Abstract
Bipolar I disorder is associated with diminished gating of the auditory evoked P50 component. P50 gating may relate to early filtering of sensory information, protecting higher-order cognitive functions. Gating of the auditory evoked N100 and P200 components has not been investigated in bipolar I disorder, although N100 and P200 gating could reflect different mechanisms and functions in the process of filtering sensory information in addition to those reflected by P50 gating. We investigated P50, N100, and P200 gating assessed with the paired-click paradigm in 22 subjects with bipolar I disorder and 54 healthy controls. Peak amplitudes and latencies were assessed at Cz for the P50, N100, and P200 components. Gating was defined as the reduction in peak amplitude from the first (S1) to the second stimulus (S2) of a stimulus pair, and expressed as gating ratio ([S2amplitude /S1amplitude]*100) and difference score (S1amplitude–S2amplitude). Group differences were detected with multivariate analyses and controlled for differences in age and ethnicity. Subjects with bipolar I disorder had higher P50, N100 and P200 ratios and lower difference scores compared with findings for controls. These findings extend the existing evidence on impaired sensory gating in bipolar I disorder beyond the P50, suggesting impaired filtering at both pre-attentive and early attentive levels in bipolar I disorder.
Keywords: Bipolar I disorder, P50 sensory gating, N100 sensory gating, P200 sensory gating
1. Introduction
Bipolar I disorder is associated with abnormal filtering of sensory information at an early, pre-attentive level as revealed by diminished gating of the auditory evoked P50 potential assessed with a paired-click paradigm (Franks et al., 1983; Adler et al., 1990; Olincy and Martin, 2005; Schulze et al., 2007). The paired-click paradigm is used to assess neuronal reactivity to redundant stimuli: a first auditory stimulus (S1) elicits a positive component at the vertex (Cz) peaking between 40 and 80 ms (P50 wave) (Picton et al., 1974), and is attenuated in response to a second identical auditory stimulus (S2) presented within 500 ms following S1 (Zouridakis and Boutros, 1992; Dolu et al., 2001). This reduction in P50 amplitude could reflect filtering, or gating, of irrelevant sensory information, potentially protecting higher-order functions from being overloaded (Freedman et al., 1991). The strength of gating of the P50 can be measured by its reduction with stimulus repetition, expressed either as the ratio between the P50 amplitude evoked by S2 divided by the amplitude evoked by S1 or as the absolute difference in amplitude between S1 and S2. A higher ratio and lower difference score are interpreted as weaker gating.
Subjects with bipolar I disorder who were in a euthymic state had a higher P50 gating ratio than healthy controls, which related to a weaker reduction of the P50 response to S2 rather than to changes in response to S1 (Olincy and Martin, 2005; Schulze et al., 2007), suggesting disinhibition of the early gating system in bipolar I disorder. The impairment in P50 gating was found regardless of treatment with antidepressants or mood stabilizers (Olincy and Martin, 2005). P50 gating was impaired in subjects with bipolar disorder whether or not there was a history of psychosis, though impairment was greater in subjects with than subjects without such a history (Olincy and Martin, 2005). Furthermore, impaired P50 gating was found in unaffected first-degree relatives of subjects with bipolar I disorder (Schulze et al., 2007). These outcomes suggest that impaired P50 gating in bipolar I disorder could be an endophenotypic marker, little affected by affective state and medication. In contrast, other studies suggested that P50 ratios were state-dependent, with impaired gating during manic episodes that improved when mania resolved (Franks et al., 1983; Adler et al., 1990). Thus, impaired P50 gating in subjects with bipolar I disorder who are in a euthymic state may be further impaired during a manic state.
Gating is not limited to the P50 component, however: repetition of auditory stimuli also elicits gating of a negative and a positive component peaking between 80 and 250 ms following auditory stimulation (Fruhstorfer et al., 1970;Müller et al., 2001; Boutros et al., 2004), which are designated N100 and P200, respectively (Picton et al., 1974). Until recently, gating of the N100 and P200 received only limited attention. However, recent studies found reduced N100 and P200 gating in schizophrenia (Boutros et al., 2004) and in cocaine abusers (Boutros et al., 2006). This is of interest as several lines of evidence suggest that gating of the N100 and P200 may reflect mechanisms of information processing that are partially distinct from those involved in gating of the P50. First, gating measures of the P50 and the N100 (Kisley et al., 2004; Hanlon et al., 2005), and P50 and P200 (Wan et al., 2007) did not correlate significantly. Secondly, there may be a stronger genetic influence in the N100 and P200 ratio than in the P50 ratio (Anokhin et al., 2007).
Here, we present results on gating of auditory evoked P50, N100, and P200 components in subjects with bipolar I disorder. Based on previous findings for P50 gating, our primary hypothesis is (1a) that P50 gating ratio will be higher in subjects with bipolar I disorder than in healthy control subjects. We further hypothesized (1b) that reduced P50 gating in subjects with bipolar I disorder would be due to reduced inhibition of the P50 following S2. In addition, although gating to P50 and N100 and P200 may be unrelated, we hypothesized (1c) that subjects with bipolar I disorder would show general disinhibition reflected by reduced gating of the N100 and P200.
We also studied effects of clinical characteristics including affective state and history of psychosis, and current pharmacological treatment. With respect to these factors, we hypothesized that: 2a) severity of manic symptoms would correlate with gating of the P50, N100, and P200 (cf. Adler et al., 1990); 2b) subjects with bipolar I disorder with a history of psychosis would show reduced gating compared with subjects without such a history, and 2c) because impaired gating was reported in euthymic subjects with bipolar I disorder, pharmacological treatment would not contribute substantially to gating impairment for the P50, N100, and P200 (cf. Olincy and Martin, 2005).
2. Methods
2.1. Subjects
Subjects with bipolar I disorder were recruited through advertisements, referral, and brochures; control subjects were recruited through advertisements. The study complied with the principles of the Declaration of Helsinki and was approved by Committee for the Protection of Human Subjects, the IRB for the University of Texas Health Science Center at Houston. After thorough explanation of the study and opportunity to have questions answered, each subject signed informed consent before participating in the study. Bipolar patients signed the informed consent when they were euthymic.
To establish diagnosis, all subjects completed the Structured Clinical Interview for DSM-IV Axis-I and Axis-II Disorders (SCID-I and SCID-II) (First et al., 1996), conducted by trained staff members and confirmed by a consensus meeting of the authors. Subjects with bipolar disorder were required to meet full DSM-IV criteria for bipolar I disorder. Controls were required to have never met diagnostic criteria for any axis I or axis II disorder. Further inclusion criteria for both groups were good hearing (at least 60 dB in each ear), normal or corrected-to-normal vision, lack of head trauma or epilepsy, and (for the control subjects) no current use of psychoactive medication.
Twenty-two subjects with bipolar I disorder and 54 healthy controls participated in the study. The groups differed significantly in age and moderately on the distribution of ethnicity, but not in level of education or distribution of gender (Table 1). The mean age of onset for subjects with bipolar I disorder was 17.2 years (S.D.=7.6, range 3–33). Fourteen subjects had a history of psychosis (two subjects provided no information); 12 subjects had a history of suicidal ideation or suicide attempts; 20 subjects met criteria for one or more comorbid Axis I (n=17) or Axis II (n=14) disorders. Diagnoses included past alcohol abuse (n=10), current alcohol abuse (n=1), current substance abuse (n=1), past substance abuse (n=11), past anorexia nervosa (n=1), anxiety/panic disorder (n=5), anti-social personality disorder (n=8), and borderline personality disorder (n=7). Two subjects had no comorbid disorders. Subjects were using no medications other than those prescribed to treat symptoms of bipolar disorder: five used no medicines, six used one medicine, and 10 subjects took two to four medicines including anticonvulsants, antipsychotics, and/or antidepressants. One patient provided no information. The medications used most frequently were valproic acid (n=12) and aripiprazole (n=5). Studies were carried out when pharmacological treatments had not changed for at least 2 weeks.
Table 1.
Demographics and affective state.
| Normal control (n=54) | Bipolar disorder (n=22) | Outcome | |
|---|---|---|---|
| Age, mean (S.D.) | 31.52 (11.02) | 38.45 (10.29) | t=−2.5, df=74, P=0.01 |
| Level of education, mean (S.D.) | 14.83 (1.98) | 14.59 (2.40) | t=0.5, df=74, P=ns. |
| Female, n (%) | 28 (51.90) | 10 (45.50) | χ2=0.3, df=1, P=ns. |
| Ethnicity | χ2=6.8, df=3, P=0.08 | ||
| African-American, n (%) | 27 (50) | 6 (27.30) | |
| Asian-American, n (%) | 6 (11.10) | 1 (4.50) | |
| European-American, n (%) | 15 (27.80) | 13 (59.10) | |
| Hispanic, n (%) | 6 (11.10) | 2 (9.10) | |
| SADS-Ca | |||
| Depression, mean (S.D.) | 1.11 (0.17) | 1.92 (0.76) | U=97.5, Z=−4.8, P<0.001 |
| Anxiety, mean (S.D.) | 1.11 (0.19) | 1.93 (0.90) | U=135.5, Z=−4.4, P<0.001 |
| Mania, mean (S.D.) | 1.06 (0.12) | 1.69 (0.65) | U=83.5, Z=−5.3, P<0.001 |
| Psychosis, mean (S.D.) | 1.00 (0) | 1.48 (0.64) | U=156, Z=−5.3, P<0.001 |
SADS-C=change version of the Schedule for Affective Disorders and Schizophrenia.
Non-parametric Mann–Whitney U test.
2.2. Procedures
Subjects underwent the SCID-I and SCID-II and a physical examination (day 1); auditory evoked potential (AEP) and laboratory tasks (day 2); and neuropsychological testing (day 3). On each day subjects were screened for use of drugs (RediCup®, Redwood Biotech, Santa Rosa) and alcohol (Alco-Sensor III, Intoximeters Inc., Saint Louis) before testing. Subjects were required to have negative drug and alcohol screenings in order to undergo any study procedure. For day 2, subjects were required to refrain from consuming caffeinated products for at least 8 h, and from smoking for at least 1 h before testing. We will only report findings for AEP data.
Psychophysiologic testing was performed at the General Clinical Research Center of the University of Texas Medical School at Houston. In addition to the AEP measures, approximately 45 min before testing, blood samples were obtained for studies to be reported elsewhere. A trained staff member (SLM) assessed state-related symptoms using the Change version of the Schedule for Affective Disorders and Schizophrenia (SADS-C) (Spitzer and Endicott, 1978), complemented by five additional mania items originally used in the SADS. The SADS-C assessed the severity of depression (12 items), anxiety (5 items), mania (10 items), and psychosis (3 items) on a six-point Likert-like scale. To compare the severity between symptoms, the scores on the subscales were expressed relative to the number of items. A score of 3 is considered somewhat symptomatic. Although the bipolar group had significantly higher scores on all of the subscales than the control group (Table 1), only four bipolar subjects had scores in a somewhat symptomatic range (higher than 3, but lower than 4) for depression (n=2) or mania (n=2); data were missing for two subjects; the remaining subjects met no criteria for a depressive or manic state (scores lower than 3).
2.3. Paired-click paradigm
Subjects were administered the paired-click paradigm. In total, 80 stimulus pairs were presented divided across two blocks that lasted 7 min each. Stimuli were 40-ms, 80-dB clicks (1000 Hz, 4 ms rise and fall times) presented binaurally through ABR headphones using STIM software (Neuroscan, Inc., El Paso). The inter-stimulus interval between S1 and S2 was 500 ms, and the interval between two consecutive pairs was 8 to 10 s, allowing full recovery of the AEP (Zouridakis and Boutros, 1992). Subjects were instructed to listen passively to the clicks, and to relax and sit quietly with their eyes open while fixing their gaze on a fixation-cross. Blinking was allowed.
2.4. Data acquisition and analysis
Raw EEG was recorded from 32 Ag/AgCl sintered electrodes attached in a Quik-cap (Neuromedics Neuroscan, El Paso) arranged according to the international 10–20 system (Jasper, 1958). Data were recorded using the Acquire module of Scan 4.3 software (NeuroScan, El Paso). Signals were continuously sampled at 1000 Hz, filtered between 0.1 and 100 Hz (AC mode), and amplified 10,000× through SynAmps amplifiers. All signals were referenced to linked electrodes attached to the mastoids. Vertical and horizontal electrooculographic activity was obtained with electrodes placed above and below the right eye, and at the canthi of both eyes, respectively. The ground electrode was attached in the cap anteriorly to F3–F4 in line with the midline electrodes. Impedances were kept below 4 kΩ.
For further analysis, raw signals were digitally filtered off-line between 1 and 50 Hz (48 dB/octave rolloff, zero-phase shift). Next, eye blinks were detected and corrected semi-automatically with a regression algorithm (cf. Semlitsch et al., 1986). In the following step, data were epoched starting 100 ms preceding the beginning of the stimulus and extending to 400 ms following stimulus presentation for S1 and S2, separately. The first 100 ms of each epoch served as baseline. Next, artifacts were detected. Trials that were contaminated with artifacts (peak-to-peak values exceeding 75 µV, muscle related activity, alpha activity contaminating more than 75% of the epoch) were rejected from further analysis together with the trial of the paired stimulus (i.e., trials were rejected as S1–S2 pairs). Prior to averaging, the 1- to 50-Hz signals of the remaining trials were filtered with different filter settings to optimize scoring of the three components: a 20-Hz low-pass filter was used to optimize scoring of the N100 and P200; a 10-Hz high-pass filter was used to optimize scoring of the P50 (Jerger et al., 1992). According to Jerger et al. (1992), the P50 ‘rides’ on the slope of the N100, attenuating the amplitude of the P50. If one of the two groups that are being investigated in this study had increased N100 amplitudes, this could have reduced apparent P50 amplitudes, washing out group differences in P50. A filter setting lower than 10 Hz would not sufficiently suppress the N100 and could subsequently reduce the apparent amplitude of the P50. Finally, averages were made for each filter setting for S1 and S2, separately. Averages were based on the same trials.
P50, N100, and P200 peak amplitudes and latencies were scored at Cz. P50, N100, and P200 components were scored relative to their preceding trough, which could be the P50 for the N100, and which was always the N100 for the P200 component. All peaks and preceding troughs were detected with an automatic peak-picking procedure implemented in the Edit module of Scan 4.3 software (Neuroscan, El Paso) using pre-set intervals: P50 was detected as the most pronounced positivity between 35 and 85 ms, the N100 was detected as the most pronounced negativity between 80 and 150 ms, and the P200 was detected as the most pronounced positivity between 150 and 250 ms (Boutros et al., 2004). Additionally, an investigator who was aware of the diagnosis of the subjects (ML) checked the marker points of the peaks and troughs and adjusted the markers if needed, following the recommendations postulated of Boutros et al. (2004). Following their recommendations, the N100 was identified first. If no N100 peak could be identified within the time-window mentioned previously (80 to 150 ms), a larger time-window was taken into account in which the most pronounced negativity with a clear frontocentral distribution was considered the N100. In this study, all N100 peaks were detected between 60 and 157 ms. The N100 had to be distinguishable from baseline activity and visible on more than one lead for the subject to be included in the final analysis. If no P50 peak was found in the initial time-window (35 to 85 ms), the P50 was identified as the most positive peak preceding the N100. If no P200 peak was found in the initial window (150 to 250 ms), the P200 was identified as the most positive peak following the N100 as long as it peaked before 300 ms. In this study, all P50 peaks but one (one subject had a P50 at 124 ms) were detected between 36 and 82 ms, and all P200 amplitudes were detected between 142 and 288 ms. Scalp distributions were taken into account to identify components that were ambiguous due to multiple peaks or small amplitudes, in which case peaks were selected with a central or frontocentral distribution. For S2 evoked components, additional constraints included the following: S2 P50 activity peaking within 10 ms of the S1 P50 (Nagamoto et al., 1991); S2 N100 and P200 activity peaking within 40 and 80 ms of the S1 N100 and P200, respectively. If no peaks occurred within those windows, the component was considered completely attenuated, unless S2 elicited clear components falling outside the windows as revealed by iso-potential maps (P50: n=5, window to 14 ms; N100: n=7, window to 89 ms; P200: n=1, window of 98 ms).
2.5. Statistical analysis
Dependent variables were peak amplitudes and latencies of P50, N100 and P200 evoked by S1 and S2 scored at Cz, P50, N100, and P200 gating ratio ([S2amplitude/S1amplitude] *100), and difference score (S1amplitude−S2amplitude, ΔS1S2) (e.g., Smith et al., 1994; Wan et al., 2007). Gating ratios higher than 2 were set at 2 (Boutros et al., 2000). A lower ratio and a higher difference score reflect better gating.
Distributions were tested for normality with a level of significance set at P=0.01 (Tabachnick and Fidell, 1989, pp. 72–73). Distributions deviating from normality (P50, N100, P200 amplitudes; P50, N100 latencies; P2 ratio; N100, P200 difference score) were normalized with a logarithmic transformation prior to data analysis. Group differences for gating ratio and difference score were tested by analysis of variance (ANOVA). Stimulus and group effects for peak amplitude and latency were tested for each component separately with repeated measures ANOVA containing Stimulus (2 levels: S1 and S2) as within-subjects factor, and Group (2 levels) as between-subjects factor. Significant Stimulus × Group interactions were tested further in two steps: 1) with ANOVAs for between-group differences for each stimulus, and 2) with separate repeated measures ANOVAs for each group to test within-group stimulus effects. Age and ethnicity were included as covariates in all analyses testing for group differences, controlling for differences on those variables. In testing effects within groups, the two covariates were not included. Outcomes were considered significant if P<0.05 (two-tailed). Group differences were additionally expressed as an effect size (ES), calculated as the mean for the bipolar group minus the mean for the control group, divided by the standard deviation weighted and pooled across the bipolar and the control group, and corrected for the difference in sample size between the groups (Lipsey and Wilson, 2001, p. 198). For all analyses, degrees of freedom were 1,73 unless stated otherwise.
3. Results
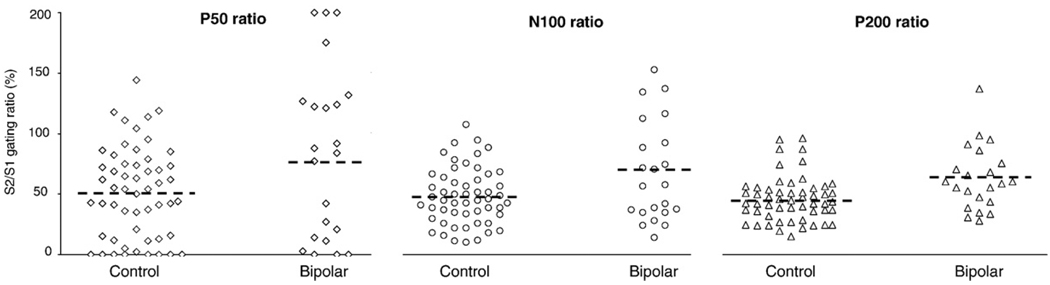
Fig. 1 shows the distributions of P50, N100, and P200 ratios separately for the control and bipolar groups.
Fig. 1.
Distributions of P50, N100, and P200 gating ratios for the control group (n=54) and the bipolar I group (n=22) scored at lead Cz.
3.1. P50
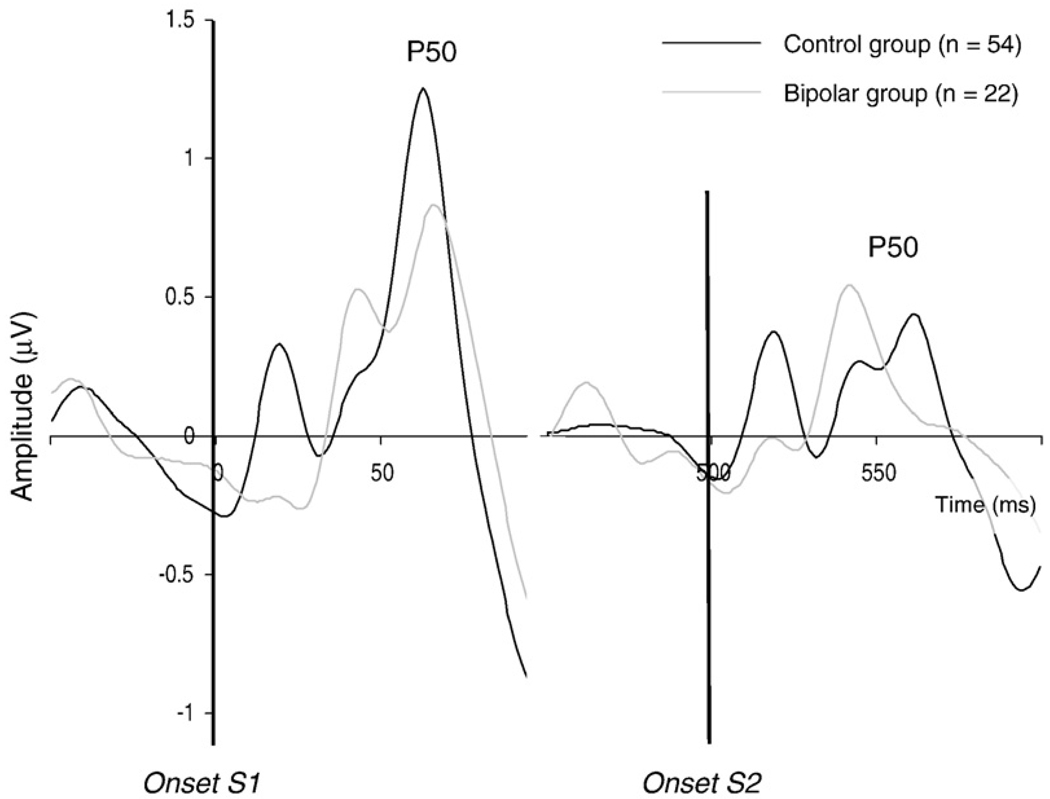
Fig. 2 shows the grand averages of the P50 evoked by S1 and S2 for the control and the bipolar group. Table 2 summarizes the results and the outcomes of the analyses. The P50 ratio was higher (ES=0.65) and the P50 difference score was significantly lower (ES=0.72) in the bipolar group than in the control group, confirming weaker P50 gating in subjects with bipolar I disorder. Repeated measures analysis for the P50 amplitude showed a significant Stimulus × Group interaction (Table 2). P50 amplitude in the bipolar group was significantly smaller than in the control group for S1 (ES=0.67), but not for S2 (ES=0.04). The amplitude for S2 compared with S1 was significantly smaller in the control group (F(1,53)=57.78, P<0.001), but not in the bipolar group (F(1,21)=2.88, P=0.104). No other significant differences were found.
Fig. 2.
P50 gating: grand average of the auditory evoked potential at Cz for S1 and S2 for the control and bipolar I groups. The potential was filtered between 10 and 50 Hz to optimize scoring of the P50 component.
Table 2.
P50 mean (S.D.): ratio (%), difference score (ms), amplitudes (µV), latencies (ms), and outcomes of statistical analysis.
| Measures | Normal controls | Bipolar disorder | Statistical outcomes | ||
|---|---|---|---|---|---|
| Group | Stimulus | Group×Stimulus | |||
| Gating | |||||
| Ratio | 51.85 (37.73) | 84.42 (70.47) | F=10.03, P=0.002 | – | – |
| ΔS1S2 | 1.25 (1.36) | 0.32 (1.01) | F=9.46, P=0.003 | – | – |
| Amplitude a | F=0.95, P=ns. | F=0.06, P=ns. | F=8.36, P=0.005 | ||
| S1 | 2.40 (1.50) | 1.55 (1.08) | F=5.84, P=0.018 | – | – |
| S2 | 1.15 (1.04) | 1.22 (1.13) | F=.37, P=ns. | – | – |
| Latency a,b | F=0.06, P=ns. | F=0.01, P=ns. | F=0.70, P=ns. | ||
| S1 | 62.02 (13.76) | 59.68 (12.08) | – | – | – |
| S2 | 60.95 (15.83) | 56.33 (14.37) | – | – | – |
ΔS1S2=difference score.
Data were log transformed prior to data analysis.
n=44 for normal control and n=18 for bipolar disorder group due to absence of S2 P50 amplitude with total suppression; df=1,58.
3.2. N100
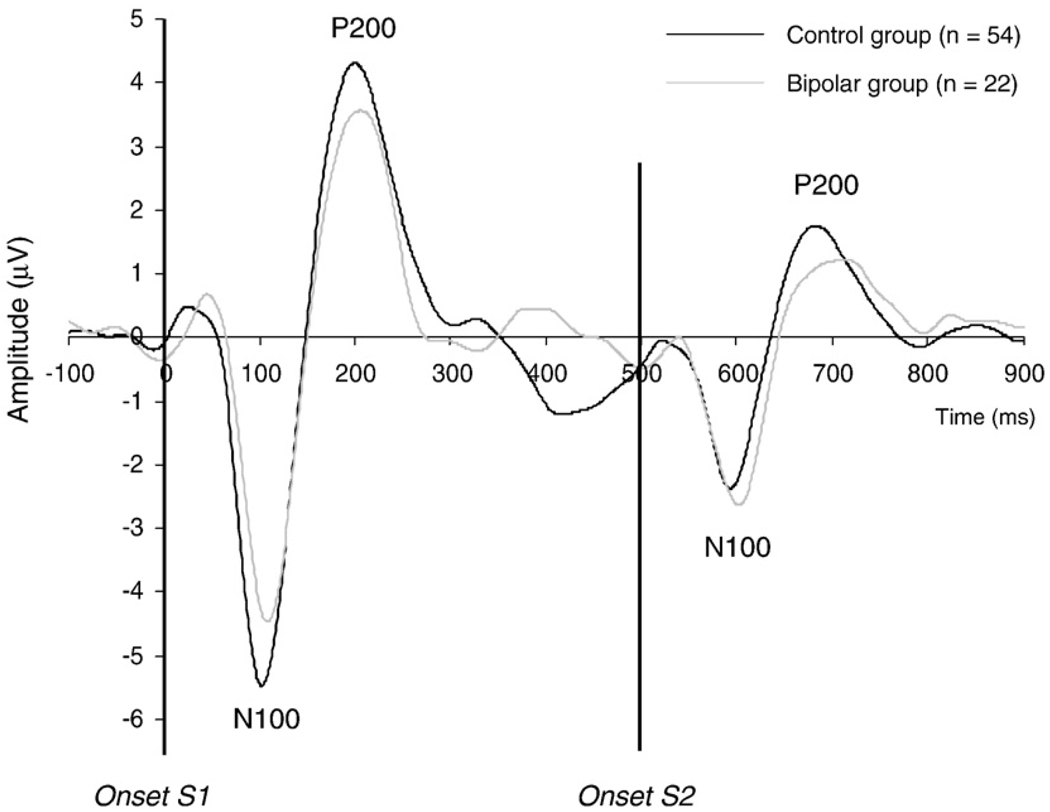
Fig. 3 shows the grand averages of the N100 and P200 evoked by S1 and S2 for the control and the bipolar group. Table 3 summarizes the results and the outcomes of the analyses for the N100. The N100 ratio was higher (ES=0.64), and the N100 difference score was significantly lower (ES=0.54) in the bipolar group than in the control group, indicating weaker N100 gating in bipolar I subjects. Repeated measures ANOVA for N100 amplitude revealed a significant Stimulus×Group interaction (Table 3). There was no group difference in N100 amplitude for S1 (ES=0.25) or S2 (ES=0.17). Testing the effect of Stimulus within each group revealed a significantly enhanced negativity for S1 compared with S2 in both the control (F(1,53)=140.70, P<0.001) and the bipolar group (F(1,21)=17.48, P<0.001). No other significant differences were found.
Fig. 3.
N100 and P200 gating: grand average of the auditory evoked potential at Cz for S1 and S2 for the control and bipolar I group. The potential was filtered between 1 and 20 Hz to optimize scoring of the N100 and P200 components.
Table 3.
N100 mean (S.D.): ratio (%), difference score (ms), amplitudes (µV), latencies (ms), and outcomes of statistical analysis.
| Measures | Normal controls | Bipolar disorder | Statistical outcomes | ||
|---|---|---|---|---|---|
| Group | Stimulus | Group×Stimulus | |||
| Gating | |||||
| Ratio | 48.50 (23.50) | 67.67 (41.70) | F=8.43, P=0.005 | – | – |
| ΔS1S2 a | −3.86 (2.94) | −2.71 (3.34) | F=6.30, P=0.014 | – | – |
| Amplitude a | F=0.08, P=ns. | F=1.84, P=ns. | F=4.96, P=0.029 | ||
| S1 | −6.93 (3.76) | −6.14 (3.65) | F=1.47, P=ns. | – | – |
| S2 | −3.06 (1.72) | −3.43 (1.98) | F=0.58, P=ns. | – | – |
| Latency a | F=0.89, P=ns. | F=0.13, P=ns. | F=1.75, P=ns. | ||
| S1 | 105.52 (14.59) | 105.14 (15.32) | – | – | – |
| S2 | 94.39 (17.20) | 97.68 (14.89) | – | – | – |
ΔS1S2=difference score.
Data were log transformed prior to data analysis.
3.3. P200
Table 4 summarizes the results of the analyses for the P200 component. The P200 ratio was higher (ES=0.79), and the P200 difference score was significantly lower (ES=0.59) in the bipolar group than in the control group, indicating weaker P200 gating in bipolar I subjects. Repeated measures analysis for P200 amplitude revealed a significant Stimulus main effect and a Stimulus × Group interaction (Table 4). There were no significant group differences for P200 amplitude for S1 (ES=0.38) or S2 (ES=0.24). Analysis for each group separately showed significantly reduced P200 amplitudes for S2 compared with S1 in both the control (F(1,53)=229.17, P<0.001) and the bipolar group (F(1,21)=36.65, P<0.001). Furthermore, a significant Group main effect and a Stimulus × Group interaction were found for P200 latency, with a significantly delayed P200 peak latency in the bipolar compared with the control group for S2 (ES=0.91) but not S1 (ES=0.25). Analysis for each group separately showed a significantly earlier latency for S2 compared with S1 in the control group (F(1,53)=28.02, P<0.001) but not in the bipolar group (F(1,21)=0.22, P=ns).
Table 4.
P200 mean (S.D.): ratio (%), difference score (ms), amplitudes (µV), latencies (ms), and outcomes of statistical analysis.
| Measures | Normal controls | Bipolar disorder | Statistical outcomes | ||
|---|---|---|---|---|---|
| Group | Stimulus | Group×Stimulus | |||
| Gating | |||||
| Ratio a | 46.48 (18.83) | 63.63 (26.70) | F=7.58, P=0.007 | – | – |
| ΔS1S2 a | 6.60 (4.82) | 4.54 (4.91) | F=4.69, P=0.034 | – | – |
| Amplitude a | F=0.33, P=ns. | F=21.06, P<0.001 | F=6.36, P=0.006 | ||
| S1 | 11.57 (6.21) | 10.11 (6.42) | F=2.53, P=ns. | – | – |
| S2 | 4.96 (2.58) | 5.57 (2.77) | F=0.27, P=ns. | – | – |
| Latency | F=4.35, P=0.04 | F=3.47, P=ns. | F=4.11, P=0.05 | ||
| S1 | 198.59 (27.65) | 205.82 (29.66) | F=0.78, P=ns. | – | – |
| S2 | 180.15 (21.80) | 203.41 (32.80) | F=8.62, P=0.004 | – | – |
ΔS1S2=difference score.
Data were log transformed prior to data analysis.
3.4. Gating and affective state, history of psychosis, and the use of medication
Associations between gating parameters and affective state were expressed as Kendall’s tau because scores on the SADS-C depression, anxiety, mania, and psychosis scales could not be corrected by logarithmic transformations. Within the bipolar I group, neither gating ratio nor the difference score correlated significantly with SADS-C depression or mania scores (tau=−0.25 to 0.26), suggesting no effect of affect on sensory gating within the relatively limited range of symptoms in this group of subjects.
Differences in gating between subgroups of bipolar I disorder were tested by calculating effect sizes because group sizes of subgroups were not large enough to reliably apply conventional statistics. Effect sizes suggested a somewhat higher P50 ratio in subjects with bipolar I disorder with a history of psychosis (n=14; ratio=94.91%, S.D.=78.88, ΔS1S2=0.19, S.D.=1.11) than subjects without a history of psychosis (n=6; ratio=52.03%, S.D.=52.72, ΔS1S2=0.75, S.D.=0.84) (ES approximately 0.54 for both measures). For the N100 and P200 gating measures, ES did not exceed 0.29.
Low ES were found for P50 gating in subjects with bipolar disorder who used medication (n=16; ratio= 79.74%, S.D.=73.81, ΔS1S2=0.35, S.D.=1.12) compared with subjects who used no medication (n=5; ratio=91.40%, S.D.=71.37, ΔS1S2=0.37, S.D.=0.74) (ES=0.17 and 0.02 for P50 ratio and difference score, respectively). Similar outcomes were found for N100 and P200 gating (ES<0.32). Furthermore, correlation analysis showed no significant correlations between gating measures and the number of medications that patients were taking.
3.5. Post-hoc analysis: relationship between P50, N100 and P200 components
The P50 ratio did not correlate significantly with the N100 or the P200 ratio in either group, but a significant correlation was found between theN100 and the P200 ratio (Table 5). For the difference scores, a significant correlation between P50 and N100, and between P50 and P200, was found for the control group, but not for the bipolar group (Table 5). There were significant correlations between P50 and N100 amplitude, and between P50 and P200 amplitude for S1 in the control group, but not in the bipolar group (Table 5). For S2, there were no significant correlations between P50 and N100 amplitude, or between P50 and P200 amplitude. All measures of the latter two components correlated significantly with each other in both groups. These findings suggest that P50 sensory gating is not directly related to sensory gating of the N100 and P200 components as reflected by amplitudes evoked by S2 (inhibition). In contrast, there is a relationship between processes reflected by amplitudes of the P50, N100 and P200 evoked by S1, but only for the control group.
Table 5.
Pearson correlation coefficients (r) between P50, N100, and P200 ratios, between difference scores, and between amplitudes for the control and bipolar groups.
| Relationship | Ratio | Difference score | ||
|---|---|---|---|---|
| Control | Bipolar | Control | Bipolar | |
| P50–N100 | 0.15 | −0.01 | 0.45** | 0.14 |
| P50–P200 | 0.15 | −0.11 | 0.42** | 0.20 |
| N100–P200 | 0.44** | 0.47** | 0.74** | 0.62** |
| S1 amplitude | S2 amplitude | |||
| Control | Bipolar | Control | Bipolar | |
| P50–N100 | 0.33* | −0.06 | −0.02 | 0.15 |
| P50–P200 | 0.34* | 0.18 | −0.07 | −0.15 |
| N100–P200 | 0.79** | 0.71** | 0.47** | 0.72** |
N100 amplitudes were log transformed prior to data analysis. N100 amplitudes were first multiplied by −1, making them positive. Thus, a positive correlation between N100 amplitude and P50/P200 amplitudes indicates that an increase in N100 peak amplitude is associated with an increase in P50/P200 amplitude.
P<0.02.
P<0.007.
4. Discussion
We investigated gating of the P50, N100, and P200 components of the auditory event-related potential in subjects with bipolar I disorder compared with controls. Regarding gating impairment, our hypotheses were that gating for all three potentials would be impaired in subjects with bipolar I disorder, with secondary hypotheses that impairments would result from decreased inhibition of S2. The results confirmed the primary hypothesis but revealed more complexity with respect to the secondary hypotheses. In a more preliminary manner, we investigated clinical relationships, hypothesizing that 2a) gating impairment would correlate with severity of mania, 2b) gating would be more impaired in subjects with a history of psychosis, and 2c) pharmacological treatment would not influence gating impairment. The results confirmed an apparent lack of medication effect, confirmed the effect of history of psychosis for P50 but not for N100 and P200 gating, but did not confirm effects of symptom severity. We will discuss potential explanations, and consequences, of these results.
4.1. P50
We expected reduced P50 gating in subjects with bipolar disorder due to diminished inhibition of the P50 evoked by S2. Our hypothesis on reduced P50 gating in subjects with bipolar I disorder was confirmed, replicating previous reports of a higher P50 gating ratio in bipolar I disorder compared with a healthy control group (Olincy and Martin, 2005; Schulze et al., 2007). Similarly, the P50 difference score was smaller in the bipolar than in the control group. Thus, our findings corroborate weaker gating of the P50 in bipolar subjects who, as a group, had a broad range of clinical characteristics. Recent studies (Olincy and Martin, 2005; Schulze et al., 2007) attributed the difference in P50 ratio to disinhibition of the P50 following S2. In contrast, we found a significant difference between the two groups for the P50 to S1 but not S2, which could reflect less activity following S1 rather than disinhibition. This finding is consistent with the origin of weaker P50 gating reported for schizophrenic subjects (Freedman et al., 1991).
4.2. N100 and P200
In this study we specifically tested whether bipolar I and control subjects differed in gating of the auditory evoked N100 and P200, with the hypothesis that gating of the N100 and P200 would be impaired in subjects with bipolar I disorder. N100 and P200 auditory gating had not been investigated in bipolar disorder. We found higher N100 and P200 ratios and smaller difference scores in bipolar subjects than in healthy controls, indicating weaker gating in euthymic bipolar patients extending beyond the early, pre-attentive level reflected by P50 gating. The fact that the gating ratio of the P50 potential does not correlate significantly with those of the N100 and P200 (Kisley et al., 2004; Hanlon et al., 2005; Wan et al., 2007) suggests that they tap into the integrity of different underlying mechanisms. However, when gating was expressed as a difference score, P50 gating correlated significantly with N100 and P200 gating, but only in the control group. This suggests that gating of the N100 and P200 may not be completely independent from gating of the P50. This relationship was due to significant correlations between amplitudes of the P50, N100 and P200 evoked by S1, and not S2. Our results showed that the correlations between S1 P50 on the one hand and S1 N100 and P200 on the other hand were not significant for subjects with bipolar I disorder, further corroborating that processing of auditory information differs between subjects with bipolar I disorder and controls.
Our results further showed that weaker gating of the N100 and P200 in subjects with bipolar I disorder was due to a combination of a diminished (but statistically non-significantl) amplitude following S1 and a larger (but also statistically non-significant) amplitude for S2. Previous studies on N100 and P200 amplitudes in patients with bipolar disorder reported conflicting outcomes. Bruder et al. (1992) reported a smaller visual evoked N100 amplitude in bipolar patients, while Hongxing et al. (2002) reported smaller auditory and visual P200 amplitudes in manic and in suicidal patients. Considering that ERP measures reflect cortical neural activation, both studies suggest less activity of neuronal populations in response to stimulation in subjects with bipolar disorder. If a stimulus (i.e., S1) elicited less activity, inhibitory mechanisms that are activated as a consequence of processing S1 (Freedman et al., 1991) would receive a smaller signal for inhibition, which could be reflected in a larger amplitude following S2. In contrast, other studies found similar amplitudes in control subjects and in bipolar subjects in a manic or mixed state for both N100 and P200 on non-target stimuli in an auditory oddball task (Muir et al., 1991; O’Donnell et al., 2004).
4.3. Effects of affective state, history of psychosis, and medication
We expected that gating would be further reduced as a function of severity of affective state, and more specifically hypomania and mania (Franks et al., 1983; Adler et al., 1990). However, we found no significant correlations between P50, N100 or P200 gating measures and affective state measured by the SADS-C, replicating Olincy and Martin (2005) on the relation between affective state and P50 gating. The state-dependent effect of mania may require severe manic symptoms (Franks et al., 1983; Adler et al., 1990). Unlike the case with Adler et al. (1990) and Franks et al. (1983), however, none of the subjects in our study were manic or even moderately hypomanic. The limited range of affective symptoms in our sample of subjects with bipolar I disorder may therefore have prevented significant correlations between P50 gating and affective state.
Our findings and those of Olincy and Martin (2005) and Schulze et al. (2007) seemingly contradict earlier reports that gating was normalized when bipolar patients in manic states entered a euthymic state (Franks et al., 1983; Adler et al., 1990). However, for the difference between the euthymic bipolar and the control group, the effect size in the latter studies was large (0.86), consistent with effect sizes reported by Olincy and Martin (2005), Schulze et al. (2007), and our own study (1.12, 0.85, and 0.65, respectively). Thus, all studies point towards weaker P50 gating in euthymic bipolar subjects.
Olincy and Martin (2005) further reported that P50 sensory gating was diminished in bipolar patients with a history of psychoses. Dividing the subjects in our sample into two subgroups and contrasting subjects with and without a history of psychosis revealed a moderate effect size towards an increased P50 gating ratio, but low power may have prevented statistical significance. Although the result should be interpreted with caution, our results replicated those of the earlier study that bipolar I subjects with a history of psychosis have weaker gating of the P50, whereas those without a history of psychosis have gating in the range of control subjects (ratio=52 vs. 51.9, for the bipolar and control group, respectively). In contrast, the effect sizes for the difference between the bipolar group with and without a history of psychosis for N100 and P200 ratios were small at best (<0.29), suggesting that N100 and P200 gating are not related to psychosis.
Finally, we expected that medication would not normalize P50, N100, and P200 gating. We confirmed our hypothesis by finding small effect sizes in differences in P50, N100, and P200 gating between subjects with bipolar I disorder using medication and those not using medication, suggesting a limited influence of medication on gating processes, which replicates the findings of Olincy and Martin (2005).
In summary, our results showed weaker gating of the P50, N100 and P200 in subjects with bipolar I disorder, revealing a global impairment in filtering incoming information in subjects with bipolar I disorder. We further showed a possible deficiency in bipolar I subjects in the integrity of connections between processes involved in stimulus processing reflected by the P50 and those reflected by the N100 and P200.
Acknowledgements
This study was supported in part by the Pat R Rutherford, Jr Chair in Psychiatry (ACS) and by NIH grants RO1-MH 69944 (ACS), RO1-DA08425 (FGM), KO2-DA00403 (FGM), RO1-MH58784 (NNB), and UL1-RR024148 (CTSA) (General Clinical Research Center, UT Houston). Thanks are due to Hima Bodagala, BA, Glenn S. Colton, PsyD, Sherine Kurian, BA, and Tony G. Zamudio, RN, BC, MSN, CRM, APRN, APMH-NP, for their help with recruitment of subjects and the General Clinical Research Center for providing research facilities and excellent nursing support.
References
- Adler LE, Gerhardt GA, Franks R, Baker N, Nagamoto H, Drebing C, Freedman R. Sensory physiology and catecholamines in schizophrenia and mania. Psychiatry Research. 1990;31:297–309. doi: 10.1016/0165-1781(90)90099-q. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Vedeniapin AB, Heath AC, Korzyukov O, Boutros NN. Genetic and environmental influences on sensory gating of mid-latency auditory evoked responses: a twin study. Schizophrenia Research. 2007;89:312–319. doi: 10.1016/j.schres.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Boutros N, Campbell D, Petrakis I, Krystal J, Caporale M, Kosten T. Cocaine use and the mid-latency auditory evoked response. Psychiatry Research. 2000;96:117–126. doi: 10.1016/s0165-1781(00)00207-9. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Korzyuko O, Oliwa G, Feingold A, Campbell D, McClain-Furmanski D, Struve F, Jansen BH. Morphological and latency abnormalities of the mid-latency auditory evoked responses in schizophrenia: a preliminary report. Schizophrenia Research. 2004;70:303–313. doi: 10.1016/j.schres.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Gooding D, Sundaresan K, Burroughs S, Johanson C. Cocaine-dependence and cocaine-induced paranoia and mid-latency auditory evoked responses and sensory gating. Psychiatry Research. 2006;145:147–154. doi: 10.1016/j.psychres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Steward JW, Towey JP, Friedman D, Tenke CE, Voglmaier MM, et al. Abnormal cerebral laterality in bipolar depression: convergence of behavioral and brain event-related potential findings. Biological Psychiatry. 1992;32:33–47. doi: 10.1016/0006-3223(92)90140-u. [DOI] [PubMed] [Google Scholar]
- Dolu N, Süer C, Özesmi Ç. A comparison of the different interpair intervals in the conditioning-testing P50 paradigms. International Journal of Psychophysiology. 2001;41:265–270. doi: 10.1016/s0167-8760(01)00134-9. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Biometrics Research Institute. New York: New York State Psychiatric Institute; 1996. Structured Clinical Interview for DSM-IVAxis I disorders—Patient Edition. [Google Scholar]
- Franks RD, Adler LE, Waldo MC, Alpert J, Freedman R. Neuropsychological studies of sensory gating in mania: comparison with schizophrenia. Biological Psychiatry. 1983;18:989–1005. [PubMed] [Google Scholar]
- Freedman R, Waldo M, Bickford-Wimer P, Nagamoto H. Elementary neuronal dysfunction in schizophrenia. Schizophrenia Research. 1991;4:233–243. doi: 10.1016/0920-9964(91)90035-p. [DOI] [PubMed] [Google Scholar]
- Fruhstorfer H, Soveri P, Järvilehto T. Short-term habituation of the auditory evoked response in man. Electroencephalography and Clinical Neurophysiology. 1970;28:153–161. doi: 10.1016/0013-4694(70)90183-5. [DOI] [PubMed] [Google Scholar]
- Hanlon FM, Miller GA, Thoma RJ, Irwin J, Jones A, Moses SN, et al. Distinct M50 andM100 auditory gating deficits in schizophrenia. Psychophysiology. 2005;42:417–427. doi: 10.1111/j.1469-8986.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- Hongxing W, Xingshi C, Peishen B, Ling Y. Sensory evoked potentials in patients with affective disorders accompanying suicidal behavior. Chinese Medical Journal (English) 2002;115:1675–1678. [PubMed] [Google Scholar]
- Jasper HH. Report of the committee on methods of clinical examination in electroencephalography. Electroencephalography and Clinical Neurophysiology. 1958;10:370–371. [Google Scholar]
- Jerger K, Biggins C, Fein G. P50 suppression is not affected by attentional manipulations. Biological Psychiatry. 1992;31:365–377. doi: 10.1016/0006-3223(92)90230-w. [DOI] [PubMed] [Google Scholar]
- Kisley MA, Noecker TL, Guinther PM. Comparison of sensory gating to mismatch negativity and self-reported perceptual phenomena in healthy adults. Psychophysiology. 2004;41:604–612. doi: 10.1111/j.1469-8986.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- Lipsey MW, Wilson DB. Practical Meta-Analysis. Thousand Oaks, CA: Sage Publications; 2001. [Google Scholar]
- Muir WJ, St Clair DM, Blackwood DH. Long-latency auditory event related potentials in schizophrenia and in bipolar and unipolar affective disorder. Psychological Medicine. 1991;21:867–879. doi: 10.1017/s003329170002986x. [DOI] [PubMed] [Google Scholar]
- Müller MM, Keil A, Kissler J, Gruber T. Suppression of the auditory middle-latency response and evoked gamma-band response in a paired-click paradigm. Experimental Brain Research. 2001;136:474–479. doi: 10.1007/s002210000597. [DOI] [PubMed] [Google Scholar]
- Nagamoto HT, Adler LE, Waldo MC, Griffith J, Freedman R. Gating of auditory response in schizophrenics and normal controls. Schizophrenia Research. 1991;4:31–40. doi: 10.1016/0920-9964(91)90007-e. [DOI] [PubMed] [Google Scholar]
- O’Donnell BF, Vohs J, Hetrick WP, Carroll CA, Shekhar A. Auditory event-related potential abnormalities in bipolar disorder and schizophrenia. International Journal of Psychophysiology. 2004;53:45–55. doi: 10.1016/j.ijpsycho.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Olincy A, Martin L. Diminished suppression of the P50 auditory evoked potential in bipolar disorder subjects with a history of psychosis. American Journal of Psychiatry. 2005;162:43–49. doi: 10.1176/appi.ajp.162.1.43. [DOI] [PubMed] [Google Scholar]
- Picton T, Hillyard S, Krausz HI, Galambos R. Human auditory evoked potentials: 1. Evaluation of components. Electroencephalography and Clinical Neurophysiology. 1974;36:179–190. doi: 10.1016/0013-4694(74)90155-2. [DOI] [PubMed] [Google Scholar]
- Schulze KK, Hall M, McDonald C, Marshall N, Walshe M, Murray RM, Bramon E. P50 auditory evoked potential suppression in bipolar disorder patients with psychotic features and their unaffected relatives. Biological Psychiatry. 2007;62:121–128. doi: 10.1016/j.biopsych.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliability and valid reduction of ocular artifacts applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Smith DA, Boutros NN, Schwarzkopf SB. Reliability of the P50 auditory event-related potential indices of sensory gating. Psychophysiology. 1994;31:495–502. doi: 10.1111/j.1469-8986.1994.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Endicott J. Biometrics Research. New York: New York State Psychiatric Institute; 1978. Schedule for Affective Disorders and Schizophrenia: Change Version. [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. 2nd ed. New York: Harper and Row; 1989. [Google Scholar]
- Wan L, Crawford HJ, Boutros N. Early and late auditory sensory gating: moderating influences from schizotypal personality, tobacco smoking status, and acute smoking. Psychiatry Research. 2007;151:11–20. doi: 10.1016/j.psychres.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Zouridakis G, Boutros NN. Stimulus parameter effects on the P50 evoked response. Biological Psychiatry. 1992;32:839–841. doi: 10.1016/0006-3223(92)90088-h. [DOI] [PubMed] [Google Scholar]