Abstract
Abnormal vascular remodeling mediated by inflammatory cells has been identified as a key pathologic component of various vascular diseases, including abdominal aortic aneurysms, brain arteriovenous malformations and atherosclerosis. Based on findings from observational studies that analysed human intracranial aneurysms and experimental studies that utilized animal models, an emerging concept suggests that a key component of the pathophysiology of intracranial aneurysms is sustained abnormal vascular remodeling coupled with inflammation. This concept may provide a new treatment strategy to utilize agents to inhibit inflammation or cytokines produced by inflammatory cells such as matrix metalloproteinases. Such an approach would aim to stabilize these vascular lesions and prevent future expansion or rupture.
Keywords: Intracranial aneurysms, inflammation, remodeling, pathophysiology
Approximately 27,000 Americans are estimated to suffer subarachnoid hemorrhage resulted from ruptured intracranial aneurysms each year1. Ruptured intracranial aneurysms often result in catastrophic consequences causing severe morbidity and high mortality2. Despite recent advancements in the diagnosis and treatment of intracranial aneurysms, mechanisms for the formation, development and subsequent rupture of intracranial aneurysms are not well understood.
Abnormal vascular remodeling mediated by inflammatory cells has been identified as a key pathologic component of various vascular diseases, including abdominal aortic aneurysms, brain arteriovenous malformations (BAVM) and atherosclerosis3–6. This concept may provide a new treatment strategy to utilize agents to inhibit inflammation or cytokines produced by inflammatory cells such as matrix metalloproteinases (MMPs). Such an approach would aim to stabilize these vascular lesions and prevent future expansion or rupture (Figure 1).
Figure 1.
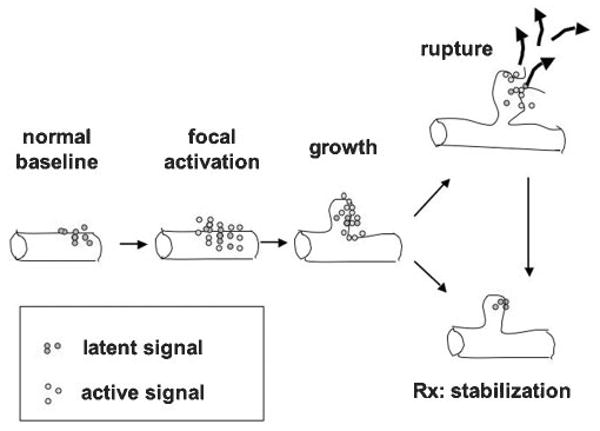
Schematic representation of hypothetic events in the development of aneurysms leading to aneurysm formation. Hemodynamic forces cause activation of factors in vessel wall, for example, MMPs. This initiation leads to lesion growth. Rupture may follow or stabilization may occur. Potential medical therapies might be aimed at promoting stabilization. Different phases may not be mechanistically determined by the same class of signals
Based on findings from observational studies that analysed human intracranial aneurysms and experimental studies that utilized animal models, an emerging concept suggests that a key component of the pathophysiology of intracranial aneurysms is sustained abnormal vascular remodeling coupled with inflammation7,8.
Hemodynamic Stress and Intracranial Aneurysms
Vascular remodeling is intimately tied to hemodynamics. Blood vessel is an active organ that can undergo a dynamic remodeling process in response to acute and chronic changes in blood flow, blood pressure and metabolism9,10.
Sustained hemodynamic stresses, especially sustained high blood flow, result in flow-induced outward remodeling, which is an adaptive process of the vascular wall to reduce wall shear stress to physiologic baseline values in response to a chronic increase in blood flow9–12. This adaptive process is characterized by an increase in luminal dimension with relatively small change in wall thickness9. Wall shear stress, a tangential force on endothelial cell lining exerted by blood flow, is a major determinant of vascular remodeling13.
Intracranial aneurysms are commonly found in the location where abnormal hemodynamic stresses are exerted on the vascular wall14. These locations include the junction of the internal carotid artery and the posterior communicating artery, the anterior communicating artery complex and the bifurcation of the basilar artery1. Vascular remodeling triggered by abnormal hemodynamic stress on the blood vessels inside the circle of Willis may play a role in the formation, development and subsequent rupture of intracranial aneurysms. Increased hemodynamic stresses especially elevated wall shear stress at the bifurcation or the outer lateral curve of blood vessels may trigger physiologic vascular remodeling as an adaptive process15.
A number of studies have estimated the hemodynamic stresses on the cerebrovasculature and aneurysmal wall by analysing either idealized aneurysm models or clinical data obtained by angiography or magnetic resonance imaging technique15–19. Various mathematic models were used to estimate the wall shear stress and pressure. These studies indeed predicted that an apex of bifurcation or the outer lateral wall of a curved blood vessel, common sites of intracranial aneurysms, have abnormally high hemodynamic stresses, i.e. high pressure and shear stress. Such hemodynamic stresses may lead to the initiation of normal adaptive outward vascular remodeling.
However, owing to complex geometry of these locations, adaptive flow-induced outward vascular remodeling becomes asymmetric remodeling that can be recognized as a pre-aneurysmal change. Once such pre-aneurysm starts forming, the distal neck of aneurysmal wall will have sustained hemodynamic stress15,17. Asymmetric and sustained outward vascular remodeling will eventually lead to the growth of aneurysms.
The ‘impact zone’, the location on the aneurysmal wall exposed to high hemodynamic stresses, can be better characterized by elevated local wall shear stress than elevated local pressure15. The flow impact results in two orthogonal forces. The direct ‘impacting force’ or ‘dynamic pressure’ at the stagnation point is perpendicular to the vessel wall and the wall shear stress, the viscous friction from the bloodstream that passes next to the stagnation point, is tangential to the wall (Figure 2).
Figure 2.
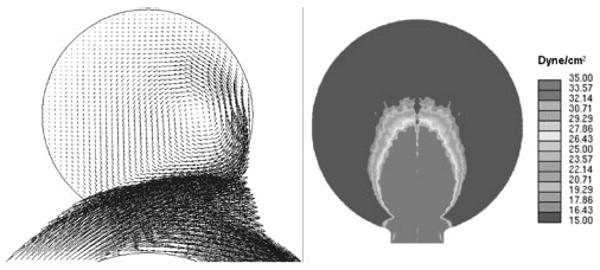
Flow impingement and the resulting ‘impact zone’ at the distal wall of an idealized saccular aneurysm on a curved vessel, obtained from computational fluid dynamics simulation: (A) Two-dimensional velocity field in the center plane. Vectors represent both magnitude and direction of velocity; (B) wall shear stress (WSS) distribution at the distal aneurysm wall. Color scale represents WSS values from below 15 (deep blue) to above 35 dynes/cm2 (bright red). The maximum WSS value is 298 dynes/cm2. Following Hoi et al.15, we define the impact zone as the area where WSS>20 dynes/cm2 (a value considered as an upper limit for normal physiologic condition)
Although it has been suggested that the ‘impacting force’ or the resulting local pressure elevation may contribute significantly to aneurysm development20–23, some studies suggested that such focal pressure elevations are rather insignificant or may not be a critical physical force. Two studies24,25 reported that the local pressure elevations at branch points and bends in arteries were ∼1 mmHg, which is only 1–2% of the peak intravascular pressure. The pressure elevation inside the aneurysmal sac produced by flow impact was found to be even weaker24. However, substantial wall shear stresses were found beside the direct impingement point: 200 dynes/cm2 at the apex of an arterial bifurcation25 and 100 dynes/cm2 at the distal neck of a saccular aneurysm15. Consistent with the common notion that the upstream triggers for flow-mediated vascular remodeling events come from endothelial cells, sensors for wall shear stress26–30, Hoi et al. define the impact zone as the vascular area that experiences a wall shear stress above the physiologic level of 20 dynes/cm2 (Ref. 15).
Another factor that sometimes is classified as a hemodynamic stress is systemic or essential hypertension, which is not the local pressure elevation owing to local hemodynamic conditions that was previously discussed. As will be discussed as either a cause or effect of the disease, systemic hypertension represents a systemic endothelial dysfunction, in addition to the purely hemodynamic effects on the vascular tree.
Systemic hypertension is a consistent risk factor for intracranial aneurysm development31,32. Like other risk factors such as smoking and genetic disposition1, it is not well understood how systemic hypertension contributes to aneurysm development. From a very basic mechanical point of view, intravascular pressure subjects the vessel wall under tensile stress33, which could cause the aneurysmal sac to enlarge if the wall is already degraded.
However, this simple view does not uniquely explain how systemic hypertension contributes to the vessel wall degradation that is essential for aneurysm initiation, growth and rupture.
Research on systemic hypertension and peripheral arteries provides some cues as to how systemic hypertension may affect the arterial wall functions.
Hypertension appears to be associated with impairment of endothelial function in its vasodilation response to acetylcholine infused into the brachial artery34,35. For example, two studies showed that endothelium-dependent vasodilation by acetylcholine is reduced in the forearm of essential and secondary hypertensive patients34,35. An altered balance of vasodilator and vasoconstrictor molecules could probably characterize hypertension-related endothelial dysfunction.
In patients with hypertension, the endothelial cell vasodilatory response that includes prostacyclin and nitric oxide production was diminished34, while the production of endothelin-136, reactive oxygen species, angiotensin II37 and other endothelium-derived constricting factors was either maintained or increased. It has also been found that chronic arterial hypertension results in altered intercellular adhesion molecule-1 (ICAM-1) expression on the endothelium, which may contribute to the abnormal inflammatory responses associated with this disease38. The altered pathologic behavior of the vasculature may contribute to intracranial aneurysm formation and development by preventing a healthy remodeling process in response to a sudden increase of blood flow.
High shear stress and hypertension alone may not be sufficient to explain the hemodynamic conditions responsible for intracranial aneurysm development. It is well established that normal vascular remodeling is an arterial adaptation to a long-term increase or decrease in wall shear stress from the baseline level of 15–20 dynes/cm2 (Ref. 39). Increased flow causes dilation of the artery until the wall shear stress is restored to baseline11,40. The remodeling process of the common carotid artery in response to high flow and shear stress is well described in the work of Sho et al.41. An increase in the early expression of MMP-2 and -9 is associated with a sudden increase in blood flow and wall shear stress41. Inhibition of MMPs by doxycycline was able to reduce outward remodeling in response to an increased blood flow12. The resulting degradation of internal elastic lamina allows the artery to be dilated and the wall shear stress to be reduced, followed by the proliferation of endothelial cells and smooth muscle cells41. This remodeling mechanism does not seem to lead to a diseased condition. Other than the degraded internal elastic lamina, the remodeled wall of the arterial tube was rather uniform and healthy with abundant endothelial and smooth muscle cells.
The morphology of the saccular intracranial aneurysm wall, nonetheless, is quite different. Besides having a disrupted internal elastic lamina, the aneurysm wall is very inhomogeneous; endothelial layer is usually disrupted; media is thinned and smooth muscle cells are scarce, evidently owing to apoptosis and possibly by inhibition of proliferation; inflammatory cells are frequently found42. Such remodeling appears to be related to the complex hemodynamic microenvironment associated with the apex or flow divider. This particular geometry harbors a ‘disturbed flow’ created by flow impingement in addition to the high shear stress (Figure 3). The high shear stress might play the critical role in degrading internal elastic lamina through the release of MMPs, but the ‘disturbed flow’ may be just as important for aneurysm development as the high shear stress.
Figure 3.
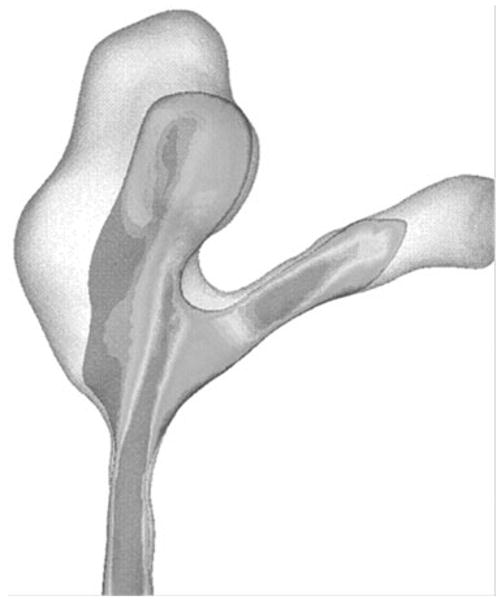
Flow velocity magnitude distribution on a cross-section of a patient's intracranial aneurysm. The lumen was reconstructed from CT images and the flow field was calculated using computational fluid dynamics simulation. The distal neck serves as a flow divider splitting the inflow into different directions, therefore creating a large spatial variation of wall shear stress, a hallmark of a ‘disturbed flow’
The ‘disturbed flow’ has been a key concept in the onset of atherosclerosis, stenosis, thrombosis and some inflammation18,39,43. In these situations, ‘disturbed flow’ is generally used to describe a recirculating flow in a cavity or wall expansion near an arterial branch. The shear stress is low owing to the slow velocity, but the change in direction is frequent44. Blood elements stay in the region for a prolonged period, greatly increasing the adhesion of elements such as platelets and monocytes to endothelial cells43. It should be noted that near the reattachment point of the recirculation bubble, the flow splits into opposite directions; therefore, the local shear stress gradient (spatial variation of shear stress) is extremely high. Increasing evidence suggests that it is not the low shear stress but the high wall shear stress gradient that triggers the modification of endothelial gene expression, causing the cascade of events that lead to atherosclerosis, stenosis and related inflammation45–47.
The hemodynamic environment associated with saccular aneurysms is also likely to constitute a ‘disturbed flow’, whereby the flow splits into opposite directions near the impingement point and produces a high spatial variation of shear stress or wall shear stress gradient. This ‘disturbed flow’ may distinguish the pathologic remodeling that leads to saccular aneurysm development from the normal outward remodeling of an arterial tube subjected to increased flow. It is possible that, together with the high shear stress and high pressure, high wall shear stress gradient mediates saccular aneurysm formation.
Fusiform aneurysms constitute ∼2% of all intracranial aneurysms. Unless the aneurysmal distention is severely asymmetric (a case bordering with the saccular aneurysms), no impinging flow is usually present and hemodynamic stress is usually low in a fusiform aneurysm19. We nevertheless find the ‘disturbed flow’–the recirculation flow in the expansion area. Such recirculation flow is similar to that in the abdominal aortic aneurysm and atherosclerosis development48. Indeed, fusiform aneurysms are known as atherosclerotic aneurysms18,49.
In order to further characterize exact relationships between hemodynamic stresses and a clinical course of intracranial aneurysms, a means to measure shear stress and pressure in the human cerebral circulation needs to be further developed19,24.
An Animal Model of Intracranial Aneurysm Induced by Hemodynamics
A critical role of hemodynamic stresses in the formation of intracranial aneurysms has been demonstrated by studies using an animal model of intracranial aneurysms. Experimentally, changing hemodynamic stresses in the circle of Willis can induce intracranial aneurysms. In animals, a combination of unilateral carotid ligation and hypertension can cause intracranial aneurysms in the circle of Willis through an increase in blood flow and local arterial pressure. This model was originally developed and has been extensively studied by a group of researchers led by Dr Nobuo Hashimoto at Department of Neurosurgery at Kyoto University in Japan. In this animal model, aneurysms were frequently discovered in areas of high hemodynamic stresses such as at (1) the bifurcation between anterior cerebral artery and olfactory artery contralateral to the ligated common carotid artery and (2) posterior communicating arteries50.
Interestingly, rats that underwent bilateral common carotid ligation displayed higher incidences of intracranial aneurysms in the posterior circulation than those that underwent unilateral ligation50. Presumably, rats that underwent bilateral common carotid artery ligation developed higher blood flow through the basilar artery to posterior communicating arteries than those with unilateral carotid ligation, because a majority of the blood flow to the brain needs to be supplied by basilar artery in the rats that underwent bilateral ligation. This finding may indicate a dose-relationship between an increase in the blood flow and the incidence of aneurysms.
Histologically, these hemodynamically-induced intracranial aneurysms in rats closely resemble intracranial aneurysms in humans. Elastic lamina in this model showed degenerative changes such as loss or fragmentation of the lamina, which are common in human intracranial aneurysms1,51. In addition, both human intracranial aneurysms and rodent experimentally-induced aneurysms showed damage to endothelial layers and thrombi in the sac52. Death of medial smooth muscle cells through apoptosis was also observed in the rat aneurysms53.
The same manipulation of intracranial hemodynamics in rats resulted in intracranial aneurysm formation in mice and primates54–57. It should be noted that some studies using these models utilized beta-aminopropionitrile (BAPN) treatment to increase the incidence of aneurysms. BAPN is an irreversible inhibitor of lysyl oxidase that inhibits the crosslinking of collagen and elastin fibers, which are critical structural components of blood vessels. Some histologic changes in the structural components of the vascular wall, such as the disruption or loss of elastic lamina in these models, may be due to the inhibition of the normal metabolism of collagen and elastin fibers by BAPN treatment.
The addition of BAPN treatment to the combination of unilateral carotid ligation and hypertension had quadrupled the incidence of aneurysms58. This may be analogous to the increased incidence of aneurysms in patients with Ehlers-Danlos syndrome, in which common defects are in collagen synthesis and metabolism.
Inflammation in Human Intracranial Aneurysms
The potential roles of inflammation in the development and rupture of intracranial aneurysms have been suggested by observational studies that analysed the presence of inflammatory cells or markers in human intracranial aneurysm tissues or serum samples from patients harboring intracranial aneurysms. Several studies reported the presence of inflammatory cells in the wall of intracranial aneurysms. Histologic analysis of intracranial aneurysms using electron microscopy showed the presence of extracellular lysosome-like granules and leukocytes near disorganized elastic lamina, indicating degradation of elastic lamina by lytic enzymes secreted by leukocytes in the vascular wall59.
Analysing mostly unruptured aneurysm tissues (23 unruptured and two ruptured aneurysms), Chyatte et al. reported the presence of macrophages/monocytes and T-lymphocytes in the aneurysmal wall7. Previously, a similar observation was attributed to potential atherosclerotic changes in part of the aneurismal sac60. However, Chyatte et al. found these inflammatory cells scattered throughout the aneurysmal wall, indicating that inflammation in intracranial aneurysms is not limited to the part of the vascular wall undergoing atherosclerotic changes but rather is a generalized reaction. Kataoka et al. examined leukocyte infiltration in relation to structural changes of the aneurysmal wall in both ruptured and unruptured intracranial aneurysms42. Half of the unruptured aneurysms (10/20) and all of the ruptured aneurysms (40/40) had been infiltrated by leukocytes. Leukocyte infiltration appeared to be associated with the damage or loss of the smooth muscle cell layer and collagen fibers.
Similar findings were reported by Frösen et al.8. In this study, 24 unruptured and 42 ruptured aneurysms were analysed. The majority of both unruptured and ruptured aneurysms showed evidence of macrophage and leukocyte infiltration, with more prominent leukocyte infiltrations in the aneurysms that had ruptured. Interestingly, in unruptured aneurysms, the areas on the vascular wall that showed macrophage and leukocyte infiltration were also free of organizing thrombosis or myointimal hyperplasia, changes often regarded as atherosclerotic. This indicates that inflammation mediated by these inflammatory cells in the aneurysmal wall is not a necessary part of atherosclerosis or the tissue repairing process after rupture.
The associations in these studies between histologic findings and rupture need to be carefully interpreted. The cross-sectional nature of these studies makes the exploration of a causal relationship between inflammation and aneurysmal rupture complex. Part of the challenge is distinguishing if structural changes in the vascular wall and leukocyte infiltration in aneurysms are due to the rupture itself or an epiphenomenon. For example, the inflammation and small degree of remodeling that follows an aneurysmal rupture could be due to the provocation of intravascular thrombosis and normal tissue repair. However, this does not explain the presence of inflammatory cells in unruptured aneurysms and as several studies have consistently found it, the majority of both ruptured and unruptured intracranial aneurysms exhibited macrophage and lymphocyte infiltration into the vascular wall7,8,42.
The presence of inflammation in intracranial aneurysms is further supported by studies that analysed cytokines related to inflammation in serum samples from patients with intracranial aneurysms. Serum elastase and collagenase levels seem to be elevated in patients with intracranial aneurysms61–65. These findings were corroborated by studies intracranial aneurysm tissues for expression or activity of collagenase and elastase. High levels of collagenases and elastases were observed in intracranial aneurysms tissues65,66. The major collagenases in intracranial aneurysm tissues seem to be MMP-2 and -9 (Ref. 67). MMP-2 and -9 are known to be produced by inflammatory cells, especially by macrophages in other vascular diseases such as abdominal aortic aneurysms5,68.
Predicting natural course of aneurysms in regard to a propensity to rupture using serum levels of these serum proteinases may be intriguing, but data for associations between rupture and the serum proteinase levels are conflicting61,62,65. To explore the relationship between the presence of serum proteinases and aneurysmal rupture, a longitudinal study following patients with unruptured aneurysms is needed.
Hemodynamic Stress and Inflammation
Hemodynamic stress can trigger an inflammatory process by activating endothelial and inflammatory cells. High shear stress, i.e. high blood flow, activates endothelial cells and up-regulates leukocyte adhesion molecules including ICAM-1 and chemokines such as monocyte chemotactic protein-1 (Refs. 69–72). These molecules attract circulating neutrophils and monocytes, which facilitate their invasion into the vascular wall and their maturation. Along with activated endothelial cells and smooth muscle cells, these inflammatory cells secrete cytokines, including MMPs and elastases, and initiate outward vascular remodeling12,69,73.
Other studies have linked the ‘disturbed flow’ pattern to the increased leukocyte adhesion to endothelial cells and ultimately to the observed inflammation and atherosclerotic lesions in the arterial wall43,74. The flow disturbance may cause an increased residence time of circulating particles in the region and increased mass transfer, therefore enhancing the interactions between circulating leukocytes and vascular endothelial cells. Furthermore, there is considerable evidence that spatial variations of shear stress in the ‘disturbed flow’ region exert significant influences on endothelial cells43,75,76. The adhesion of monocytes on endothelial cells occurs preferentially in the vicinity of the reattachment point in a recirculating flow77. The large shear stress gradient not only increases monocyte adhesion to endothelial cells43 but also causes a net migration of endothelial cells away from the region of high shear gradient47.
We have pointed out that both saccular and fusiform aneurysms experience a ‘disturbed flow’. The flow splitting at the impingement point of the inflow jet or the reattachment point of recirculating flow creates large gradients in fluid shear stress on the luminal surface of the aneurysm, the neck and possibly the immediately adjacent parent vessel. Such large shear gradients may be responsible for monocyte adhesion. In addition, according to the study of Munn et al.78, a flow velocity component perpendicular to the vascular wall can cause a cell flux and therefore lead to direct invasion of large blood elements into the wall. Flow impingement in saccular aneurysms provides such perpendicular velocity to the wall, which, coupled with the possible endothelial migration away from this region of large spatial gradient of shear stress, may further facilitate leukocyte invasion.
Furthermore, both saccular and fusiform aneurysms contain recirculating flow inside their cavities, where the long residence time of particles enhances the mass transfer between the circulating particles and the vascular endothelial layer. The recirculation zone (cavity) is therefore the pro-atherosclerotic area.
Hemodynamics, Vascular Remodeling, Inflammation and the Development of Intracranial Aneurysms
Based on experimental and observational findings previously discussed, a concept that views the formation of intracranial aneurysms as a sustained and focalized vascular remodeling emerges (Figure 4). While normal outward vascular remodeling in response to an increase in blood flow is a healthy adaptive process to normalize the wall shear stress, aneurysm development is a pathologic manifestation of failure to re-establish homeostasis under hemodynamic insults.
Figure 4.
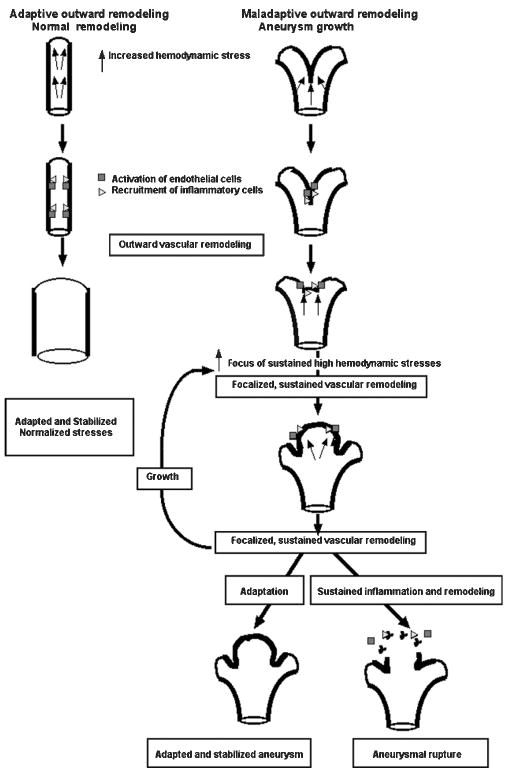
Schematic representation of normal outward vascular remodeling under laminar flow and pathologic vascular remodeling leading to aneurysm formation at a flow divider. Increased hemodynamic stresses activate endothelial cells, which cause recruitment and activation of inflammatory cells and secretion of MMPs to degrade the internal elastic lamina. These processes initiate outward vascular remodeling. In normal vascular remodeling, the luminal diameter increases. The vessel enlargement brings the shear stress down to baseline level; therefore, blood vessels will be adapted and stabilized. However, during aneurysm formation, outward vascular remodeling becomes asymmetric or focalized. Flow impingement creates a complex hemodynamic environment with spatially varying shear stress and increased invasion of inflammatory cells. As the micro-aneurysm grows, part of the wall will continue to experience high shear stress. Focalized vascular remodeling continues with increasing participation of inflammation, which could repair the wall, stabilize the aneurysm or further degrade it, leading to rupture
Inflammation has been found to play an important role in this process, whether by contributing to the initiation of the cascade for the outpouching process, attempting to repair the wound8 or accelerating the degradation of the wall en route to aneurysm rupture42.
Intracranial aneurysm formation may start as an otherwise normal outward vascular remodeling at a bifurcation point or curve. However, because of the particular geometry of the affected blood vessels (branching point or curved blood vessel), outward vascular remodeling during the development of intracranial aneurysms may become asymmetric or focalized. The focus of the vascular remodeling causes pre-aneurysmal change and leads to microaneurysm formation, a small bulging of the blood vessel. As the micro-aneurysm grows, part of it will be subjected to sustained high shear stress15. In this area, endothelial cells are kept either activated or damaged, causing the accumulation of inflammatory cells. Focalized vascular remodeling continues with increasing inflammation. When the inflammation is intense, major structural components of the vascular wall may be destroyed and lead to aneurysm rupture. A hallmark of the pathophysiology of intracranial aneurysms may be a sustained, focalized outward vascular remodeling coupled with inflammation.
Genetic factors, such as certain single nucleotide polymorphisms or other underlying diseases including hypertension and atherosclerosis, may have a role in accentuating the sustained vascular remodeling and inflammation79,80.
Related Cerebrovascular Conditions: BAVM
There may be a parallel in the inflammation observed in the walls of aneurysms operative in patients harboring BAVM. Lacking intervening capillaries, BAVM function as arteriovenous shunts in the cerebro-circulation. Abnormal blood vessels in BAVM (nidal vessels) are exposed to high blood flow and possibly high shear stress81. Although their genesis is not well understood, there is growing evidence that suggests a role for sustained vascular remodeling and inflammation in the pathophysiology of BAVM82–87.
Genetic and tissue analysis in BAVM patients show an interesting link between inflammatory cytokines and the clinical behavior of BAVM. Interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α) are potent inflammatory cytokines that can serve as tumor and endothelial cell growth factors. The GG genotype of the IL-6-174G>C promoter polymorphism is associated with clinical presentation of intracranial hemorrhage (ICH) in BAVM patients86. Further, TNF-α-238G>A promoter polymorphism is associated with an increased risk of new hemorrhage in the natural course of BAVM patients87.
Chen et al. investigated whether tissue IL-6 expression was associated with IL-6-174G>C genotype and IL-6 was linked to putative downstream targets involved in angiogenesis and vascular instability85. They found that the highest IL-6 protein levels in BAVM tissue were associated with IL-6-174GG genotype. IL-6 induced MMP-3 and -9 expression and activity in normal mouse brain and increased proliferation and migration of cerebral endothelial cells. Together, these results suggest that the IL-6 genotype associated with ICH appears to have functional differences in IL-6 tissue expression, consistent with the hypothesis that inflammatory processes induce angiogenic activity and possibly contribute to BAVM ICH.
Because BAVM tissue expresses both MMP-9 (Ref. 3) and IL-6 (Ref. 85), the question arises as to from which cell population those cytokines were primarily derived. Preliminary evidence suggests that it is by infiltrating leukocytes, in particular neutrophils85.
Taken together, it appears that a combination of genetic and local environmental factors helps shape the BAVM lesional phenotype and appears to be responsible for some portions of the clinical behavior. More studies need to be conducted to dissect their operative mechanisms as well as their relationship to the actual pathogenesis of the disease. In any event, there may be a broad overlap in the inflammatory contribution to the phenotype of both aneurysms and BAVM.
Future Directions
Findings from observational and experimental studies suggest roles for vascular remodeling and inflammation in the pathophysiology of intracranial aneurysms. However, there is a lack of study linking these two processes, i.e. vascular remodeling and inflammation in this pathology. A recent advancement in image acquisition and analysis techniques that enable a longitudinal follow-up of changes in geometry of intracranial aneurysms and local hemodynamic stresses in humans will help us to study the effects of changing hemodynamic stresses on aneurysmal growth and subsequent rupture18. Animal models, especially hemodynamically-induced intracranial aneurysms, will be invaluable for establishing a causal relationship among hemodynamic stresses, inflammation and aneurysm growth.
Management of unruptured intracranial aneurysms still remains controversial. In some patients with unruptured aneurysms, the risk of surgical treatment may be higher than the risk of rupture88. There are limited treatment options for some patients with giant aneurysms. New treatments targeting vascular remodeling and inflammation through inhibition of MMPs or other cytokines may be offered to these subsets of patients.
Acknowledgments
We thank Voltaire Gunglab for the assistance in preparation of the manuscript. Portions of this work were supported by the National Institutes of Health Grants NS44155 (WLY, TH), NS27713 (WLY), NS34949 (WLY), NS37921 (WLY) and NS047-242 (HM).
References
- 1.Schievink WI. Intracranial aneurysms. N Engl J Med. 1997;336:28–40. doi: 10.1056/NEJM199701023360106. [DOI] [PubMed] [Google Scholar]
- 2.Juvela S. Treatment options of unruptured intracranial aneurysms. Stroke. 2004;35:372–374. doi: 10.1161/01.STR.0000115299.02909.68. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto T, Wen G, Lawton MT, et al. Abnormal expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in brain arteriovenous malformations. Stroke. 2003;34:925–931. doi: 10.1161/01.STR.0000061888.71524.DF. [DOI] [PubMed] [Google Scholar]
- 4.Knox JB, Sukhova GK, Whittemore AD, et al. Evidence for altered balance between matrix metalloproteinases and their inhibitors in human aortic diseases. Circulation. 1997;95:205–212. doi: 10.1161/01.cir.95.1.205. [DOI] [PubMed] [Google Scholar]
- 5.Goodall S, Crowther M, Hemingway DM, et al. Ubiquitous elevation of matrix metalloproteinase-2 expression in the vasculature of patients with abdominal aneurysms. Circulation. 2001;104:304–309. doi: 10.1161/01.cir.104.3.304. [DOI] [PubMed] [Google Scholar]
- 6.Loftus IM, Naylor AR, Goodall S, et al. Increased matrix metalloproteinase-9 activity in unstable carotid plaques. A potential role in acute plaque disruption. Stroke. 2000;31:40–47. doi: 10.1161/01.str.31.1.40. [DOI] [PubMed] [Google Scholar]
- 7.Chyatte D, Bruno G, Desai S, et al. Inflammation and intracranial aneurysms. Neurosurgery. 1999;45:1137–1146. doi: 10.1097/00006123-199911000-00024. [DOI] [PubMed] [Google Scholar]
- 8.Frosen J, Piippo A, Paetau A, et al. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: Histological analysis of 24 unruptured and 42 ruptured cases. Stroke. 2004;35:2287–2293. doi: 10.1161/01.STR.0000140636.30204.da. [DOI] [PubMed] [Google Scholar]
- Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330:1431–1438. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 10.De Mey JG, Schiffers PM, Hilgers RH, et al. Toward functional genomics of flow-induced outward remodeling of resistance arteries. Am J Physiol Heart Circ Physiol. 2005;288:H1022–H1027. doi: 10.1152/ajpheart.00800.2004. [DOI] [PubMed] [Google Scholar]
- 11.Kamiya A, Togawa T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol. 1980;239:H14–H21. doi: 10.1152/ajpheart.1980.239.1.H14. [DOI] [PubMed] [Google Scholar]
- 12.Tronc F, Mallat Z, Lehoux S, et al. Role of matrix metalloproteinases in blood flow-induced arterial enlargement: Interaction with NO. Arterioscler Thromb Vasc Biol. 2000;20:E120–E126. doi: 10.1161/01.atv.20.12.e120. [DOI] [PubMed] [Google Scholar]
- 13.Tzima E, Del Pozo MA, Kiosses WB, et al. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J. 2002;21:6791–6800. doi: 10.1093/emboj/cdf688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellegala DB, Day AL. Ruptured cerebral aneurysms. N Engl J Med. 2005;352:121–124. doi: 10.1056/NEJMp048301. [DOI] [PubMed] [Google Scholar]
- 15.Hoi Y, Meng H, Woodward SH, et al. Effects of arterial geometry on aneurysm growth: Three-dimensional computational fluid dynamics study. J Neurosurg. 2004;101:676–681. doi: 10.3171/jns.2004.101.4.0676. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez CF, Cho YI, Ortega HV, et al. Intracranial aneurysms: Flow analysis of their origin and progression. AJNR Am J Neuroradiol. 1992;13:181–188. [PMC free article] [PubMed] [Google Scholar]
- 17.Foutrakis GN, Yonas H, Sclabassi RJ. Saccular aneurysm formation in curved and bifurcating arteries. AJNR Am J Neuroradiol. 1999;20:1309–1317. [PMC free article] [PubMed] [Google Scholar]
- 18.Jou LD, Wong G, Dispensa B, et al. Correlation between lumenal geometry changes and hemodynamics in fusiform intracranial aneurysms. AJNR Am J Neuroradiol. 2005;26:2357–2363. [PMC free article] [PubMed] [Google Scholar]
- 19.Jou LD, Quick CM, Young WL, et al. Computational approach to quantifying hemodynamic forces in giant cerebral aneurysms. AJNR Am J Neuroradiol. 2003;24:1804–1810. [PMC free article] [PubMed] [Google Scholar]
- 20.Steiger HJ. Pathophysiology of development and rupture of cerebral aneurysms. Acta Neurochir Suppl (Wien) 1990;48:1–57. [PubMed] [Google Scholar]
- 21.Sekhar LN, Heros RC. Origin, growth, and rupture of saccular aneurysms: A review. Neurosurgery. 1981;8:248–260. doi: 10.1227/00006123-198102000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson GG. Physical factors in the initiation, growth, and rupture of human intracranial saccular aneurysms. J Neurosurg. 1972;37:666–677. doi: 10.3171/jns.1972.37.6.0666. [DOI] [PubMed] [Google Scholar]
- 23.Stehbens WE. Etiology of intracranial berry aneurysms. J Neurosurg. 1989;70:823–831. doi: 10.3171/jns.1989.70.6.0823. [DOI] [PubMed] [Google Scholar]
- 24.Shojima M, Oshima M, Takagi K, et al. Role of the bloodstream impacting force and the local pressure elevation in the rupture of cerebral aneurysms. Stroke. 2005;36:1933–1938. doi: 10.1161/01.STR.0000177877.88925.06. [DOI] [PubMed] [Google Scholar]
- 25.Meng H, Metaxa E, Wang Z, et al. Vascular response to impinging blood flow. Present at 2005 BMES Annual Fall Meeting; Baltimore, MD, USA. 2005. [Google Scholar]
- 26.Traub O, Berk BC. Laminar shear stress: Mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol. 1998;18:677–685. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- 27.Chien S, Li S, Shyy YJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31:162–169. doi: 10.1161/01.hyp.31.1.162. [DOI] [PubMed] [Google Scholar]
- 28.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies PF, Barbee KA, Volin MV, et al. Spatial relationships in early signaling events of flow-mediated endothelial mechanotransduction. Annu Rev Physiol. 1997;59:527–549. doi: 10.1146/annurev.physiol.59.1.527. [DOI] [PubMed] [Google Scholar]
- 30.Shyy JY. Mechanotransduction in endothelial responses to shear stress: Review of work in Dr. Chien's laboratory. Biorheology. 2001;38:109–117. [PubMed] [Google Scholar]
- 31.Krex D, Schackert HK, Schackert G. Genesis of cerebral aneurysms–an update. Acta Neurochir (Wien) 2001;143:429–448. doi: 10.1007/s007010170072. [DOI] [PubMed] [Google Scholar]
- 32.Inci S, Spetzler RF. Intracranial aneurysms and arterial hypertension: A review and hypothesis. Surg Neurol. 2000;53:530–540. doi: 10.1016/s0090-3019(00)00244-5. [DOI] [PubMed] [Google Scholar]
- 33.Meng H, Feng Y, Woodward SH, et al. Mathematical model of the rupture mechanism of intracranial saccular aneurysms through daughter aneurysm formation and growth. Neurol Res. 2005;27:459–465. doi: 10.1179/016164105X25171. [DOI] [PubMed] [Google Scholar]
- 34.Taddei S, Virdis A, Mattei P, et al. Vasodilation to acetylcholine in primary and secondary forms of human hypertension. Hypertension. 1993;21:929–933. doi: 10.1161/01.hyp.21.6.929. [DOI] [PubMed] [Google Scholar]
- 35.Panza JA, Quyyumi AA, Brush JE, Jr, et al. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 36.Savoia C, Schiffrin EL. Significance of recently identified peptides in hypertension: Endothelin, natriuretic peptides, adrenomedullin, leptin. Med Clin North Am. 2004;88:39–62. doi: 10.1016/s0025-7125(03)00122-6. [DOI] [PubMed] [Google Scholar]
- 37.Sowers JR. Hypertension, angiotensin II, and oxidative stress. N Engl J Med. 2002;346:1999–2001. doi: 10.1056/NEJMe020054. [DOI] [PubMed] [Google Scholar]
- 38.Komatsu S, Panes J, Russell JM, et al. Effects of chronic arterial hypertension on constitutive and induced intercellular adhesion molecule-1 expression in vivo. Hypertension. 1997;29:683–689. doi: 10.1161/01.hyp.29.2.683. [DOI] [PubMed] [Google Scholar]
- 39.Wootton DM, Ku DN. Fluid mechanics of vascular systems, diseases, and thrombosis. Annu Rev Biomed Eng. 1999;1:299–329. doi: 10.1146/annurev.bioeng.1.1.299. [DOI] [PubMed] [Google Scholar]
- 40.Zarins CK, Zatina MA, Giddens DP, et al. Shear stress regulation of artery lumen diameter in experimental atherogenesis. J Vasc Surg. 1987;5:413–420. [PubMed] [Google Scholar]
- 41.Sho E, Sho M, Singh TM, et al. Arterial enlargement in response to high flow requires early expression of matrix metalloproteinases to degrade extracellular matrix. Exp Mol Pathol. 2002;73:142–153. doi: 10.1006/exmp.2002.2457. [DOI] [PubMed] [Google Scholar]
- 42.Kataoka K, Taneda M, Asai T, et al. Structural fragility and inflammatory response of ruptured cerebral aneurysms. A comparative study between ruptured and unruptured cerebral aneurysms. Stroke. 1999;30:1396–1401. doi: 10.1161/01.str.30.7.1396. [DOI] [PubMed] [Google Scholar]
- 43.Chiu JJ, Chen CN, Lee PL, et al. Analysis of the effect of disturbed flow on monocytic adhesion to endothelial cells. J Biomech. 2003;36:1883–1895. doi: 10.1016/s0021-9290(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 44.Cheng CP, Herfkens RJ, Taylor CA. Abdominal aortic hemodynamic conditions in healthy subjects aged 50–70 at rest and during lower limb exercise: In vivo quantification using MRI. Atherosclerosis. 2003;168:323–331. doi: 10.1016/s0021-9150(03)00099-6. [DOI] [PubMed] [Google Scholar]
- 45.Chiu JJ, Wang DL, Chien S, et al. Effects of disturbed flow on endothelial cells. J Biomech Eng. 1998;120:2–8. doi: 10.1115/1.2834303. [DOI] [PubMed] [Google Scholar]
- 46.Nagel T, Resnick N, Dewey CF, Jr, et al. Vascular endothelial cells respond to spatial gradients in fluid shear stress by enhanced activation of transcription factors. Arterioscler Thromb Vasc Biol. 1999;19:1825–1834. doi: 10.1161/01.atv.19.8.1825. [DOI] [PubMed] [Google Scholar]
- 47.Tardy Y, Resnick N, Nagel T, et al. Shear stress gradients remodel endothelial monolayers in vitro via a cell proliferation-migration–loss cycle. Arterioscler Thromb Vasc Biol. 1997;17:3102–3106. doi: 10.1161/01.atv.17.11.3102. [DOI] [PubMed] [Google Scholar]
- 48.Egelhoff CJ, Budwig RS, Elger DF, et al. Model studies of the flow in abdominal aortic aneurysms during resting and exercise conditions. J Biomech. 1999;32:1319–1329. doi: 10.1016/s0021-9290(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 49.Nakatomi H, Segawa H, Kurata A, et al. Clinicopathological study of intracranial fusiform and dolichoectatic aneurysms: Insight on the mechanism of growth. Stroke. 2000;31:896–900. doi: 10.1161/01.str.31.4.896. [DOI] [PubMed] [Google Scholar]
- 50.Hashimoto N, Handa H, Nagata I, et al. Experimentally induced cerebral aneurysms in rats: Part V. Relation of hemodynamics in the circle of Willis to formation of aneurysms. Surg Neurol. 1980;13:41–45. [PubMed] [Google Scholar]
- 51.Hazama F, Kataoka H, Yamada E, et al. Early changes of experimentally induced cerebral aneurysms in rats. Light-microscopic study. Am J Pathol. 1986;124:399–404. [PMC free article] [PubMed] [Google Scholar]
- 52.Hashimoto N, Handa H, Hazama F. Experimentally induced cerebral aneurysms in rats: Part II. Surg Neurol. 1979;11:243–246. [PubMed] [Google Scholar]
- 53.Kondo S, Hashimoto N, Kikuchi H, et al. Apoptosis of medial smooth muscle cells in the development of saccular cerebral aneurysms in rats. Stroke. 1998;29:181–188. doi: 10.1161/01.str.29.1.181. [DOI] [PubMed] [Google Scholar]
- 54.Sadamasa N, Nozaki K, Hashimoto N. Disruption of gene for inducible nitric oxide synthase reduces progression of cerebral aneurysms. Stroke. 2003;34:2980–2984. doi: 10.1161/01.STR.0000102556.55600.3B. [DOI] [PubMed] [Google Scholar]
- 55.Morimoto M, Miyamoto S, Mizoguchi A, et al. Mouse model of cerebral aneurysm: Experimental induction by renal hypertension and local hemodynamic changes. Stroke. 2002;33:1911–1915. doi: 10.1161/01.str.0000021000.19637.3d. [DOI] [PubMed] [Google Scholar]
- 56.Hashimoto N, Kim C, Kikuchi H, et al. Experimental induction of cerebral aneurysms in monkeys. J Neurosurg. 1987;67:903–905. doi: 10.3171/jns.1987.67.6.0903. [DOI] [PubMed] [Google Scholar]
- 57.Coutard M. Experimental cerebral aneurysms in the female heterozygous blotchy mouse. Int J Exp Pathol. 1999;80:357–367. doi: 10.1046/j.1365-2613.1999.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hashimoto N, Handa H, Hazama F. Experimentally induced cerebral aneurysms in rats: Part III. Pathology. Surg Neurol. 1979;11:299–304. [PubMed] [Google Scholar]
- 59.Cajander S, Hassler O. Enzymatic destruction of the elastic lamella at the mouth of cerebral berry aneurysm? An ultrastructural study with special regard to the elastic tissue. Acta Neurol Scand. 1976;53:171–181. doi: 10.1111/j.1600-0404.1976.tb04335.x. [DOI] [PubMed] [Google Scholar]
- 60.Kosierkiewicz TA, Factor SM, Dickson DW. Immunocytochemical studies of atherosclerotic lesions of cerebral berry aneurysms. J Neuropathol Exp Neurol. 1994;53:399–406. doi: 10.1097/00005072-199407000-00012. [DOI] [PubMed] [Google Scholar]
- 61.Gaetani P, Grazioli V, Tancioni F, et al. Abnormalities of collagen cross-linkage in posterior communicating artery aneurysms: A preliminary study. Neurol Res. 1996;18:541–545. doi: 10.1080/01616412.1996.11740467. [DOI] [PubMed] [Google Scholar]
- 62.Baker CJ, Fiore A, Connolly ES, Jr, et al. Serum elastase and alpha-1-antitrypsin levels in patients with ruptured and unruptured cerebral aneurysms. Neurosurgery. 1995;37:56–61. doi: 10.1227/00006123-199507000-00008. [DOI] [PubMed] [Google Scholar]
- 63.Chyatte D, Lewis I. Gelatinase activity and the occurrence of cerebral aneurysms. Stroke. 1997;28:799–804. doi: 10.1161/01.str.28.4.799. [DOI] [PubMed] [Google Scholar]
- 64.Todor DR, Lewis I, Bruno G, et al. Identification of a serum gelatinase associated with the occurrence of cerebral aneurysms as pro-matrix metalloproteinase-2. Stroke. 1998;29:1580–1583. doi: 10.1161/01.str.29.8.1580. [DOI] [PubMed] [Google Scholar]
- 65.Gaetani P, Rodriguez y Baena R, Tartara F, et al. Metalloproteases and intracranial vascular lesions. Neurol Res. 1999;21:385–390. doi: 10.1080/01616412.1999.11740948. [DOI] [PubMed] [Google Scholar]
- 66.Bruno G, Todor R, Lewis I, et al. Vascular extracellular matrix remodeling in cerebral aneurysms. J Neurosurg. 1998;89:431–440. doi: 10.3171/jns.1998.89.3.0431. [DOI] [PubMed] [Google Scholar]
- 67.Kim SC, Singh M, Huang J, et al. Matrix metalloproteinase-9 in cerebral aneurysms. Neurosurgery. 1997;41:642–666. doi: 10.1097/00006123-199709000-00027. [DOI] [PubMed] [Google Scholar]
- 68.Thompson RW, Parks WC. Role of matrix metalloproteinases in abdominal aortic aneurysms. Ann NY Acad Sci. 1996;800:157–174. doi: 10.1111/j.1749-6632.1996.tb33307.x. [DOI] [PubMed] [Google Scholar]
- 69.Hoefer IE, van Royen N, Rectenwald JE, et al. Arteriogenesis proceeds via ICAM-1/Mac-1-mediated mechanisms. Circ Res. 2004;94:1179–1185. doi: 10.1161/01.RES.0000126922.18222.F0. [DOI] [PubMed] [Google Scholar]
- 70.Tzima E, del Pozo MA, Shattil SJ, et al. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001;20:4639–4647. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shyy JY, Chien S. Role of integrins in cellular responses to mechanical stress and adhesion. Curr Opin Cell Biol. 1997;9:707–713. doi: 10.1016/s0955-0674(97)80125-1. [DOI] [PubMed] [Google Scholar]
- 72.Shyy YJ, Hsieh HJ, Usami S, et al. Fluid shear stress induces a biphasic response of human monocyte chemotactic protein 1 gene expression in vascular endothelium. Proc Natl Acad Sci USA. 1994;91:4678–4682. doi: 10.1073/pnas.91.11.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tronc F, Wassef M, Esposito B, et al. Role of NO in flow-induced remodeling of the rabbit common carotid artery. Arterioscler Thromb Vasc Biol. 1996;16:1256–1262. doi: 10.1161/01.atv.16.10.1256. [DOI] [PubMed] [Google Scholar]
- 74.Hinds MT, Park YJ, Jones SA, et al. Local hemodynamics affect monocytic cell adhesion to a three-dimensional flow model coated with E-selectin. J Biomech. 2001;34:95–103. doi: 10.1016/s0021-9290(00)00139-1. [DOI] [PubMed] [Google Scholar]
- 75.DePaola N, Gimbrone MA, Jr, Davies PF, et al. Vascular endothelium responds to fluid shear stress gradients. Arterioscler Thromb. 1992;12:1254–1257. doi: 10.1161/01.atv.12.11.1254. [DOI] [PubMed] [Google Scholar]
- 76.Davies PF. Spatial hemodynamics, the endothelium, and focal atherogenesis: A cell cycle link? Circ Res. 2000;86:114–116. doi: 10.1161/01.res.86.2.114. [DOI] [PubMed] [Google Scholar]
- 77.Barber KM, Pinero A, Truskey GA. Effects of recirculating flow on U-937 cell adhesion to human umbilical vein endothelial cells. Am J Physiol. 1998;275:H591–H599. doi: 10.1152/ajpheart.1998.275.2.H591. [DOI] [PubMed] [Google Scholar]
- 78.Munn LL, Melder RJ, Jain RK. Analysis of cell flux in the parallel plate flow chamber: Implications for cell capture studies. Biophys J. 1994;67:889–895. doi: 10.1016/S0006-3495(94)80550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peters DG, Kassam A, St Jean PL, et al. Functional polymorphism in the matrix metalloproteinase-9 promoter as a potential risk factor for intracranial aneurysm. Stroke. 1999;30:2612–2616. doi: 10.1161/01.str.30.12.2612. [DOI] [PubMed] [Google Scholar]
- 80.Ruigrok YM, Rinkel GJ, Wijmenga C. Genetics of intracranial aneurysms. Lancet Neurol. 2005;4:179–189. doi: 10.1016/S1474-4422(05)01015-X. [DOI] [PubMed] [Google Scholar]
- 81.Young WL. Intracranial arteriovenous malformations: Pathophysiology and hemodynamics (chapter 6) In: Jafar JJ, Awad IA, Rosenwasser RH, editors. Vascular malformations of the central nervous system. New York, NY, USA: Lippincott Williams & Wilkins; 1999. pp. 95–126. [Google Scholar]
- 82.Hashimoto T, Lam T, Boudreau NJ, et al. Abnormal balance in the angiopoietin-tie2 system in human brain arteriovenous malformations. Circ Res. 2001;89:111–113. doi: 10.1161/hh1401.094281. [DOI] [PubMed] [Google Scholar]
- 83.Hashimoto T, Emala CW, Joshi S, et al. Abnormal pattern of Tie-2 and vascular endothelial growth factor receptor expression in human cerebral arteriovenous malformations. Neurosurgery. 2000;47:910–918. doi: 10.1097/00006123-200010000-00022. [DOI] [PubMed] [Google Scholar]
- 84.Hashimoto T, Mesa-Tejada R, Quick CM, et al. Evidence of increased endothelial cell turnover in brain arteriovenous malformations. Neurosurgery. 2001;49:124–131. doi: 10.1097/00006123-200107000-00019. [DOI] [PubMed] [Google Scholar]
- 85.Chen Y, Fan YF, Zhu Y, et al. Matrix metalloproteinase-9 is associated with myeloperoxidase expression in BAVM. J Neurosurg Anesthesiol. 2005;11:221. [Google Scholar]
- 86.Pawlikowska L, Tran MN, Achrol AS, et al. Polymorphisms in genes involved in inflammatory and angiogenic pathways and the risk of hemorrhagic presentation of brain arteriovenous malformations. Stroke. 2004;35:2294–2300. doi: 10.1161/01.STR.0000141932.44613.b1. [DOI] [PubMed] [Google Scholar]
- 87.Achrol AS, Pawlikowska L, McCulloch CE, et al. Tumor necrosis factor-alpha-238G>A promoter polymorphism is associated with increased risk of new hemorrhage in the natural course of patients with brain arteriovenous malformations. Stroke. 2006;37:231–234. doi: 10.1161/01.STR.0000195133.98378.4b. [DOI] [PubMed] [Google Scholar]
- 88.Wiebers DO, Whisnant JP, Huston J, 3rd, et al. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]


