous renal replacement therapy in sepsis: The peak concentration hypothesis. Artif Organs 2003; 27:792–801.
An Individualized Approach to the Management of Sepsis
What then determines the nature of the host response to infection? Certainly, age (increased mortality at the extremes of age – both young and old) [11, 20], gender (increased severity of illness and mortality in males compared to females) [86, 87], nutritional status (increased morbidity and mortality with malnutrition or obesity) [88–91], and the presence or absence of co-morbid conditions (e.g., immunosuppression secondary to chemotherapy, solid organ transplantation, or bone marrow transplantation) [11, 20] significantly impacts the host response to infection, but these factors clearly are not all that determines this response. Critical care physicians have all been frustrated by the common scenario in which two patients of similar age and having similar co-morbidities are treated in an identical manner, yet only one of the two patients survives, while the other patient progresses to multiple organ system failure and death. The answer to why the one patient survives and the other patient does not may be found in their genetic make-up. In other words, our genes may affect the way we respond to infection. While no clear “sepsis gene” has yet been identified, genetic factors undoubtedly play an important role in the pathophysiology of sepsis [92]. Perhaps the best evidence of the importance of genetics in determining the nature of the response to infection provided to date is the landmark study published by Sorensen and associates [93] in 1988. These investigators conducted a longitudinal cohort study involving over 900 adopted children born between 1924 and 1926. The adopted children and both their biologic and adoptive parents were followed until 1982. The death of a biologic parent before age 50 years resulted in a significantly increased risk of death in the adopted children (R.R. 1.71, 95% C.I. 1.14 to 2.57) for all causes. Of greater interest, if a biologic parent died of infection before the age of 50 years, the relative risk of death from infectious causes in the child was highly significant, with a relative risk of 5.81 (95% C.I. 2.47 to 13.7). In contrast, the death of an adoptive parent from infectious causes did not confer a greater risk of death in the adopted child.
A gene polymorphism is defined as the regular occurrence, in a population, of two or more alleles at a particular chromosome location. The most frequent type of gene polymorphism is called a single nucleotide polymorphism (SNP). A SNP is a substitution, deletion, or insertion of a single nucleotide that occurs in approximately 1 per every 200–300 base pairs of human DNA, which may or may not result in a biologically significant alteration in protein. It is believed that approximately 10% of all SNPs found in the human genome result in either a change in expression or function of a protein [94]. Pertinent to the present discussion, several SNPs in candidate proteins involved in the host immune response to sepsis have been described [84]. These SNPs may have a profound impact on the nature of the host response to infection and may determine the type of treatment that is necessary for a particular patient. Hopefully, further research in this area will lead to a more individualized approach to the management of critically ill patients with sepsis and septic shock, taking into account both the genetic make-up of the host, as well as the characteristics of the immune response, i.e. predominantly pro-inflammatory or predominantly anti-inflammatory. We believe that a strategy based upon “the right therapy, at the right time, in the right patient” will achieve the best possible outcome.
Why Previous Treatments Have Failed
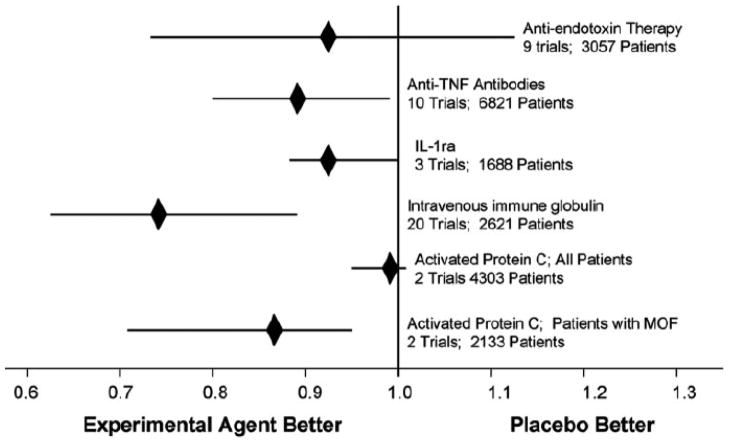
Several experts have speculated on the reasons as to why the vast majority of therapeutic trials in sepsis have failed [23, 95–97]. One commonly cited reason is the sheer complexity of the sepsis phenotype. How could a single therapeutic agent targeted at just one particular mediator be expected to impact outcome when viewed in context of the fact that several hundred genes are differentially expressed in sepsis [68–71, 98, 99], many of which involved in redundant and overlapping pathways. Upon closer inspection, however, there appears to be some room for optimism. For most of the aforementioned studies, there is a consistent, albeit small, absolute reduction in mortality of about 2–4% Fig (2). Unfortunately, in most, if not all of these studies, this reduction in mortality is not statistically significant. A landmark study by Eichacker and colleagues [100] suggested that the efficacy of anti-inflammatory or mediator-directed therapeutic agents in critically ill patients with sepsis may depend to a greater extent on the individual’s severity of illness. In other words, there may be some level of risk to these agents, such that the risk of harm is unacceptably increased in patients with a decreased severity of illness and correspondingly low risk of mortality. Conversely, if the severity of illness in a particular patient corresponds to a high risk of mortality, therapy directed at the host response may, in fact, be beneficial. Early studies using a human monoclonal antibody (HA-1A) directed against endotoxin [101], the p55 TNF receptor fusion protein (lenercept) [102], and recombinant IL-1Ra [103] were able to demonstrate either a trend towards or statistically significant reduction in mortality when patients were stratified by severity of illness. This concept is further supported by the findings of the MONARCS study [29], in which mortality was significantly improved when afelimomab was administered to critically ill patients stratified to a high risk of mortality, defined as a serum IL-6 concentration above 1,000 pg/mL.
Figure 2.
Pooling data from clinical trials of five different therapeutic strategies targeting microbial products or endogenous mediators of the host response reveal a consistent signal, suggesting clinical benefit. IL-1ra, IL-1R antagonist; MOF, multiple organ failure. Copied with permission from [23]
In further support of the above concept put forth by Eichacker and colleagues, our group recently measured serum levels of IL-8 in a cohort of critically ill children with septic shock [104]. A serum IL-8 ≤ 220 pg/mL obtained within 24 h of admission to the pediatric intensive care unit (PICU) had a 95% negative predictive value for 28-day mortality. In other words, the vast majority of children with IL-8 levels less than 220 pg/mL will survive with conventional therapy alone. We suggest that a serum IL-8 level ≤ 220 pg/mL could be used to exclude critically ill children with sepsis from interventional trials, as these children would be expected to survive with conventional therapy alone. A similar approach with IL-8 or other relevant biomarkers could be used in studies performed in critically ill adults with sepsis.
The Host Innate Immune Response
The host response to an infectious challenge may be broadly classified into the innate immune system and the acquired, or adaptive immune system. The innate immune system is the first line of defense against pathogens, while the adaptive immune system relies upon the proliferation of T and B lymphocytes specific to a particular pathogen. Importantly, the innate immune response is present prior to pathogen exposure and is generally not further enhanced by pathogen exposure. In contrast, the adaptive immune response is specifically stimulated by exposure to pathogens (and other foreign antigens), is highly pathogen-specific, and increases in magnitude, potency, and efficiency with repeated exposure to a given pathogen. Thus, whereas the innate immune response can be thought as a first line of relatively non-specific defense, the adaptive immune response can be thought of as a long term and highly specific mechanism of defense.
The innate immune response begins with the recognition of a repertoire of molecular motifs found on pathogens, known as pathogen-associated molecular patterns (PAMPs) by a surprisingly limited number of highly conserved pattern recognition receptors (PRRs). The family of PRR, which include the Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain (NOD) receptors are crucial to the recognition of pathogens by the cells of the innate immune system. These PRR recognize both endogenous and exogenous “danger signals” [105], which include PAMPs such as lipopolysaccharide (LPS), lipoteichoic acid, and peptidoglycan [106, 107], as well as endogenous danger signals [108], including uric acid [109] and extracellular heat shock proteins [110].
At present, ten human TLRs have been identified [111–116]. These TLRs recognize a wide array of molecular patterns from bacteria, fungi, yeast, and viruses (Table 4) and undoubtedly play a major role in the pathophysiology of sepsis. The Toll-like receptors are members of the Toll/IL-1 receptor (TIR) superfamily of receptors (which include the Toll-like receptors, IL-1 receptor, and IL-18) which all share to the highly conserved TIR homology domain [117, 118]. The TIR domain is necessary for propagating the pro-inflammatory signal from the cell membrane to additional intracellular signaling domains via interaction with the TIR domain on other adaptor proteins. These adaptor proteins include, MyD88 (necessary for MyD88-dependent signaling in all TLR, with the exception of TLR-3), TIRAP/Mal (TLR-2 and TLR-4 signaling), TICAM-1 (TLR-3 and MyD88-independent TLR-4 signaling), and TRAM (MyD88-independent TLR-4 signaling) [108].
Table 4.
Toll-like receptors and their agonists
| TOLL-LIKE RECEPTOR | LIGAND |
|---|---|
| TLR1 | Triacycl lipopeptides (Bacteria) |
| TLR2 | Peptidoglycan (Gram-positive bacteria) |
| Lipoteichoic acid (Gram-positive bacteria) | |
| Lipopolysaccharide (Leptospira interrogans) | |
| Lipopolysaccharide (Porphyrmonas gingivalis) | |
| Zymosan (Fungi) | |
| Heat shock protein (Hsp) 70 (Host origin)* | |
| TLR3 | Double-stranded RNA (Viruses) |
| TLR4 | Lipopolysaccharide (Gram-negative bacteria) |
| Taxol (Plants) | |
| Fusion protein (Respiratory syncytial virus) | |
| HMGB-1 (Host origin) | |
| Hsp 60 (Chlamydia pneumoniae)* | |
| Hsp70 (Host origin)* | |
| Hyaluronic acid (Host origin) | |
| Fibrinogen (Host origin) | |
| Fibronectin A domain (Host origin) | |
| TLR5 | Flagellin (Bacteria) |
| TLR6 | Diacyl lipopeptides (Mycoplasma) |
| Lipoteichoic acid (Gram-positive bacteria) | |
| Zymosan (Fungi) | |
| TLR7 | Imidazoquinoline (Synthetic compound) |
| Single-stranded RNA (Viruses) | |
| TLR8 | Imidazoquinoline (Synthetic compound) |
| Single-stranded RNA (Viruses) | |
| TLR9 | CpG-containing DNA (Bacteria and viruses) |
| TLR10 | ? |
Some of the reported effects of extracellular heat shock proteins may be due to endotoxin contamination of these recombinant proteins.
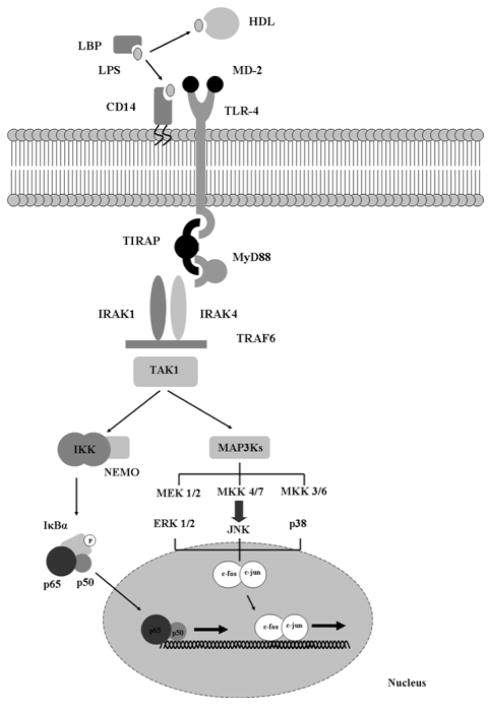
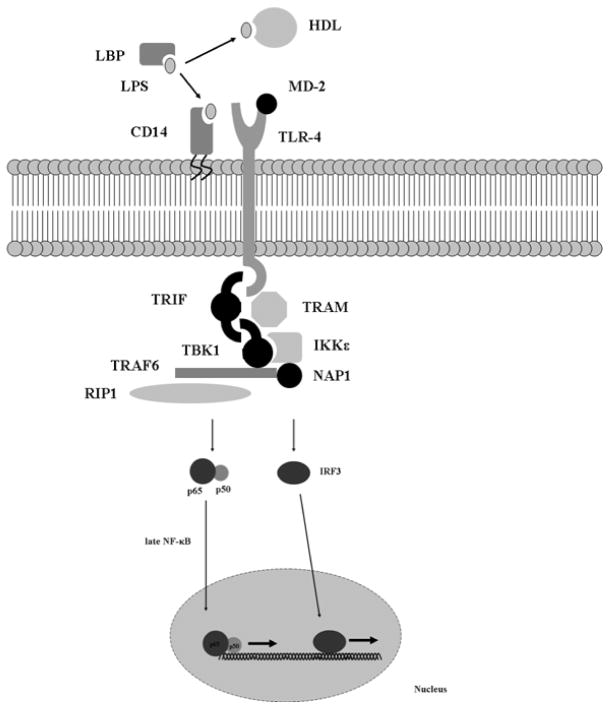
The signal transduction pathways that result in LPS-mediated pro-inflammatory gene expression are perhaps the best characterized of the innate immune response [119–121]. Recognition of LPS or endotoxin, a component of the outer cell membrane of gram-negative bacteria by TLR-4 results in a massive cytokine release responsible for most of the signs and symptoms of gram-negative septic shock. Conversely, mice that carry an inherited mutation in the TLR-4 receptor are resistant to these effects [122, 123]. As mentioned briefly above, two major pathways of LPS signal transduction have been characterized – the MyD88-dependent pathway Fig (3) and the MyD88-independent pathway Fig (4). The MyD88-dependent pathway culminates in activation of the transcription factor, NF-κB. While other transcription factors (e.g, activating protein-1, AP-1) are certainly involved in LPS-mediated pro-inflammatory signaling, NF-κB appears to act as a master control switch for the activation of several pro-inflammatory genes that play a major pathophysiologic role in sepsis (Table 5) [124]. Conversely, the MyD88-independent pathway culminates in the activation of interferon (IFN)-inducible genes, such as IP10 and glucocorticoid-attenuated response gene 16 (GARG16) via the transcription factor, IFN regulatory factor 3 (IRF-3). The MyD88-independent activation also results in a delayed, late activation of NF-κB-dependent pro-inflammatory gene expression.
Figure 3.
LPS signal transduction via the MyD88-dependent pathway.
Figure 4.
LPS signal transduction via the MyD88-independent pathway.
Table 5.
Genes regulated by the transcription factor, NF-κB
| Cytokines and Chemokines |
| Tumor necrosis factor-α |
| Interleukins-1, -2, -3, -6, -8, and –12 |
| RANTES |
| Eotaxin |
| Gro-α, -β, and -γ |
| Macrophage inhibitory protein 1alpha(MIP-1α) |
| Macrophage chemotactic protein 1 (MCP-1) |
| Cell Adhesion Molecules |
| Intracellular adhesion molecule 1 (ICAM-1) |
| Vascular cell adhesion molecule 1 (VCAM-1) |
| E-selectin |
| Growth Factors |
| Granulocyte-macrophage colony-stimulating factor (GM-CSF) |
| Granulocyte colony-stimulating factor (G-CSF) |
| Macrophage colony-stimulating factor (M-CSF) |
| Miscellaneous |
| Inducible Nitric oxide synthase |
| C reactive protein (CRP) |
| 5-lipoxygenase |
| Inducible cyclo-oxygenase 2 |
NF-κB belongs to the Rel family of transcription factors, which share common structural motifs for dimerization and DNA binding. Five known subunits belong to the mammalian NF-κ B/Rel family: c-Rel, NF-κB1 (p50/p105), NF-κB2 (p52/p100), rel A (p65), and rel B. “NF-κB” consists of two such subunits arranged as either homodimers (e.g. p50/p50) or heterodimers (e.g. p65/p50). The most common form of activated NF-κB consists of a p65 (Rel A) and p50 heterodimer [125–127]. Other subunit combinations, however, are known to exist and appear to have different DNA binding characteristics that can impart an additional level of gene regulation by the NF-κB pathway [125].
NF-κB is predominantly localized to the cytoplasm compartment of the cell in an inactive state, bound to a related inhibitory protein known as IκB. Several forms of IκB exist, all characterized by the presence of five to seven conserved domains known as ankyrin repeats. By way of these ankyrin repeats, IκB physically masks the nuclear translocation sequence (NLS) of the p65 subunit of NF-κB, though the NLS of the p50 subunit remains exposed. Nuclear export sequences on IκB, coupled with the NLS on p50 lead to constant shuttling of the IκB/NF-κB complex between the nucleus and cytoplasm, although the localization of this complex under steady-state conditions appears to be predominantly in the cytoplasm [126, 128]. The canonical pathway for activation of NF-κB occurs when IκB is phosphorylated by IκB kinase (IKK). Activation of IKK occurs in response to a variety of proinflammatory signals such as LPS, TNF-α, interleukin-1β, oxidants, bacteria, and viruses. Phosphorylated IκB is targeted for rapid ubiquitination, which results in degradation by the 26S proteasome. Degradation of IκB unmasks the NLS of NF-κB, favoring nuclear localization of NF-κB. Once in the nucleus, NF-κB is free to direct the transcription of target genes [125, 126].
Targeting the Host Innate Immune Response
Corticosteroids
As mentioned in the preceding paragraphs above, corticosteroids have been used in the management of critically ill patients with sepsis [129]. The approach until the early 1980’s focused on administering very high, supraphysiologic doses of corticosteroids in an attempt to block the host inflammatory response – consistent with the prevailing theory of the pathophysiology of sepsis at the time (above). Unfortunately, large, multicenter, randomized, placebo-controlled trials failed to show any benefits to this practice [130, 131]. Two subsequent meta-analyses [132, 133] failed to demonstrate any benefit to high-dose corticosteroid administration in this patient population, and the practice was largely abandoned [134].
Recently, there has been a resurgence of interest in the use of corticosteroids in the management of critically ill patients with septic shock. Following the promising results of smaller studies that suggested a benefit to moderate-dose corticosteroids [135, 136], Annane and co-workers [39] conducted a multicenter, randomized, placebo-controlled, double-blind trial comparing the use of stress-dose hydrocortisone (50 mg i.v. every 6 hours) and fludrocortisones (50 μg once daily) or placebo in critically ill adults with septic shock and relative adrenal insufficiency (as determined by an inadequate response to ACTH). While the trial suffered from some methodologic concerns [129], there was a significant reduction in the duration of vasopressor therapy and 28-day mortality in patients with relative adrenal insufficiency who were randomized to the treatment group.
The results of the Corticosteroid Therapy of Septic Shock (CORTICUS) trial [40] were recently reported, in which hydrocortisone treatment shortened the duration of time to shock reversal in patients with an inadequate cortisol response to ACTH, as well as those patients who did respond with an adequate cortisol response to ACTH. However, there was no difference in 28-day mortality in either the responder group or non-responder group in patients randomized to hydrocortisone treatment versus placebo. Unfortunately, the trial was underpowered to detect a difference in mortality, as the trial was prematurely terminated after only 500 of the planned 800 subjects were enrolled due to slow enrollment. It appears then that the jury is still out on this type of therapy, and further studies are warranted.
Endogenous immunomodulatory and anti-microbial peptides
On-going translational research efforts are focused on the TLR-4 pathway, as well as additional [137–139] mammalian Toll-like receptor pathways and their activation by pathogen ligands in the hopes of identifying potential therapeutic targets for the treatment of sepsis. Several endogenous compounds that negatively modulate LPS-TLR-4 signal transduction exist and impart an additional level of regulation to pro-inflammatory gene expression (Table 6) [140–154]. These compounds would appear to be attractive therapeutic targets for critically ill patients with sepsis. In addition, there is growing interest in anti-microbial peptides, endogenous compounds such as lysozymes, lactoferrin, defensins, and cathelicidins that play a major role in innate immunity [155–157]. Similarly, anti-microbial peptides derived from amphibians have been tested in pre-clinical models and appear to directly inhibit the effects of LPS [158–160].
Table 6.
Selected endogenous modulators of the host innate immune response
| MODULATOR | SITE OF ACTION | MECHANISM OF ACTION |
|---|---|---|
| sTLR-4[142] | Extracellular | “sink” for LPS |
| sST2 [143] | Extracellular | ? ↓ TLR-4 expression |
| sCD14 [144, 164] | Extracellular | “sink” for LPS |
| RP105 [145] | Membrane | TLR-4 homolog |
| SIGIRR [146, 147] | Membrane | Inhibition of MyD88, IRAK, TRAF6 |
| TRAIL-R [148] | Membrane | Stabilization of IκBα |
| A20 [137, 149] | Intracellular | Removes ubiquitin from TRAF6 |
| ABIN-3 [138] | Intracellular | Inhibition of NF-κB |
| ATF-3 [139, 150] | Intracellular | Alters chromatin structure |
| β-arrestin [141, 151] | Intracellular | Inhibition of TRAF6 |
| Dok-1/Dok-2 [152] | Intracellular | Inhibition of ERK |
| IRAK-M [153] | Intracellular | Inhibition of IRAK-4/IRAK-1 |
| Monarch-1 [154] | Intracellular | Inhibition of IRAK-4/IRAK-1 |
| MyD88s [140] | Intracellular | Inhibition of IRAK-4/IRAK-1 |
sTLR-4, soluble Toll-like receptor-4; sST2, ; sCD14, soluble CD14; SIGIRR, single Ig IL-1 receptor-related molecule; TRAIL-R, TRAIL receptor, ABIN-3, A20-binding inhibitor of NF-κB activation; ATF-3, activating transcription factor-3; Dok-1/2, downstream of tyrosine kinase-1/2; IRAK, IL-1R-associated kinase
LPS, LBP, and sCD14
Several novel therapeutic agents directed at the LPS-TLR-4 signal transduction pathway have been tested in pre-clinical models of sepsis. One attractive strategy is to target the LPS molecule and neutralize it before it activates the host innate immune response [161, 162]. For example, analogs of lipid-binding protein (LBP), which shuttles LPS to the TLR-4 complex, have been shown to block LPS-LBP interactions and effectively inhibit LPS-mediated pro-inflammatory gene expression [163]. Similarly, treatment with recombinant soluble CD14 (sCD14) improves outcome in a murine model of endotoxic shock [164], and a monoclonal antibody directed at CD14 (membrane-bound) has been tested in phase I clinical trials [165]. Leturcq et al recently filed a patent on the use of monoclonal antibodies directed against CD14 [166] Recently, studies have shown that high-density lipoprotein (HDL) can act as a “sink” for LPS and prevent LPS-mediated pro-inflammatory gene expression both in vitro and in vivo [19, 167]. HDL may have other immunomodulatory effects aside from direct LPS binding. Collectively, these studies have opened up an entire line of investigation into the role of lipoproteins and pharmacologic agents that target lipoproteins (e.g. HMG-CoA reductase inhibitors) in the management of patients with sepsis [167, 168].
Endogenous danger signals
The fact that LPS is not readily measured or detectable in the majority of patients who present to the ICU with sepsis casts some doubt on therapeutic strategies targeting this molecule. In many cases, the early surge in pro-inflammatory cytokines has already abated by the time these critically ill patients are diagnosed with sepsis and treatment is initiated. However, as mentioned earlier, recent studies suggest that endogenous danger signals, including several members of the family of proteins known as heat shock proteins (Hsp10, Hsp60, Hsp72, and Hsp90) and high mobility group box-1 (HMGB-1), also activate the host innate immune response via TLR-4 [169, 170]. HMGB-1 appears to be a relatively late mediator of sepsis and would appear to be an attractive therapeutic target [171, 172].
Several clinical studies have recently been published in which extracellular heat shock proteins are directly targeted for therapy. For example, a human recombinant antibody directed against yeast-derived Hsp90 (Mycograb, Neutec-Pharma, Manchester, UK) derived [173] has been used to treat critically ill patients with invasive candidiasis [174, 175]. In this study, recombinant antibody to Hsp90 plus lipid-associated amphotericin B produced significant clinical and culture-confirmed improvement in outcome for patients with invasive candidiasis. Phase I studies of 17-allylaminogeldanamycin (17-AAG), an inhibitor of tumor-derived Hsp90 have been recently completed in both children [176, 177] and adults [178] with solid tumors. Chaperonin 10 (Hsp10) was recently used to treat 23 adults with moderate to severe active rheumatoid arthritis in a randomized, double-blind, multicenter clinical trial. Biweekly administration of chaperonin 10 was well tolerated and effective in reducing the symptoms of rheumatoid arthritis, at least in the short term [179]. DiaPep277, a peptide derived from Hsp60, has been found to slow the deterioration of beta-cell function after the clinical onset of diabetes in preclinical studies and several small clinical trials [180]. Collectively, these studies support the overall concept of targeting endogenous danger signals in critically ill patients with sepsis.
TLR-4
Given the importance of the TLR-4 in initiating and propagating the host innate immune response, there is growing interest in targeting TLR-4 and other Toll receptors in patients with sepsis. As an example, E5564 (Eritoran), a structural analog of the lipid A portion of the LPS molecue has been shown to inhibit TLR-4-mediated proinflammatory gene expression in vitro and in vivo [181–183]. A recent phase II trial of eritoran in patients undergoing cardiopulmonary bypass (CPB) did not appear to prevent the inflammatory response and attendant organ dysfunction and injury following CPB, though the drug was shown to be safe and without overt toxicity [184]. Further studies will be required to establish both the safety and efficacy of this therapeutic approach in critically ill patients with severe sepsis and septic shock. To this end, a phase III, randomized, placebo-controlled trial of eritoran in critically ill adults with severe sepsis (ACCESS – “A controlled comparison of eritoran tetrasodium and placebo in patients with severe sepsis”) is currently enrolling patients with an estimated completion date of June, 2010 (NCT00334828). The future of this kind of therapeutic strategy, in which pharmacologic agents are directed at the TLR-4 receptor or other similar PRR will depend to a great extent on the success or failure of this trial.
NF-κB
It is clear that the NF-κB pathway is linked to the dysregulated inflammation that is characteristic of sepsis. Many of the genes that comprise the complex network contributing to this dysregulated inflammation, both on the pro-inflammatory and anti-inflammatory side, are regulated at the transcriptional level by NF-κB [58, 124, 185, 186]. The NF-κB pathway would then appear to be a logical therapeutic target for the treatment of critically ill patients with sepsis [185]. Several compounds appear to inhibit NF-κB in vitro and in vivo [185, 187], most notably the salicylates, non-steroidal anti-inflammatory drugs (NSAIDS), corticosteroids, and anti-oxidants. In addition, several natural compounds, including curcumin [188, 189], sesquiterpene lactones [190–192], and tea polyphenols [193], such as epigallocatechin-3-gallate (EGCG) [194–196] and theaflavin [197] have been shown to inhibit NF-κB activation. However, several recent studies have led to questions surrounding this kind of therapeutic strategy [198]. For example, given its critical role in the innate and adaptive immune responses to infection, inhibition of NF-κB may worsen pathogen clearance and increase the risk of mortality from overwhelming infection. Alternatively, NF-κB plays an important anti-apoptotic role (see below) – inhibition may then have unintended and untoward effects on cell survival and function. Finally, other studies suggest that NF-κB has a critical anti-inflammatory function in preventing an overwhelming host inflammatory response to infectious challenge [199–202]. Obviously, further studies, both in the pre-clinical and clinical setting will be necessary to further validate this therapeutic approach.
Current and Future Developments
While the majority of clinical studies of so-called mediator-directed therapy in critically ill patients with sepsis have been unsuccessful, there is continued optimism and support for this kind of therapeutic strategy. However, the “one size fits all” approach that has been utilized in the clinical design of these studies will not work in the future. We need to address the role of host factors, such as age, gender, presence of co-morbid conditions, and genetic predisposition in determining the individual response to therapy. When designing clinical trials, critically ill patients should be stratified according to severity of illness in order to maximize the signal-to-noise ratio of new therapeutic agents. Ideally, those patients who would generally survive with standard therapy alone should not be included in such studies [104]. Further, we need to recognize that immunomodulation as a therapeutic strategy encompasses both augmentation of the immune response in critically ill patients with the immunoparalysis phenotype, as well as suppression of the immune response in those patients with a predominantly pro-inflammatory phenotype. Ideally, new diagnostic tools and biomarkers will be developed, such that these two diametrically opposing phenotypes can be easily and quickly identified at the bedside. Finally, it is likely that one particular therapeutic agent directed against any one mediator is not likely to be successful. Given the inherent complexity and redundancy of the host inflammatory response, future management strategies will likely encompass the use of multiple, synergistic agents acting upon different steps in the mediator cascade [23, 96]. Regardless of these challenges, this kind of management approach seems both reasonable and feasible
Acknowledgments
Supported by the National Institutes of Health, 5KO8GM077432 (DSW), 1R03HD058246 (DSW), 5R01GM064619 (HRW), and 5R01GM067202 (BZ)
References
- 1.ACCP, SCCM. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74. [PubMed] [Google Scholar]
- 2.Marshall JC. SIRS and MODS: What is their relevance to the science and practice of intensive care? Shock. 2000;14:586–9. [PubMed] [Google Scholar]
- 3.Baue AE. A debate on the subject “Are SIRS and MODS important entities in the clinical evaluation of patients?” The con position. Shock. 2000;14:590–3. doi: 10.1097/00024382-200014060-00003. [DOI] [PubMed] [Google Scholar]
- 4.Vincent JL. Dear SIRS, I’m sorry to say that I don’t like you. Crit Care Med. 1997;25:372–4. doi: 10.1097/00003246-199702000-00029. [DOI] [PubMed] [Google Scholar]
- 5.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 6.Denoix PX. Enquete permanent dans les centres anticancereaux. Bull Inst Natl Hyg. 1946;1:70–5. [PubMed] [Google Scholar]
- 7.Opal SM. Concept of PIRO as a new conceptual framework to understand sepsis. Pediatr Crit Care Med. 2005;6:S55–S60. doi: 10.1097/01.PCC.0000161580.79526.4C. [DOI] [PubMed] [Google Scholar]
- 8.Heron M. Deaths: Leading causes for 2004. Natl Vital Stat Rep. 2007;56:1–95. [PubMed] [Google Scholar]
- 9.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 10.Finfer S, Bellomo R, Lipman J, French C, Dobb G, Myburgh J. Adult population incidence of severe sepsis in Australian and New Zealand Intensive Care Units. Intensive Care Med. 2004;30:589–96. doi: 10.1007/s00134-004-2157-0. [DOI] [PubMed] [Google Scholar]
- 11.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: A trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–50. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 13.Ponce de Leon-Rosales SP, Molinar-Ramos F, Dominquez-Cherit G, Rangel-Frausto MS, Vazquez-Ramos VG. Prevalence of infections in intensive care units in Mexico: A multicenter study. Crit Care Med. 2000;28:1316–21. doi: 10.1097/00003246-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Alberti C, Brun-Buisson C, Burchardi H, Martin C, Goodman S, Artigas A, et al. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002;28:108–21. doi: 10.1007/s00134-001-1143-z. [DOI] [PubMed] [Google Scholar]
- 15.Padkin A, Goldfrad C, Brady A, Young D, Black N, Rowan K. Epidemiology of severe sepsis occurring in the first 24 hrs in intensive care units in England, Wales, and Northern Ireland. Crit Care Med. 2003;31:2332–8. doi: 10.1097/01.CCM.0000085141.75513.2B. [DOI] [PubMed] [Google Scholar]
- 16.Silva E, Pedro Mde A, Sogayar AC, Mohovic T, Silva CL, Janiszewski M, et al. Brazilian Sepsis Epidemiological Study (BASES study) Crit Care. 2004;8:R251–R60. doi: 10.1186/cc2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng B, Xie G, Yao S, Wu X, Guo Q, Gu M, et al. Epidemiology of severe sepsis in critically ill surgical patients in ten university hospitals in China. Crit Care Med. 2007;35:2538–46. doi: 10.1097/01.CCM.0000284492.30800.00. [DOI] [PubMed] [Google Scholar]
- 18.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: Results of the SOAP study. Crit Care Med. 2006;34:344–53. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 19.Murch O, Collin M, Hinds CJ, Thiemermann C. Lipoproteins in inflammation and sepsis. I. Basic science. Intensive Care Med. 2007;33:13–24. doi: 10.1007/s00134-006-0432-y. [DOI] [PubMed] [Google Scholar]
- 20.Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167:695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- 21.Rice TW. Treatment of severe sepsis: Where next? Current and future treatment approaches after the introduction of drotrecogin alfa. Vasc Health Risk Manag. 2006;2:3–18. doi: 10.2147/vhrm.2006.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trzeciak S, Cinel I, Dellinger RP, Shapiro NI, Arnold RC, Parrillo JE, et al. Resuscitating the microcirculation in sepsis: The central role of nitric oxide, emerging concepts for novel therapies, and challenges for clinical trials. Acad Emerg Med. 2008;15:399–413. doi: 10.1111/j.1553-2712.2008.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall JC. Sepsis: Rethinking the approach to clinical research. J Leukoc Biol. 2008;83:471–82. doi: 10.1189/jlb.0607380. [DOI] [PubMed] [Google Scholar]
- 24.Derkx B, Wittes J, McCloskey R. Randomized, placebo-controlled trial of HA-1A, a human monoclonal antibody to endotoxin, in children with meningococcal septic shock. European Pediatric Meningococcal Septic Shock Trial Study Group. Clin Infect Dis. 1999 Apr;28(4):770–7. doi: 10.1086/515184. [DOI] [PubMed] [Google Scholar]
- 25.Giroir BP, Quint PA, Barton P, Kirsch EA, Kitchen L, Goldstein B, et al. Preliminary evaluation of recombinant amino-terminal fragment of human bactericidal/permeability-increasing protein in children with severe meningococcal sepsis. Lancet. 1997 Nov 15;350(9089):1439–43. doi: 10.1016/s0140-6736(97)06468-4. [DOI] [PubMed] [Google Scholar]
- 26.Levin M, Quint PA, Goldstein B, Barton P, Bradley JS, Shemie SD, et al. Recombinant bactericidal/permeability-increasing protein (rBPI21) as adjunctive treatment for children with severe meningococcal sepsis: A randomized trial. Lancet. 2000;356:961–7. doi: 10.1016/s0140-6736(00)02712-4. [DOI] [PubMed] [Google Scholar]
- 27.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 28.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001 Mar 8;344(10):699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 29.Panacek EA, Marshall JC, Albertson TE, Johnson DH, Johnson S, MacArthur RD, et al. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab′)2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit Care Med. 2004;32:2173–82. doi: 10.1097/01.ccm.0000145229.59014.6c. [DOI] [PubMed] [Google Scholar]
- 30.Marik PE, Varon J. Goal-directed therapy for severe sepsis. N Engl J Med. 2002;346:1025. doi: 10.1056/NEJM200203283461314. [DOI] [PubMed] [Google Scholar]
- 31.Abroug F, Besbes L, Nouira S. Goal-directed therapy for severe sepsis. N Engl J Med. 2002;346:1025–6. [PubMed] [Google Scholar]
- 32.Sarkar S, Kupfer Y, Tessler S. Goal-directed therapy for severe sepsis. N Engl J Med. 2002;346:1026. [PubMed] [Google Scholar]
- 33.Donnino MW, Talmor D. A central venous pressure goal of 8–12 mm Hg for all patients in septic shock. Crit Care Med. 2007;35:1441–2. doi: 10.1097/01.CCM.0000260961.31428.06. [DOI] [PubMed] [Google Scholar]
- 34.Burton TM. New therapy for sepsis infections raises hope but many questions. The Wall Street Journal. 2008 [cited 2008 August 14]; Available from: http://online.wsj.com/article/SB12186719036438865.html?mod=dist_smartbrief#articleTabs%3 Darticle.
- 35.Han YY, Carcillo JA, Dragotta MA, Bills DM, Watson RS, Westerman ME, et al. Early reversal of pediatric-neonatal septic shock by community physicians is associated with improved outcome. Pediatrics. 2003;112:793–9. doi: 10.1542/peds.112.4.793. [DOI] [PubMed] [Google Scholar]
- 36.de Oliveira CF, de Oliveira DS, Gottschald AF, Moura JD, Costa GA, Ventura AC, et al. ACCM/PALS haemodynamic support guidelines for paediatric septic shock: An outcomes comparison with and without monitoring of central venous oxygen saturation. Intensive Care Med. 2008;34:1065–75. doi: 10.1007/s00134-008-1085-9. [DOI] [PubMed] [Google Scholar]
- 37.Nadel S, Goldstein B, Williams MD, Dalton H, Peters M, Macias WL, et al. Drotrecogin alfa (activated) in children with severe sepsis: A multicentre phase III randomised controlled trial. Lancet. 2007;369:836–43. doi: 10.1016/S0140-6736(07)60411-5. [DOI] [PubMed] [Google Scholar]
- 38.Finfer S, Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, et al. Design, conduct, analysis and reporting of a multi-national placebo-controlled trial of activated protein C for persistent septic shock. Intensive Care Med. 2008;34:1935–47. doi: 10.1007/s00134-008-1266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–71. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 40.Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–24. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 41.Laupland KB, Kirkpatrick AW, Delaney A. Polyclonal intravenous immuneglobulin for the treatment of severe sepsis and septic shock in critically ill adults: A systematic review and meta-analysis. Crit Care Med. 2007;35:2686–92. [PubMed] [Google Scholar]
- 42.Levi M, de Jonge E, van der Poll T. Rationale for restoration of physiological anticoagulant pathways in patients with sepsis and disseminated intravascular coagulation. Crit Care Med. 2001;29:S90–S4. doi: 10.1097/00003246-200107001-00028. [DOI] [PubMed] [Google Scholar]
- 43.Feistritzer C, Wiedermann CJ. Effects of anticoagulant strategies on activation of inflammation and coagulation. Expert Opin Biol Ther. 2007;7:855–70. doi: 10.1517/14712598.7.6.855. [DOI] [PubMed] [Google Scholar]
- 44.Cornet AD, Smit EG, Beishuizen A, Groeneveld AB. The role of heparin and allied compounds in the treatment of sepsis. Thromb Haemost. 2007;98:579–86. [PubMed] [Google Scholar]
- 45.Oberholzer A, Oberholzer C, Minter RM, Moldawer LL. Considering immunomodulatory therapies in the septic patient: Should apoptosis be a potential therapeutic target? Immunol Lett. 2001;75:221–4. doi: 10.1016/s0165-2478(00)00307-2. [DOI] [PubMed] [Google Scholar]
- 46.Oberholzer C, Oberholzer A, Clare-Salzler M, Moldawer LL. Apoptosis in sepsis: A new target for therapeutic exploration. FASEB J. 2001;15:879–92. doi: 10.1096/fj.00-058rev. [DOI] [PubMed] [Google Scholar]
- 47.Ayala A, Wesche-Soldato DE, Perl M, Lomas-Neira JL, Swan R, Chung C-S. Blockade of apoptosis as a rational therapeutic strategy for the treatment of sepsis. Novartis Found Symp. 2007;280:37–164. doi: 10.1002/9780470059593.ch4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hotchkiss RS, Coopersmith CM, Karl IE. Prevention of lymphocyte apoptosis - a potential treatment of sepsis? Clin Infect Dis. 2005;41:S465–S9. doi: 10.1086/431998. [DOI] [PubMed] [Google Scholar]
- 49.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–22. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 50.Berger MM. Antioxidant micronutrients in major trauma and burns: Evidence and practice. Nutr Clin Pract. 2006;21:438–49. doi: 10.1177/0115426506021005438. [DOI] [PubMed] [Google Scholar]
- 51.Berger MM, Chiolero RL. Antioxidant supplementation in sepsis and systemic inflammatory response syndrome. Crit Care Med. 2007;35:S584–S90. doi: 10.1097/01.CCM.0000279189.81529.C4. [DOI] [PubMed] [Google Scholar]
- 52.Eaton S. The biochemical basis of antioxidant therapy in critical illness. Proc Nutr Soc. 2006;65:242–9. doi: 10.1079/pns2006501. [DOI] [PubMed] [Google Scholar]
- 53.Heyland DK, Dhaliwal R, Suchner U, Berger MM. Antioxidant nutrients: A systematic review of trace elements and vitamins in the critically ill patient. Intensive Care Med. 2005;31:327–37. doi: 10.1007/s00134-004-2522-z. [DOI] [PubMed] [Google Scholar]
- 54.Roth E, Manhart N, Wessner B. Assessing the antioxidant status in critically ill patients. Curr Opin Clin Nutr Metab Care. 2004;7:161–8. doi: 10.1097/00075197-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 55.Hauser B, Bracht H, Matejovic M, Radermacher P, Venkatesh B. Nitric oxide synthase inhibition in sepsis? Lessons learned from large-animal studies. Anesth Analg. 2005;101:488–98. doi: 10.1213/01.ANE.0000177117.80058.4D. [DOI] [PubMed] [Google Scholar]
- 56.Cauwels A. Nitric oxide in shock. Kidney Int. 2007;72:557–65. doi: 10.1038/sj.ki.5002340. [DOI] [PubMed] [Google Scholar]
- 57.Harbrecht BG. Therapeutic use of nitric oxide scavengers in shock and sepsis. Curr Pharm Design. 2006;12:3543–9. doi: 10.2174/138161206778343000. [DOI] [PubMed] [Google Scholar]
- 58.Hazelzet J, Driessen GJA, Abboud P, Wheeler DS, Shanley TP, Wong HR. Sepsis. In: Wheeler DS, Wong HR, Shanley TP, editors. Pediatric Critical Care Medicine: Basic Science and Clinical Evidence. London, UK: Springer-Verlag London Limited; 2007. pp. 1421–44. [Google Scholar]
- 59.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 60.Shanley TP, Hallstrom C, Wong HR. Sepsis. In: Fuhrman BP, Zimmerman JJ, editors. Pediatric Critical Care Medicine. 3. St. Louis: Mosby ; 2006. pp. 1474–93. [Google Scholar]
- 61.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003 Jan 9;348(2):138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 62.Marshall JC, Vincent JL, Fink MP, Cook DJ, Rubenfeld G, Foster D, et al. Measures, markers, and mediators: Toward a staging system for clinical sepsis. A report of the Fifth Toronto Sepsis Roundtable, Toronto, Ontario, Canada, October 25–26, 2000. Crit Care Med. 2003;31:1560–7. doi: 10.1097/01.CCM.0000065186.67848.3A. [DOI] [PubMed] [Google Scholar]
- 63.Lorente JA, Marshall JC. Neutralization of tumor necrosis factor in preclinical models of sepsis. Shock. 2005;24 (Suppl):107–19. doi: 10.1097/01.shk.0000191343.21228.78. [DOI] [PubMed] [Google Scholar]
- 64.Clark IA. How TNF was recognized as a key mechanism of disease. Cytokine Growth Factor Rev. 2007;18:335–43. doi: 10.1016/j.cytogfr.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 65.McKenna JM, Papa PW, Sakata ST, Erdman PE, Packard GK inventors. Inhibitors of TNFalpha, PDE4, and B-RAF, compositions thereof and methods of use therewith. 2008 [Google Scholar]
- 66.Vincent JL, Sun Q, Dubois MJ. Clinical trials of immunomodulatory therapies in severe sepsis and septic shock. Clin Infect Dis. 2002;34:1084–93. doi: 10.1086/339549. [DOI] [PubMed] [Google Scholar]
- 67.Fannin RD, Auman JT, Bruno ME, Sieber SO, Ward SM, Tucker CJ, et al. Differential gene expression profiling in whole blood during acute systemic inflammation in lipopolysaccharide-treated rats. Physiol Genomics. 2005;21:92–104. doi: 10.1152/physiolgenomics.00190.2004. [DOI] [PubMed] [Google Scholar]
- 68.Brownstein BH, Logvinenko T, Lederer JA, Cobb JP, Hubbard WJ, Chaudry IH, et al. Commonality and differences in leukocyte gene expression patterns among three models of inflammation and injury. Physiol Genomics. 2006;24:298–309. doi: 10.1152/physiolgenomics.00213.2005. [DOI] [PubMed] [Google Scholar]
- 69.Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, et al. A network-based analysis of systemic inflammation in humans. Nature. 2005 Oct 13;437(7061):1032–7. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 70.Wong HR, Shanley TP, Sakthivel B, Cvijanovich N, Lin R, Allen GL, et al. Genome-level expression profiles in pediatric septic shock indicate a role for altered zinc homeostasis in poor outcome. Physiol Genomics. 2007 Jul 18;30(2):146–55. doi: 10.1152/physiolgenomics.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shanley TP, Cvijanovich N, Lin R, Allen GL, Thomas NJ, Doctor A, et al. Genome-Level Longitudinal Expression of Signaling Pathways and Gene Networks in Pediatric Septic Shock. Mol Med. 2007 Sep;13(9–10):495–508. doi: 10.2119/2007-00065.Shanley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Felmet KA, Hall MW, Clark RS, Jaffe R, Carcillo JA. Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J Immunol. 2005 Mar 15;174(6):3765–72. doi: 10.4049/jimmunol.174.6.3765. [DOI] [PubMed] [Google Scholar]
- 73.Ronco C, Tetta C, Mariano F, Wratten ML, Bonello M, Bordoni V, et al. Interpreting the mechanisms of continuous renal replacement therapy in sepsis: The peak concentration hypothesis. Artif Organs. 2003;27:792–801. doi: 10.1046/j.1525-1594.2003.07289.x. [DOI] [PubMed] [Google Scholar]
- 74.Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177:1967–74. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 75.Ashare A, Powers LS, Butler NS, Doerschug KC, Monick MM, Hunninghake GW. Anti-inflammatory response is associated with mortality and severity of infection in sepsis. Am J Physiol Lung Cell Mol Physiol. 2005;288:L633–L40. doi: 10.1152/ajplung.00231.2004. [DOI] [PubMed] [Google Scholar]
- 76.Docke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, et al. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med. 1997 Jun;3(6):678–81. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 77.Volk HD, Reinke P, Krausch D, Zuckermann H, Asadullah K, Muller JM, et al. Monocyte deactivation - rationale for a new therapeutic strategy in sepsis. Intensive Care Med. 1996;22 (Suppl 4):S474–S81. doi: 10.1007/BF01743727. [DOI] [PubMed] [Google Scholar]
- 78.Kox WJ, Bone RC, Krausch D, Docke WD, Kox SN, Wauer H, et al. Interferon gamma-1b in the treatment of compensatory anti-inflammatory response syndrome. A new approach: Proof of principle. Arch Intern Med. 1997;157:389–93. [PubMed] [Google Scholar]
- 79.Bundschuh DS, Barsig J, Hartung T, Randow F, Docke WD, Volk HD, et al. Granulocyte-macrophage colony-stimulating factor and IFN-gamma restore the systemic TNF-alpha response to endotoxin in lipopolysaccharide-desensitized mice. J Immunol. 1997;158:2862–71. [PubMed] [Google Scholar]
- 80.Pugin J. Immunostimulation is a rational therapeutic strategy in sepsis. Novartis Found Symp. 2007;280:21–36. 160–4. [PubMed] [Google Scholar]
- 81.Nelson LA. Use of granulocyte-macrophage colony-stimulating factor to reverse anergy in otherwise immunologically healthy children. Ann Allergy Asthma Immunol. 2007;98:373–82. doi: 10.1016/S1081-1206(10)60885-X. [DOI] [PubMed] [Google Scholar]
- 82.Spight D, Trapnell B, Zhao B, Berclaz P, Shanley TP. Granulocyte-macrophage-colony-stimulating factor-dependent peritoneal macrophage responses determine survival in experimentally induced peritonitis and sepsis in mice. Shock. 2008;30:434–42. doi: 10.1097/SHK.0b013e3181673543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Flohe SB, Agrawal H, Flohe S, Rani M, Bangen JM, Schade FU. Diversity of interferon gamma and granulocyte-macrophage colony-stimulating factor in restoring immune dysfunction of dendritic cells and macrophages during polymicrobial sepsis. Mol Med. 2008;14:247–56. doi: 10.2119/2007-00120.Flohe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wheeler DS, Wong HR. Genetic approach to pediatric septic shock. Personalized Med. 2008;5:249–63. doi: 10.2217/17410541.5.3.249. [DOI] [PubMed] [Google Scholar]
- 85.Monneret G, Venet F, Pachot A, Lepape A. Monitoring immune dysfunctions in the septic patient: A new skin for the old ceremony. Mol Med. 2008;14:64–78. doi: 10.2119/2007-00102.Monneret. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marriott I, Huet-Hudson YM. Sexual dimorphism in innate immune responses to infectious organisms. Immunol Res. 2006;34:177–92. doi: 10.1385/IR:34:3:177. [DOI] [PubMed] [Google Scholar]
- 87.Choudhry MA, Bland KI, Chaudry IH. Trauma and immune response - effect of gender differences. Injury. 2007;38:1382–91. doi: 10.1016/j.injury.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carcillo JA. Reducing the global burden of sepsis in infants and children: A clinical practice research agenda. Pediatr Crit Care Med. 2005;6:S157–S64. doi: 10.1097/01.PCC.0000161574.36857.CA. [DOI] [PubMed] [Google Scholar]
- 89.Cave MC, Hurt RT, Frazier TH, Matheson PJ, Garrison RN, McClain CJ, et al. Obesity, inflammation, and the potential application of pharmaconutrition. Nutr Clin Pract. 2008;23:16–34. doi: 10.1177/011542650802300116. [DOI] [PubMed] [Google Scholar]
- 90.Nguyen TH, Nguyen TL, Lei HY, Lin YS, Le BL, Huang KJ, et al. Association between sex, nutritional status, severity of dengue hemorrhagic fever, and immune status in infants with dengue hemorrhagic fever. Am J Trop Med Hyg. 2005;72:370–4. [PubMed] [Google Scholar]
- 91.Sakr Y, Madl C, Filipescu D, Moreno R, Groeneveld J, Artigas A, et al. Obesity is associated with increased morbidity but not mortality in critically ill patients. Intensive Care Med. 2008;34:1999–2009. doi: 10.1007/s00134-008-1243-0. [DOI] [PubMed] [Google Scholar]
- 92.Cooke GS, Hill AV. Genetics of susceptibility to human infectious disease. Nat Rev Genet. 2001;2:967–77. doi: 10.1038/35103577. [DOI] [PubMed] [Google Scholar]
- 93.Sorensen TI, Nielsen GG, Andersen PK, Teasdale TW. Genetic and environmental influences on premature death in adult adoptees. N Engl J Med. 1988 Mar 24;318(12):727–32. doi: 10.1056/NEJM198803243181202. [DOI] [PubMed] [Google Scholar]
- 94.Arcaroli J, Fessler MB, Abraham E. Genetic polymorphisms in sepsis. Shock. 2005;24:300–12. doi: 10.1097/01.shk.0000180621.52058.e1. [DOI] [PubMed] [Google Scholar]
- 95.Carlet J, Cohen J, Calandra T, Opal SM, Masur H. Sepsis: Time to reconsider the concept. Crit Care Med. 2008;36:964–6. doi: 10.1097/CCM.0B013E318165B886. [DOI] [PubMed] [Google Scholar]
- 96.Marshall JC. Such stuff as dreams are made on: Mediator-directed therapy in sepsis. Nat Rev Drug Discov. 2003;2:391–405. doi: 10.1038/nrd1084. [DOI] [PubMed] [Google Scholar]
- 97.Sweeney DA, Danner RL, Eichacker PQ, Natanson C. Once is not enough: Clinical trials in sepsis. Intensive Care Med. 2008;34:1955–60. doi: 10.1007/s00134-008-1274-6. [DOI] [PubMed] [Google Scholar]
- 98.Cobb JP, Mindrinos MN, Miller-Graziano C, Calvano SE, Baker HV, Xiao W, et al. Application of genome-wide expression analysis to human health and disease. Proc Natl Acad Sci U S A. 2005 Mar 29;102(13):4801–6. doi: 10.1073/pnas.0409768102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wong HR. Pediatric septic shock treatment: new clues from genomic profiling. Pharmacogenomics. 2007 Oct;8(10):1287–90. doi: 10.2217/14622416.8.10.1287. [DOI] [PubMed] [Google Scholar]
- 100.Eichacker PQ, Parent C, Kalil A, Esposito C, Cui X, Banks SM, et al. Risk and the efficacy of antiinflammatory agents: Retrospective and confirmatory studies of sepsis. Am J Respir Crit Care Med. 2002;166:1197–205. doi: 10.1164/rccm.200204-302OC. [DOI] [PubMed] [Google Scholar]
- 101.Ziegler EJ, Fisher CJ, Jr, Sprung CL, Straube RC, Sadoff JC, Foulke GE, et al. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N Engl J Med. 1991 Feb 14;324(7):429–36. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]
- 102.Abraham E, Glauser MP, Butler T, Garbino J, Gelmont D, Laterre PF, et al. p55 Tumor necrosis factor receptor fusion protein in the treatment of patients with severe sepsis and septic shock. A randomized controlled multicenter trial. Ro 45–2081 Study Group. Jama. 1997;277(19):1531–8. [PubMed] [Google Scholar]
- 103.Fisher CJ, Jr, Dhainaut JF, Opal SM, Pribble JP, Balk RA, Slotman GJ, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. Jama. 1994;271(23):1836–43. [PubMed] [Google Scholar]
- 104.Wong HR, Cvijanovich N, Wheeler DS, Bigham MT, Monaco M, Odoms K, et al. Interleukin-8 as a stratification tool for interventional trials involving pediatric septic shock. Am J Respir Crit Care Med. 2008;178:276–82. doi: 10.1164/rccm.200801-131OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Matzinger P. An innate sense of danger. Semin Immunol. 1998;10:399–415. doi: 10.1006/smim.1998.0143. [DOI] [PubMed] [Google Scholar]
- 106.Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 107.Franchi L, McDonald C, Kanneganti T-D, Amer A, Nunez G. Nucleotide-binding oligomerization domain-like receptors: Intracellular pattern recognition molecules for pathogen detection and host defense. J Immunol. 2006;177:3507–13. doi: 10.4049/jimmunol.177.6.3507. [DOI] [PubMed] [Google Scholar]
- 108.Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol. 2008;214:161–78. doi: 10.1002/path.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–21. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 110.El Mezayen R, El Gazzar M, Seeds MC, McCall CE, Dreskin SC, Nicolls MR. Endogenous signals released from necrotic cells augment inflammatory responses to bacterial endotoxin. Immunol Lett. 2007;111:36–44. doi: 10.1016/j.imlet.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–7. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 112.Modlin RL, Brightbill HD, Godowski PJ. The toll of innate immunity on microbial pathogens. N Engl J Med. 1999 Jun 10;340(23):1834–5. doi: 10.1056/NEJM199906103402312. [DOI] [PubMed] [Google Scholar]
- 113.Underhill DM. Toll-like receptors and microbes take aim at each other. Curr Opin Immunol. 2004 Aug;16(4):483–7. doi: 10.1016/j.coi.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 114.Kopp EB, Medzhitov R. The Toll-like receptor family and control of innate immunity. Curr Opin Immunol. 1999;11:13–8. doi: 10.1016/s0952-7915(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 115.Gordon S. Pattern recognition receptors: Doubling up for the innate immune response. Cell. 2002;111:927–30. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- 116.Akira S, Takeda K. Toll-like receptor signalling. Nat Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 117.Jefferies C, O’Neill LAJ. Signal transduction pathway activated by Toll-like receptors. Mod Asp Immunobiol. 2002;2:169–75. [Google Scholar]
- 118.O’Neill LO. The Toll/interleukin-1 receptor domain: A molecular switch for inflammation and host defence. Biochem Soc Trans. 2000;28:557–63. doi: 10.1042/bst0280557. [DOI] [PubMed] [Google Scholar]
- 119.Lakhani SA, Bogue CW. Toll-like receptor signaling in sepsis. Curr Opin Pediatr. 2003;15:278–82. doi: 10.1097/00008480-200306000-00009. [DOI] [PubMed] [Google Scholar]
- 120.Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Lab Invest. 2006;86:9–22. doi: 10.1038/labinvest.3700366. [DOI] [PubMed] [Google Scholar]
- 121.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–51. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 122.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 123.Qureshi ST, Lariviere L, Levegue G, Clermont S, Moore KJ, Gros P, et al. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–25. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zingarelli B. Nuclear factor-kappaB. Crit Care Med. 2005;33:S414–S6. doi: 10.1097/01.ccm.0000186079.88909.94. [DOI] [PubMed] [Google Scholar]
- 125.Ghosh S, May MJ, Koop EB. NF-kappaB and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 126.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 127.Hayden MS, West AP, Ghosh S. SnapShot: NF-kappaB signaling pathways. Cell. 2006;127:1286–7. doi: 10.1016/j.cell.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 128.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 129.Mesotten D, Vanhorebeek I, Van den Berghe G. The altered adrenal axis and treatment with glucocorticoids during critical illness. Nat Clin Pract Endocrinol Metab. 2008;4:496–505. doi: 10.1038/ncpendmet0921. [DOI] [PubMed] [Google Scholar]
- 130.Group. VASSCS. Effect of high-dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. N Engl J Med. 1987;317:659–65. doi: 10.1056/NEJM198709103171102. [DOI] [PubMed] [Google Scholar]
- 131.Bone RC, Fisher CJ, Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987;317:653–8. doi: 10.1056/NEJM198709103171101. [DOI] [PubMed] [Google Scholar]
- 132.Lefering R, Neugebauer EA. Steroid controversy in sepsis and septic shock: A meta-analysis. Crit Care Med. 1995;23:1294–303. doi: 10.1097/00003246-199507000-00021. [DOI] [PubMed] [Google Scholar]
- 133.Cronin L, Cook DJ, Carlet J, Heyland DK, King D, Lansang MA, et al. Corticosteroid treatment for sepsis: A critical appraisal and meta-analysis of the literature. Crit Care Med. 1995;23:1430–9. doi: 10.1097/00003246-199508000-00019. [DOI] [PubMed] [Google Scholar]
- 134.Zimmerman JJ. A history of adjunctive glucocorticoid treatment for pediatric sepsis: Moving beyond steroid pulp fiction toward evidence-based medicine. Pediatr Crit Care Med. 2007;8:530–9. doi: 10.1097/01.PCC.0000288710.11834.E6. [DOI] [PubMed] [Google Scholar]
- 135.Bollaert PE, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A. Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit Care Med. 1998;26:645–50. doi: 10.1097/00003246-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 136.Briegel J, Forst H, Haller M, Schelling G, Kilger E, Kuprat G, et al. Stress dose of hydrocortisone reverse hyperdynamic septic shock: A prospective, randomized, double-blind, single-center study. Crit Care Med. 1999;27:723–32. doi: 10.1097/00003246-199904000-00025. [DOI] [PubMed] [Google Scholar]
- 137.O’Reilly SM, Moynagh PN. Regulation of Toll-like receptor 4 signaling by A20 zinc finger protein. Biochem Biophys Res Comm. 2003;303:586–93. doi: 10.1016/s0006-291x(03)00389-9. [DOI] [PubMed] [Google Scholar]
- 138.Wullaert A, Verstrepen L, Van Huffel C, Adib-Conquy M, Cornelis S, Kreike M, et al. LIND/ABIN-3 is a novel lipopolysaccharide-inducible inhibitor of NF-kappaB activation. J Biol Chem. 2007;282:81–90. doi: 10.1074/jbc.M607481200. [DOI] [PubMed] [Google Scholar]
- 139.Gilchrist M, Thorsson V, Li B, Rust AG, Korb M, Roach JC, et al. Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature. 2006;441:173–8. doi: 10.1038/nature04768. [DOI] [PubMed] [Google Scholar]
- 140.Burns K, Janssens S, Brissoni B, Olivos N, Beyaert R, Tschopp J. Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J Exp Med. 2003;197:263–8. doi: 10.1084/jem.20021790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang Y, Tang Y, Teng L, Wu Y, Zhao X, Pei G. Association of beta-arrestin and TRAF6 negatively regulates Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2006;7:139–47. doi: 10.1038/ni1294. [DOI] [PubMed] [Google Scholar]
- 142.Iwami KI, Matsuguchi T, Masuda A, Kikuchi T, Musikachareoen T, Yoshikai Y. Cutting edge: Naturally occurring soluble form of mouse Toll-like receptor 4 inhibits lipopolysaccharide signaling. J Immunol. 2000;165:6682–6. doi: 10.4049/jimmunol.165.12.6682. [DOI] [PubMed] [Google Scholar]
- 143.Sweet MJ, Leung BP, Kang D, Sogaard M, Schulz K, Trajkovic V, et al. A novel pathway regulating lipopolysaccharide-induced shock by ST2/T1 via inhibition of Toll-like receptor 4 expression. J Immunol. 2001;166:6633–9. doi: 10.4049/jimmunol.166.11.6633. [DOI] [PubMed] [Google Scholar]
- 144.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–3. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 145.Divanovic S, Trompette A, Petiniot LK, Allen JL, Flick LM, Belkaid Y, et al. Regulation of TLR4 signaling and the host interface with pathogens and danger: The role of RP105. J Immunol. 2007;82:265–71. doi: 10.1189/jlb.0107021. [DOI] [PubMed] [Google Scholar]
- 146.Wald D, Qin J, Zhao Z, Qian Y, Naramura M, Tian L, et al. SIGIRR, a negative regulator of Toll-like receptor-interleukin-1 receptor signaling. Nat Immunol. 2003;4:920–7. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- 147.Qin J, Qian Y, Grace C, Li X. SIGIRR inhibits interleukin-1 receptor- and toll-like receptor 4-mediated signaling through different mechanisms. J Biol Chem. 2005;280:25233–41. doi: 10.1074/jbc.M501363200. [DOI] [PubMed] [Google Scholar]
- 148.Diehl GE, Yue HY, Hsieh K, Kuang AA, Ho M, Morici LA, et al. TRAIL-R as a negative regulator of innate immune cell responses. Immunity. 2004;21:877–89. doi: 10.1016/j.immuni.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 149.Cooper JT, Stroka DM, Brostjan C, Palmetschofer A, Bach FH, Ferran C. A20 blocks endothelial cell activation through a NF-kappaB-dependent mechanism. J Biol Chem. 1996;271:18068–73. doi: 10.1074/jbc.271.30.18068. [DOI] [PubMed] [Google Scholar]
- 150.Whitmore MM, Iparraguirre A, Kubelka L, Weninger W, Hai T, Williams BR. Negative regulation of TLR-signaling pathways by activating transcription factor-3. J Immunol. 2007;179:3622–30. doi: 10.4049/jimmunol.179.6.3622. [DOI] [PubMed] [Google Scholar]
- 151.Fan H, Luttrell LM, Tempel GE, Senn JJ, Halushka PV, Cook JA. Beta-arrestins 1 and 2 differentially regulate LPS-induced signaling and proinflammatory gene expression. Mol Immunol. 2007;44:3092–9. doi: 10.1016/j.molimm.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shinohara H, Inoue A, Toyama-Sorimachi N, Nagai Y, Yasuda T, Suzuki H, et al. Dok-1 and Dok-2 are negative regulators of lipopolysaccharide-induced signaling. J Exp Med. 2005;201:333–9. doi: 10.1084/jem.20041817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kobayashi K, Hernandez LD, Galan JE, Janeway CAJ, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 154.Williams KL, Lich JD, Duncan JA, Reed W, Rallabhandi P, Moore C, et al. The CATERPILLAR protein monarch-1 is an antagonist of toll-like receptor, tumor necrosis factor alpha, and Mycobacterium tuberculosis-induced proinflammatory signals. J Biol Chem. 2005;280:39914–24. doi: 10.1074/jbc.M502820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cole AM, Waring AJ. The role of defensins in lung biology and therapy. Am J Respir Med. 2002;1:249–59. doi: 10.1007/BF03256616. [DOI] [PubMed] [Google Scholar]
- 156.Beisswenger C, Bals R. Antimicrobial peptides in lung inflammation. Chem Immunol Allergy. 2005;86:55–71. doi: 10.1159/000086651. [DOI] [PubMed] [Google Scholar]
- 157.Hiemstra PS. The role of epithelial beta-defensins and cathelicidins in host defense of the lung. Exp Lung Res. 2007;33:537–42. doi: 10.1080/01902140701756687. [DOI] [PubMed] [Google Scholar]
- 158.Giacometti A, Cirioni O, Ghiselli R, Mocchegiani F, Silvestri C, Orlando F, et al. Amphibian peptides prevent endotoxemia and bacterial translocation in bile duct-ligated rats. Crit Care Med. 2006;34:2415–20. doi: 10.1097/01.CCM.0000231879.11963.EB. [DOI] [PubMed] [Google Scholar]
- 159.Mangoni ML, Marcellini HG, Simmaco M. Biological characterization and modes of action of temporins and bombinins H, multiple forms of short and mildly cationic anti-microbial peptides from amphibian skin. J Pept Sci. 2007;13:603–13. doi: 10.1002/psc.853. [DOI] [PubMed] [Google Scholar]
- 160.Cirioni O, Ghiselli R, Orlando F, Silvestri C, De Luca S, Salzano AM, et al. Efficacy of the amphibian peptide distinctin in a neutropenic mouse model of staphylococal sepsis. Crit Care Med. 2008;36:2629–33. doi: 10.1097/CCM.0b013e318184430d. [DOI] [PubMed] [Google Scholar]
- 161.Andra J, Gutsmann T, Garidel P, Brandenburg K. Mechanisms of endotoxin neutralization by synthetic cationic compounds. J Endotoxin Res. 2006;12:261–77. doi: 10.1179/096805106X118852. [DOI] [PubMed] [Google Scholar]
- 162.Bosshart H, Heinzelmann M. Targeting bacterial endotoxin: Two sides of a coin. Ann N Y Acad Sci. 2007;1096:1–17. doi: 10.1196/annals.1397.064. [DOI] [PubMed] [Google Scholar]
- 163.Arana Mde J, Vallespi MG, Chinea G, Vallespi GV, Rodriguez-Alonso I, Garay HE, et al. Inhibition of LPS-responses by synthetic peptides derived from LBP associates with the ability of the peptides to block LBP-LPS interactions. J Endotoxin Res. 2003;9:281–91. doi: 10.1179/096805103225002520. [DOI] [PubMed] [Google Scholar]
- 164.Haziot A, Rong GW, Lin XY, Silver J, Goyert SM. Recombinant soluble CD14 prevents mortality in mice treated with endotoxin (lipopolysaccharide) J Immunol. 1995;154:6529–32. [PubMed] [Google Scholar]
- 165.Reinhart K, Gluck T, Ligtenberg J, Tschaikowsky K, Bruining A, Bakker J, et al. CD14 receptor occupancy in severe sepsis: Results of a phase I clinical trial with a recombinant chimeric CD14 monoclonal antibody (IC14) Crit Care Med. 2004;32:1100–8. doi: 10.1097/01.ccm.0000124870.42312.c4. [DOI] [PubMed] [Google Scholar]
- 166.Leturcq DJ, Moriart AM, Ulevitch RJ, Tobias PS, Mathison JC inventors. Methods and compositions for inhibiting CD14 mediated cell activation. 2006 [Google Scholar]
- 167.Wendel M, Paul R, Heller AR. Lipoproteins in inflammation and sepsis. II. Clinical aspects. Intensive Care Med. 2007;33:25–35. doi: 10.1007/s00134-006-0433-x. [DOI] [PubMed] [Google Scholar]
- 168.Wu A, Hinds CJ, Thiemermann C. High-density lipoproteins in sepsis and septic shock: Metabolism, actions, and therapeutic applications. Shock. 2004;21:210–21. doi: 10.1097/01.shk.0000111661.09279.82. [DOI] [PubMed] [Google Scholar]
- 169.Wheeler DS, Wong HR. Heat shock response and acute lung injury. Free Radic Biol Med. 2007;42:1–14. doi: 10.1016/j.freeradbiomed.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: Endogenous danger signaling. Mol Med. 2008;14:476–84. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 172.Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Matthews RC, Rigg G, Hodgetts S, Carter T, Chapman C, Gregory C, et al. Preclinical assessment of the efficacy of mycograb, a human recombinant antibody against fungal HSP90. Antimicrob Agents Chemother. 2003;47:2208–16. doi: 10.1128/AAC.47.7.2208-2216.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pachl J, Svoboda P, Jacobs F, Vandewoude K, van der Hoven B, Spronk P, et al. A randomized, blinded, multicenter trial of lipid-associated amphotericin B alone versus in combination with an antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin Infect Dis. 2006;42:14014–1413. doi: 10.1086/503428. [DOI] [PubMed] [Google Scholar]
- 175.Sutherland A, Ellis D. Treatment of a critically ill child with disseminated Candida glabrata with a recombinant human antibody specific for fungal heat shock protein 90 and liposomal amphotericin B, caspofungin, and voriconazole. Pediatr Crit Care Med. 2008;9:e23–e5. doi: 10.1097/PCC.0b013e31817286e8. [DOI] [PubMed] [Google Scholar]
- 176.Bagatell R, Gore L, Egorin MJ, Ho R, Heller G, Boucher N, et al. Phase I pharmacokinetic and pharmacodynamic study of 17-N-allylamino-17-demethoxyygeldanamycin in pediatric patients with recurrent or refractory solid tumors: A pediatric oncology experimental therapeutics investigators consortium study. Clin Cancer Res. 2007;13:1783–8. doi: 10.1158/1078-0432.CCR-06-1892. [DOI] [PubMed] [Google Scholar]
- 177.Weigel BJ, Blaney SM, Reid JM, Safgren SL, Bagatell R, Kersey J, et al. A phase I study of 17-allylaminogeldanamycin in relasped/refractory pediatric patients with solid tumors: a Children’s Oncology Group study. Clin Cancer Res. 2007;13:1789–93. doi: 10.1158/1078-0432.CCR-06-2270. [DOI] [PubMed] [Google Scholar]
- 178.Neckers L, Neckers K. Heat-shock protein 90 inhibitors as novel cancer chemotherapeutic agents. Expert Opin Emerg Drugs. 2002 Oct;7(2):277–88. doi: 10.1517/14728214.7.2.277. [DOI] [PubMed] [Google Scholar]
- 179.Vanags D, Williams B, Johnson B, Hall S, Nash P, Taylor A, et al. Therapeutic efficacy and safety of chaperonin 10 in patients with rheumatoid arthritis: A double-blind randomised trial. Lancet. 2006;368:855–63. doi: 10.1016/S0140-6736(06)69210-6. [DOI] [PubMed] [Google Scholar]
- 180.Raz I, Avron A, Tamir M, Metzger M, Symer L, Eldor R, et al. Treatment of new-onset type 1 diabetes with peptide DiaPep277 is safe and associated with preserved beta-cell function: Extension of a randomized, double-blind, phase II trial. Diabetes Metab Res Rev. 2007;23:292–8. doi: 10.1002/dmrr.712. [DOI] [PubMed] [Google Scholar]
- 181.Lynn M, Rossignol DP, Wheeler JL, Kao RJ, Perdomo CA, Noveck R, et al. Blocking of responses to endotoxin by E5564 in healthy volunteers with experimental endotoxemia. J Infect Dis. 2003;187:631–9. doi: 10.1086/367990. [DOI] [PubMed] [Google Scholar]
- 182.Rossignol DP, Lynn M. Anatagonism of in vivo and ex vivo response to endotoxin by E5564, a synthetic lipid A analogue. J Endotoxin Res. 2002;8:483–8. doi: 10.1179/096805102125001127. [DOI] [PubMed] [Google Scholar]
- 183.Hawkins LD, Christ WJ, Rossignol DP. Inhibition of endotoxin response by synthetic TLR4 antagonists. Curr Top Med Chem. 2004;4:1147–71. doi: 10.2174/1568026043388123. [DOI] [PubMed] [Google Scholar]
- 184.Bennet-Guerrero E, Grocott HP, Levy JH, Stierer KA, Hogue CW, Cheung AT, et al. A phase II, double-blind, placebo-controlled, ascending-dose study of eritoran (E5564), a lipid antagonist, in patients undergoing cardiac surgery with cardiopulmonary bypass. Anesth Analg. 2007;104:378–83. doi: 10.1213/01.ane.0000253501.07183.2a. [DOI] [PubMed] [Google Scholar]
- 185.Zingarelli B, Sheehan M, Wong HR. Nuclear factor-kappaB as a therapeutic target in critical care medicine. Crit Care Med. 2003;31:S105–S11. doi: 10.1097/00003246-200301001-00015. [DOI] [PubMed] [Google Scholar]
- 186.Abraham E. Nuclear factor-kappaB and its role in sepsis-associated organ failure. J Infect Dis. 2003;187:S364–S9. doi: 10.1086/374750. [DOI] [PubMed] [Google Scholar]
- 187.D’Acquisto F, May MJ, Ghosh S. Inhibition of nuclear factor kappa B: An emerging theme in anti-inflammatory therapies. Mol Interv. 2002;2:22–35. doi: 10.1124/mi.2.1.22. [DOI] [PubMed] [Google Scholar]
- 188.Jagetia GC, Aggarwal BB. “Spicing up” of the immune system by curcumin. J Clin Immunol. 2007;27:19–35. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- 189.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: From ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–52. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sheehan M, Wong HR, Hake PW, Malhotra V, O’Connor M, Zingarelli B. Parthenolide, an inhibitor of the nuclear factor-kappaB pathway, ameliorates cardiovascular derangement and outcome in endotoxic shock in rodents. Mol Pharmacol. 2002;61:953–63. doi: 10.1124/mol.61.5.953. [DOI] [PubMed] [Google Scholar]
- 191.Sheehan M, Wong HR, Hake PW, Zingarelli B. Parthenolide improves systemic hemodynamics and decreases tissue leukosequestration in rats with polymicrobial sepsis. Crit Care Med. 2003;31:2263–70. doi: 10.1097/01.CCM.0000085186.14867.F7. [DOI] [PubMed] [Google Scholar]
- 192.Sheehan M, Wong HR, Hake PW, Zingarelli B. Protective effects of isohelenin, an inhibitor of nuclear factor kappaB, in endotoxic shock in rats. J Endotoxin Res. 2002;8:99–107. doi: 10.1179/096805102125000236. [DOI] [PubMed] [Google Scholar]
- 193.Wheeler DS, Wheeler WJ. The medicinal chemistry of tea. Drug Dev Res. 2004;61:45–65. [Google Scholar]
- 194.Yang F, de Villiers WJ, McClain CJ, Varilek GW. Green tea polyphenols block endotoxin-induced tumor necrosis factor-production and lethality in a murine model. J Nutr. 1998;128:2334–40. doi: 10.1093/jn/128.12.2334. [DOI] [PubMed] [Google Scholar]
- 195.Chen PC, Wheeler DS, Malhotra V, Odoms K, Denenberg AG, Wong HR. A green tea-derived polyphenol, epigallocatechin-3-gallate, inhibits IkappaB kinase activation and IL-8 gene expression in respiratory epithelium. Inflammation. 2002;26:233–41. doi: 10.1023/A:1019718718977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wheeler DS, Catravas JD, Odoms K, Denenberg A, Malhotra V, Wong HR. Epigallocatechin-3-gallate, a green tea-derived polyphenol, inhibits IL-1-beta-dependent proinflammatory signal transduction in cultured respiratory epithelial cells. J Nutr. 2004;134:1039–44. doi: 10.1093/jn/134.5.1039. [DOI] [PubMed] [Google Scholar]
- 197.Aneja R, Odoms K, Denenberg AG, Wong HR. Theaflavin, a black tea extract, is a novel anti-inflammatory compound. Crit Care Med. 2004;32:2097–103. doi: 10.1097/01.ccm.0000142661.73633.15. [DOI] [PubMed] [Google Scholar]
- 198.Uwe S. Anti-inflammatory interventions and NF-kappaB signaling: Potential applications and risks. Biochem Pharmacol. 2008;75:1567–79. doi: 10.1016/j.bcp.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 199.Alvira CM, Abate A, Yang G, Dennery PA, Rabinovitch M. Nuclear factor-kappaB activation in neonatal mouse lung protects against lipopolysaccharide-induced inflammation. Am J Respir Crit Care Med. 2007;175:805–15. doi: 10.1164/rccm.200608-1162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gadjeva M, Tomczak MF, Zhang M, Wang YY, Dull K, Rogers AB, et al. A role for NF-kappaB subunits p50 and p65 in the inhibition of lipopolysaccharide-induced shock. J Immunol. 2004;173:5786–93. doi: 10.4049/jimmunol.173.9.5786. [DOI] [PubMed] [Google Scholar]
- 201.Li X, Cui X, Li Y, Fitz Y, Hsu L, Eichacker PQ. Parthenolide has limited effects on nuclear factor-kappaB increases and worsens survival in lipopolysacchardie-challenged C57BL/6J mice. Cytokine. 2006;33:299–308. doi: 10.1016/j.cyto.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 202.Su J, Li X, Cui X, Yi L, Fitz Y, Hsu L, et al. Ethyl pyruvate decreased early nuclear factor-kappB levels but worsened survival in lipopolysaccharide-challenged mice. Crit Care Med. 2008;36:1059–67. doi: 10.1097/CCM.0B013E318164403B. [DOI] [PubMed] [Google Scholar]
- 203.Marik PE, Zaloga GP. Hypothermia and cytokines in septic shock. Norasept II Study Investigators. North American study of the safety and efficacy of murine monoclonal antibody to tumor necrosis factor for the treatment of septic shock. Intensive Care Med. 2000 Jun;26(6):716–21. doi: 10.1007/s001340051237. [DOI] [PubMed] [Google Scholar]
- 204.Abraham E, Anzueto A, Gutierrez G, Tessler S, San Pedro G, Wunderink R, et al. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet. 1998 Mar 28;351(9107):929–33. [PubMed] [Google Scholar]
- 205.Fisher CJ, Jr, Agosti JM, Opal SM, Lowry SF, Balk RA, Sadoff JC, et al. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N Engl J Med. 1996 Jun 27;334(26):1697–702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 206.Pittet D, Harbarth S, Suter PM, Reinhart K, Leighton A, Barker C, et al. Impact of immunomodulating therapy on morbidity in patients with severe sepsis. Am J Respir Crit Care Med. 1999 Sep;160(3):852–7. doi: 10.1164/ajrccm.160.3.9809033. [DOI] [PubMed] [Google Scholar]
- 207.Butty VL, Roux-Lombard P, Garbino J, Dayer JM, Ricou B. Anti-inflammatory response after infusion of p55 soluble tumor necrosis factor receptor fusion protein for severe sepsis. Eur Cytokine Netw. 2003 Jan-Mar;14(1):15–9. [PubMed] [Google Scholar]
- 208.Reinhart K, Wiegand-Lohnert C, Grimminger F, Kaul M, Withington S, Treacher D, et al. Assessment of the safety and efficacy of the monoclonal anti-tumor necrosis factor antibody-fragment, MAK 195F, in patients with sepsis and septic shock: a multicenter, randomized, placebo-controlled, dose-ranging study. Crit Care Med. 1996 May;24(5):733–42. doi: 10.1097/00003246-199605000-00003. [DOI] [PubMed] [Google Scholar]
- 209.McCloskey RV, Straube RC, Sanders C, Smith SM, Smith CR. Treatment of septic shock with human monoclonal antibody HA-1A. A randomized, double-blind, placebo-controlled trial. CHESS Trial Study Group. Ann Intern Med. 1994 Jul 1;121(1):1–5. doi: 10.7326/0003-4819-121-1-199407010-00001. [DOI] [PubMed] [Google Scholar]
- 210.The French National Registry of HA-1A (Centoxin) in septic shock. A cohort study of 600 patients. The National Committee for the Evaluation of Centoxin. Arch Intern Med. 1994 Nov 14;154(21):2484–91. [PubMed] [Google Scholar]
- 211.Kett DH, Quartin AA, Sprung CL, Fisher CJ, Jr, Pena MA, Heard SO, et al. An evaluation of the hemodynamic effects of HA-1A human monoclonal antibody. Crit Care Med. 1994 Aug;22(8):1227–34. doi: 10.1097/00003246-199408000-00005. [DOI] [PubMed] [Google Scholar]
- 212.van Hout BA, Birnie E, Redekop WK, Lorijn RH, Bossuyt PM, Rutten FF. Cost-effectiveness of HA-1A treatment for patients with sepsis. Prog Clin Biol Res. 1994;388:435–43. [PubMed] [Google Scholar]
- 213.Schulman KA, Glick HA, Rubin H, Eisenberg JM. Cost-effectiveness of HA-1A monoclonal antibody for gram-negative sepsis. Economic assessment of a new therapeutic agent. Jama. 1991 Dec 25;266(24):3466–71. [PubMed] [Google Scholar]
- 214.Quintiliani R, Cooper B, Maderazo E, Nightingale C. Comparison of the efficacy, safety, and therapeutic usage of HA-1A and E5 in the treatment of gram-negative sepsis. Hosp Formul. 1992 Jan;27(1):49–52. 4, 7–60. [PubMed] [Google Scholar]
- 215.Bone RC, Balk RA, Fein AM, Perl TM, Wenzel RP, Reines HD, et al. A second large controlled clinical study of E5, a monoclonal antibody to endotoxin: results of a prospective, multicenter, randomized, controlled trial. The E5 Sepsis Study Group. Crit Care Med. 1995 Jun;23(6):994–1006. doi: 10.1097/00003246-199506000-00003. [DOI] [PubMed] [Google Scholar]
- 216.Angus DC, Birmingham MC, Balk RA, Scannon PJ, Collins D, Kruse JA, et al. E5 murine monoclonal antiendotoxin antibody in gram-negative sepsis: a randomized controlled trial. E5 Study Investigators. Jama. 2000 Apr 5;283(13):1723–30. doi: 10.1001/jama.283.13.1723. [DOI] [PubMed] [Google Scholar]
- 217.Greenman RL, Schein RM, Martin MA, Wenzel RP, MacIntyre NR, Emmanuel G, et al. A controlled clinical trial of E5 murine monoclonal IgM antibody to endotoxin in the treatment of gram-negative sepsis. The XOMA Sepsis Study Group. Jama. 1991 Aug 28;266(8):1097–102. [PubMed] [Google Scholar]
- 218.Fisher CJ, Jr, Opal SM, Lowry SF, Sadoff JC, LaBrecque JF, Donovan HC, et al. Role of interleukin-1 and the therapeutic potential of interleukin-1 receptor antagonist in sepsis. Circ Shock. 1994;44(1):1–8. [PubMed] [Google Scholar]
- 219.Lopez A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004 Jan;32(1):21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- 220.Lauterbach R, Pawlik D, Kowalczyk D, Ksycinski W, Helwich E, Zembala M. Effect of the immunomodulating agent, pentoxifylline, in the treatment of sepsis in prematurely delivered infants: a placebo-controlled, double-blind trial. Crit Care Med. 1999 Apr;27(4):807–14. doi: 10.1097/00003246-199904000-00042. [DOI] [PubMed] [Google Scholar]
- 221.LaRosa SP, Opal SM. Tissue factor pathway inhibitor and antithrombin trial results. Crit Care Clin. 2005 Jul;21(3):433–48. doi: 10.1016/j.ccc.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 222.Freeman BD, Zehnbauer BA, Buchman TG. A meta-analysis of controlled trials of anticoagulant therapies in patients with sepsis. Shock. 2003 Jul;20(1):5–9. doi: 10.1097/01.shk.0000068327.26733.10. [DOI] [PubMed] [Google Scholar]
- 223.Fourrier F, Chopin C, Huart JJ, Runge I, Caron C, Goudemand J. Double-blind, placebo-controlled trial of antithrombin III concentrates in septic shock with disseminated intravascular coagulation. Chest. 1993 Sep;104(3):882–8. doi: 10.1378/chest.104.3.882. [DOI] [PubMed] [Google Scholar]
- 224.Eisele B, Lamy M. Clinical experience with antithrombin III concentrates in critically ill patients with sepsis and multiple organ failure. Semin Thromb Hemost. 1998;24(1):71–80. doi: 10.1055/s-2007-995825. [DOI] [PubMed] [Google Scholar]
- 225.Warren BL, Eid A, Singer P, Pillay SS, Carl P, Novak I, et al. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. Jama. 2001;286(15):1869–78. doi: 10.1001/jama.286.15.1869. [DOI] [PubMed] [Google Scholar]
- 226.Inthorn D, Hoffmann JN, Hartl WH, Muhlbayer D, Jochum M. Antithrombin III supplementation in severe sepsis: beneficial effects on organ dysfunction. Shock. 1997;8(5):328–34. doi: 10.1097/00024382-199711000-00003. [DOI] [PubMed] [Google Scholar]
- 227.Ilias W, List W, Decruyenaere J, Lignian H, Knaub S, Schindel F, et al. Antithrombin III in patients with severe sepsis: a pharmacokinetic study. Intensive Care Med. 2000 Jun;26(6):704–15. doi: 10.1007/s001340051236. [DOI] [PubMed] [Google Scholar]
- 228.Eisele B, Lamy M, Thijs LG, Keinecke HO, Schuster HP, Matthias FR, et al. Antithrombin III in patients with severe sepsis. A randomized, placebo- controlled, double-blind multicenter trial plus a meta-analysis on all randomized, placebo-controlled, double-blind trials with antithrombin III in severe sepsis. Intensive Care Med. 1998;24(7):663–72. doi: 10.1007/s001340050642. [DOI] [PubMed] [Google Scholar]
- 229.Abraham E, Reinhart K, Opal S, Demeyer I, Doig C, Rodriguez AL, et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: A randomized, controlled trial. JAMA. 2003;290:238–47. doi: 10.1001/jama.290.2.238. [DOI] [PubMed] [Google Scholar]
- 230.Abraham E, Reinhart K, Svoboda P, Seibert A, Olthoff D, Dal Nogare A, et al. Assessment of the safety of recombinant tissue factor pathway inhibitor in patients with severe sepsis: a multicenter, randomized, placebo- controlled, single-blind, dose escalation study. Crit Care Med. 2001;29(11):2081–9. doi: 10.1097/00003246-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 231.Schuster DP, Metzler M, Opal S, Lowry S, Balk R, Abraham E, et al. Recombinant platelet-activating factor acetylhydrolase to prevent acute respiratory distress syndrome and mortality in severe sepsis: Phase IIb, multicenter, randomized, placebo-controlled, clinical trial. Crit Care Med. 2003 Jun;31(6):1612–9. doi: 10.1097/01.CCM.0000063267.79824.DB. [DOI] [PubMed] [Google Scholar]
- 232.Opal S, Laterre PF, Abraham E, Francois B, Wittebole X, Lowry S, et al. Recombinant human platelet-activating factor acetylhydrolase for treatment of severe sepsis: results of a phase III, multicenter, randomized, double-blind, placebo-controlled, clinical trial. Crit Care Med. 2004 Feb;32(2):332–41. doi: 10.1097/01.CCM.0000108867.87890.6D. [DOI] [PubMed] [Google Scholar]
- 233.Zeiher BG, Steingrub J, Laterre PF, Dmitrienko A, Fukiishi Y, Abraham E. LY315920NA/S-5920, a selective inhibitor of group IIA secretory phospholipase A2, fails to improve clinical outcome for patients with severe sepsis. Crit Care Med. 2005 Aug;33(8):1741–8. doi: 10.1097/01.ccm.0000171540.54520.69. [DOI] [PubMed] [Google Scholar]
- 234.Jenkins JK, Carey PD, Byrne K, Sugerman HJ, Fowler AA., 3rd Sepsis-induced lung injury and the effects of ibuprofen pretreatment. Analysis of early alveolar events via repetitive bronchoalveolar lavage. Am Rev Respir Dis. 1991;143(1):155–61. doi: 10.1164/ajrccm/143.1.155. [DOI] [PubMed] [Google Scholar]
- 235.Haupt MT, Jastremski MS, Clemmer TP, Metz CA, Goris GB. Effect of ibuprofen in patients with severe sepsis: a randomized, double-blind, multicenter study. The Ibuprofen Study Group. Crit Care Med. 1991 Nov;19(11):1339–47. doi: 10.1097/00003246-199111000-00006. [DOI] [PubMed] [Google Scholar]