Abstract
Objective
To advance knowledge regarding strategies for treating selective serotonin reuptake inhibitor (SSRI)–resistant depression in adolescents, we conducted a randomized controlled trial evaluating alternative treatment strategies. In primary analyses, cognitive-behavioral therapy (CBT) combined with medication change was associated with higher rates of positive response to short-term (12-week) treatment than medication alone. This study examines predictors and moderators of treatment response, with the goal of informing efforts to match youths to optimal treatment strategies.
Method
Youths who had not improved during an adequate SSRI trial (N = 334) were randomized to an alternative SSRI, an alternative SSRI plus CBT, venlafaxine, or venlafaxine plus CBT. Analyses examined predictors and moderators of treatment response.
Results
Less severe depression, less family conflict, and absence of nonsuicidal self-injurious behavior predicted better treatment response status. Significant moderators of response to CBT + medication (combined) treatment were number of comorbid disorders and abuse history; hopelessness was marginally significant. The CBT/combined treatment superiority over medication alone was more evident among youths who had more comorbid disorders (particularly attention-deficit/hyperactivity disorder and anxiety disorders), no abuse history, and lower hopelessness. Further analyses revealed a stronger effect of combined CBT + medication treatment among youths who were older and white and had no nonsuicidal self-injurious behavior and longer prestudy pharmacotherapy.
Conclusions
Combined treatment with CBT and antidepressant medication may be more advantageous for adolescents whose depression is comorbid with other disorders. Given the additional costs of adding CBT to medication, consideration of moderators in clinical decision making can contribute to a more personalized and effective approach to treatment.
Keywords: depression, adolescents, treatment-resistant, cognitive-behavioral therapy
Despite major advances in treatments for adolescent depression, roughly 40% of adolescents with major depression do not adequately improve with first-step treatments.1 The high level of impairment, morbidity, and suicide risk associated with inadequately treated depression in adolescents underscores the critical need for research to guide treatment strategies when first-step treatments fail.1
The Treatment of Resistant Depression in Adolescents (TORDIA) study is the first study to examine second-step treatment strategies for depressed adolescents. Because medication is often easier to access than evidence-based psychosocial treatments and selective serotonin reuptake inhibitors (SSRIs) are a predominant treatment strategy,1 TORDIA focused on youths with SSRI treatment-resistant depression. Results indicated that, at the end of 12 weeks of short-term treatment, youths receiving combined cognitive-behavioral therapy (CBT) and a medication switch (either an alternative SSRI or venlafaxine) were significantly more likely to show a positive (versus inadequate) treatment response, as compared with a switch in medication alone.2 The two medication strategies (SSRI versus venlafaxine) yielded similar response rates.2
Although the TORDIA results support the use of combined CBT and medication treatment as a second-step treatment, CBT is often unavailable and increases treatment costs.3 Moreover, TORDIA response rates were still 54.8% for CBT/combined treatment and 40.5% for medication switch alone, underscoring the need for further clarification of prognostic indicators.
A key question for the practicing clinician is how to select the optimal treatment strategy for an individual youth based on an initial pretreatment evaluation. Clinical care would be advanced by knowledge regarding predictor variables (variables that predict a positive versus negative response to any treatment and provide general prognostic information) and moderator variables (variables that indicate which youths are most likely to benefit from one treatment versus another) and can be used clinically to select an optimal treatment strategy for a particular youth given his or her baseline characteristics/circumstances.4 The identification of predictors and moderators of treatment response is particularly critical for treatment nonresponders because these youths and families have already experienced unremitting depression that can lead to demoralization and treatment nonadherence.
Despite limited information on populations of treatment nonresponders, some information is available on predictors and moderators of response to initial (first-step) depression treatments. In the Treatment of Adolescent Depression Study (TADS), which compared response to placebo, CBT, fluoxetine, and combined CBT plus fluoxetine, predictors of poor outcome included older age, more chronic depression, severe suicidal ideation, comorbid diagnoses, comorbid anxiety disorder, functional impairment, hopelessness, and lower expectancies for treatment benefits.5 In other studies, poorer response to fluoxetine was predicted by more severe depression, comorbidity and family discord.6 Poorer response to psychosocial treatment has been predicted by measures of depression severity, comorbid attention-deficit/hyperactivity disorder (ADHD), hopelessness, poorer coping skills, impairment, and family dysfunction.7–10 Moderators in the TADS were as follows: depression severity, with mild to moderately depressed youths having a significantly better response to combined CBT and fluoxetine versus fluoxetine alone, whereas the combination was not superior to fluoxetine alone in more severely depressed participants; family income, with youths from high-income families (>$75,000 per year) showing the greatest benefit from CBT relative to placebo; and depressive cognitive distortions, with higher levels of cognitive distortion associated with more benefits from the addition of CBT to fluoxetine versus fluoxetine alone.5 Other studies of moderators of CBT response have found that CBT was more beneficial, relative to supportive therapy, among youths with suicidal ideation and comorbid anxiety; whereas abuse histories and maternal depression were associated with CBT being no better than comparison treatments.8,11,12
This is the first study to examine predictors and moderators of treatment response among youths with SSRI treatment–resistant depression. Candidate explanatory variables were conceptualized within a stress-vulnerability theoretical model that emphasizes the interaction of individual vulnerability factors and stress/adverse environmental circumstances in predicting outcome and treatment response. Therefore, we examined both individual-level variables posited to reflect vulnerability factors (e.g., depression severity, hopelessness, other comorbid/co-occurring disorders, suicidality/self-injurious behavior, functioning/impairment, demographics) and environmental stresses (e.g., abuse history, family conflict). We test two major hypotheses. First, to be consistent with the literature on predictors of outcome for first-step SSRI and CBT treatments, we hypothesized that favorable treatment response versus inadequate response will be associated with lower levels of baseline depression severity, chronicity, comorbidity, functional impairment, and family stress.5–10 Second, to be consistent with the results of TADS5 and the NIMH Collaborative Study13,14 that found CBT to have weaker effects among more severely depressed patients as a first-step treatment, we predict that the benefits of adding CBT in the combined CBT plus medication switch condition will be lower in more severely depressed youths in our treatment-resistant youths. We also conduct exploratory analyses examining other demographic, clinical, and service use variables.
Method
Because the study design and 12-week outcome results are described elsewhere,2 we provide only a brief overview below. The study was reviewed by each site's local institutional review board. In accordance with local institutional review board regulations, all subjects gave informed assent/consent (as appropriate), and parents gave informed consent.
Participants
Participants were 334 adolescents (drawn from 6 study sites) aged 12 to 18 years who were in active treatment with an SSRI for moderate to severe DSM-IV15 major depressive disorder. Additional inclusion criteria were as follows: significant depression, indexed by a Children's Depression Rating Scale-Revised (CDRS-R)16 total score of 40 or higher and a Clinical Global Impression-Severity subscale of 4 or higher (moderate or high severity)17; and 6 weeks of “adequate” SSRI treatment (a dose equivalent of 20 mg of fluoxetine) or longer, plus 2 weeks at a dose equivalent of 40 mg of fluoxetine (if tolerated). Exclusion criteria were as follows: 2 or more previous “adequate” SSRI trials; previous nonresponse to venlafaxine (≥4 weeks at a dose of ≥150 mg); previous CBT trial, with more than 6 sessions; on medications with psychoactive properties, excluding some study-allowed medications at stable doses (≥12 weeks' duration); diagnoses of bipolar I or II, psychosis, autism, eating disorders, substance abuse or dependence, and hypertension (diastolic blood pressure ≥90); and female subjects who were pregnant, breastfeeding, or not reliably using contraception.
The sample had a mean age of 16 years (SD 1.6 years), 70% were female subjects, and 84% were white (5% Hispanic/Latino, 5% biracial, 3% black, 2% Asian, and 2% other). The median annual family income was $61,000 (SD $55,823). The subjects had moderately severe and chronic depression (mean CDRS-R 59, SD 10; 56% duration of 2 years or longer). Co-occurring nondepressive diagnoses were observed in 51.7% of the youths: anxiety disorders (38.9%), conduct or oppositional disorders (9.6%), and ADHD (16.6%). Histories of nonsuicidal self-injurious behavior (NSSI) occurred in 36.7% of the youths; 14.9% reported suicide attempts, and 58.5% presented with clinically significant suicidal ideation (Suicide Ideation Questionnaire-JR [SIQ]18 ≥31). Duration of prestudy treatment was a median of 17 weeks for SSRI treatment and a median of 8 sessions in the previous 12 weeks for psychotherapy.
Intake and Enrollment
Participants entered the study for a first assessment, continued on their prestudy medication regimen for another 2 weeks, and were reassessed. At this second baseline assessment, the youths exhibiting continuing SSRI treatment resistance/high levels of depressive symptoms (CDRS-R ≥40, and decrease in CDRS-R scores from assessments 1 to 2 was <30%) were offered enrollment, randomized to treatment condition, and given study treatment.2
Randomization and Treatment
The participants were randomly assigned to one of four conditions: medication therapy with switch to second SSRI, medication therapy with venlafaxine, CBT/combined treatment with a switch to a second SSRI, or CBT/combined treatment with a switch to venlafaxine. Using a variant of Efron's biased coin toss,19 randomization was balanced both within and across sites on incoming treatment medication, co-occurring anxiety, chronic depression (duration ≥24 months), and suicidal ideation (BDI item 9 ≥2). The participants randomized to the SSRI condition were switched to fluoxetine if they were initially treated with paroxetine or paroxetine if they were initially treated with fluoxetine or randomized to either fluoxetine or paroxetine if they were initially treated with citalopram, sertraline, or fluvoxamine. Midway through the study when concerns emerged regarding the efficacy and safety of paroxetine,20 the youths who would have been switched to paroxetine were switched to citalopram. During a 2-week period, the participants were tapered to discontinuation from their initial medication, and the dosage of the study medication was gradually increased to the equivalent of 20 mg of fluoxetine (for SSRIs) or 150 mg of venlafaxine, with an option to increase to the equivalent of 40 mg of fluoxetine or 225 mg of venlafaxine if there was insufficient clinical improvement. As reported previously, treatment groups were similar in their baseline demographic and clinical characteristics and rates of treatment completion. Among the youths receiving CBT/combined treatment, the number of CBT sessions was comparable for the youths in the SSRI and venlafaxine groups. The participants were assessed for 12-week outcome regardless of whether they completed treatment, with follow-up data available on 287 (85.9%) of the participants.2,21
The TORDIA CBT was delivered in a flexible manner, with modules selected based on the case conceptualization, reviewed in biweekly CBT conference calls. The CBT modules targeted cognitive restructuring, behavior activation, emotion regulation, social skills, and problem solving, as well as parent-child sessions to improve support, decrease criticism, and improve family communication and problem solving.2 The youths received 12 to 15 sessions during a 12-week period, 3 to 6 of which included parents (mean 8.3 sessions, median 9 sessions).
Removal from the protocol occurred if participants developed any study exclusionary criteria, were treatment nonadherent (missed ≥3 sessions without notification), or needed/chose to receive treatment prohibited by the protocol.
Assessments
Primary Outcome Variable
As in our previous article,2 the primary outcome for this study was “adequate clinical response” at 12 weeks, defined based on independent evaluator best estimate ratings of depressive symptoms on the CDRS-R interview16 (range 17–113) and clinical improvement on the Clinical Global Impressions-Improvement Subscale (CGI-I; range 1 [very much improved]–7 [very much worse]).17 Adequate clinical response was defined as a CGI-I score of 2 or lower and an improvement in the CDRS-R of 50% or greater; participants not meeting these criteria were classified as nonresponders. Interrater reliability was high: CDRS-R, intraclass correlation = 0.85; 95% confidence interval (CI) 0.80–0.89 (n = 324); CGI-I (≤2 versus >2), κ = 0.85; 95% CI 0.72–0.98; p < .01 (n = 176).
Baseline Measures Used in Analyses
Depression Severity Indicators
Indicators of baseline depression severity included CDRS-R16 and self-reported depressive symptoms on the Beck Depression Inventory (BDI) Depression.22 The chronicity indicator was duration of depressive episode based on the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present Episode and Lifetime version (K-SADS-PL).23
Comorbidity
Co-occurring DSM-IV diagnoses were evaluated using the K-SADS-PL23 and grouped as follows: anxiety disorders (generalized anxiety, separation anxiety, social phobia, panic, agoraphobia, or posttraumatic stress disorder), disruptive behavior disorders (conduct, oppositional), and ADHD. We examined each diagnostic pattern (e.g., whether the youths had co-occurring anxiety, disruptive behavior, and ADHD) and the total number of diagnostic groupings (scale of 0–3) as an index of overall comorbidity. Although youths meeting criteria for substance abuse disorders were excluded, the Drug Use Screening Inventory24 indexed substance use–related impairment.
Suicidality/Self-Injurious Behavior
Severity of suicidal ideation was assessed using the SIQ.18 History of previous suicide attempts and NSSI were assessed using the K-SADS-PL.23 Suicide attempt was defined as “self-harm with actual or inferred intent to die” and coded using the suicide history form.25
Functioning/Impairment
The functional impairment indicator was independent evaluator ratings on the Children's Global Assessment Scale (CGAS)26 with scores ≥ 70 (range 1–100), indicating adequate functioning.
Demographic and Other Variables
Age, sex, race, parental education, and income were assessed by parent and youth report. Hopelessness was measured by the Beck Hopelessness Scale (BHS).27 Family stress was assessed using youth report on the Conflict Behavior Questionnaire.28 Total number of months of prestudy antidepressant medication treatment was assessed using the Child and Adolescent Service Assessment.29
Statistical Analyses
The primary outcome variable was clinical response at week 12. All primary analyses were intent-to-treat and used last observation carried forward. To be consistent with Kraemer et al.,4 predictors were defined as baseline (before randomization) variables that had a main effect on treatment outcome regardless of treatment assignment. Analyses of predictors proceeded in three steps: independent sample t test and Pearson χ2 were conducted to examine which of the candidate explanatory variables were associated with an adequate versus inadequate treatment response; variables significantly associated with outcome were entered into a logistic regression predicting treatment response, controlling for age, sex, race, and site; and logistic regression with a backward stepping procedure was used to identify the most parsimonious set of predictors among the variables that were significant at this second analysis step. All of the terms that were significant in the second analysis step were included in the model and were removed based on their p values in a descending order. Only those variables with p ≤ .10 were maintained in the model.
Moderators, defined as baseline (before randomization) variables that had interactive effects with treatment assignment on outcome,4 were examined using backward stepwise logistic regression models that included the candidate explanatory variable, medication type, CBT/combined, and the interaction terms. A significant treatment by baseline variable interaction with main effects included in the model is indicative of moderation.4 Because these were exploratory analyses, we also report treatment by variable interactions that were significant without the main effect in the model as long as the direction of association did not change once main effects were added.
Presented results include cases with complete data, for the corresponding analysis. Analyses were repeated, imputing missing values using multiple imputations (STATA 9.0, STATACorp LP, College Station, TX), with near-identical results. Analyses were conducted using SPSS 14.0 (SPSS, Chicago, IL). Because of the exploratory nature of these analyses, despite multiple comparisons, α was set at .05 (two-sided). Presented p values require cautious interpretation.
Results
Predictors
Nonresponse (versus an adequate treatment response) was significantly associated with higher baseline depression severity (indexed by CDRS-R, BDI, or episode duration/chronicity) and greater impairment (lower CGAS; Table 1 columns 2 and 3). Nonresponders also reported significantly higher baseline levels of suicidal ideation (SIQ) and hopelessness (BHS), were more likely to have histories of NSSI, and reported more severe family conflict (Conflict Behavior Questionnaire-Adolescent Report [CBQ-A]) and fewer months of preenrollment SSRI medication treatment. Logistic regression adjusting for site, age, sex, and race (Table 1, column 4) confirmed that the likelihood of response significantly increased as scores on baseline BDI, SIQ, BHS, or CBQ-A decreased and CGAS impairment levels decreased (higher CGAS scores). The most parsimonious predictors, along with CBT treatment were as follows: lower baseline scores on the BDI and CBQ-A and the absence of NSSI (Table 2).
TABLE 1.
Predictors of Adequate Versus Inadequate Treatment Response: Comparison of Nonresponders and Responders on Candidate Explanatory Variables and Logistic Regression Models Predicting Treatment Response From Each Explanatory Variablea
| Nonresponders (n = 175), f (%) or Mean ± SD | Responders (na = 159) Odds Ratio, f (%) or Mean ± SD | Total Sample (N = 334) Odds Ratio* (95% Confidence Interval) | |
|---|---|---|---|
| Demographics | |||
| Site | |||
| Sex (male) | 53 (30.3%) | 48 (30.2%) | — |
| Age | 15.8 ± 1.5 | 15.9 ± 1.6 | — |
| Race (white) | 140 (80%) | 137 (86.2%) | — |
| Depression | |||
| CDRS-R | 59.9 ± 11.1 | 57.6 ± 9.5* | 0.83 (0.66–1.04)† |
| BDI | 22.6 ± 12.2 | 18.2 ± 11.6** | 0.65 (0.50–0.85)** |
| Duration current episode | 24.6 ± 23.2 | 20.1 ± 16.6* | 0.80 (0.63–1.02)† |
| Suicidality/self-injurious behavior | — | ||
| SIQ | 45.0 ± 23.3 | 38.0 ± 20.6** | 0.70 (0.56–0.87)** |
| NSSI | 79 (46.2%) | 46 (29.3%)** | 0.39 (0.23–0.64)** |
| Suicide attempt | 42 (24.1%) | 37 (23.3%) | |
| Hopelessness (BHS) | 11.4 ± 5.7 | 9.5 ± 5.3** | 0.71 (0.56–0.89)** |
| Comorbidity | |||
| Drug use (DUSI) | 12.8 ± 20.4 | 9.0 ± 16.8† | 0.81 (0.65–1.01)† |
| Anxiety | 63 (36.6%) | 56 (36.1%) | — |
| ADHD | 25 (14.3%) | 27 (17.3) | — |
| Conduct/oppositional | 17 (9.8%) | 16 (10.2%) | — |
| No. comorbid disorders | |||
| 0 | 82 (48.0%) | 76 (50.0%) | — |
| 1 | 77 (45.0%) | 57 (37.5%) | |
| ≥2 | 12 (7.0%) | 19 (12.5%) | |
| Functional status | |||
| CGAS | 49.5 ± 7.6 | 51.8 ± 7.6** | 1.31 (1.03–1.67)* |
| Family | |||
| CBQ-A | 10.1 ± 6.2 | 7.6 ± 6.0** | 0.66 (0.52–0.83)** |
| Abuse | 47 (27.6%) | 34 (21.7%) | — |
| No. months' medication | 7.6 ± 9.3 | 10.7 ± 14.1* | 1.27 (0.98–1.64)† |
Note: Odds ratios for continuous variables reflect a 1 SD change in the baseline variable. ADHD = attention-deficit/hyperactivity disorder; BDI = Beck Depression Inventory; BHS = Beck Hopelessness Scale; CBQ-A = Conflict Behavior Questionnaire-Adolescent Report; CDRS-R = Children's Depression Rating Scale-Revised; CGAS = Children's Global Adjustment Scale; DUSI = Drug Use Screening Inventory; f = frequency; NSSI = nonsuicidal self-injurious behavior; SIQ = Suicidal Ideation Questionnaire-JR.
Adequate versus inadequate treatment response defined as Clinical Global Impressions-Improvement Subscale score of 2 or lower plus improvement in CDRS-R of 50% or greater. Analyses adjust for site, age, sex, and race. Odds ratios are shown only for variables associated with response status at p < .10.
p < .05;
p < .01;
p < .001;
p < .10.
TABLE 2.
Final Step of Backward Logistic Regression Predicting to Adequate Versus Inadequate Treatment Responsea
| β | SE | Wald χ2 | df | p | Odds Ratio | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Site | 8.00 | 5.00 | .16 | |||||
| Age | 0.04 | 0.08 | 0.31 | 1.00 | .58 | 1.05 | 0.89 | 1.24 |
| Sex | 0.38 | 0.28 | 1.84 | 1.00 | .18 | 1.46 | 0.84 | 2.53 |
| Race | 0.28 | 0.33 | 0.73 | 1.00 | .39 | 1.33 | 0.69 | 2.54 |
| CBT | 0.71 | 0.24 | 8.60 | 1.00 | .00 | 2.04 | 1.27 | 3.29 |
| BDI | −0.02 | 0.01 | 3.79 | 1.00 | .05 | 0.76 | 0.57 | 1.00 |
| CBQ-A | −0.05 | 0.02 | 5.32 | 1.00 | .02 | 0.74 | 0.57 | 0.96 |
| NSSI | −0.77 | 0.27 | 8.06 | 1.00 | .00 | 0.46 | 0.27 | 0.79 |
Note: Hosmer and Lemeshow test, χ28 = 12.5, p = .13. BDI = Beck Depression Inventory; CBQ-A = Conflict Behavior Questionnaire-Adolescent Report; CBT = cognitive-behavioral therapy; NSSI = nonsuicidal self-injurious behavior.
Site, age, sex, and race were forced into the final step of the backward logistic regression.
Moderators
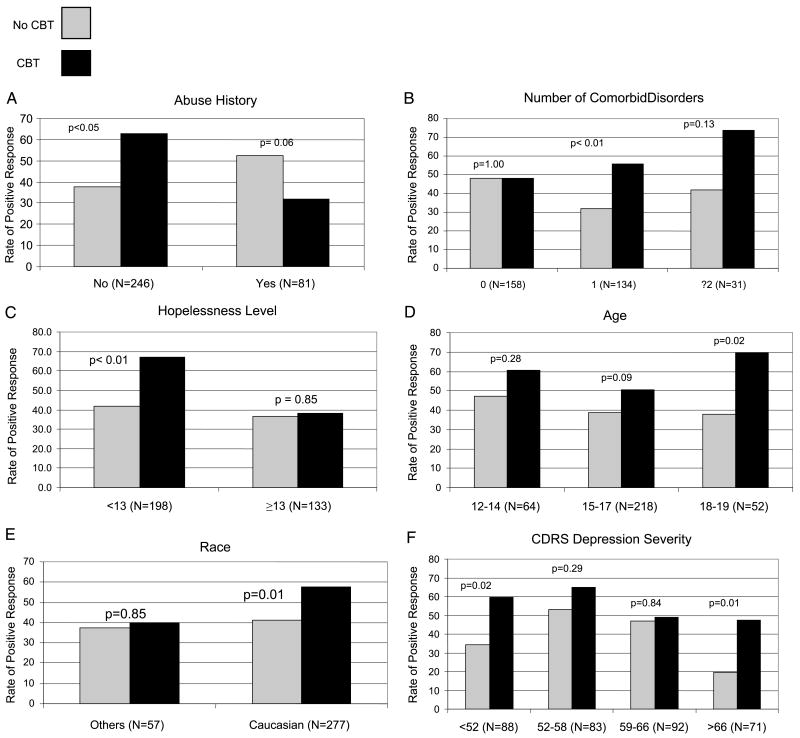
As shown in Table 3 and Figure 1, when main effects were forced into the model, response versus nonresponse to CBT/combined treatment was significantly more likely among youths with no abuse histories and more comorbid disorders, with a marginally significant effect for comorbid ADHD. Lower hopelessness was marginally associated with better response to CBT. None of these moderators were significantly associated with CBT dose (number of sessions received [p > .05]).
TABLE 3.
Results of Logistic Regression Models Exploring the Degree to Which Each of the Candidate Baseline Explanatory Variables Moderated Adequate Versus Inadequate Treatment Response
| Main Effects in Model | Main Effects Not in Modela | |||
|---|---|---|---|---|
| CBT/combined | ||||
| Abuse | 0.15 | <0.001 | — | |
| No. comorbidities | 2.30 | 0.02 | — | |
| Comorbid ADHD | 3.47 | 0.056 | 2.71 | 0.02 |
| Hopelessness (BHS) | 0.93 | 0.08 | 0.90 | 0.001 |
| Age | 1.01 | 0.01 | ||
| White vs. minority | 1.99 | 0.01 | ||
| CDRS-R | 1.01 | 0.01 | ||
| NSSI | 0.39 | 0.01 | ||
| Previous treatment | 1.05 | 0.01 | ||
| Comorbid anxiety | 2.71 | 0.01 | ||
| Medication | ||||
| BDI | 0.96 | 0.02 | ||
Note: Only significant moderators are listed, p < .05. ADHD = attention-deficit/hyperactivity disorder; BDI = Beck Depression Inventory; BHS = Beck Hopelessness Scale; CBT = cognitive-behavioral therapy; CDRS-R = Children's Depression Rating Scale-Revised; NSSI = nonsuicidal self-injurious behavior.
Reported only when candidate variable was not significant (p < .05) with main effects in the model.
Fig. 1.
Response rates for youths receiving CBT/combined treatment versus medication switch only stratified by: abuse history (A), number of comorbid disorders (B), hopelessness level (C), age (D), race (E), and CDRS depression severity level (F). CBT = cognitive-behavioral therapy; CDRS-R = Children's Depression Rating Scale-Revised. Note: BHS cut-score of 13 based on Brent et al.8
Results of more exploratory analyses (without forcing main effects in the model) are shown in Table 3, columns 4 and 5. Analyses of subgroup differences, defined based on quartile splits for continuous variables (Fig. 1D–F), revealed that CBT/combined treatment showed greater superiority over medication without CBT for youths with older age (age 18–19 years: odds ratio [OR] 3.7; 95% CI 1.2–12.0 [p < .02]; age 15–17 years: OR 1.6; 95% CI 0.9–2.7 [p < .09]), white status (OR 1.9; 95% CI 1.2–3.1 [p < .01]), co-occurring anxiety disorders (OR 2.7; 95% CI 1.3–5 [p < .01]), no NSSI (OR 2.2; 95% CI 1.3–3.9 [p < .005]), and longer duration of previous medication treatment (>11.06 months: OR 2.3; 95% CI 0.9–5.7 [p = .07]). Only ORs with p < .10 were reported.
Figure 1F illustrates results for depression severity as indicated by the CDRS-R. A significant effect emerged, with youths showing more benefits from CBT/combined treatment at the lowest and highest levels of CDRS-R severity (CDRS-R <52: OR 2.8; 95% CI 1.2–6.8 [p < .02]; CDRS-R >66: OR 3.8; 95% CI 1.3–11.2 [p < .01]). At all levels, however, CBT/combined treatment was associated with greater likelihood of response, albeit sometimes escaping statistical significance.
Despite the absence of a medication effect in primary analyses,2 exploratory analyses examined potential moderators of medication effects. With main effects in the model, self-reported depression on the BDI was a significant moderator; venlafaxine was more beneficial than SSRIs among the youths with lower baseline BDI severity levels (first quartile of BDI distribution) but not at higher levels (BDI <10: OR 2.5; 95% CI 1.0–6.6 [p = .055]; BDI = 11–19: OR 0.4; 95% CI 0.2–1.1 [p = .07]; BDI = 20–28: OR 1.6; 95% CI 0.7–3.9 [p = .28]; BDI >28: OR 0.5; 95% CI 0.2–1.2 [p = .11]).
Discussion
In this first study of the treatment of SSRI-resistant depression in adolescents, many predictors of treatment response were similar to those identified in studies of first-step treatments. Depression severity, hopelessness,5,8,10 suicidal ideation,5,10 youth-reported family conflict,6,7 and functional impairment5,10 were associated with nonresponse. Significant moderators of CBT/combined treatment were a history of abuse and number of comorbid/co-occurring disorders, with a marginal effect for hopelessness; self-reported depression moderated response to venlafaxine versus an SSRI. We place these findings within the context of the study limitations and extant literature and then discuss clinical significance and research implications.
Although the TORDIA sample was relatively large, power was limited for tests of moderation. The geographic diversity of the sample is a study strength, although ethnic/racial diversity was limited, reducing our ability to examine ethnic/racial differences. Exclusion criteria, such as substance abuse limit generalizability to samples with these comorbidities. Because TORDIA did not include a placebo, or CBT added to the initial SSRI medication conditions, future research is needed to determine whether results were due to CBT or CBT combined with a medication switch. The study was designed to evaluate combination therapy with CBT, selected as the most established psychosocial treatment; whether our results will generalize to alternative psychosocial treatments needs examination. This study reports secondary exploratory analyses; although, in some cases, findings were replications of previously reported results, other results require replication and confirmation.
We replicated previous findings that indices of severity and chronicity predict a poorer response to treatment.5,8,9 This highlights the importance of early detection and treatment, before patients' conditions become chronic and more intractable to intervention, and also the need to develop effective interventions for severely ill youth. This could include longer and more intense treatment, augmentation of antidepressant treatment with a mood stabilizer, or use of other types of intervention that have different and more rapid mechanisms of actions.30
Additional predictors emerged for this treatment-resistant sample, where we included a broader group of more chronic and severely ill youths relative to other samples. A history of NSSI was a poor prognostic indicator. This is a marker for difficulty with emotion regulation, which often accompanies chronic depression.31 The positive prognostic power of longer duration of medication treatment may reflect a commitment and/or positive expectancy for treatment as these youths and families continued in treatment despite minimal benefits. Alternatively, the longer time in treatment may screen out youths who are likely to respond to pharmacotherapy alone, leaving a group who require a change in treatment strategy. In contrast to TADS and other first-step treatment studies,5,8 our study did not find that older age predicted poorer response, nor did our study find evidence of moderation due to family income, although substantial missing data on our income variable may have affected results.
The number of comorbid diagnoses was a positive moderator of CBT/combined treatment effects in TORDIA, with stronger CBT/combined treatment effects among youths with more comorbid disorders, ADHD, and a trend to better outcome with anxiety disorders. These results are consistent with previous results from first-step psychosocial treatment studies in which CBT was most beneficial among youths with comorbid anxiety8 and ADHD10 but in contrast to TADS,5 in which comorbidity was a negative predictor and did not moderate treatment outcome. Perhaps, in our SSRI-resistant population, CBT provided a frame-work for dealing with difficulties associated with comorbid conditions that medication alone did not (e.g., problem solving, social skills training), which could have an impact on a range of disorders/adjustment problems. This finding supports the use of CBT/combined treatment with the more heterogeneous and complex patients seen in community settings,32 especially because comorbidity has been reported to be a negative prognostic factor and because comorbities with ADHD and anxiety are common in community clinical settings.
We found a weaker CBT/combined treatment effect among youths with histories of abuse and higher hopelessness—a measure of negative cognitions about the future and one component of the depressive triad of negative beliefs about the self, life, and future. The results regarding abuse are consistent with previous articles in depressed adolescents,11 although chronically depressed adults with maltreatment histories have been shown to preferentially respond to psychotherapy versus medication.33 These results suggest the need to better understand the seemingly greater resistance to depression-focused CBT among youths with abuse histories. The moderation of CBT/combined treatment response by hopelessness is also consistent with some, but not all, studies of CBT5,8,10 and suggests the need for interventions targeting hopelessness more specifically, such as developing a “Hope Box” with reminders of reasons for living and cues for generating more optimistic thoughts.34,35
Contrary to our prediction based on first-step treatment research,5,13,14 significant benefits of CBT/combined treatment were found for youths with the most severe depressive symptoms, as well as less severe symptoms. Thus, in the context of initial SSRI treatment resistance, the addition of CBT seems to yield benefits even among the most severely ill youths.
Exploratory analyses (meaning that formal criteria for moderation were not met4) indicated that response to CBT was greater among older youths, perhaps because of increasing ability to use cognitive strategies with age and developmental maturation. However, TORDIA youths ranged from 12 to 18 years old; alternative psychosocial treatment strategies rooted in knowledge regarding the developmental needs/functioning of younger youths might have yielded greater gains.36–39 The CBT/combined treatment was significantly more beneficial than medication alone among whites, but these benefits were not detected among minority participants. It could be that the TORDIA CBT required adaptation to better meet the needs of minority youths.34,40 Future studies are needed with larger minority samples and to evaluate strategies for balancing the need to maintain treatment fidelity while allowing for clinical flexibility and individual tailoring to meet diverse patient needs.
Findings regarding moderators of medication response must be interpreted cautiously because of the absence of an overall effect for medication. In contrast to our prediction that a switch to venlafaxine would be overall more efficacious than a switch to an SSRI, we found similar response rates to venlafaxine and SSRIs.2 Self-reported depression on the BDI was the only variable to emerge as a significant moderator, a significant advantage for venlafaxine versus SSRIs emerged only at the lowest levels of self-reported depression (BDI <10), and SSRIs were marginally more beneficial than venlafaxine at higher BDI levels. Given our finding of more side effects with venlafaxine,2 these results support the choice of an SSRI switch in a treatment-resistant population, although this issue requires additional research.
In conclusion, treatment nonresponse is a common problem in clinical practice: first-step treatments yield minimal benefits for 40% of youths, and TORDIA results indicate that nonresponse to second-step treatments is also common. Given the greater costs involved in adding CBT to pharmacotherapy,3 it is important for patients, parents, providers, and health care organizations to know when these costs are likely to be most beneficial and when continued emphasis on medication monotherapy is likely to be equally beneficial. We found moderators of CBT/combined treatment that may aid in personalizing treatment and in highlighting areas requiring further treatment development work. Our data support the benefits of CBT/combined treatment among youths with more comorbid disorders, suggesting that this strategy may be particularly cost-effective in community settings where youths frequently present with multiple diagnoses. The poorer response to CBT/combined treatment among youths with abuse histories and high levels of hopelessness may indicate that different treatment approaches are required for youths with these features. If confirmed by future studies, our findings can contribute to a more personalized approach to the treatment of depression in adolescence.
Acknowledgments
Dr. Keller has reported research support from Pfizer. He is a consultant to Abbott, CENEREX, Cephalon, Cypress Bioscience, Cyberonics, Forest Laboratories, Janssen, JDS, Medtronic, Organon, Novartis, Pfizer, Roche, Solvay, and Wyeth. Dr. Keller has served on the advisory boards of Abbott Laboratories, Bristol-Myers Squibb, CENEREX, Cyberonics, Cypress Bioscience, Forest Laboratories, Janssen, Neuronetics, Novartis, Organon, and Pfizer. Dr. McCracken has received research support from Eli Lilly, McNeil, Bristol-Myers Squibb, and Shire. He is a consultant to Shire, Eli Lilly, McNeil, Pfizer, Janssen, Johnson & Johnson, Novartis, and Wyeth. Dr. Wagner has served as a consultant to and/or served on the advisory boards of Abbott Laboratories, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Forest Laboratories, GlaxoSmithKline, Janssen, Johnson & Johnson, Novartis, Organon, Ortho-McNeil, Otsuka, Pfizer, Solvay, UCB Pharma, and Wyeth-Ayers; and receives research support from the National Institute of Mental Health.
This study was supported by NIMH grants MH61835 (Pittsburgh), MH61856 (Galveston), MH61864 (UCLA), MH61869 (Portland), MH61958 (Dallas), and MH62014 (Brown) and the Advanced Center for Early-Onset Mood and Anxiety Disorders (MH66371, PI: D.B.).
The authors thank the youth, families, staff, and colleagues who made this project possible. The opinions and assertions contained in this article are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of Health and Human Services, the National Institutes of Health, or the National Institute of Mental Health.
Footnotes
Disclosure: Dr. Asarnow consults on cognitive-behavioral therapy and cognitive-behavioral therapy for depression, previously consulted on an unrestricted grant from Pfizer, and receives unrestricted research funding from Philip Morris; a family member receives funding from Bristol-Myers Squibb. Dr. Birmaher has participated in forums sponsored by Solvay Pharmaceuticals and Abcomm. He has presented on bipolar disorders in children at a meeting sponsored by Solvay. Dr. Birmaher has also received royalties from Random House, has participated in regional advisory board meetings for Jazz, and has provided training on the K-SADS-PL rating scale to Shire. Dr. Emslie receives research support from NIMH, Shire, Somerset, Forest Laboratories, and Biobehavioral Diagnostics; is a consultant to Eli Lilly, Forest, Pfizer, Validus Pharmaceuticals, Wyeth-Ayerst, Shire, and Biobehavioral Diagnostics. The other authors report no conflicts of interest.
References
- 1.Birmaher B, Brent D. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. [March 10, 2008]; doi: 10.1097/chi.0b013e318145ae1c. http://www.aacap.org/galleries/PracticeParameters/InPress_2007_Depressivedisorders.pdf. [DOI] [PubMed]
- 2.Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression. The TORDIA randomized controlled trial. JAMA. 2008;299:901–913. doi: 10.1001/jama.299.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domino ME, Burns BJ, Silva SG, et al. Cost-effectiveness of treatments for adolescent depression: results from TADS. Am J Psychiatry. 2008;165:588–596. doi: 10.1176/appi.ajp.2008.07101610. [DOI] [PubMed] [Google Scholar]
- 4.Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59:877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 5.Curry J, Rohde P, Simons A, et al. Predictors and moderators of acute outcome in the Treatment for Adolescents with Depression Study (TADS) J Am Acad Child Adolesc Psychiatry. 2006;45:1427–1439. doi: 10.1097/01.chi.0000240838.78984.e2. [DOI] [PubMed] [Google Scholar]
- 6.Emslie GJ. Fluoxetine in child and adolescent depression: acute and maintenance treatment. Depression Anxiety. 1998;7:32–39. doi: 10.1002/(sici)1520-6394(1998)7:1<32::aid-da4>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Birmaher B, Brent DA, Kolko D, et al. Clinical outcome after short-term psychotherapy for adolescents with major depressive disorder. Arch Gen Psychiatry. 2000;57:29–36. doi: 10.1001/archpsyc.57.1.29. [DOI] [PubMed] [Google Scholar]
- 8.Brent DA, Kolko DJ, Birmaher B, et al. Predictors of treatment efficacy in a clinical trial of three psychosocial treatments for adolescent depression. J Am Acad Child Adolesc Psychiatry. 1998;37:906–914. doi: 10.1097/00004583-199809000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Clarke GN, Hops H, Lewinsohn PM, Andrews J, Seeley JR, Williams J. Cognitive-behavioral group treatment of adolescent depression: prediction of outcome. Behav Ther. 1992;23:341–354. [Google Scholar]
- 10.Rohde P, Seeley JR, Kaufman NK, Clarke GN, Stice E. Predicting time to recovery among depressed adolescents treated in two psychosocial group interventions. J Consult Clin Psychol. 2006;74:80–88. doi: 10.1037/0022-006X.74.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbe RP, Bridge JA, Birmaher B, Kolko DJ, Brent DA. Lifetime history of sexual abuse, clinical presentation, and outcome in a clinical trial for adolescent depression. J Clin Psychiatry. 2004;65:77–83. doi: 10.4088/jcp.v65n0113. [DOI] [PubMed] [Google Scholar]
- 12.Barbe RP, Bridge J, Birmaher B, Kolko D, Brent DA. Suicidality and its relationship to treatment outcome in depressed adolescents. Suicide Life Threat Behav. 2004;34:44–55. doi: 10.1521/suli.34.1.44.27768. [DOI] [PubMed] [Google Scholar]
- 13.Elkin I, Shea MT, Watkins JT, et al. National Institute of Mental Health Treatment of Depression Collaborative Research Program. General effectiveness of treatments. Arch Gen Psychiatry. 1989;46:971–982. doi: 10.1001/archpsyc.1989.01810110013002. discussion 983. [DOI] [PubMed] [Google Scholar]
- 14.Sotsky SM, Glass DR, Shea MT, et al. Patient predictors of response to psychotherapy and pharmacotherapy: findings in the NIMH Treatment of Depression Collaborative Research Program. Am J Psychiatry. 1991;148:997–1008. doi: 10.1176/ajp.148.8.997. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Washington: American Psychiatric Press; 1994. [Google Scholar]
- 16.Poznanski EO, Freeman LN, Mokros HB. Children's Depression Rating Scale–Revised. Psychopharmacol Bull. 1984;21:979–989. [Google Scholar]
- 17.Guy W. ECDEU Assessment Manual for Psychopharmacology. 2nd. Washington: US Government Printing Office; 1976. [Google Scholar]
- 18.Reynolds WM, Mazza JJ. Assessment of suicidal ideation in inner-city children and young adolescents: reliability and validity of the Suicidal Ideation Questionnaire-JR. School Psych Rev. 1999;28:17–30. [Google Scholar]
- 19.Begg CB, Iglewicz B. A treatment allocation procedure for sequential clinical trials. Biometrics. 1980;36:81–90. [PubMed] [Google Scholar]
- 20.U.S. Food and Drug Administration (FDA) FDA statement regarding the antidepressant paxil for pediatric depression. FDA Talk Paper. [May 23, 2008]; http://www.fda.gov/bbs/topics/ANSWERS/2003/ANS01230.html.
- 21.Spirito A, Brent D, Emslie G, et al. Site differences and their possible sources in the outcomes of a multisite clinical trial: The Treatment of SSRI Resistant Depression in Adolescents (TORDIA) 2008 In preparation. [Google Scholar]
- 22.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 23.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Kirisci L, Mezzich A, Tarter R. Norms and sensitivity of the adolescent version of the Drug Use Screening Inventory. Addict Behav. 1995;20:149–157. doi: 10.1016/0306-4603(94)00058-1. [DOI] [PubMed] [Google Scholar]
- 25.Beck AT, Beck R, Kovacs M. Classification of suicidal behaviors: I. Quantifying intent and medical lethality. Am J Psychiatry. 1975;132:285–287. doi: 10.1176/ajp.132.3.285. [DOI] [PubMed] [Google Scholar]
- 26.Shaffer D, Gould M, Brasic J, et al. Children's Global Adjustment Scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 27.Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: the Hopelessness Scale. J Consult Clin Psychol. 1974;42:861–865. doi: 10.1037/h0037562. [DOI] [PubMed] [Google Scholar]
- 28.Robin AL, Foster SL. Negotiating Parent-Adolescent Conflict: A Behavioral Family Systems Approach. New York: Guilford; 1989. [Google Scholar]
- 29.Ascher BH, Farmer EMZ, Burns BJ, Angold A. The Child and Adolescent Services Assessment (CASA): description and psychometrics. J Emot Behav Disord. 1996;4:12–20. [Google Scholar]
- 30.Zarate CA, Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 31.Nock MK, Wedig MM, Holmberg EB, Hooley JM. The emotion reactivity scale: development, evaluation, and relation to self-injurious thoughts and behaviors. Behav Ther. 2008;39:107–116. doi: 10.1016/j.beth.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Asarnow JR, Jaycox L, Duan N, et al. Effectiveness of a quality improvement intervention for adolescent depression in primary care clinics: a randomized controlled trial. JAMA. 2005;293:311–319. doi: 10.1001/jama.293.3.311. [DOI] [PubMed] [Google Scholar]
- 33.Nemeroff CB, Heim CM, Thase ME, et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci U S A. 2003;100:14293–14296. doi: 10.1073/pnas.2336126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asarnow J, Berk M, Baraff LJ. Family intervention for suicide prevention: a specialized emergency department intervention for suicidal youth. Prof Psychol Res Pr. In press. [Google Scholar]
- 35.Brown GK, Ten Have T, Henriques GR, Xie SX, Hollander JE, Beck AT. Cognitive therapy for the prevention of suicide attempts: a randomized controlled trial. JAMA. 2005;294:563–570. doi: 10.1001/jama.294.5.563. [DOI] [PubMed] [Google Scholar]
- 36.Asarnow JR, Scott CV, Mintz J. Cognitive-behavioral treatments and family interventions for children with depression. A combined cognitive-behavioral family education intervention for depression in children: a treatment development study. Cognit Ther Res. 2002;26:221–229. [Google Scholar]
- 37.Stark KD, Herren J, Fisher M. Treatment of childhood depression. In: Abela JRZ, Hankin BJ, editors. Handbook of Depression in Children and Adolescents. New York: Guilford; 2008. [Google Scholar]
- 38.Weisz JR, Thurber CA, Sweeney L, Proffitt VD, LeGagnoux GL. Brief treatment of mild-to-moderate child depression using primary and secondary control enhancement training. J Consult Clin Psychol. 1997;65:703–707. doi: 10.1037//0022-006x.65.4.703. [DOI] [PubMed] [Google Scholar]
- 39.Tompson MC, Pierre CB, Haber FM, Fogler JM, Groff AR, Asarnow JR. Family-focused treatment for childhood-onset depressive disorders: results of an open trial. Clin Child Psychol Psychiatry. 2007;12:403–420. doi: 10.1177/1359104507078474. [DOI] [PubMed] [Google Scholar]
- 40.Miranda J, Bernal G, Lau A, Kohn L, Hwang WC, LaFromboise T. State of the science on psychosocial interventions for ethnic minorities. Annu Rev Clin Psychol. 2005;1:113–142. doi: 10.1146/annurev.clinpsy.1.102803.143822. [DOI] [PMC free article] [PubMed] [Google Scholar]