Abstract
Context
Although cigarette smokers have an increased risk of developing multiple sclerosis (MS), the effect of smoking on MS progression remains uncertain.
Objectives
To establish the relationship between cigarette smoking and MS progression using clinical and MRI outcomes
Design
Cross-sectional survey and longitudinal follow-up for an average of 3.29 years, ending January 15, 2008.
Setting
Partners MS Center (Boston, MA), a referral center for MS patients
Patients
1465 patients with clinically definite MS (25.1% men) with mean baseline age of 42.0 years (range: 16–75) and disease duration of 9.4 years (range: 0–50.4) -- 780 (53.2%) patients were never smokers, 428 (29.2%) ex-smokers, and 257 (17.5%) were current smokers.
Main Outcome Measures
Smoking groups were compared in terms of baseline clinical and MRI characteristics as well as progression and sustained progression on the expanded disability status scale (EDSS) at 2 years and 5 years and the time until conversion to secondary progressive MS. In addition, the rate of on-study change in the brain parenchymal fraction (BPF) and T2 hyperintense lesion volume was compared.
Results
Current smokers had significantly worse disease at baseline than never-smokers in terms of EDSS (adjusted p<0.0001), multiple sclerosis severity score (adjusted p<0.0001), and BPF (adjusted p=0.004). In addition, current smokers were significantly more likely to have primary progressive MS (adjusted odds ratio=2.41; 95% CI: 1.09, 5.34). In longitudinal analyses, smokers converted from relapsing remitting MS to secondary progressive MS faster than never-smokers (HR for current smokers versus never smokers, =2.50, 95% CI: 1.42, 4.41) and had a faster rate of increase in the T2 lesion volume (p=0.017) and a faster rate of decrease in BPF (p=0.021).
Conclusion
Our data suggest that cigarette smoke has an adverse influence on MS progression and accelerates the conversion from a relapsing-remitting to a progressive course.
Keywords: Disease progression, MRI, Multiple sclerosis, Smoking
Introduction
Cigarette smokers have a higher risk of developing multiple sclerosis (MS) than never-smokers 1, and smokers with clinically isolated syndromes (CIS) may convert to clinically definite MS sooner than non-smokers 2, but whether smoking has adverse effects on MS progression remains uncertain. In a study relying on prospectively collected smoking information on 179 patients with relapsing-remitting MS (RRMS), Hernan et al 3 found that ever smokers with RRMS converted to secondary progressive (SP) MS at a faster rate than never smokers. In contrast, Koch et al 4, in a retrospective study including 364 patients (164 of which had RRMS) found that cigarette smoking was not significantly associated with the development of secondary progressive MS or progression of clinical disability as measured by the expanded disability status scale (EDSS). Neither study reported whether smoking was associated with magnetic resonance imaging (MRI) markers of disease severity.
We therefore examined whether MS progresses faster in smokers than in non-smokers in a population of over 1,400 patients followed by serial clinical and brain MRI measures for an average of over 3 years in an MS clinic in Boston, MA.
Methods
Subjects
The study population comprised 1465 out of 1745 patients who completed a self-administered smoking questionnaire handed out to patients at the time of their visit to the Partners MS Center at the Brigham and Women’s Hospital between 2/13/2006 and 8/29/2007. To collect smoking history from the majority of patients regularly followed at the clinic, the questionnaires were distributed systematically to patients. Patients were eligible for the study if they had a diagnosis of clinically definite MS by McDonald criteria 5, 6 at the time of their last visit to the clinic. Excluded patients either never developed definite MS during follow-up (180), were missing smoking history (74), were exclusively pipe or cigar smokers (8), were missing disease category or age at baseline (16) or had errors in data entry (2). At baseline, patients were classified as RR (n=1020), SP (n=212), primary progressive (PP) (n=63), progressive relapsing (PR) (n=24), CIS (n=106), unspecified demyelinating disease (n=39) or suspected MS (n=1). The average number of follow up visits was 6.80 (SD=3.63) with irregular intervals between visits. The duration of clinical follow-up ranged from 0 to 7.63 years (mean (SD): 3.29 (1.81) years; median: 3.63 years). Eighty-five patients only had a baseline clinical visit.
Smoking history
Information on smoking history included current smoking status, age of starting and quitting, and average number of cigarettes smoked per day. Smoking status at the start of clinical and MRI observation for patients was determined using the age at the time of visit and the ages of smoking initiation and smoking cessation. Since some patients had significant time between the baseline MRI and clinical measurements, the smoking status at baseline for the analysis of clinical and MRI characteristics was determined separately.
Clinical outcomes
The three clinical outcomes of interest in our study were the expanded disability status scale (EDSS) score, the multiple sclerosis severity score (MSSS), which is a combination of the EDSS and the disease duration,7 and the patient’s disease category as classified by the physician. The MSSS was calculated using the global MSSS table provided in the original paper. The MSSS was only used for cross-sectional analyses in our work, but the change in the EDSS and the change in the disease category were used to define progression as described in the statistical analysis section.
MRI protocol
Patients were scanned with a T2-weighted axial dual echo protocol covering the whole brain (TR=3000 ms, TE=30/80 ms, 192 phase encoding steps, 256×256×54 voxels with 0.93 × 0.93×3 mm3 nominal voxel size and no interslice gaps). All baseline and follow-up scans were performed on the same 1.5 Tesla machine (GE Signa, General Electrics, Milwaukee, WI). MRI scans were available for 1045 patients; the mean number of scans per patient was 3.76 (SD=2.60), and the average follow-up period between the first and the last scan was 2.82 years (SD=2.42, range=0–13.3). Spinal cord imaging was not available on all patients, so no analysis of spinal cord data is included in this report.
Tissue class segmentation
An iterative combination of nearest neighbor multi-channel clustering (expectation-maximization (EM)-concept) and template-driven segmentation 8 was applied to segment white matter, gray matter, cerebrospinal fluid and hyperintense white matter signal abnormalities. The addition of a template-driven strategy to the EM-criterion showed excellent accuracy and robustness.9 The segmentation derived whole brain T2 hyperintense lesion volume and brain parenchymal fraction (BPF), a marker of whole brain atrophy. 9
Study design
The relationship between smoking behavior and MS progression was independently examined in cross-sectional and longitudinal analyses. In the cross-sectional analyses, we examined whether smoking history up to the time of the baseline visit or MRI (i.e. the first recorded clinical or imaging examination at the MS Partners Center) was related to MS severity at baseline. In longitudinal analyses, we examined whether the smoking history up to baseline contributed to predict the future progression of MS over the period of follow-up.
Statistical analysis
The baseline demographic, clinical and MRI characteristics were compared using Kruskal-Wallis, Wilcoxon and Chi-square tests when appropriate. To adjust for potential confounders, rank ANCOVA was used for comparisons involving EDSS or MSSS and linear regression on appropriately transformed variables was used for the MRI characteristics. 10 Potential confounders were age, disease duration from first symptom and gender. In addition, ever smokers were broken into three categories based on the smoking severity at study entry: <=3, between 3 and 20, >20 pack years. Baseline comparisons of these groups were conducted controlling for age, disease duration, and gender. Finally, the proportion of patients who had PPMS was compared across the smoking groups.
The association between smoking and the time to conversion from RRMS to SPMS was examined using a Cox proportional hazards model controlling for baseline age, disease duration, gender and treatment status. Treatment status at baseline was defined as the presence or absence of primary therapy (interferon-beta or glatiramer acetate) and presence or absence of secondary therapy (all other MS treatments). Patient were censored at their last available clinical visit if they had not yet converted. Further, we compared the proportion of patients who progressed on the EDSS after two years and after five years across the smoking groups. Progression on EDSS was defined as an increase of at least 1 point when baseline EDSS was less than 6, or an increase of at least 0.5 point when baseline EDSS was at least 6. Sustained progression, defined as progression on EDSS maintained for two observations spaced at least 6 months apart, was also investigated. The comparisons of EDSS progression were conducted using logistic regression controlling for baseline age, disease duration, gender and treatment status.
For longitudinal MRI measurements, mixed effects models controlling for baseline age, disease duration, gender, and disease course (RR/SP, PP/PR) were fit to the BPF and the log-transformed T2 lesion volume. Extreme changes in the BPF (>5% change on consecutive measurements) were excluded from the analyses because these were likely the results of technical errors. This reduced the sample size from 3932 scans on 1045 patients to 3887 scans on 1040 patients for the BPF analysis. All scans were used for the T2 lesion volume analysis. In all group comparisons, smoking status was defined at baseline even though a subset of patients changed group; given the small number of patients who switched, any bias introduced by this assumption is likely small.
Results
Baseline characteristics
At baseline, the patient population consisted of 257 current smokers (17.5%), 428 ex-smokers (29.2%), and 780 never smokers (53.2%). Only seven of the never smokers began smoking during follow-up, and 57 of the current smokers stopped smoking during follow-up. The baseline characteristics of the three groups are summarized in Table 1. As compared with never smokers, the EDSS was significantly higher in current smokers (p, adjusted for age, gender, and disease duration, <0.0001), but not significantly different in ex-smokers (adjusted p=0.22). Similar results were seen in comparisons for the MSSS controlling for age and gender (p<0.0001 for comparisons of current smokers vs. never smokers, p=0.47 for ex-smokers vs. never smokers). These results were unchanged after controlling for disease course at first visit. Comparisons of ex-smokers and current smokers are provided in an on-line supplement to this paper.
Table 1.
Baseline characteristics for the smoking groups.
| Current smokers | Ex-smokers | Non-smokers | Univariate p-value + | Adjusted p-value* | |
|---|---|---|---|---|---|
| Number of patients | 257 | 428 | 780 | ||
| Age (mean +/− SD) | 39.2 +/− 10.5 | 46.4 +/− 10.7 | 40.5 +/− 10.9 | <0.0001 | |
| Disease duration from first symptom (mean +/− SD) | 7.93 +/− 8.60 | 11.7 +/− 10.2 | 8.59 +/− 8.52 | <0.0001 | |
| Gender (F, M) | 196, 61 | 312, 116 | 589, 191 | 0.52 | |
| BPF (mean +/− SD) | 0.869 +/− 0.047 | 0.858 +/−0.047 | 0.876 +/−0.045 | <0.0001 | 0.009 |
| T2 lesion volume (cm3) (mean +/− SD) | 4.70 +/− 4.59 | 5.09 +/− 4.26 | 4.13 +/− 3.64 | 0.002 | 0.0081 |
| EDSS (median, IQR) | 2 (1,3.5) | 2 (1,3.5) | 1.5 (1,3) | <0.0001 | <0.0001 |
| MSSS (mean +/− SD) | 4.15 +/− 2.90 | 3.68 +/− 2.71 | 3.32 +/− 2.74 | 0.0003 | 0.0001 |
| Type of MS (RR, SP, PP, other) | (179, 28, 12, 38) | (270, 84, 31, 43) | (571, 100, 20, 89) | <0.0001 | |
| One year follow-up (%) | 85.6 | 84.6 | 83.6 | ||
| Number of follow-up visits (mean +/− SD) | 6.59 +/− 3.41 | 6.69 +/− 3.57 | 6.93 +/− 3.74 | 0.37 | |
| IFN-GA treatment (Y/N) | 114/143 | 228/200 | 379/401 | 0.068 | |
| Other treatments (Y/N) | 18/239 | 35/393 | 41/739 | 0.13 |
Key: BPF=brain parenchymal fraction; EDSS=expanded disability status score; MSSS=multiple sclerosis severity score; RR=relapsing remitting; SP=secondary progressive; PP=primary progressive; IFN-GA =interferon-beta or glatiramer acetate; Other treatments= cyclophosphamide, natalizumab, rituximab, daclizumab, mitoxantrone or methotrexate;
= p-values are for 2 degree of freedom comparison of 3 groups;
= p-values are for 2 degree of freedom comparison of 3 groups adjusting for age, gender and disease duration as appropriate
Given the relationship between smoking and clinical status, the effect of severity of smoking was investigated by comparing patients based on pack years smoked at study entry. Ever smokers were classified into 3 groups as described previously (Table 2). The EDSS was significantly lower in the light smoking group compared to the moderate smoking group (adjusted p=0.040) and compared to the heavy smoking group (adjusted p=0.025). The MSSS was also significantly higher in the heavy smoking group compared to the light smoking group (adjusted p=0.038) and the moderate smoking group (adjusted p=0.048).
Table 2.
Baseline characteristics for the patients based on smoking duration (smokers only).
| 3 pack years or less | Between 3 and 20 pack years | More than 20 pack years | Univariate p-value+ | Adjusted p-value* | |
|---|---|---|---|---|---|
| Number of patients | 163 | 327 | 164 | ||
| Age (mean +/− SD) | 38.9 +/− 11.2 | 42.6 +/− 10.7 | 50.5 +/− 9.0 | <0.0001 | |
| Disease duration from first symptom (mean +/− SD) | 8.60 +/− 8.68 | 10.03 +/− 9.73 | 12.50 +/−10.73 | 0.0013 | |
| Gender (F/M) | 124, 39 | 255, 72 | 108, 56 | 0.013 | |
| BPF (mean +/− SD) | 0.873 +/−0.049 | 0.864 +/−0.046 | 0.847 +/−0.045 | <0.0001 | 0.53 |
| T2 lesion volume (cm3) (mean +/− SD) | 4.64 +/− 3.75 | 5.02 +/− 4.65 | 5.10 +/− 4.55 | 0.72 | 0.84 |
| EDSS (median, IQR) | 1.5 (1,3) | 2 (1,3.5) | 2 (3, 6) | <0.0001 | 0.011 |
| MSSS (mean +/−SD) | 3.31 +/− 2.76 | 3.77 +/− 2.76 | 4.57 +/− 2.76 | 0.0002 | 0.019 |
| Type of MS (RR, SP, PP, other) | (119, 16, 8, 20) | (211, 59, 17, 40) | (101, 32, 16, 15) | 0.044 |
Key: BPF=brain parenchymal fraction; EDSS=expanded disability status score; MSSS=multiple sclerosis severity score; RR=relapsing remitting; SP=secondary progressive; PP=primary progressive;
= p-values are for 2 degree of freedom comparison of 3 groups;
= p-values are for 2 degree of freedom comparison of 3 groups adjusting for age, gender and disease duration as appropriate. 25 patients had missing number of cigarettes smoked per day and an additional six patients stopped smoking prior to the study but were missing the age of smoking initiation. All of these patients are not included in this table.
The probability of a PP course of MS, as determined at the time of the baseline visit, compared to an initially relapsing course of MS (RRMS or SPMS at baseline visit) was higher among current smokers (odds ratio, adjusted for age, disease duration and sex, = 2.42; 95% CI: 1.09, 5.35) or ex-smokers (adjusted odds ratio = 1.91; 95% CI: 1.02, 3.58) than never smokers.
The higher degree of MS severity among smokers was also seen in analyses of MRI factors. Current smokers had significantly lower BPF than never smokers (adjusted p=0.0042), although no significant difference was observed between ex-smokers and never smokers (adjusted p= 0.060). The T2 lesion volume was significantly higher in ex-smokers compared to never smokers (adjusted p=0.002), and there was no significant difference between current and never smokers (adjusted p=0.22). There was no significant association between of pack years of smoking and either MRI measure after adjusting for age, disease duration and sex.
Longitudinal analysis
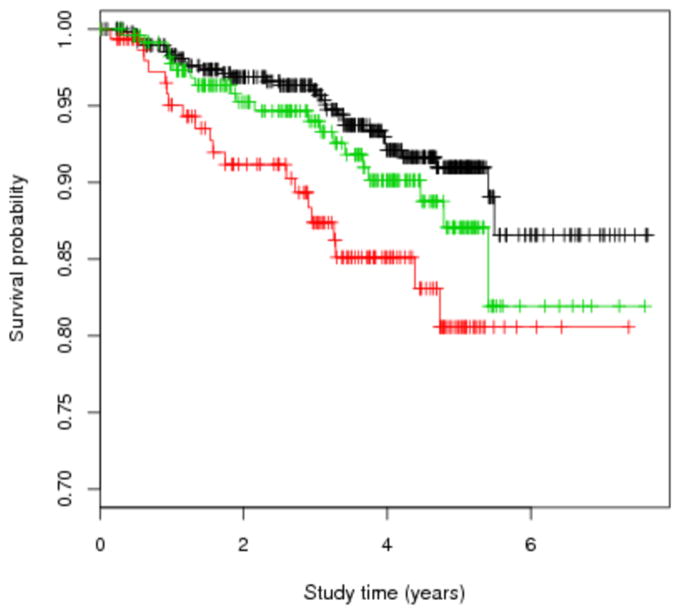
The survival analysis for conversion from RRMS to SPMS included 891 patients, of which 154 were current smokers, 237 ex-smokers, and 500 never smokers at baseline. During an average follow-up of 3.34 years (SD=1.70, median=3.56), conversion to SPMS occurred in 72 patients (20 smokers, 20 ex-smokers, and 32 never smokers; Figure 1). The conversion from RRMS to SPMS occurred at faster rate in current smokers than in never smokers (adjusted hazard ratio= 2.50, 95% CI: 1.42, 4.41), but was similar in ex-smokers and never smokers (1.05, 95% CI: 0.59, 1.84). Similar results were obtained in analyses controlling for baseline EDSS (adjusted hazard ratio = 2.08, 95% CI: 1.15, 3.77 for current smokers vs. never smokers; adjusted hazard ratio = 0.95, 95% CI: 0.54, 1.68 for ex-smokers vs. never smokers). In contrast, we found no association between smoking status and EDSS progression at the end of 2 years; the proportion who progressed was 23.3% among smokers, 30.8% among ex-smokers, and 26.0% among never smokers (p, adjusted for baseline age, disease duration, gender and treatment, = 0.57). The probability of sustained progression was also not significantly different across the groups (adjusted p=0.53). Similar results were found in terms of progression at five years. Results were similar in analyses restricted to patients with RRMS at baseline.
Figure 1. Kaplan-Meier curve for time until conversion from relapsing-remitting to secondary progressive MS.
Legend: Smoking status was defined at study entry. Current smokers progressed significantly faster than the never smokers (p=0.0016). Red=current smokers, green=ex-smokers, black=never smokers.
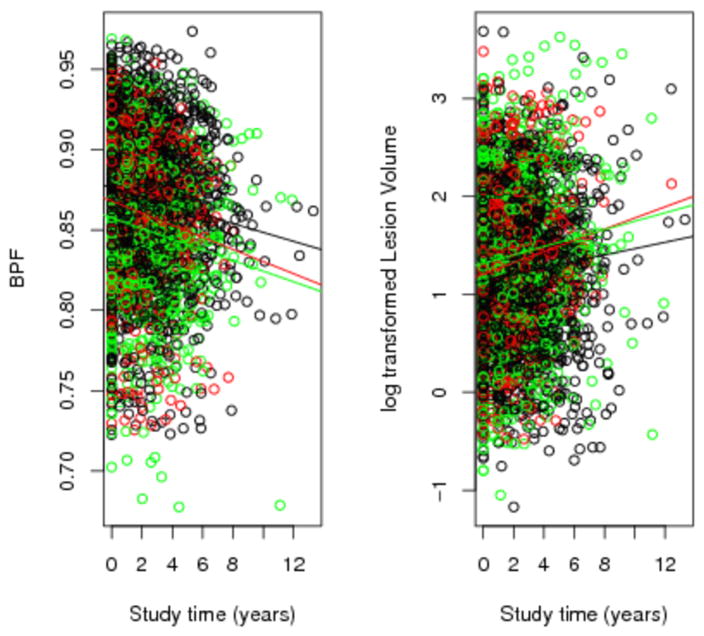
Finally, the progression of patients in terms of BPF and T2 lesion volume was investigated (Figure 2). Current smokers compared to never smokers had a significantly greater increase in the T2 lesion volume (p, adjusted for baseline age, disease duration, gender, and disease course = 0.017) and a significantly greater decrease in the BPF (adjusted p = 0.021), but the past smokers and never smokers were not significantly different on either measure.
Figure 2. Change in the brain parenchymal fraction (BPF) and log-transformed T2 lesion volume over time.
Legend: The unadjusted trend over time is provided. The change over time in the BPF was significantly greater in current smokers compared to never smokers (adjusted p=0.021). The change over time in the T2 lesion volume was significantly greater in current smokers compared to the never smokers (adjusted p=0.017). Red=current smokers, green=ex-smokers, black=never smokers.
Discussion
An aggravation of MS symptoms soon after smoking has been reported in several early studies 11–17, but only two previous investigations have examined whether smoking adversely affects MS progression. Hernan et al., in a case-control investigation nested within the UK General Practice Research Database, reported a three-fold higher rate of conversion in ever smokers as compared to never smokers. 3 Those results, however, were based on a relatively small sample --179 RRMS patients, of which only 20 converted to SPMS during the follow-up. Further, no MRI results were available. Koch et al. examined the relationship between smoking and MS progression within a database comprising clinical information collected prospectively since 1985 on 672 patients attending the MS clinic of the University Medical Center Groningen.4 Smoking information was collected by mailed questionnaires or telephone interviews in 2006, and was available for 364 patients. In this population, smoking was not associated with the rate of conversion from RRMS to SPMS or with time from disease onset to EDSS scores 4.0 or 6.0. The reasons for the conflicting results between the two studies are not entirely clear, but a potential source of bias in the Groningen study is that it was conducted over 20 years after the recruitment of the first MS patients into the database, and a substantial proportion had died or could not be contacted to complete the smoking questionnaire.
The present investigation, because of the substantially larger sample size, had more power to assess the relationship between smoking and MS progression than the previous studies combined. Further strengths include a detailed smoking history in almost all patients, and the availability of multiple clinical examinations and standardized MRI assessment during the follow-up. Although the smoking history was collected retrospectively, smoking behavior is usually well recalled and bias from misclassification of smoking status is thus likely to be small. The potential bias associated with sicker patients starting to smoke seems likely to be small as well. Another possible source of bias, which could affect this as well as previous studies, is a preferential selection or retention in the study population of smokers with more severe or more rapidly progressing MS, or never smokers with less severe or less rapidly progressing MS. This occurrence cannot be excluded, but it seems unlikely. As in all observational studies, we cannot exclude the possibility that the results presented are confounded by unknown factors. These may include genetic mutations that predispose to both addictive behavior and MS severity, and other behavioral or environmental exposures, including alcohol consumption, that were not collected on our patients. Finally, our study was conducted among patients seen at a specialized MS clinic and therefore the results may not be generalized to all MS patients. However, the fact that similar results on smoking and rates of conversion to SPMS were found in the study by Hernan et al., which was conducted in a population-based cohort of MS patients, adds to the generalizability of this finding.
The results of numerous studies, including four rigorous prospective investigations and a population survey 18, provide strong support for smoking as a risk factor for MS development. 1 That this association reflects a causal effect is suggested, albeit not proven, by the fact that the risk of MS increases with the duration and intensity of smoking and declines with time since quitting smoking. 19 Further, preliminary results suggest that exposure to passive smoking may also increase the risk of MS. 20, 21 The present findings provide additional evidence that smoking may adversely affect the underlying disease process in MS, and suggest that these adverse effects of smoking may extend from the period preceding the clinical onset of MS to at least the time of conversion to a progressive course in the case of MS with RR onset. Further, the observation that the rate of conversion from RRMS to SPMS was significantly higher in current smokers than in ex-smokers provides evidence that the adverse effects of smoking may be at least in part reverted by quitting.
An adverse effect of smoking on MS progression would be consistent with the results of experimental studies and of epidemiological investigations of other neurodegenerative or autoimmune diseases. Components of cigarette smoke may have neurotoxic effects 22 and tobacco smoke components such as cyanides have been associated with demyelination in animals. 23, 24 Other chemicals in smoke can compromise the blood-brain barrier (e.g. nicotine)25 or have immunomodulatory effects. 26, 27 Further, cigarette smoking contains nitric oxide (NO), and nicotine may induce the release of NO in the CNS 3; NO metabolites in the CSF may contribute to axonal degeneration and are associated with MS progression.28 In some previous studies of healthy individuals, smokers were found to have smaller gray matter volume 29 and a higher brain lesion load 30. Similar effects in individuals with MS may explain the baseline difference in the BPF between smokers and never-smokers in our study, and may possibly contribute to accelerate the conversion to a progressive phase. In epidemiological studies, a neurotoxic effect of tobacco smoke is supported by its association with optic neuropathy. 31 On the other hand, the association between smoking and an increased risk of several autoimmune diseases, including rheumatoid arthritis, systemic lupus erythematosus, Graves’ hyperthyroidism, and primary biliary cirrhosis 32, suggests that the effects of smoking may be at least in part related to its effects on the immune system. Finally, cigarette smoke increases the frequency and duration of respiratory infections 33, which have been linked to MS risk and to the occurrence of MS relapses. 34
Three additional limitations should be considered when interpreting our results. First, because our study population did not include healthy controls, we could not determine the specific effect of smoking on MRI measures in MS patients; some general consequences of smoking could have been mistakenly attributed to MS progression. Second, as mentioned in the methods section, spinal cord imaging was not available on all of our patients, and therefore we cannot determine whether smoking has adverse effects on the spinal cord. Third, although smokers had higher disease severity than non-smokers according to both clinical and imaging measures, a dose response effect was only evident on clinical progression; this result should be validated in an outside sample.
Conclusion
In summary, the results of this large and in part prospective investigation support the hypothesis that cigarette smoking has an adverse effect on MS progression as measured by clinical and MRI outcomes. Although causality remains to be proven, these findings suggest that individuals with MS who quit smoking may not only reduce their risk of smoking related diseases, but also delay the progression of MS itself.
Acknowledgments
The authors report no conflicts of interest with regards to the presented work. The work was supported by the Partners MS Center and NINDS/NIH Molecular Epidemiology of EBV and Multiple Sclerosis, R01 NS047467 (AA). The following authors contributed to conception and design (CRGG, AA), to acquisition of data (EA, CRGG, TC, BIG, GB, MH, LS, JM, AMB, YD, RB, SK, HW, AA), to analysis and interpretation of data (BCH, EA, CRGG, TC, RB, AA), to drafting the manuscript (BCH, EA, AA), and to critical revision of the manuscript (BCH, EA, CRGG, TC, BIG, GB, MH, LS, JM, AMB, YD, RB, SK, HW, AA). AA had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We would also like to acknowledge Mariann Polgar-Turcsanyi, Sandra Cook, Karen Himmelberger, and Leslie Unger for help in preparation of the data/manuscript
References
- 1.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: Noninfectious factors. Ann Neurol. 2007;61(6):504–13. doi: 10.1002/ana.21141. [DOI] [PubMed] [Google Scholar]
- 2.Di Pauli F, Reindl M, Ehling R, et al. Smoking is a risk factor for clinically definite multiple sclerosis. Mult Scler. 2008;14(8):1026–30. doi: 10.1177/1352458508093679. [DOI] [PubMed] [Google Scholar]
- 3.Hernan MA, Jick SS, Logroscino G, et al. Cigarette smoking and the progression of multiple sclerosis. Brain. 2005;128(Pt 6):1461–5. doi: 10.1093/brain/awh471. [DOI] [PubMed] [Google Scholar]
- 4.Koch M, van Harten A, Uyttenboogaart M, De Keyser J. Cigarette smoking and progression in multiple sclerosis. Neurology. 2007;69(15):1515–20. doi: 10.1212/01.wnl.0000277658.78381.db. [DOI] [PubMed] [Google Scholar]
- 5.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121–7. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 6.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58(6):840–6. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 7.Roxburgh RH, Seaman SR, Masterman T, et al. Multiple Sclerosis Severity Score: using disability and disease duration to rate disease severity. Neurology. 2005;64(7):1144–51. doi: 10.1212/01.WNL.0000156155.19270.F8. [DOI] [PubMed] [Google Scholar]
- 8.Warfield SK, Kaus M, Jolesz FA, Kikinis R. Adaptive, template moderated, spatially varying statistical classification. Med Image Anal. 2000;4(1):43–55. doi: 10.1016/s1361-8415(00)00003-7. [DOI] [PubMed] [Google Scholar]
- 9.Wei X, Warfield SK, Zou KH, et al. Quantitative analysis of MRI signal abnormalities of brain white matter with high reproducibility and accuracy. J Magn Reson Imaging. 2002;15(2):203–9. doi: 10.1002/jmri.10053. [DOI] [PubMed] [Google Scholar]
- 10.Stokes ME, Davis CS, Koch GG. Categorical data analysis using the SAS system. 2. Wiley-SAS; 2001. [Google Scholar]
- 11.Franklin CR, Brickner RM. Vasospasm associated with multiple sclerosis. Arch Neruol Psychiatry. 1947;58:125–62. doi: 10.1001/archneurpsyc.1947.02300310003001. [DOI] [PubMed] [Google Scholar]
- 12.Spillane J. The effect of nicotine on spinocerebellar ataxia. Br Med J. 1955;2:1345–51. doi: 10.1136/bmj.2.4952.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MB, BS Smoking and multiple sclerosis (Letter) Br Med J. 1964;1:773. [Google Scholar]
- 14.Perkin GD, Bowden P, Rose FC. Smoking and optic neuritis. Postgrad Med J. 1975;51(596):382–5. doi: 10.1136/pgmj.51.596.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perkin GD, Rose FC. Uhthoff’s syndrome. Br J Ophthalmol. 1976;60(1):60–3. doi: 10.1136/bjo.60.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emre M, de Decker C. Effects of cigarette smoking on motor functions in patients with multiple sclerosis. Arch Neurol. 1992;49(12):1243–7. doi: 10.1001/archneur.1992.00530360041015. [DOI] [PubMed] [Google Scholar]
- 17.Emre M, de Decker C. Nicotine and CNS. Neurology. 1987;37(12):1887–8. doi: 10.1212/wnl.37.12.1887-b. [DOI] [PubMed] [Google Scholar]
- 18.Riise T, Nortvedt MW, Ascherio A. Smoking is a risk factor for multiple sclerosis. Neurology. 2003;61(8):1122–4. doi: 10.1212/01.wnl.0000081305.66687.d2. [DOI] [PubMed] [Google Scholar]
- 19.Hernán MA, Olek MJ, Ascherio A. Cigarette smoking and incidence of multiple sclerosis. Am J Epidemiol. 2001;154(1):69–74. doi: 10.1093/aje/154.1.69. [DOI] [PubMed] [Google Scholar]
- 20.Mikaeloff Y, Caridade G, Tardieu M, Suissa S. Parental smoking at home and the risk of childhood-onset multiple sclerosis in children. Brain. 2007;130(Pt 10):2589–95. doi: 10.1093/brain/awm198. [DOI] [PubMed] [Google Scholar]
- 21.Sundstrom P, Nystrom L, Hallmans G. Smoke exposure increases the risk for multiple sclerosis. Eur J Neurol. 2008;15(6):579–83. doi: 10.1111/j.1468-1331.2008.02122.x. [DOI] [PubMed] [Google Scholar]
- 22.Smith ADM, Duckett S, Waters AH. Neuropathological changes in chronic cyanide intoxication. Nature. 1963;200:179–81. doi: 10.1038/200179a0. [DOI] [PubMed] [Google Scholar]
- 23.Bass NH. Pathogenesis of myelin lesions in experimental cyanide encephalopathy. A microchemical study. Neurology. 1968;18(2):167–77. doi: 10.1212/wnl.18.2.167. [DOI] [PubMed] [Google Scholar]
- 24.Lessell S. Experimental cyanide optic neuropathy. Arch Ophthalmol. 1971;86(2):194–204. doi: 10.1001/archopht.1971.01000010196014. [DOI] [PubMed] [Google Scholar]
- 25.Chen JL, Wei L, Bereczki D, et al. Nicotine raises the influx of permeable solutes across the rat blood-brain barrier with little or no capillary recruitment. J Cereb Blood Flow Metab. 1995;15(4):687–98. doi: 10.1038/jcbfm.1995.85. [DOI] [PubMed] [Google Scholar]
- 26.Sopori ML, Kozak W. Immunomodulatory effects of cigarette smoke. J Neuroimmunol. 1998;83(1–2):148–56. doi: 10.1016/s0165-5728(97)00231-2. [DOI] [PubMed] [Google Scholar]
- 27.Francus T, Klein RF, Staiano-Coico L, Becker CG, Siskind GW. Effects of tobacco glycoprotein (TGP) on the immune system. II. TGP stimulates the proliferation of human T cells and the differentiation of human B cells into Ig secreting cells. J Immunol. 1988;140(6):1823–9. [PubMed] [Google Scholar]
- 28.Rejdak K, Eikelenboom MJ, Petzold A, et al. CSF nitric oxide metabolites are associated with activity and progression of multiple sclerosis. Neurology. 2004;63(8):1439–45. doi: 10.1212/01.wnl.0000142043.32578.5d. [DOI] [PubMed] [Google Scholar]
- 29.Gallinat J, Meisenzahl E, Jacobsen LK, et al. Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci. 2006;24(6):1744–50. doi: 10.1111/j.1460-9568.2006.05050.x. [DOI] [PubMed] [Google Scholar]
- 30.Fukuda H, Kitani M. Cigarette smoking is correlated with the periventricular hyperintensity grade of brain magnetic resonance imaging. Stroke. 1996;27(4):645–9. doi: 10.1161/01.str.27.4.645. [DOI] [PubMed] [Google Scholar]
- 31.The Cuba Neuropathy Field Investigation Team. Epidemic optic neuropathy in Cuba--clinical characterization and risk factors. The Cuba Neuropathy Field Investigation Team. N Engl J Med. 1995;333(18):1176–82. doi: 10.1056/NEJM199511023331803. [DOI] [PubMed] [Google Scholar]
- 32.Costenbader KH, Karlson EW. Cigarette smoking and autoimmune disease: what can we learn from epidemiology? Lupus. 2006;15(11):737–45. doi: 10.1177/0961203306069344. [DOI] [PubMed] [Google Scholar]
- 33.Graham NM. The epidemiology of acute respiratory infections in children and adults: a global perspective. Epidemiol Rev. 1990;12:149–78. doi: 10.1093/oxfordjournals.epirev.a036050. [DOI] [PubMed] [Google Scholar]
- 34.Sibley WA, Bamford CR, Clark K. Clinical viral infections and multiple sclerosis. Lancet. 1985;1(8441):1313–5. doi: 10.1016/S0140-6736(85)92801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]