Abstract
E-cadherin is thought to mediate intercellular adhesion in the mammalian epidermis and in hair follicles as the adhesive component of adherens junctions. We have tested this role of E-cadherin directly by conditional gene ablation in the mouse. We show that postnatal loss of E-cadherin in keratinocytes leads to a loss of adherens junctions and altered epidermal differentiation without accompanying signs of inflammation. Overall tissue integrity and desmosomal structures were maintained, but skin hair follicles were progressively lost. Tumors were not observed and β-catenin levels were not strongly altered in the mutant skin. We conclude that E-cadherin is required for maintaining the adhesive properties of adherens junctions in keratinocytes and proper skin differentiation. Furthermore, continuous hair follicle cycling is dependent on E-cadherin.
Keywords: adherens junction/desmosomes/E-cadherin/epidermis/fibrosis
Introduction
Mammalian skin is composed of three main layers, the epidermis, dermis and adipose layers. The epidermis forms a physical barrier protecting the organism from the environment and consists of tightly packed sheets of keratinocytes. Fundamental to the formation of epidermis and hair follicles is the ability of cells to attach to each other, thus enabling them to withstand mechanical stress. Cadherins and integrins are key components of the various adhesive structures that allow dynamic cell–cell and cell–extracellular matrix interactions during differentiation and migration (Hodivala and Watt, 1994; Zhu and Watt, 1996; Fuchs and Raghavan, 2002).
In the skin, during terminal differentiation of keratinocytes, a cornified epithelium is established. The close juxtapositioning of keratinocytes in the epidermis requires a pool of different structures that are responsible for adherence of these cells but also allow the dynamic changes that occur during further differentiation. The interplay between different adherens structures and a large ensemble of specific adhesion molecules provide the basis for the integrity of the epidermal compartment. Four different adherens structures are found in the epidermis: desmosomes (reviewed by Garrod et al., 2002), tight junctions (TJs; reviewed by Tsukita and Furuse, 2002), hemidesmosomes (Schaapveld et al., 1998; Nievers et al., 1999) and adherens junctions (AJs; reviewed by Perez-Moreno et al., 2003). Transgenic approaches in mice have demonstrated that the functional integrity of the epidermal compartment is crucially dependent on several proteins mediating adhesion, including desmocollin in desmosomes (Chidgey et al., 2001), claudin 1 in tight junctions (Furuse et al., 2002), and β1 integrin at the basolateral surfaces of keratinocytes (Brakebusch et al., 2000). Depending on the gene disrupted and the structures affected, the consequences of gene ablation ranged from severe epidermal defects to the loss of terminal differentiation and lack of correct hair follicle formation and hair growth.
AJs in the epidermis are formed mainly by classical cadherins, of which E-cadherin is the major representative (Yap et al., 1997; Jamora and Fuchs, 2002). E-cadherin is a transmembrane molecule that links neighboring cells through homophilic interactions. The intracellular tail of E-cadherin binds β-catenin, which itself binds α-catenin. This interaction mediates the coupling of the intracellular actin cytoskeleton of neighboring cells (Aberle et al., 1996; Braga, 2000; Jamora and Fuchs, 2002). Deficiency of α-catenin in mice resulted in deficits of epidermal intercellular tightness and numerical reduction of AJs (Vasioukhin et al., 2001a). Loss of β-catenin had a major impact on hair follicle morphogenesis, terminal differentiation of keratinocytes and the balance between cell death and proliferation (Huelsken and Birchmeier, 2001; Huelsken et al., 2001). β-catenin has a dual function, affecting both cell adhesion and intracellular signaling acting via the canonical Wnt/Lef pathway (Huelsken and Behrens, 2000; Korswagen et al., 2000). Whether and how E-cadherin, the major partner of β-catenin at the plasma membrane, influences each of these functions in keratinocytes is currently unclear since it has been difficult to discriminate between adhesion and signaling function of β-catenin.
Disruption of E-cadherin in murine embryonic stem cells abrogated cell aggregation and was lethal in early embryogenesis (Larue et al., 1994). Experiments in vitro suggested that E-cadherin is involved in the control of cell migration and cell shape (Larue et al., 1996; Ohsugi et al., 1997). Conditional gene targeting revealed that ablation of E-cadherin has no major impact on myelination or myelin maintenance, despite its normally high expression between uncompacted myelin membrane lamellae of myelinated peripheral nerves. However, AJs were lost in the outer mesaxon, a specialized myelin compartment (Young et al., 2002). Specific lack of E-cadherin in the lactating murine mammary gland affected the terminal differentiation program and caused increased premature cell death, preventing these mice from nurse-feeding their litters (Boussadia et al., 2002).
In this report, we used the Cre/loxP system (Gu et al., 1994) to examine the functional consequences of induced E-cadherin deficiency in the postnatal murine epidermis. This was achieved using Cre recombinase-expressing mice in which Cre expression is under the control of the Krox20 cis-acting regulatory elements and expression is observed in the epidermis, in hair follicles of murine back skin and in whisker follicles, among other sites (Gambardella et al., 2000). We found and describe a critical role for E-cadherin in epidermal intercellular adherence and in hair follicle morphogenesis in vivo.
Results
Ablation of E-cadherin in the epidermis and hair follicles of mutant mice
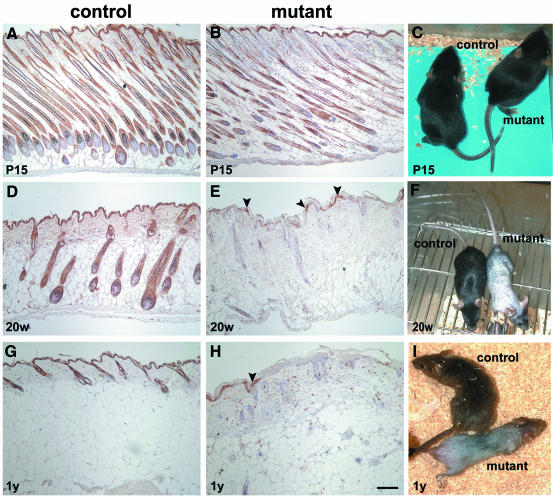
Mice with the genotype Krox20+/Cre; E-cad–/lox were viable and showed no clinical dermal abnormalities up to the age of 30 days (Figure 1C). Then, the fur of the back became less shiny and, within the next 10 days, a progressive hair loss occurred that was clearly evident at the age of 20 weeks (Figure 1F). Hair loss was not complete until late adult stages (Figure 1I) and was most pronounced on the back. Palpation of the skin revealed reduced elasticity in the back skin of mutants. In older mice, superficial skin defects most probably due to mechanical stress were common. Whisker hairs were curled and much shorter than in control animals (data not shown). Immunohisto chemistry revealed reduced E-cadherin by postnatal day (P) 15, predominantly in the upper parts of the hair follicles. No reduction was apparent at this age in the epidermis or the bulbar region of the hair follicle (Figure 1A and B). By 20 weeks of age, E-cadherin was absent in most areas of the epidermis (∼80% of the total area; Figure 1D and E). In 1-year-old mutants, E-cadherin deficiency extended to >90% of the epidermis (Figure 1G and H). Remaining hair follicles were completely devoid of E-cadherin already at the age of 20 weeks (Figure 1E).
Fig. 1. Ablation of E-cadherin in back skin epidermis and in hair follicles of mutant mice. Epidermis of back skin of P15 control (A) and mutant (B) mice shows a reduction of E-cadherin immunoreactivity in the upper parts of the hair follicles of mutant mice, while no difference can be detected in the epidermis. At the age of 20 weeks (20w), mutant mice show a massive reduction (78%) of the E-cadherin immuno-positive area within the epidermis, and the hair follicles have lost E-cadherin completely (E). In control animals, E-cadherin was detected throughout the epidermis and in all hair follicles (D). One-year-old (1y) mutant mice displayed a further increase in E-cadherin-negative areas (92%) (H). In control animals, E-cadherin staining was localized in the epidermis and hair follicles (G). Phenotypically, mutant mice were indistinguishable from control mice at P15 (C). Hair loss was already close to maximal at 20 weeks (F) and progressed slightly (I). The age of the animals is indicated in the lower left corner of each figure. Arrowheads indicate remaining E-cadherin-positive epidermal cells in mutant animals. Scale bar in (H) (also for A, B, D, E and G): 100 µm.
Western blot analysis confirmed the loss of E-cadherin in back skin and, as a control, in the sciatic nerve of 20-week-old mutants (Figure 2; Young et al., 2002). Tail skin showed normal E-cadherin expression, while no E-cadherin was detectable in whole brain lysates in either mutants or control animals. β-Catenin levels were not strongly affected by the loss of E-cadherin, although minor alterations may have escaped our analysis (Figure 2).
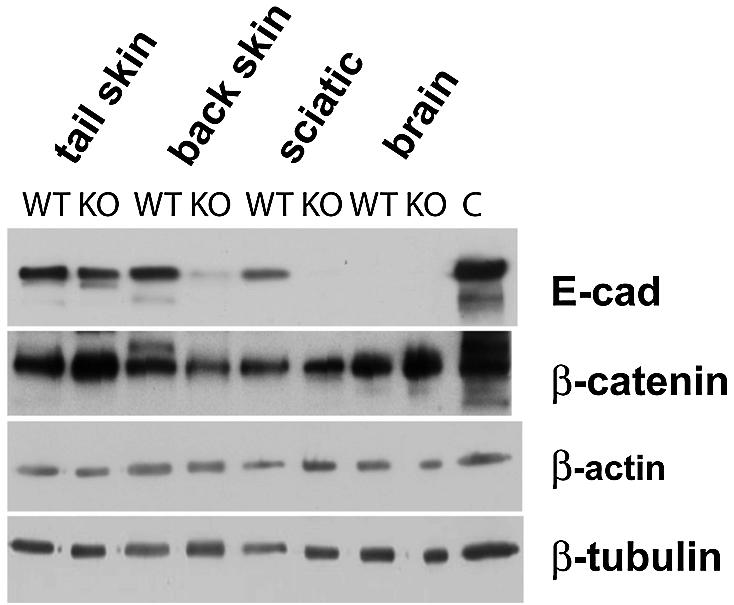
Fig. 2. Western blot analysis of E-cadherin expression in different tissues from 20-week-old control (WT) and mutant (KO) mice. Protein was extracted from back skin, tail skin, sciatic nerve and whole brain from control and mutant mice. E-cadherin levels were strongly reduced in back skin and sciatic nerve of mutant animals. Tail skin showed comparable amounts of E-cadherin.
Krox20Cre expression in the epidermis and hair follicles during early postnatal development
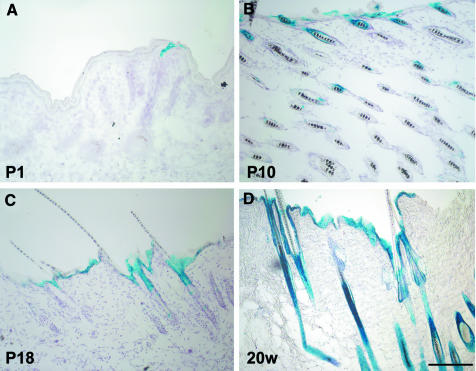
Next, we wanted to relate the observed loss of E-cadherin to the expression pattern of the Cre recombinase during skin development. For this, we intercrossed Krox20Cre/+ animals with a β-galactosidase (β-gal) Cre-reporter ROSA26R line (Soriano, 1999). β-Gal expression, as indicated by X-gal staining, was first detected by P1 in very few small epidermal areas of back skin (Figure 3A). At P10 and P18, β-gal was found mainly in the upper parts of the hair follicles and in epidermal regions next to hair follicles (Figure 3B and C). In back skin of adult mice (20 weeks old), β-gal was present throughout the entire hair follicles and in almost the entire epidermis (Figure 3D), although occasional small regions of the latter remained devoid of β-gal expression (see also Gambardella et al., 2000). Analysis of footpad skin and tail skin revealed no expression of β-gal, neither in the epidermis nor in the dermis. However, thin nerves in footpad skin showed β-gal activity (data not shown).
Fig. 3. Monitoring of recombination in Krox20Cre/+; R26R reporter mice by X-gal staining for β-galactosidase (β-gal) activity in the back skin at different postnatal developmental stages. At P1, recombination had occurred within only very small epidermal patches located near the hair follicle (A). In later stages (P10 and P18), the upper parts of the hair follicles show β-gal-positive cells, and β-gal-positive patches within the epidermis increased (B and C). At the age of 20 weeks (20w), all hair follicles and most parts of the epidermis show β-gal-positive cells indicating recombination (D). Scale bar in (D) (also for A–C): 200 µm.
Ablation of E-cadherin induces loss of adherens junctions and intercellular tightness in the epidermis and hair follicles
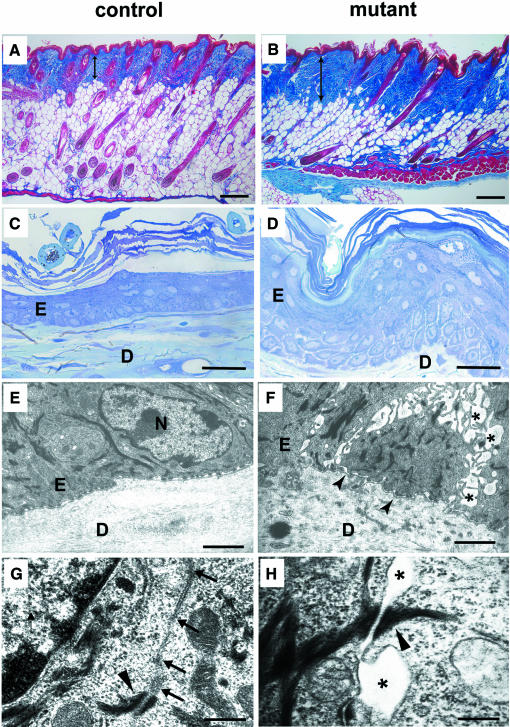
Morphological analysis of back skin from adult mutant mice (20 weeks old) showed an altered dermal tissue due to a slight fibrosis (Figure 4B) that was not observed in control animals (Figure 4A). Signs of dermal fibrosis were a conspicuous replacement of fatty (adipose) tissue by connective tissue and more pronounced collagen staining in the mutants. Hair follicle examination in the anagen stage of hair growth showed some remaining follicles with bulbs reaching the muscular layer (Figure 4B). The number of hair follicles appeared reduced. Detailed quantification was not carried out due to de-synchronization of the hair cycle at this age. Light microscopic analysis of semithin sections also revealed a thickening of the epidermis (strong acanthosis) and areas of mild parakeratosis (Figure 4C and D, and data not shown). In addition, we observed a dramatic loss of intercellular tightness of basal keratinocytes and in the cell layers lying just above. Based on these findings, we examined the basal cell layer at the ultrastructural level (Figure 4E and F). As suggested by light microscopy, the basal cells were not as tightly packed in the mutant tissue as in controls. Instead, the mutant cells showed filopodia-like projections reminiscent of the proposed intermediate stage in the process of joining sheets of epithelia (reviewed by Jamora and Fuchs, 2002). Desmosomes were still intact in the mutant tissue, appeared morphologically normal, and mediated cell–cell contacts (Figure 4G and H), while AJs could not be detected in most of the regions examined. Immunogold labeling confirmed the loss of E-cadherin in the affected areas (data not shown). Hemidesmosomal contacts between basal keratinocytes and the basement membrane were similar in control (Figure 4E) and mutant animals (Figure 4F). However, the basement membrane separating the epidermal compartment from the dermis was much more undulated in mutants, somewhat reminiscent of β1 integrin-deficient animals, although blistering and reduced hemidesmosomes were the main feature observed in those animals (Figure 4F; Brakebusch et al., 2000).
Fig. 4. Morphological changes resulting from ablation of the E-cadherin gene in the skin and hair follicles in adult 20-week-old mice. Masson trichrome staining revealed increased collagen deposition (indicated by the double arrowed line), and a reduction of hair follicles in mutant animals (B) in comparison with control animals (A). In contrast to control epidermis (C), mutant animals displayed strong epidermal acanthosis and mild parakeratosis (D). A widening of intercellular spaces was evident in the basal cell layer and the layers just above. On the ultrastructural level, widening of intracellular spaces was seen in the basal epidermal cell layer in back skin of mutant mice (asterisks) (F). Basally localized hemidesmosomes were detectable in control (E) as in mutant animals (F), but the basement membrane was undulated in mutant animals, as indicated by arrowheads (F). At higher magnification, loss of adherens junctions was seen in mutant animals indicated by asterisks (H), while control mice showed normal adherens junctions (G; arrows). Desmosomes in the epidermis of mutant animals were preserved (H; arrowhead). E = epidermis, D = dermal compartment, N = nucleus. Scale bars in (A–D), 10 µm; (E and F), 2 µm; (G and H), 200 nm.
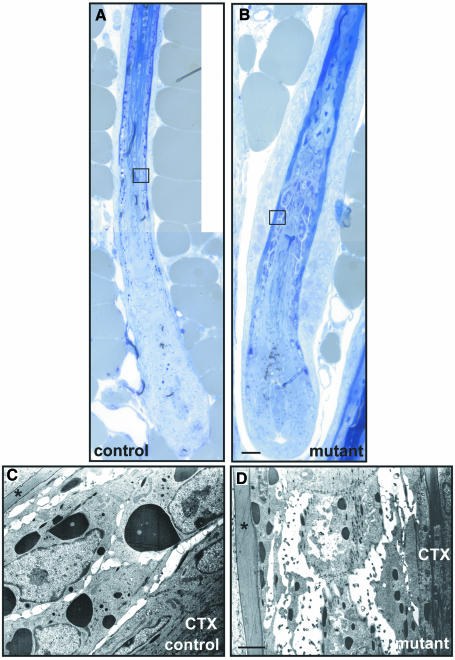
In the remaining hair follicles of back skin from adult mutant mice, we observed, similarly to the changes in the epidermis of the back skin, a reduction of intercellular tightness in the Huxley layer of the inner root sheath (IRS). Intercellular spaces were increased and the depositions of trichohyalin granula were reduced (Figure 5C and D). Furthermore, the outer root sheath (ORS) appeared to contain an increased number of cell layers in the remaining hair follicles compared with control animals (Figure 5A and B).
Fig. 5. Loss of intercellular tightness between cells of the Huxley layer of the IRS in back skin. Analysis of the remaining hair follicles in the back skin of 20-week-old mutant mice revealed a loss of intercellular tightness in the Huxley layer of the IRS (B and D). As in adult control animals, the Henle layer (indicated by asterisks) was not disturbed and the cortex of the hair was normal (A and B). Trichohyalin granula were massively reduced in mutant mice. Note the supernumery cells in the ORS of mutant mice (B). CTX = cortex of the hair shaft. Scale bar in (B), 50 µm (for A and B); (D) 2 µm (for C and D).
Ablation of E-cadherin alters epidermal differentiation
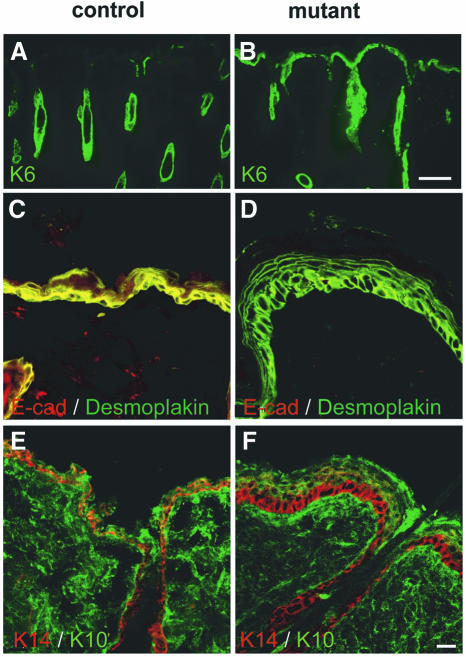
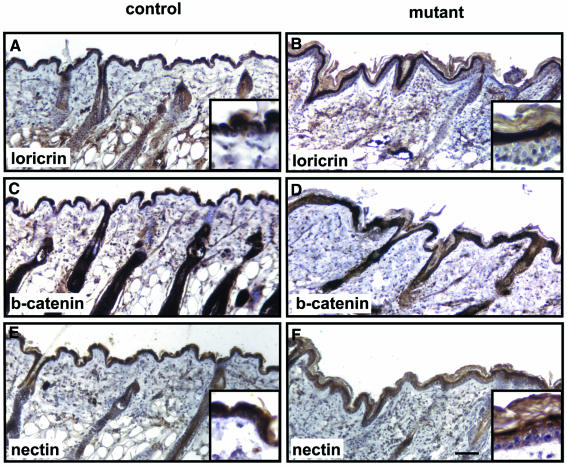
To determine whether the loss of E-cadherin affects the normal program of keratinocyte differentiation, we examined the potential expression of K6, a protein normally not expressed in the adult interfollicular epidermis. We found that the loss of E-cadherin caused robust ectopic expression of K6 in the epidermis of the back skin of adult mutant mice (Figure 6A and B) while the basal K14 and the suprabasal K10 were expressed normally (Figure 6E and F). Note that in both normal and mutant mice, K14 expression was restricted to the basal layer. This layer, however, was easier to distinguish in the mutant mice due to the enlarged size of the basal cells. Staining for loricin, a major cornified envelope protein of the epidermis, however, revealed a clearly increased number of loricrin-negative suprabasal cells in the mutant animals (Figure 7A and B). Desmoplakin expression was comparable between mutant and control tissue (Figure 6C and D), as was desmoglein (data not shown). Nectin 1α was expressed similarly to loricrin, with additional low expression in the basal cell layer. Whether there was a significant reduction of nectin 1α in the mutant basal layer could not be assessed quantitatively in a conclusive way (Figure 7E and F). β-Catenin was expressed in all epidermal cell layers in the mutant mice (Figure 7C and D).
Fig. 6. Keratinocyte differentiation in adult E-cadherin mutant mice. Sections from back skin of control (A, C and E) and mutant (B, D and F) mice were stained with antibodies against K6 (green), E-cadherin (E-cad; red), desmoplakin (green), K14 (red) and K10 (green) as indicated. K6 expression was restricted to the hair follicle in control mice (A), whereas in mutant mice it was also ectopically found in the interfollicular epidermis (B). Desmoplakin was localized in all epidermal layers in control (C) and in mutant mice (D). K14 was found in the basal layer in back skin of control mice (E) and mutants (F). The extended expression of K14 in the mutants reflects mainly the enlargement (hypertrophy) of the basal cell layer. Note the acanthosis of the epidermis in all sections from mutant mice. Scale bar in (B), 100 µm (for A and B); (F), 50 µm (for C–F).
Fig. 7. Epidermal expression of loricrin, β-catenin and nectin 1α in back skin of mutant E-cadherin-deficient mice. The epidermis of back skin from 20-week-old mutant mice showed loricrin-negative supernumery cells above the basal keratinocyte layer (A and B). β-catenin was expressed throughout the epidermis in control and mutant animals (C and D). Additional nectin 1α-negative (or low expressing) cells were found in the suprabasal region of mutant animals (F) compared with control animals (E). Scale bar in (F), 100 µm (also for A–E).
Morphology of whisker hair follicles is dramatically disturbed in E-cadherin-deficient mice
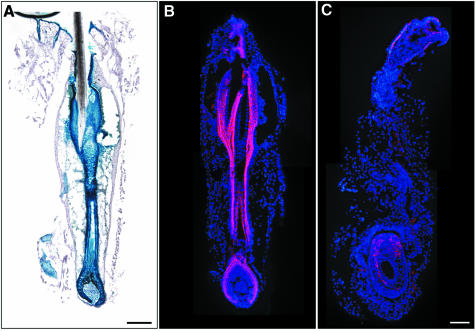
To follow-up on the near absence of whiskers in E-cadherin mutant mice, we first analyzed β-gal expression in micro-dissected whisker follicles of Krox20Cre/+; ROSA26R mutants. β-Gal expression was observed throughout all layers of whisker follicles during the anagen phase of whisker follicle growth (Figure 8A). This pattern was analogous to the expression of E-cadherin in wild-type mice (Figure 8B). In Krox20+/Cre; E-cad–/lox animals, only some small residual patches of E-cadherin were left, while the morphology of the whisker follicle was dramatically distorted, precluding a useful more detailed analysis (Figure 8C).
Fig. 8. Abnormal morphology in micro-dissected whisker follicles of adult (20-week-old) mutant mice. Recombination was detectable throughout the entire whisker follicle in Krox20Cre/+; R26R β-gal-reporter mice (A). E-cadherin expression was observed in control animals in the outer root sheath and parts of the inner root sheath (pink; B). In mutant animals, E-cadherin was almost totally lost and only a few E-cadherin-positive cells remained in the hair bulb (pink, C). Sections in (B) and (C) were counterstained with DAPI to visualize nuclei. Scale bar in (A and B), 50 µm (for A–C).
Reduced proliferation in hair follicles of E-cadherin-deficient mice
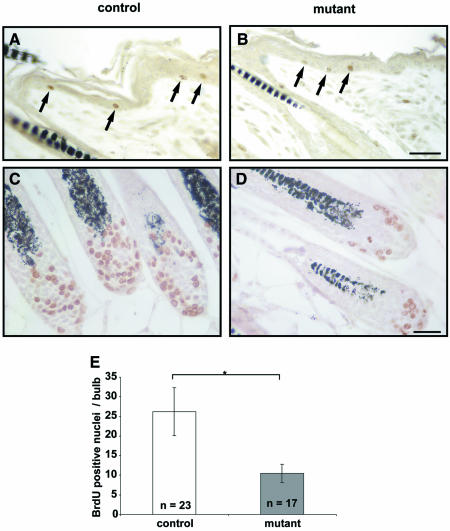
To analyze whether altered proliferation contributes to the observed phenotype in the epidermis and the hair follicle (Boussadia et al., 2002), we carried out bromodeoxyuridine (BrdU) labeling assays on back skin of mutant and control mice. Initially, we did not detect differences in proliferation in the epidermis of back skin of adult animals (data not shown). Therefore, we performed BrdU labeling of back skin in P15 animals to analyze possible early proliferation defects. There was no striking difference between mutant and control animals in the proliferation of epidermal keratinocytes (Figure 9A and B). In contrast, a significant decrease in the number of BrdU-labeled cells was found in the bulbs of hair follicles in back skin of mutant mice (Figure 9C–E).
Fig. 9. Reduced proliferation in hair bulbs of 15-day-old E-cadherin-deficient mice. Some BrdU-labeled proliferating cells were observed in the epidermis of control (A) and mutant animals (B). In the hair bulbs, the number of proliferating cells was significantly reduced in mutant animals (D) compared with control animals (C). (E) Quantitative analysis of three independent experiments. Cells of medially cut bulbs were counted. The asterisk indicates statistical significance (P < 0.005). Scale bar in (B), 100 µm (for A and B), (D), 20 µm (for C and D).
Discussion
E-cadherin deficiency causes loss of adherens junctions but not tumorigenic transformation of keratinocytes
In this report, we show that ablation of the E-cadherin gene in the postnatal epidermis causes severe disruption of AJs. Thus, our findings demonstrate conclusively that E-cadherin function is required for the maintenance of the skin in vivo. Somewhat surprisingly, however, despite the lack of AJs, the desmosomes (and with them the main mechanical integrity of the skin) were preserved. These observations make it extremely interesting to use the E-cadherin mutant animals in wounding or friction paradigms to test the interdependence in the formation of AJs and desmosomes in vivo.
The alterations in the epidermis of E-cadherin-deficient mice resemble squamous metaplasia, similar to the changes caused by the ablation of α-catenin (Vasioukhin et al., 2001a). However, E-cadherin-deficient animals did not develop skin tumors and no upregulation of active mitogen-activated protein kinase was observed (data not shown). The latter is comparable with the findings in desmoplakin null animals that have no functional desmosomes (Vasioukhin et al., 2001b). We conclude that the loss of some adherence structures in the epidermis per se does not necessarily lead to uncontrolled cell proliferation and each protein involved appears to have its specific impact on cell migration, differentiation and the cell cycle.
E-cadherin is required for correct differentiation of epidermal keratinocytes
During the course of terminal differentiation, keratinocytes go through a series of changes in gene expression that are well characterized. Basal keratinocytes express K5 and K14. As they detach from the basement membrane and migrate upward into the spinous layer, expression of these specific keratins ceases and upregulation of K1 and K10 is found. K14 and K10 were expressed normally in the mutant skin. In contrast, K6 was upregulated in interfollicular epidermal areas of E-cadherin-deficient mice. This indicates altered differentiation, as also described in animals lacking β1-integrin (Brakebusch et al., 2000) or β-catenin (Huelsken et al., 2001). Upregulation of K6 has been found in diseased tissue, such as in psoriasis (Stoler et al., 1989; Thewes et al., 1991) and in skin tumors (Tinsley et al., 1992; Caulin et al., 1993), but it may generally mark altered keratinocyte differentiation since increased expression of K6 also correlates with epidermal hypoproliferation in mouse models (Sellheyer et al., 1993). The acanthosis of the epidermis in E-cadherin-deficient animals is probably not the result of keratinocyte proliferation (although we cannot exclude some contribution). Our results rather suggest that the differentiation process is delayed or altered otherwise as indicated by increased layers of partially differentiated keratinocytes as suggested by the loricrin and nectin 1 expression patterns. Furthermore, we may also speculate that altered keratinocyte differentiation could lead to spatially and/or temporally altered secretion of cytokines that may in turn stimulate the observed fibrosis (Munz et al., 1999).
The morphology and maintenance of hair follicles is dependent on E-cadherin
E-cadherin-deficient mice showed loss of hair follicles of back skin and distorted morphology of whisker hair follicles consistent with the previously described E-cadherin expression in the murine hair follicles (Muller-Rover et al., 1999). Starting with the second cycle of growth, E-cadherin deficiency induced a loss of hair follicles in back skin while in regions that still expressed E-cadherin, such as tail skin, hair follicles developed normally and were not reduced in number. This phenomenon has also been observed in several other mutant mice (Yamanishi, 1998). Unfortunately, a comparison with mutant mouse lines deficient for β-catenin or α-catenin is not yet possible since the currently described mutant mice die during the first days of postnatal development and, thus, hair growth could not be examined (Huelsken et al., 2001; Vasioukhin et al., 2001a). However, a number of mutant mice carrying mutations in genes for important developmental regulators, including Lef1 (van Genderen et al., 1994; DasGupta and Fuchs, 1999) and Shh (Wang et al., 2000), also show disturbed hair follicle biology. In the E-cadherin-deficient mice, the lack of AJs is a likely contributor to the disturbed maintenance of hair follicles. Importantly, we did not observe an enrichment of inflammatory cells in the dermis of 20-week-old (or younger) mutants, thus indicating no major inflammatory component that contributes to the observed hair loss (data not shown) such as described in β1-integrin-deficient mice (Brakebusch et al., 2000).
Whisker hair follicles appear to be particularly dependent on E-cadherin since its deficiency caused a strongly impaired morphology and composition of cell layers, while the number of whisker follicles was not reduced.
Altered cell proliferation and cell death as a consequence of the loss of E-cadherin may contribute to the loss of hair follicles in their renewal phase (Boussadia et al., 2002). Our preliminary analysis suggests that at least altered proliferation is involved since E-cadherin deficiency caused a strong reduction in the proliferation of matrix cells. It will be extremely interesting and challenging to elucidate this process further in the different phases of hair follicle development (reviewed in Stenn and Paus, 2001).
Is E-cadherin deficiency in mouse skin a potential disease model?
In Hailey–Hailey disease, a benign familial form of pemphigous and acantholysis, basal keratinocytes show a widening of intercellular spaces similar to the phenotype of epidermal E-cadherin-deficient mice (Burge, 1992). In this inherited skin disease, desmosomes are not affected, though E-cadherin expression is disturbed significantly in keratinocytes of patients (Burge and Schomberg, 1992; Hakuno et al., 2000). Further analysis will be required to determine a potential relationship to this and related skin disorders.
Materials and methods
Generation of conditional E-cadherin-deficient mice
To generate conditional E-cadherin-deficient mice, mice were bred as described (Young et al., 2002). Briefly, Krox20Cre/+; E-cad–/wt animals carrying the Krox20-Cre allele (Voiculescu et al., 2000) and the E-cadherin null allele (Larue et al., 1994) were inter-crossed. These mice were bred subsequently with animals homozygous for a floxed E-cadherin allele (E-cadlox/lox) (Boussadia et al., 2002). KroxCre/+; E-cad–/lox (mutant) were compared with Krox20Cre/+; E-cad+/lox littermates (control). Animals were genotyped as described (Young et al., 2002). The analysis of conditional E-cadherin-deficient animals was performed on a mixed C57BL/6/129 genetic background. The ROSA26R reporter transgenic line, kindly provided by Dr P.Soriano (Soriano, 1999), was used to monitor Cre expression and recombination.
Histology and immunohistochemistry
For histology and immunohistochemistry, back skin was taken from the interscapular region (Paus et al., 1999). Tissue was fixed in 4% paraformaldehyde (PFA) in 0.1 M cacodylate buffer pH 7.4 overnight and subsequently embedded in paraffin. Paraffin sections were cut in sections of 7 µm thickness for Masson trichrome staining. For immunohistochemistry, animals were anesthesized by intraperitoneal injection of sodium pentobarbital (100–150 µg/g body weight) and subsequently transcardially perfused with 0.25 mg/ml heparin in phosphate-buffered saline (PBS) followed by 4% PFA in 0.1 M cacodylate buffer pH 7.4 for 20 min. Tissue was embedded in optimal cutting compound (OCT), and cryosections of 10 µm thickness were cut. Vibrissae hair follicles were micro-dissected from animals, fixed by perfusion according to the scheme above, and subsequently embedded in OCT for longitudinal sectioning.
Sections were re-hydrated with PBS for 10 min, followed by blocking unspecific binding with 10% goat serum, Fab fragments of goat antibodies against mouse IgG (H + L, 0.13 mg/ml), and IgM chain-specific antibodies (0.14 mg/ml) (both Jackson Laboratories, West Grove, PA) in 0.1% Triton X-100. Incubation with different combinations of primary antibodies was carried out for 1–2 h at room temperature or at 4°C overnight depending on the antibody. Mouse monoclonal antibodies against K10 (Babco, Richmond, CA), claudin 1 (Zymed, St Louis, MO), ZO1 (Zymed) and nectin 1 (Santa Cruz Biotechnology, CA), rat monoclonal antibodies against E-cadherin (Vestweber and Kemler, 1984) and rabbit polyclonal antibodies against K6 (Babco), K14, desmoplakin (both from Serotec, Dottikon, Switzerland) and loricrin (kindly provided by Dr Daniel Hohl, Lausanne) were used. After incubation with the primary antibody, sections were washed three times for 5 min in PBS containing 0.1% Tween-20 (Sigma). As secondary antibodies, donkey fluorescein isothiocyanate (FITC)–anti-rabbit, goat Cy3–anti-rat and goat Cy3–anti-mouse antibodies (both Jackson Laboratories), or anti-rabbit A594 antibodies and anti-mouse A488 antibodies (both Molecular Probes, Leiden) were used. For peroxidase stainings, the StrepABComplex/HRP duet kit was used following the manufacturer’s instructions (Dako). Incubation was carried out for 1 h at room temperature. Primary and secondary antibodies were diluted in blocking buffer without Fab fragments. After the incubation with secondary antibodies, sections were washed in PBS and subsequently mounted in anti-fade AF1 (Citifluor, Canterbury, UK). In all experiments, appropriate controls with ‘secondary antibodies only’ incubations have been carried out and evaluated in parallel. All experiments were performed with at least three animals of each genotype.
Photographs were taken with conventional fluorescence microscopy using a Hamamatsu Colour chilled 3CCD Camera, SPOT II camera system (Visitron, München) or on a Leica confocal laser-scanning microscope (DM IRB/E true confocal scanner TCS NT). Figures were assembled with Adobe Photoshop 4.0.
BrdU labeling
To analyze keratinocyte proliferation, BrdU in vivo labelling was done in three P15 animals. BrdU (100 µg/ml) was injected 2 h prior to sacrifice. Back skin was dissected and fixed as described above. Tissue was embedded in paraffin. After dewaxing, sections were incubated with a peroxidase-conjugated monoclonal antibody against BrdU (Roche Diagnostics Ltd, Rotkreuz, Switzerland). Visualization was performed using the diaminobenzidine (DAB) peroxidase substrate kit (Vector Laboratories, Burlingame, CA).
Electron microscopy
For electron microscopy, three 20-week-old mutant animals and corresponding control littermates were used. After anesthesia and initial perfusion with 0.25 mg/ml heparin in 0.1 M PBS, a fixative solution containing 4% PFA, 2% glutaraldehyde in 0.1 M sodium cacodylate buffer pH 7.4 was applied. Back skin was dissected as described above, post-fixed for 24 h at 4°C, osmicated for 2 h in 2% osmium tetroxide at room temperature, washed in distilled H2O several times, dehydrated through graded acetone dilutions, once with 4% uranyl acetate in 70% acetone overnight and finally embedded in Spurr’s medium as described previously (Martini et al., 1995; Young et al., 2002). Semithin 0.5 µm sections were cut on an Ultracut 200 microtome (Leica, Wetzlar, Germany). Ultrathin sections were cut at 90 nm thickness, mounted on copper grids and contrasted with uranyl citrate and lead citrate. Photographs were taken on a JEOL 200C electron microscope.
Quantification and statistical analysis
Quantification of E-cadherin deficiency within the epidermis was performed by measuring areas devoid of E-cadherin immunoreactivity per visual field in three independent preparations. BrdU labeling was quantified in three independent experiments.
Statistical analysis was performed with the Mann–Whitney U-test (two-sided) using Statview 4.0 software. P-values of ≤0.05 were considered to be statistically significant.
Western blot analysis
Skin and nerve tissues were prepared and lysed in 0.3 ml of lysis buffer (50 mM Tris–HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1.0% SDS) using a Dounce homogenizer followed by three 20 s pulses with an ultrasonic tip. Cell debris was removed by centrifugation for 15 min at 30 000 g. The protein concentration was then determined using the micro BCA kit (Pierce). Taking 10 µg of protein lysate per lane, SDS loading buffer was added, and samples were boiled for 5 min before loading on a 10% SDS–polyacrylamide gel for electrophoresis. For immunoblotting, the resolved proteins were transferred to an Immobilon P membrane (Millipore) and incubated with antibodies against E-cadherin, β-catenin, plakoglobin (the latter both from Transduction Laboratories), tubulin or β-actin (both from Sigma Aldrich, St Louis, MO). The membranes were then washed three times with 10 mM Tris–HCl pH 7.4, 150 mM NaCl, 0.05% Tween-20 (TBST) and incubated with a goat-anti-mouse IgG-horseradish peroxidase-conjugated second antibody (1:20 000) for 1 h, followed by three washes with TBST. Finally, the membranes were developed with a chemiluminescence kit according to the manufacturer’s instructions (Pierce).
Acknowledgments
Acknowledgements
We thank P.Soriano for R26R mice, D.Hohl for antibodies, L.Gambardella and Y.Barrandon for expert advice in the preparation of hair follicles and vibrissae, J.Nowitzki, D.Nergenau and M.Apel for excellent technical assistance, S.Werner for excellent advice on the work and the manuscript, and E.B.Ringelstein and F.Stögbauer for critical reading of the manuscript. This work was supported by a research fellowship to P.Y. from the Deutsche Forschungsgemeinschaft, Bonn (YO 48/1-1), a grant of the commission ‘Innovative Medizinische Forschung’ (IMF, YO 120111), the National Center for Competence in Research ‘Neural Plasticity and Repair’ and the Swiss National Science Foundation.
References
- Aberle H., Schwartz,H. and Kemler,R. (1996) Cadherin–catenin complex: protein interactions and their implications for cadherin function. J. Cell. Biochem., 61, 514–523. [DOI] [PubMed] [Google Scholar]
- Boussadia O., Kutsch,S., Hierholzer,A., Delmas,V. and Kemler,R. (2002) E-cadherin is a survival factor for the lactating mouse mammary gland. Mech. Dev., 115, 53–62. [DOI] [PubMed] [Google Scholar]
- Braga V. (2000) Epithelial cell shape: cadherins and small GTPases. Exp. Cell Res., 261, 83–90. [DOI] [PubMed] [Google Scholar]
- Brakebusch C. et al. (2000) Skin and hair follicle integrity is crucially dependent on β1 integrin expression on keratinocytes. EMBO J., 19, 3990–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge S.M. (1992) Hailey–Hailey disease: the clinical features, response to treatment and prognosis. Br. J. Dermatol., 126, 275–282. [DOI] [PubMed] [Google Scholar]
- Burge S.M. and Schomberg,K.H. (1992) Adhesion molecules and related proteins in Darier’s disease and Hailey–Hailey disease. Br. J. Dermatol., 127, 335–343. [DOI] [PubMed] [Google Scholar]
- Caulin C., Bauluz,C., Gandarillas,A., Cano,A. and Quintanilla,M. (1993) Changes in keratin expression during malignant progression of transformed mouse epidermal keratinocytes. Exp. Cell Res., 204, 11–21. [DOI] [PubMed] [Google Scholar]
- Chidgey M. et al. (2001) Mice lacking desmocollin 1 show epidermal fragility accompanied by barrier defects and abnormal differentiation. J. Cell Biol., 155, 821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta R. and Fuchs,E. (1999) Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development, 126, 4557–4568. [DOI] [PubMed] [Google Scholar]
- Fuchs E. and Raghavan,S. (2002) Getting under the skin of epidermal morphogenesis. Nature Rev. Genet., 3, 199–209. [DOI] [PubMed] [Google Scholar]
- Furuse M., Hata,M., Furuse,K., Yoshida,Y., Haratake,A., Sugitani,Y., Noda,T., Kubo,A. and Tsukita,S. (2002) Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J. Cell Biol., 156, 1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambardella L., Schneider-Maunoury,S., Voiculescu,O., Charnay,P. and Barrandon,Y. (2000) Pattern of expression of the transcription factor Krox-20 in mouse hair follicle. Mech. Dev., 96, 215–218. [DOI] [PubMed] [Google Scholar]
- Garrod D.R., Merritt,A.J. and Nie,Z. (2002) Desmosomal adhesion: structural basis, molecular mechanism and regulation (review). Mol. Membr. Biol., 19, 81–94. [DOI] [PubMed] [Google Scholar]
- Gu H., Marth,J.D., Orban,P.C., Mossmann,H. and Rajewsky,K. (1994) Deletion of a DNA polymerase β gene segment in T cells using cell type-specific gene targeting. Science, 265, 103–106. [DOI] [PubMed] [Google Scholar]
- Hakuno M., Shimizu,H., Akiyama,M., Amagai,M., Wahl,J.K., Wheelock,M.J. and Nishikawa,T. (2000) Dissociation of intra- and extracellular domains of desmosomal cadherins and E-cadherin in Hailey–Hailey disease and Darier’s disease. Br. J. Dermatol., 142, 702–711. [DOI] [PubMed] [Google Scholar]
- Hodivala K.J. and Watt,F.M. (1994) Evidence that cadherins play a role in the downregulation of integrin expression that occurs during keratinocyte terminal differentiation. J. Cell Biol., 124, 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J. and Behrens,J. (2000) The Wnt signalling pathway. J. Cell Sci., 113, 35–45. [DOI] [PubMed] [Google Scholar]
- Huelsken J. and Birchmeier,W. (2001) New aspects of Wnt signaling pathways in higher vertebrates. Curr. Opin. Genet. Dev., 11, 547–553. [DOI] [PubMed] [Google Scholar]
- Huelsken J., Vogel,R., Erdmann,B., Cotsarelis,G. and Birchmeier,W. (2001) β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell, 105, 533–545. [DOI] [PubMed] [Google Scholar]
- Jamora C. and Fuchs,E. (2002) Intercellular adhesion, signalling and the cytoskeleton. Nature Cell Biol., 4, E101–E108. [DOI] [PubMed] [Google Scholar]
- Korswagen H.C., Herman,M.A. and Clevers,H.C. (2000) Distinct β-catenins mediate adhesion and signalling functions in C.elegans. Nature, 406, 527–532. [DOI] [PubMed] [Google Scholar]
- Larue L., Ohsugi,M., Hirchenhain,J. and Kemler,R. (1994) E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc. Natl Acad. Sci. USA, 91, 8263–8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue L., Antos,C., Butz,S., Huber,O., Delmas,V., Dominis,M. and Kemler,R. (1996) A role for cadherins in tissue formation. Development, 122, 3185–3194. [DOI] [PubMed] [Google Scholar]
- Martini R., Zielasek,J., Toyka,K.V., Giese,K.P. and Schachner,M. (1995) Protein zero (P0)-deficient mice show myelin degeneration in peripheral nerves characteristic of inherited human neuropathies. Nature Genet., 11, 281–286. [DOI] [PubMed] [Google Scholar]
- Muller-Rover S., Tokura,Y., Welker,P., Furukawa,F., Wakita,H., Takigawa,M. and Paus,R. (1999) E- and P-cadherin expression during murine hair follicle morphogenesis and cycling. Exp. Dermatol., 8, 237–246. [DOI] [PubMed] [Google Scholar]
- Munz B. et al. (1999) Overexpression of activin A in the skin of transgenic mice reveals new activities of activin in epidermal morphogenesis, dermal fibrosis and wound repair. EMBO J., 18, 5205–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievers M.G., Schaapveld,R.Q. and Sonnenberg,A. (1999) Biology and function of hemidesmosomes. Matrix Biol., 18, 5–17. [DOI] [PubMed] [Google Scholar]
- Ohsugi M., Larue,L., Schwarz,H. and Kemler,R. (1997) Cell-junctional and cytoskeletal organization in mouse blastocysts lacking E-cadherin. Dev. Biol., 185, 261–271. [DOI] [PubMed] [Google Scholar]
- Paus R. et al. (1999) A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J. Invest. Dermatol., 113, 523–532. [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M., Jamora,C. and Fuchs,E. (2003) Sticky business: orchestrating cellular signals at adherens junctions. Cell, 112, 535–548. [DOI] [PubMed] [Google Scholar]
- Schaapveld R.Q. et al. (1998) Hemidesmosome formation is initiated by the β4 integrin subunit, requires complex formation of β4 and HD1/plectin and involves a direct interaction between β4 and the bullous pemphigoid antigen 180. J. Cell Biol., 142, 271–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellheyer K., Bickenbach,J.R., Rothnagel,J.A., Bundman,D., Longley,M.A., Krieg,T., Roche,N.S., Roberts,A.B. and Roop,D.R. (1993) Inhibition of skin development by overexpression of transforming growth factor β1 in the epidermis of transgenic mice. Proc. Natl Acad. Sci. USA, 90, 5237–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. (1999) Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genet., 21, 70–71. [DOI] [PubMed] [Google Scholar]
- Stenn K.S. and Paus,R. (2001) Controls of hair follicle cycling. Physiol. Rev., 81, 449–494. [DOI] [PubMed] [Google Scholar]
- Stoler A., Duvic,M. and Fuchs,E. (1989) Unusual patterns of keratin expression in the overlying epidermis of patients with dermatofibromas: biochemical alterations in the epidermis as a consequence of dermal tumors. J. Invest. Dermatol., 93, 728–738. [DOI] [PubMed] [Google Scholar]
- Thewes M., Stadler,R., Korge,B. and Mischke,D. (1991) Normal psoriatic epidermis expression of hyperproliferation-associated keratins. Arch. Dermatol. Res., 283, 465–471. [DOI] [PubMed] [Google Scholar]
- Tinsley J.M., Fisher,C. and Searle,P.F. (1992) Abnormalities of epidermal differentiation associated with expression of the human papillomavirus type 1 early region in transgenic mice. J. Gen. Virol., 73, 1251–1260. [DOI] [PubMed] [Google Scholar]
- Tsukita S. and Furuse,M. (2002) Claudin-based barrier in simple and stratified cellular sheets. Curr. Opin. Cell Biol., 14, 531–536. [DOI] [PubMed] [Google Scholar]
- van Genderen C., Okamura,R.M., Farinas,I., Quo,R.G., Parslow,T.G., Bruhn,L. and Grosschedl,R. (1994) Development of several organs that require inductive epithelial– mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev., 8, 2691–2703. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V., Bauer,C., Degenstein,L., Wise,B. and Fuchs,E. (2001a) Hyperproliferation and defects in epithelial polarity upon conditional ablation of α-catenin in skin. Cell, 104, 605–617. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V., Bowers,E., Bauer,C., Degenstein,L. and Fuchs,E. (2001b) Desmoplakin is essential in epidermal sheet formation. Nature Cell Biol., 3, 1076–1085. [DOI] [PubMed] [Google Scholar]
- Vestweber D. and Kemler,R. (1984) Rabbit antiserum against a purified surface glycoprotein decompacts mouse preimplantation embryos and reacts with specific adult tissues. Exp. Cell Res., 152, 169–178. [DOI] [PubMed] [Google Scholar]
- Voiculescu O., Charnay,P. and Schneider-Maunoury,S. (2000) Expression pattern of a Krox-20/Cre knock-in allele in the developing hindbrain, bones and peripheral nervous system. Genesis, 26, 123–126. [DOI] [PubMed] [Google Scholar]
- Wang L.C. et al. (2000) Regular articles: conditional disruption of hedgehog signaling pathway defines its critical role in hair development and regeneration. J. Invest. Dermatol., 114, 901–908. [DOI] [PubMed] [Google Scholar]
- Yamanishi K. (1998) Gene-knockout mice with abnormal epidermal and hair follicular development. J. Dermatol. Sci., 18, 75–89. [DOI] [PubMed] [Google Scholar]
- Yap A.S., Brieher,W.M. and Gumbiner,B.M. (1997) Molecular and functional analysis of cadherin-based adherens junctions. Annu. Rev. Cell. Dev. Biol., 13, 119–146. [DOI] [PubMed] [Google Scholar]
- Young P., Boussadia,O., Berger,P., Leone,D.P., Charnay,P., Kemler,R. and Suter,U. (2002) E-cadherin is required for the correct formation of autotypic adherens junctions of the outer mesaxon but not for the integrity of myelinated fibers of peripheral nerves. Mol. Cell. Neurosci., 21, 341–351. [DOI] [PubMed] [Google Scholar]
- Zhu A.J. and Watt,F.M. (1996) Expression of a dominant negative cadherin mutant inhibits proliferation and stimulates terminal differentiation of human epidermal keratinocytes. J. Cell Sci., 109, 3013–3023. [DOI] [PubMed] [Google Scholar]