Abstract
Interactions between dipalmitoylphosphatidylcholine (DPPC) and dipalmitoylphosphatidylserine (DPPS), combined both as binary lipid bilayer assemblies and separately, under the influence of divalent Mg2+, a membrane bilayer fusogenic agent, are reported. Infrared vibrational spectroscopic analyses of the lipid acyl chain methylene symmetric stretching modes indicate that aggregates of the two phospholipid components exist as domains heterogeneously distributed throughout the binary bilayer system. In the presence of Mg2+, DPPS maintains an ordered orthorhombic subcell gel phase structure through the phase transition temperature, while the DPPC component is only minimally perturbed with respect to the gel to liquid crystalline phase change. The addition of Mg2+ induces a reorganization of the lipid domains in which the gel phase acyl chain planes rearrange from an hexagonal configuration toward a triclinic, parallel chain subcell. Examination of the acyl chain methylene deformation modes at low temperatures allows a determination of DPPS microdomain sizes, which decrease in size upon the addition of DPPC-d62 in the absence of Mg2+. On adding Mg2+, a uniform DPPS domain size is observed in the binary mixtures. In either the presence or absence of Mg2+, DPPC-d62 aggregates remain in a configuration for which microdomain sizes are not spectroscopically measurable. Analysis of the acyl chain methylene deformation modes for DPPC-d62 in the binary system suggests that clusters of the deuterated lipids are distributed throughout the DPPS matrix. Light scattering and fluorescence measurements indicate that Mg2+ induces both the aggregation and the fusion of the lipid assemblies as a function of the ratio of DPPS to DPPC. The structural reorganizations of the lipid microdomains within the DPPS/DPPC bilayer are interpreted in the context of current concepts regarding lipid bilayer fusion.
Keywords: Lipid microdomains, vesicle fusion, vibrational spectroscopy, light scattering, fluorescence, Mg
1. Introduction
The role of lipids in regulating cellular membranes has evolved greatly from the model of a homogeneous, fluid barrier supporting proteins to current concepts in which lipid and protein microdomains control cellular activities. Although controversial, a current concept invokes microdomain complexes, commonly referred to as “rafts”, to explain a variety of membrane associated phenomena.1–5 Efforts to detect and to characterize these lipid raft domains in intact membranes led to the consideration that numerous bilayer microdomains, in addition to raft-type structures, exist in biological membranes, thus emphasizing the “mosaicism” of the Singer-Nicholson model to better reflect the current understanding of membrane bilayer architecture.5 The existence of raft-like domains is derived from the observation of detergent insoluble membrane fractions6 even though it remains difficult to observe directly these microdomains in intact cellular systems. Model systems, however, demonstrate the chemical interactions giving rise to membrane microdomains, and provide progress toward understanding the effects of domain organizational changes as they relate to complex biological phenomena.7–13
Since domains may form in response to a stimulus,1 issues arise in relation to specific stimulus driven bilayer reorganizations, such as biomembrane fusion. Membrane bilayer fusion represents important processes in many biochemical and biophysical events, including cellular signaling and trafficking. Both theoretical14–16 and experimental models for elucidating lipid bilayer fusion have been proposed. A classic lipid vesicle fusion assay monitors the exchange of encapsulated fluorescent dyes between phosphatidylserine (PS) vesicles in the presence of Ca2+ and Mg2+ cations.17 Bilayer fusion has also been observed from vesicles formed from oppositely charged fluorescent lipids.18 A common characteristic of these vesicle fusion assays is that increased concentrations of zwitterionic lipids, such as phosphatidylcholine (PC), inhibit PS fusion, or only allows a fusion of the outer leaflet without bridging the vesicle’s internal contents.17,18 Changes in lipid composition, such as differing length acyl chain lengths19 or the inclusion of unsaturated chain lipid species,20 are reported to modulate fusion properties; however other perturbations, as, for example, changes in pH,21,22 the insertion of either small peptides23 or polyethyleneglycol molecules,24,25 also affect the fusion process.
Experimental studies generally assess either the success or failure of fusion; however, experimental evidence concerning specific lipid interactions within the bilayer during the fusion process is limited. Towards this end, numerous approaches, in addition to the fluorescence experiments noted above, have been attempted, including, for example, electrical conductance measurements,26 calorimetry,23,27 and light scattering methods.28,29 Lateral phase separation has been studied for various lipids associated with the fusion process.19,30,31 X-ray diffraction experiments have successfully detected partial fusion, or hemifusion, events in lipid bilayer assemblies 32,33. Vibrational infrared spectroscopic techniques, however, suggest an additional and potentially important mechanistic approach toward characterizing bilayer fusion properties, particularly, since lipid acyl chain packing configurations34 and temperature dependent rearrangements can be readily gauged.27 Specifically, infrared spectroscopy has been used successfully to monitor phase changes associated with lipid bilayer inhomogenieties and headgroup/ interfacial – ion interactions.27,35,36 For bovine brain DPPS assemblies, an infrared spectroscopic analysis of changes in the phosphate bands indicated that divalent cations bind to the phosphate in a bidentate configuration; upon binding Ca2+, a fusogenic agent, the gel to liquid crystalline phase transition of bovine brain DPPS was observed to be suppressed to temperatures greater than 100 °C.37
In the present study we apply infrared spectroscopic techniques to examine the binary DPPS/DPPC bilayer system both for assessing bilayer microdomain formation and for determining lipid acyl chain reorganizations within the membrane’s hydrophobic core in response to a divalent cation fusion catalyst, namely, Mg2+. In our model systems, we utilize disaturated palmitoyl acyl chain lipids in order to facilitate microdomain characterizations. In particular, the observed bilayer reorganizations reveal putative interactions involved in disrupting the bilayer membrane as the fusion process progresses. We augment the infrared spectroscopic measurements with dynamic light scattering studies and fluorescence determinations for further characterization of the bilayer fusion event.
2. Experimental Methods
Materials
1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-dipalmitoyl-d62-sn-glycero-3- phosphocholine (DPPC-d62), and 1,2-dipalmitoyl-sn-glycero-3-[phospho-l-serine] (sodium salt) (DPPS) were purchased from Avanti Polar Lipids (Alabaster, AL). All multilamellar and unilamellar lipid bilayer assemblies were prepared using ultra pure water (KD Medical, Columbia, MD). Stock solutions of molecular biology grade 1.0 M MgCl2 and 0.5 M ethylenediaminetetraacetic acid (EDTA, pH = 8.0) in H2O were obtained from Quality Biological, Inc. (Gaithersburg, MD) and diluted as necessary. HPLC grade chloroform was procured from Sigma-Aldrich (St. Louis, MO).
Sample Preparation
Multilamellar lipid vesicles (MLVs) were prepared from appropriate mole fractions of DPPS and DPPC lipids. For infrared spectroscopic experiments, DPPC-d62 was used to permit chain discrimination from the DPPS components. Mixed lipid systems were prepared from stock solutions in chloroform. Chloroform was removed by flowing nitrogen gas over the samples followed by maintaining the mixtures under vacuum overnight. Lipid mixtures were dispersed in either ultrapure H2O or phosphate buffered saline, pH 7–7.4, to produce MLVs with approximately 25% (wt.%) lipid. For infrared experiments, MLV dispersions were sandwiched between two ZnSe windows and transferred to the infrared spectrometer. For light scattering and vesicle fusion assays, the MLVs were diluted to 1% (wt. %) and sequentially extruded through 1.0 µm, 0.4 µm, 0.2 µm, and 0.1 µm Whatman Nuclepore Track-Etch polycarbonate membranes (Whatman plc., Kent, UK) to obtain unilamellar vesicles (SUVs) of approximately 100 nm in diameter.
Infrared Spectroscopy
Temperature dependent infrared vibrational spectra were collected using a liquid nitrogen gas exchange cryostat mounted on a DA-3 Bomem infrared spectrometer. Infrared spectra were acquired with 0.5 cm−1 spectral resolution and using Hamming apodization. Infrared spectra of the lipid MLVs were acquired at 25 °C. Domain size and organization were charactized in detail using a rapid quenching spectroscopic methodology described previously.38 Briefly, lipids were rapidly cooled from the liquid crystalline to the gel phase, reaching a final −120 °C within minutes. Correlation field splitting parameters involving the methylene deformation modes were obtained at −120 °C; the samples were then heated at various temperature intervals for collecting spectra to ascertain temperature dependent spectral changes and to obtain phase transition information.
Infrared spectroscopic correlation field splitting parameters were determined from curve deconvolutions corresponding to minima observed in the second derivative spectra in the 1470 cm−1 and 1090 cm−1 spectral regions representative of the methylene deformation modes for the normal protonated and perdeuterated acyl chain systems, respectively. Melting curves were generated from the temperature dependent frequencies of the 2850 cm−1 and 2090 cm−1 methylene symmetric stretching modes of the respective lipid species. Peak frequencies were determined to better than ± 0.1 cm−1 from the fits of Lorentzian bandshapes with appropriate baseline corrections. Peak fitting was accomplished using Grams 7.0 (Thermo-Galatic Inc.). Gel to liquid crystalline phase transition temperatures (Tms) and phase transition intervals (ΔT) for each melting curve were determined using a previously described two-state model.39
Dynamic Light Scattering
To measure the extent of irreversible vesicle aggregation, a light scattering size assay was employed. Particle size measurements were obtained using a Malvern Instruments LTD, Zetasizer Nano (Malvern, UK). SUVs prepared by extrusion were diluted to a concentration of 0.45 mg/mL (total lipid weight to volume). 1 mL of lipid solution was added to a standard 1 cm cuvette. The initial vesicle size distribution was obtained. Samples were prepared with an initial polydispersity index (PDI) of 0.1 or better. Mg2+ titration was performed by addition of either a single 100 µL aliquot or a series of 10 × 10 µL aliquots of 10 mM MgCl solution to the cuvette containing SUVs. After each addition the sample was gently stirred and a particle size measurement obtained. After the addition of 100 µL of Mg2+, the solution was back titrated with the addition of 20 µL of 100 mM EDTA solution (pH=8.0), and the particle size distribution was measured. All measurements were determined at 25 °C.
The data were analyzed using the Dispersion Technology Software v.5.1 beta (Malvern Instruments, LTD.) Dynamic light scattering data were collected at a scattering angle of 173 degrees from a laser source of 633 nm wavelength. The particle size distribution was derived from the diffusion coefficient distribution, which is characterized by fitting a set of exponential decays to the first order homodyne correlation function, with non-negativity and smoothness constraints. The algorithm used is capable of quantifying multimodal decay spectra, such as those occurring in particle size distributions, subject to the constraint that the sizes are separated from an adjacent component by a factor of approximately 2.40 It is also necessary to verify that sample concentrations avoid complications that arise from multiple scattering and particle interactions. Such ideal behavior is determined by experimental measurements over a range of concentrations for the dispersed phase and by selecting the range over which the result is independent of sample concentration. In this work the peak representing the most substantial size component (or the only component) was used to characterize the ‘particle size’ of the sample.
Fluorescence Imaging
Multilamellar vesicles were prepared in 0.1 M saline solutions containing 0.1 mM of either 6-carboxyfluorescein (6-CF) or sulforhodamine 101 (SR101) fluorescent indicators (Sigma Aldrich, St. Louis, MO). 5 µm vesicles were prepared by extrusion of the multilamellar vesicles in the fluorescent molecule solution through a polycarbonate membrane with 5 µm pore size. The fluorescent indicator containing vesicles were then isolated from excess fluorescent indicator by passing the vesicle solution through a Sephadex G-25 Superfine column (GE Healthcare, Piscataway, NJ). The 6-CF and SR101 containing vesicles were combined in the presence of 1 mM Mg2+. After 15 min, the mixed vesicle solution was suspended in 2% agarose and imaged under a microscope. The fluorescence assay was performed at room temperature, nominally 21–25 °C.
3. Results
A. Phase Transition Characteristics
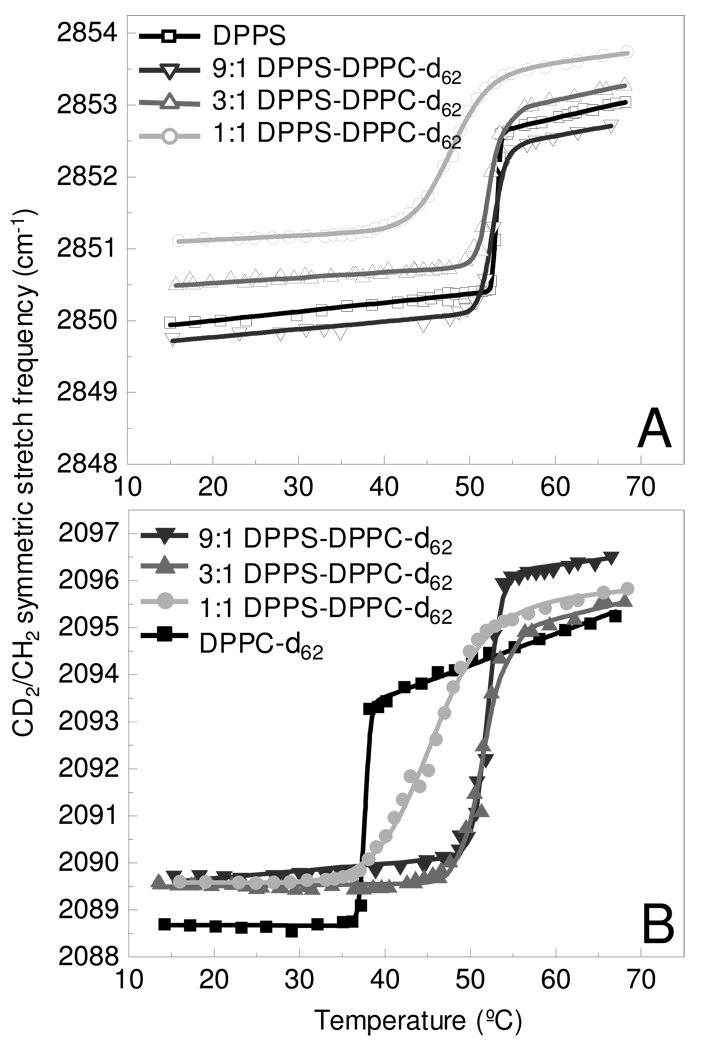
Melting curves were constructed from the changes in the frequencies of the acyl chain methylene symmetric stretching modes for multilamellar assemblies of both pure DPPS and DPPC, as well as mixed lipid systems comprised of 9:1, 3:1, and 1:1 mole fractions of DPPS to DPPC-d62, respectively. The perdeuterated DPPC acyl chains permit discrimination between the DPPC and DPPS components. The DPPS acyl chain methylene symmetric stretching modes at 2850 cm−1 shift to 2090 cm−1 in the deuterated DPPC system. The gel to liquid crystalline phase transition curves, shown in figure 1, plot the changes in the symmetric stretching mode frequencies as a function of temperature for DPPS (Fig 1A) and DPPC-d62 (Fig 1B) in the pure and binary systems.
Figure 1.
Melting curves determine from the methylene symmetric stretching modes are shown for the DPPS MLVs (A) and DPPC-d62 MLVs (B) components in the binary bilayer assemblies. Melting curves are shown for the a pure lipid assembly (black) and mixed lipid assemblies comprised of 9:1 (dk. gray), 3:1 (gray), and 1:1 (lt. gray) DPPS – DPPC-d62, respectively. Lines represent the fit to a two-state model. 39
Several trends are evident in Figure 1A. First, the methylene symmetric stretching mode frequencies are slightly lower both in the gel and liquid crystalline phase for the 9:1 DPPS-DPPC mixture in comparison to pure DPPS, indicating a more ordered acyl chain system. In contrast, increasing amounts of DPPC-d62 in the binary mixtures increases the frequency of the methylene symmetric stretching modes in both the gel and liquid crystalline phases. This increased frequency in the binary systems is commonly associated with increasing chain disorder in comparison to pure DPPS, but has also been attributed to changes in vibrational coupling associated with isotopic dilution.41 A second trend evident in Figure 1A is the shift in the gel to liquid crystalline Tm of the DPPS component with increasing DPPC-d62 content. Table 1 records the phase transition temperature, Tm, and transition width, ΔT values determined for all samples explored in this study. The gel to liquid crystalline transition is observed to be sharp, occurring over less than 1° C, for pure DPPS, but broadens with increased DPPC-d62 content. In the 1:1 mixtures, the melting transition is observed over a temperature interval of nearly 10°.
Table 1.
Gel to liquid crystalline phase change temperatures and melting transition widths.
| DPPS | DPPC-d62 | DPPS + Mg2+ | DPPC-d62 + Mg2+ | |||||
|---|---|---|---|---|---|---|---|---|
| Tm (°C)a | ΔT (°C)b | Tm (°C)a | ΔT (°C)b | Tm (°C)a | ΔT (°C)b | Tm (°C)a | ΔT (°C)b | |
| DPPS | 53.2 ± 0.1 | 0.8 ± 0.1 | NA | NA | -- | -- | NA | NA |
| 9 :1 DPPS- DPPC-d62 |
52.7 ± 0.1 | 2.6 ± 0.1 | 52.0 ± 0.2 | 3.2 ± 0.2 | -- | -- | 50.3 ± 0.2 | 8.9 ± 0.4 |
| 3 :1 DPPS- DPPC-d62 |
52.2 ± 0.1 | 2.6 ± 0.1 | 51.4 ± 0.2 | 5.0 ± 0.1 | -- | -- | 50.3 ± 0.1 | 10.7 ± 0.1 |
| 1 :1 DPPS- DPPC-d62 |
47.8 ± 0.1 | 8.5 ± 0.1 | 45.2 ± 0.2 | 12.8 ± 0.1 | -- | -- | 43.7 ± 0.1 | 7.5 ± 0.2 |
| DPPC-d62 | NA | NA | 37.6 ± 0.1 | 0.7 ± 0.5 | NA | NA | 40.1 ± 0.1 | 1.8 ± 0.4 |
Tm- Phase transition temperature
ΔT – Melting transition width
The phase transition profiles for the DPPC-d62 component of the binary system are plotted in Figure 1B. Contrary to the trend observed for DPPS, the gel phase disorder, which is slightly greater than pure DPPC-d62, remains nearly constant for the DPPS-DPPC-d62 mixtures. DPPC-d62 shows an increase in Tm, compared to the pure MLV, with increasing DPPS content and is consistent with the Tms of the DPPS component. The Tms, summarized in Table 1, indicate that the phase transition temperatures of DPPC-d62 in the binary systems are significantly higher than pure DPPC-d62. In the binary systems, the DPPC-d62 Tm is observed to occur at temperatures slightly below the DPPS melting transition. In the 1:1 system, the melting temperature of DPPC-d62 is observed about 2.5° below that of the DPPS component. A similar result was reported for the Tms of a 1:1 mixture of perdeuterated dimyristoylphosphatidylcholine (DMPC-d54) and dimyristoylphosphatidylserine (DMPS).27 While the exact Tms are slightly different for the DPPS and DPPC-d62 components, the shapes of the melting curves exhibit strong similarities. The narrow widths of the melting transitions, ΔT, indicate that the pure lipid systems show a high degree of cooperativity. The broad transitions observed in the mixed lipid systems indicate a loss of cooperativity. A similar melting transition for a 1:1 mixture of DPPS and DPPC was observed over the same broad temperature window (ca. 10°) using calorimetry,23 as was observed in our study. The broadened temperature profiles for the binary systems also agree with the phase diagram reported from electron paramagnetic resonance experiments.31 The Tm’s for the pure lipid components are consistent with values previously reported.42 The broadening of the phase transition widths observed in melting curves for the binary systems is consistent with the formation of lipid microdomain components that undergo their phase transitions at slightly different temperatures. The general Tms for the binary system are driven by the phase transition for the DPPS component. Interestingly, Monte-Carlo43,44 and molecular dynamics45 simulations suggest PS and PC mixtures exhibit non-ideal mixing which leads to lipid clustering. The ΔTs observed in the infrared spectroscopic melting curves supports the idea of randomly distributed lipid clusters comprising the bilayer assemblies.
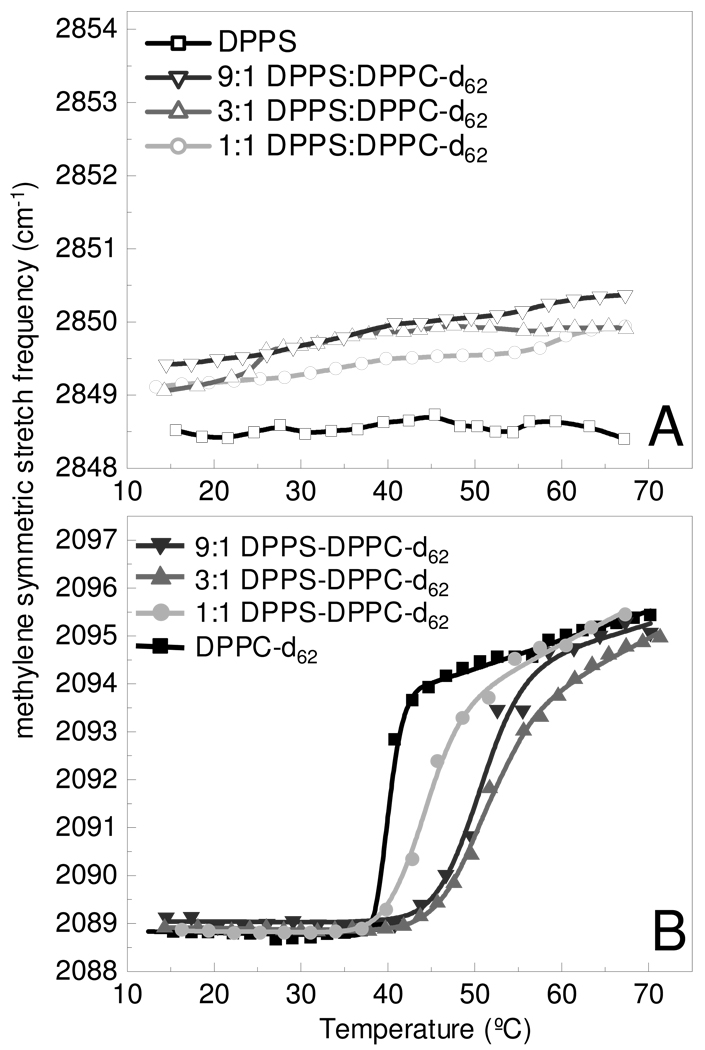
The addition of Mg2+, shown in Figure 2, has dramatic effects on the phase transition behavior, particularly that of DPPS (Fig 2A). Lipid samples were prepared in MgCl2 solution, to yield a lipid to Mg2+ mole ratio of 1:1.5, providing a slight molar excess of Mg2+. In the presence of Mg2+, DPPS acyl chains maintain an ordered gel phase across the conventional phase transition temperature that occurs without Mg2+. Conversely, the DPPC-d62 acyl chains continue to exhibit a gel to liquid crystalline phase change, as shown in Figure 2B, indicating independent DPPC-d62 microdomains. In Table 1, the Tms of the DPPC-d62 component is observed at temperatures 1–1.5° lower in the binary lipid systems containing Mg2+, but 2.5° higher in the pure DPPC-d62 + Mg2+ system. This 2.5° elevated melting temperature for pure DPPC-d62 is consistent with the behavior of lipids in the presence of salts.42 The width of the DPPC-d62 melting transitions is observed to be broader in the presence of Mg2+, despite the close correspondence in Tms to the melting curves without Mg2+. These increased ΔT values indicate that the DPPC-d62 component lipids behave less cooperatively and more independently than the DPPS component in the presence of Mg2+; however, the elevated Tms for DPPC-d62 components in the binary systems suggest that the DPPS matrix exerts a force on the DPPC microdomains inhibiting disorder until higher temperatures are obtained. This further implies that DPPC lipid clusters remain randomly distributed throughout the DPPS matrix. Had Mg2+ induced a complete phase separation, one would expect see the DPPC-d62 acyl chains behave in a fashion similar to pure DPPC-d62. Experiments performed using additional monovalent Na+ and K+ salts, showed no significant differences in the phase transition curves from that of pure H2O.
Figure 2.
Melting curves in the presence of Mg2+ are determined from the methylene symmetric stretching modes are shown for the DPPS MLVs (A) and DPPC-d62 MLVs (B) components in the binary bilayer assemblies. Melting curves are shown for the a pure lipid assembly (black) and mixed lipid assemblies comprised of 9:1 (dk. gray), 3:1 (gray), and 1:1 (lt. gray) DPPS – DPPC-d62, respectively. The lines in panel B represent the fit to a two-state model.39
Inspection of the phosphate asymmetric stretching mode provides insight into the nature of Mg2+ binding to the DPPS present in the bilayer assemblies. In aqueous assemblies, this phosphate stretching mode is observed as a broad band at 1224 cm−1 (see Supporting Information). Addition of Mg2+ results in a shift in this mode to 1242 cm−1 (see Supporting Information). This frequency shift is known to correspond to binding between the phosphate moiety and a divalent cation, which also results in dehydration of the phosphate in the lipid headgroup.37 No shift is observed for pure DPPC-d62 assemblies (see Supporting Information), further indicating the Mg2+ interacts specifically with DPPS.
B. Lipid Bilayer Microdomain Characterization in MLVs
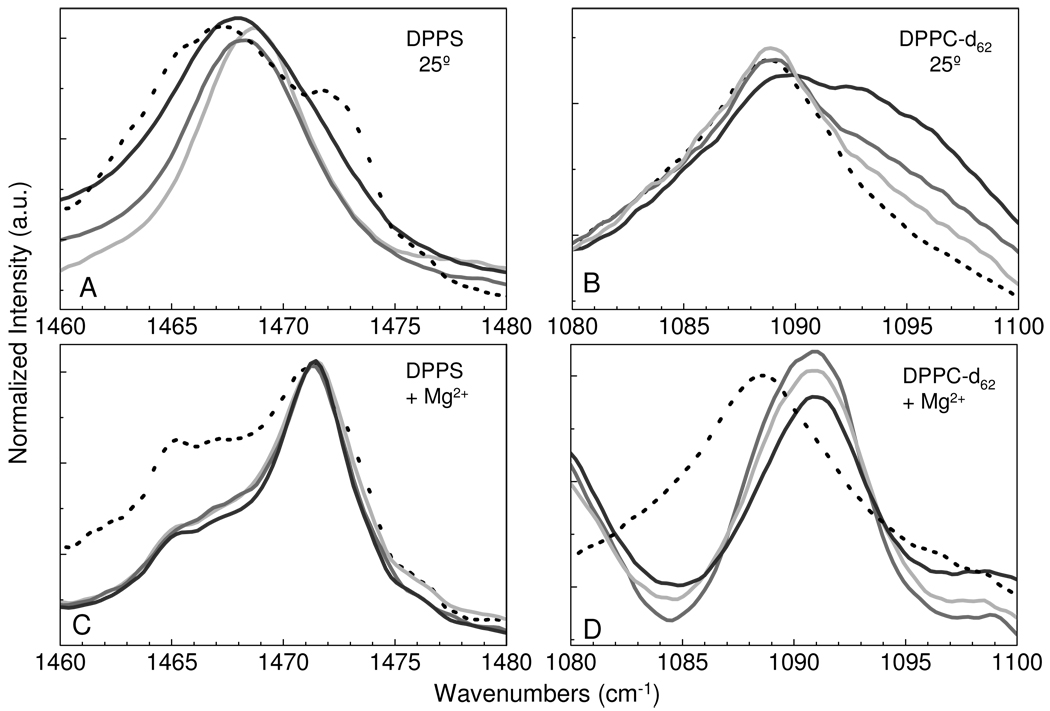
Examination of the methylene deformation modes provides insight into the acyl chain packing properties and the existence of lipid microdomains. In Figure 3, the methylene deformation modes at ~1467 cm−1 for DPPS and ~1090 cm−1 for DPPC-d62 are plotted at 25 °C for the different lipid assemblies with and without Mg2+. Figure 3A for pure DPPS at 25 °C, shows a chain packing configuration to be a convolution of hexagonal chain organization, represented by a peak at 1467 cm−1, and an orthorhombic subcell, which results in a split peak accounting for the shoulders at higher and lower frequencies. The addition of DPPC-d62 disrupts the gel phase orthorhombic chain packing order in which the frequency of the methylene deformation modes shift to higher frequencies with increasing amounts of DPPC-d62, from 1467 cm−1 for the hexagonal subcell of pure DPPS to 1469 cm−1 for the 1:1 mixture with DPPC-d62. Examination of the deuterated methylene deformation modes of DPPC-d62 in Figure 3B shows only a broad single peak at 1089 cm−1, representative of a hexagonally organized gel phase chain subcell. In Figure 3B the spectra are normalized to the 1089 cm−1 peak intensity. For the 9:1 DPPS-DPPC-d62 mixture, the low signal attendant to the small DPPC-d62 component is barely visible above the background; however, the peak position is clearly determined from a second derivative spectral analysis.
Figure 3.
For the binary systems the infrared spectra of the methylene deformation modes are shown at 25 °C for DPPS MLVs (A), DPPC-d62 MLVs (B), DPPS MLVs + Mg2+ (C), and DPPC-d62 MLVs +Mg2+ (D). Pure lipid MLV assemblies are represented by the black broken line, while the mixed lipid MLV assemblies are represented as follows: 9:1 (dk. gray), 3:1 (gray), and 1:1 (lt. gray) of DPPS – DPPC-d62, respectively.
The addition of Mg2+ dramatically alters the acyl chain packing characteristics as seen in Figures 3C and 3D. Two spectral features are worth particular note in Figure 3C. First, definitive splitting, representative of orthorhombic subcell lipid packing arrangements, is clearly observed in the pure DPPS system, replacing the hexagonal packing system originally observed in the absence of Mg2+. A small hexagonal spectral component is still evident, although largely diminished. Shoulders are observed in the binary assemblies, representative of splitting patterns. Second, the DPPS-DPPC-d62 mixtures all exhibit nearly identical spectra. DPPC-d62 in Figure 3D demonstrates a different behavior than that observed for DPPS in that the pure DPPC-d62 assembly is unaffected by the addition of Mg2+. The mixed DPPS-DPPC-d62 systems exhibit a single peak shifted to 1092 cm−1. Previous studies indicate that in protonated lipid systems the occurrence of a single peak at 1473 cm−1 for the methylene deformation mode, compared to 1467 cm−1 for an hexagonal chain packing arrangement, is indicative of a triclinic subcell in which the acyl chain planes are arranged in a parallel manner.46 Accounting for the expected shift in frequency attendant to isotopic substitution, the peak at 1092 cm−1 of DPPC-d62 corresponds well with the triclinic subcell organization.
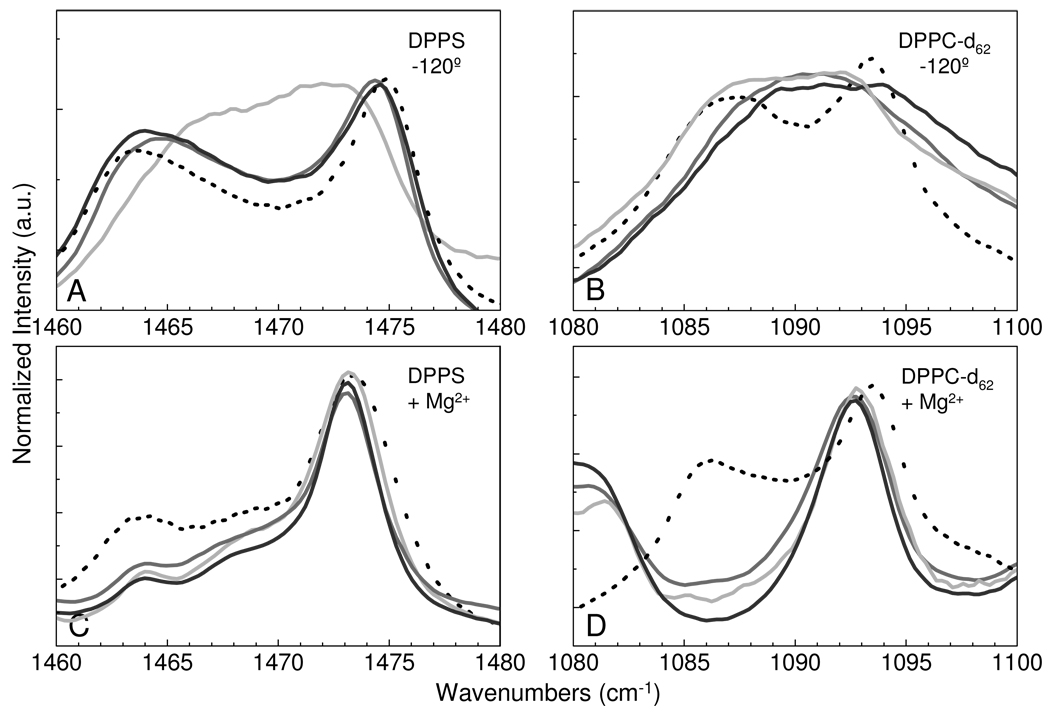
Correlation field splitting parameters for the methylene modes are enhanced at low temperatures. That is, in order to capture the liquid crystalline clustering characteristics, we employed the rapid quenching methodology from the high temperature phase, which was previously established for investigation of lipid microdomains,38 to obtain correlation field splitting parameters from spectra at −120 °C. Figure 4 displays the low temperature spectra of the methylene deformation mode both in the absence (Figs 4A and 4B) and presence (Fig 4C and 4D) of Mg2+ for the DPPS and DPPC-d62 chains, respectively. As expected, DPPS in Figure 4A exhibits an orthorhombic chain packing arrangement, as evidenced by the observation of correlation field splitting components, in all samples. Microdomain sizes can be estimated from the relationship:
| (Eq. 1) |
where Δν is the observed correlation field splitting and Δ ν0 is the reference splitting observed from a pure assembly.47 The number of interacting acyl chains (N) indicates that the microdomain sizes decrease as the percentage of DPPS decreases. In the 9:1 DPPS-DPPC-d62 assembly, the observed splitting is too large to accurately assess a domain size.47 At lower DPPS concentrations, microdomains of 30 and 8 acyl chains are observed for the 3:1 and 1:1 DPPS-DPPC-d62 systems, respectively. The setting angle, the angle of the acyl chain carbon-carbon backbone plane with respect to the orthorhombic subcell axis, can be determined from the intensity ratio of the correlation field splitting components as follows48:
| (Eq. 2) |
In equation 2, the Ia and Ib are the intensities of the high and low frequency splitting components, respectively. The intensities of the correlation field splitting components indicate setting angles representative of a slightly distorted orthorhombic subcell with respect to the ideal orthorhombic subcell setting angle of 45°. The low temperature correlation field splitting parameters, determined from peak deconvolutions, are listed in Table 2. The broad bandshapes observed in Figure 4B for DPPC-d62 in the 9:1 and 3:1 mixed lipid assemblies are not amenable to deconvolution to determine splitting components. However, a splitting is observed for the 1:1 mixed assembly and for the pure DPPC-d62 methylene deformation modes. The lack of splitting in the 9:1 and 3:1 mixed systems indicates that the DPPC-d62 lipid molecules are spatially isolated from each other or arranged in a random configuration that does not give rise correlation field interactions. In the 1:1 DPPS-DPPC-d62 mixture, there is some evidence of a small peak splitting, indicating that at higher DPPC concentrations splitting parameters would be observed.
Figure 4.
For the binary systems the infrared spectra of the methylene deformation modes are shown at − 120 °C for DPPS MLVs (A), DPPC-d62 MLVs (B), DPPS MLVs + Mg2+ (C), and DPPC-d62 MLVs +Mg2+ (D). Pure lipid MLV assemblies are represented by the black broken line, while the mixed lipid MLV assemblies are represented as follows: 9:1 (dk. gray), 3:1 (gray), and 1:1 (lt. gray) of DPPS – DPPC-d62, respectively.
Table 2.
Methylene deformation mode correlation field splitting parameters at −120 °C, calculated domain sizes and setting angles.
| υa | υb | Δυ | Na | Ia | Ib | Ia/Ib | θb | |
|---|---|---|---|---|---|---|---|---|
| DPPS | 1474.7 ± 0.1 | 1463.5 ± 0.1 | 11.2 ± 0.1 | -- | 0.134 ± 0.002 | 0.079 ± 0.09 | 1.70 ± 0.09 | 37° |
| 9 :1 | 1474.7 ± 0.1 | 1463.5 ± 0.1 | 11.2 ± 0.1 | >100 | 0.16 ± 0.04 | 0.12 ± 0.03 | 1.3 ± 0.5 | 41° |
| 3 :1 | 1474.6 ± 0.1 | 1464.7 ± 0.1 | 9.9 ± 0.4 | 30 | 0.34 ± 0.05 | 0.4 ± 0.04 | 0.9 ± 0.2 | 50° |
| 1 :1 | 1473.5 ± 0.1 | 1465.9 ± 0.1 | 7.6 ± 0.4 | 8 | 0.11 ± 0.1 | 0.13 ± 0.03 | 0.8 ± 0.4 | 50° |
| DPPC-d62 | 1093.5 ± 0.1 | 1086.0 ± 0.1 | 7.5 ± 0.1 | -- | 0.42 ± 0.01 | 0.35 ± 0.01 | 1.20 ± 0.04 | 42° |
| 9 :1 | 1091.1 ± 0.1 | -- | -- | c | -- | -- | -- | -- |
| 3 :1 | 1089.3 ± 0.1 | -- | -- | c | -- | -- | -- | -- |
| 1 :1 | 1092.3 ± 0.1 | 1087.3 ± 0.1 | 5.1 ± 0.3 | 7 | 0.17 ± 0.03 | 0.28 ± 0.08 | 0.6 ± 0.2 | 52° |
| +Mg2+ | ||||||||
| DPPS | 1473.3 ± 0.1 | 1463.4 ± 0.1 | 9.9 ± 0.1 | 30 | 0.15 ± 0.01 | 0.04 ± 0.01 | 3.8 ± 0.3 | 27° |
| 9 :1 | 1473.2 ± 0.1 | 1463.8 ± 0.1 | 9.4 ± 0.1 | 20 | 0.53 ± 0.01 | 0.06 ± 0.01 | 8.9 ± 0.3 | 19° |
| 3 :1 | 1473.1 ± 0.1 | 1463.7 ± 0.1 | 9.4 ± 0.1 | 20 | 0.49 ± 0.09 | 0.07 ± 0.01 | 7 ± 1 | 20° |
| 1 :1 | 1473.3 ± 0.1 | 1463.8 ± 0.1 | 9.5 ± 0.1 | 22 | 0.47 ± 0.01 | 0.06 ± 0.01 | 7.5 ± 0.6 | 20° |
| DPPC-d62 | 1093.6 ± 0.2 | 1085.8 ± 0.1 | 7.8 ± 0.2 | -- | 0.20 ± 0.03 | 0.14 ± 0.02 | 1.4 ± 0.3 | 40° |
| 9 :1 | 1092.6 ± 0.1 | -- | -- | d | 0.37 ± 0.01 | -- | -- | -- |
| 3 :1 | 1092.5 ± 0.2 | -- | -- | d | 0.5 ± 0.1 | -- | -- | -- |
| 1 :1 | 1092.8 ± 0.1 | -- | -- | d | 0.39 ± 0.02 | -- | -- | -- |
Calculated domain size, where N represents the number of interacting acyl chains.
Calculated setting angle, where θ is related to the angle between the lipid acyl chain plane and the orthorhombic subcell axis.
The broad peak observed is not amenable to analysis of correlation field splitting components used to determine microdomain sizes.
Infrared spectroscopy cannot determine cluster sizes for lipid triclinic subcells.
Analysis of the low temperature correlation field splitting parameters in the presence of Mg2+ shows significant changes in the correlation field splitting components of DPPS (Fig. 4C). Both the magnitude of the splitting and the intensity of the splitting components are altered. From the N values tabulated in Table 2, Mg2+ addition results in the clustering of DPPS lipids. In the pure lipid system with added Mg2+, microdomains of approximately 30 acyl chains are observed, while the mixed lipid systems with Mg2+ show a uniform domain size of approximately 20 acyl chains. The low frequency correlation field splitting component shows decreased intensity, resulting in a decreased setting angle for the DPPS systems. Interestingly, the high frequency correlation field splitting component is observed at 1473 cm−1. The observation of a single, unsplit, methylene deformation mode at this frequency indicates a triclinic chain plane subcell packing configuration. The dramatic change in setting angle in conjunction with observation of this 1473 cm−1 frequency suggests that Mg2+ induces the clustered DPPS chain planes to orient more parallel to each other.
Figure 4D shows the effect of Mg2+ addition on DPPC-d62. The pure DPPC systems shows little change relative to the Mg2+ free system. This is consistent with the melting curve data above. The mixed lipid assemblies show an interesting change similar to their behavior at 25 °C, in which the deuterated methylene deformation modes are observed as a single peak shifted to a higher frequency. Accounting for the change in reduced mass attendant to the DPPC-d62 moiety, this shift is that which is expected for the triclinic, parallel chain plane, subcell arrangement. While the conformation of the dilute DPPC-d62 assemblies did not show strong organization in the absence of Mg2+, in the presence of Mg2+ the DPPC-d62 component clearly demonstrates a triclinic organization in all the binary assemblies. Since the DPPC-d62 lipid shows no specific interaction with Mg2+, the change in DPPC-d62 acyl chain organization in the mixed assemblies must arise from forces imposed on the bilayer by the DPPS Mg2+ interaction. Thus, the acyl chains of DPPC in the mixed systems remain randomly incorporated in the DPPS matrix and cluster in an orientation dictated by the DPPS acyl chains interacting with Mg2+. Since the clusters exhibit a triclinic chain plane structure, infrared spectroscopy cannot determine a DPPC-d62 microdomain size.
C. Aggregation and Fusion Properties of Single Shell Vesicles
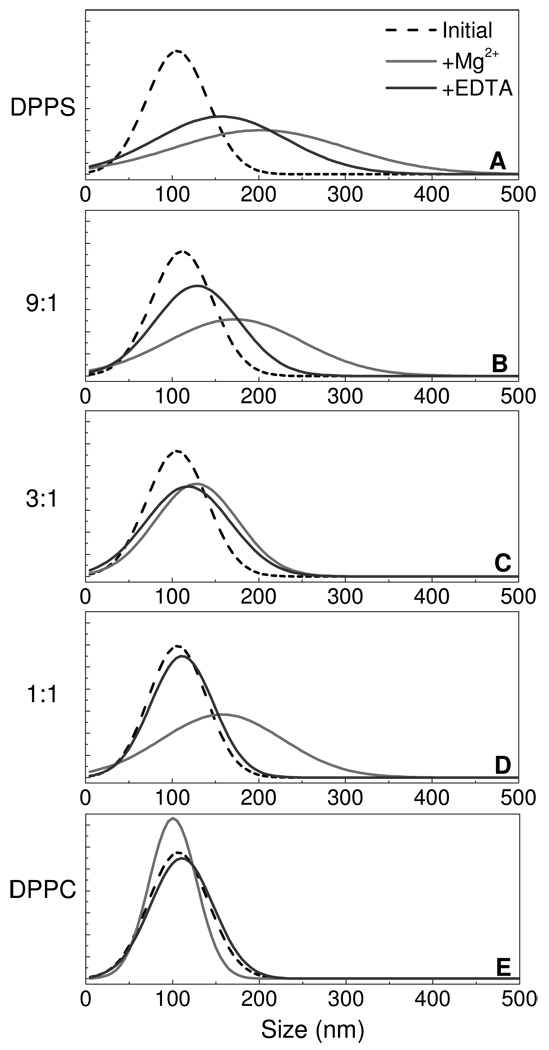
At 25 °C, DPPC and DPPS exist in a hydrated gel phase. To assess the reversibility of aggregation and possible fusion for these lipid assemblies, 100 µL of 10 mM Mg2+ was added in a single addition, followed by back titration with excess EDTA. Figure 5 displays the effect of Mg2+ and EDTA on the observed vesicle size distribution for each lipid system. In particular, all 5 samples show similar initial distributions of 100 nm single shell vesicles. The addition of Mg2+ results in an aggregation trend similar to that observed in the step-wise titration. The width of the distributions suggests that multi-aggregates may be forming, which appear to be more prevalent with increased DPPS concentration. EDTA is strong metal chelating agent, with a Mg2+ binding constant more than 2 orders of magnitude stronger than DPPS. Addition of excess EDTA is expected to remove Mg2+ from solution and to permit an assessment of aggregation reversibility. The addition of excess EDTA (relative to added Mg2+) results in a slight size decrease for DPPS as well as the 9:1 and 3:1 DPPS-DPPC mixed vesicles. Upon standing for several hours, no further decrease in size was observed. The inability to recover the initial vesicle size distributions after treatment with EDTA implies vesicle fusion. The 1:1, DPPS: DPPC, system showed reversible aggregation, returning back to the original size distribution suggesting that in the 1:1 DPPS to DPPC mixed vesicle ensemble, Mg2+ induces vesicle aggregation without a fusion event. As expected, the Mg2+ or EDTA additions show little effect on the DPPC control sample. It has been previously demonstrated using a fluorescence-based mixing assay that increasing the amount of phosphatidylcholine within phosphatidylserine vesicles inhibits vesicle fusion;17 the results presented here are consistent with the previous fluorescence data.
Figure 5.
The single shell size distributions for DPPS (A), the mixed lipid vesicle assemblies comprised of 9:1 (B), 3:1 (C), and 1:1 (D) DPPS – DPPC, respectively, and DPPC single shell vesicles (E) are plotted. The initial distribution is plotted in black (--), the distribution measured following the addition 1 mM Mg2+ is shown in gray (−), and the distribution resulting from the subsequent addition of excess EDTA is shown in dk. gray (−).
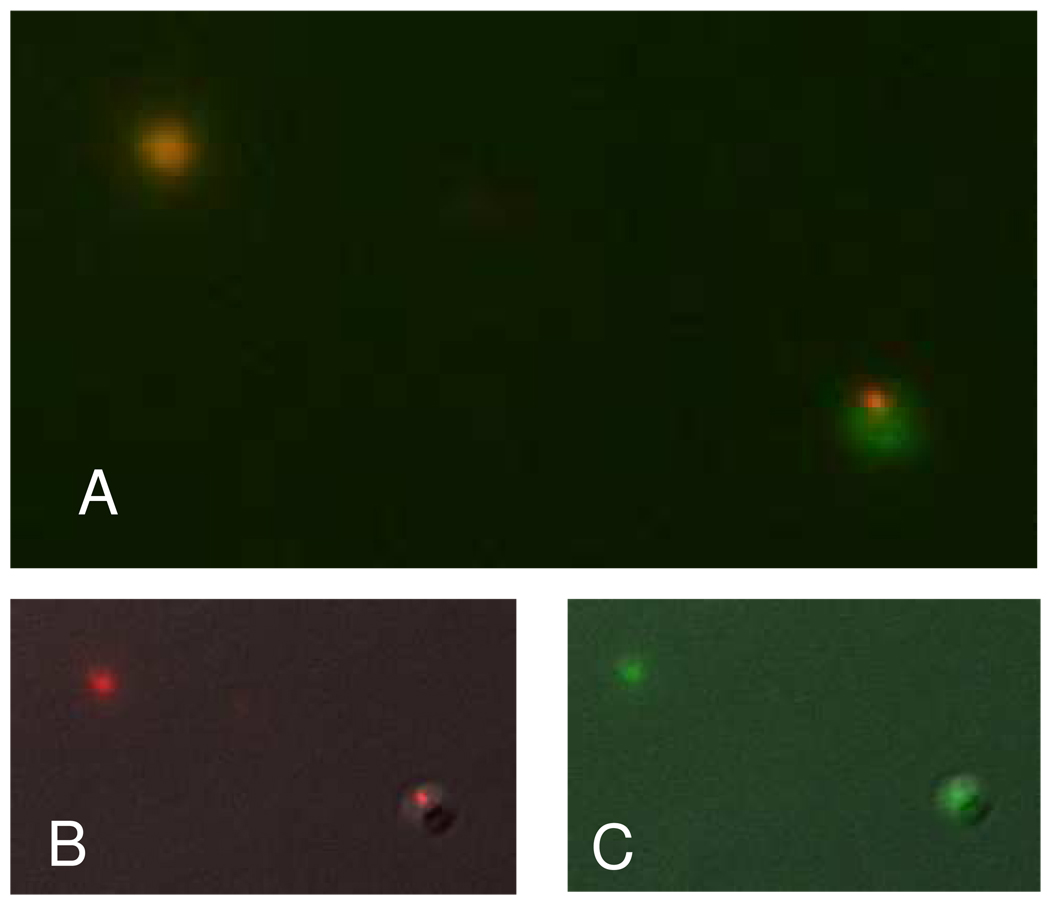
A fluorescent mixing experiment verified that both aggregation and fusion indeed occur. Figure 6 shows vesicle products that were imaged after mixing DPPS vesicles containing 6-CF and SR101 fluorescent indicators in the presence of 1 mM Mg2+. In Figure 6a, it was observed that some vesicles showed colocalization of the fluorescent dyes indicating fusion. Aggregation was also observed, consistent with the light scattering results. Colocalization of these dyes was verified by examining the individual channels for SR101 (Figure 6b) and 6-CF (figure 6c). Single shell DPPS and binary DPPS/DPPC vesicles were observed to coalesce upon the addition of Mg2+ by light scattering. The addition of EDTA, with a substantially stronger affinity for Mg2+ than either lipid, effectively removes the Mg2+ from solution. The fluorescence data indicates the irreversible increase in vesicle size under these conditions corresponds to vesicle fusion. The behavior observed from the gel phase di-saturated lipids is consistent with the behavior reported for the fluid phase bovine brain PS model systems reported previously.17,37
Figure 6.
The fluorescence image (A) obtained from a mixture of vesicles containing either 6-CF or SR101 combined in the presence of Mg2+ indicates both fusion and aggregation occurring as evidenced by the colocalization of the dyes but also the observation of aggregation products. The individual fluorescence signals are shown for SR101 (B) and 6-CF (C).
4. Discussion
The existence and behavior of membrane bilayer lipid microdomains and acyl chain plane reorganizations provide additional prospectives in our understanding of biomembrane fusion processes. Earlier studies investigating divalent cation induced PS and PC phase separation as a mechanism for fusion suggested that the concentration of PS lipids in in-vivo membranes was too low to drive the fusion process.19 With the formation of lipid microdomains, however, the local, increased concentrations of specfic lipid components could allow fusion to occur. Previous reports indicate that the lipid bilayer must undergo significant changes in the kinetic steps toward complete fusion, some of which appear to incur substantial energetic penalties. Bilayer alterations toward complete fusion involve the initial contact between bilayers (or stalk formation), which entails the lipids to undergo significant conformational changes which could include bilayer stretching, chain tilting and bilayer bending deformations for accomplishing fusion without creating energetically unfavorable voids.49 Since PS containing lipid vesicles are known to fuse in presence of Mg2+,17 microdomain formation and subtle chain plane rearrangements provide a rationale for a detailed molecular basis for lipid bilayers undergoing fusion in a protein free model system.
There exists a long history of investigations into the binding of divalent cations to PS containing lipid.assemblies related to bilayer fusion.17,19,50–57 Ca2+, is reported to be a critical component in the membrane fusion process. Studies have shown that SNARE mediated bilayer fusion is dependent upon the presence of Ca2+ to complete the fusion process.54,58,59 In another biological system, a Ca2+ release has been shown to trigger the release of sea urchin oocytes through via an excytotic membrane fusion event.60 Both Ca2+ and Mg2+ are known to promote the fusion of lipid vesicles. It is now clear that molecular interactions involving the divalent cation are involved in the destabilization of the lipid bilayer necessary for fusion and that the binding of divalent cations to the lipid bilayer causes this destabilization.50,57 Additionally, it has been reported that the destabilization arises from the displacement of water resulting from the strong binding of Ca2+ with phosphate moieties across the fusing bilayers.54
Our data provides new insights regarding the molecular mechanisms leading to a destabilization of the lipid bilayer. Our results agree with previous reports of PS lipids binding to Ca2+ in that with the inclusion of Mg2+, the DPPS lipids maintain an ordered packing conformation to elevated temperatures.37,61 Changes observed in the methylene deformation modes of the saturated acyl chains allow an assessment of the domain structure within the bilayer as a result of the binding of Mg2+ to the DPPS component. Our infrared spectroscopic measurements indicate specifically both a size and organizational change within the DPPS domains, as Mg2+ appears to organize the DPPS lipids into clusters of 10–15 lipids. The infrared spectra support Mg2+ binding to the phosphate of DPPS to form these micro-clusters. Within these DPPS domains there exists a packing configuration where the acyl chains exhibit a setting angle, related to the angle between the acyl chain planes and the orthorhombic subcell axis, approaching 20° as opposed to 40–50° in the absence Mg2+. Despite no evidence of a direct interaction between DPPC and Mg2+, The DPPC-d62 lipid domains also exhibit a change in acyl chain organization. That is, the frequency shift observed in the deuterated methylene deformation modes indicates that the DPPC lipids align with their acyl chain planes oriented in a parallel manner. In the absence of direct interaction between DPPC and Mg2+, the observed DPPC realignment likely results from a lateral force exerted within the bilayer arising from the DPPS/Mg2+ interaction. It has been noted that an important step in progressing from partial, or hemifusion, to complete fusion is an increase in the lateral tension within the lipid bilayer.49 The increased free energy associated with bilayer tension is believed to destabilize the bilayer resulting in spontaneous pore formation. The infrared spectroscopic results presented here support such a mechanism; namely, when Mg2+ binds to DPPS to form a lipid cluster, increase in bilayer tension occurs. Pore formation arises when a critical concentration of DPPS domains are formed. The fusion mechanism suggested by the infrared measurements is supported by the light scattering and fluorescence measurements. In our lipid assemblies the degree of fusion observed in the light scattering studies is correlated with the percentage of DPPS relative to DPPC. At high DPPC concentrations, the threshold tension is not achieved, resulting only in vesicle aggregation, as observed, for example, in the 1:1 DPPS/DPPC assemblies.
Most reports of fusion in the literature involve Ca2+ as the fusion catalyst. The differences between Ca2+ and Mg2+ become important in model systems with respect to their binding to PS headgroups. It has been previously suggested that Ca2+ binds PS headgroups in a “trans” geometry, while Mg2+ creates a “cis” lipid complex.55 The trans complex implicated binding through the carbonyl groups;55 however, previous infrared spectroscopic studies showed the formation of bidentate Ca-PO4 complex between Ca2+ and PS lipids.37 The spectroscopic results presented here indicate that Mg2+ binds to PS in a similar fashion as Ca2+. The reported Ca2+/PS binding constant is reported to be double the Mg2+/PS binding constant.55 In the presence of Ca2+ PS lipids are reported to undergo a rapid phase separation, demixing in binary lipid model systems.19,43,62 The systems reported in the present study do not indicate phase separation in the presence of Mg2+. Specifically, the infrared spectroscopic melting curves in the presence of Mg2+ (Fig. 2) support a heterogeneous distribution of DPPC and DPPS lipid domains. This difference in Ca2+ and Mg2+ phase seperation behavior is likely related to the binding constant differences. The strong Ca2+/PS binding results in virtually no free PS lipids when sufficient Ca is present; however, the lower Mg2+/PS binding constant results in a fraction of unbound PS lipids in excess Mg2+. It has been recognized that a principal difference between Ca2+ and Mg2+, as reflected by the difference in binding constants, is the observed kinetics; such that Mg2+/ PS binding occurs slower or requires higher cation concentrations.50,55 The differences between Ca2+ and Mg2+ have been evaluated with respect to the surface tension needed to promote fusion, where the critical surface tensions needed were identical in both cases.57 By avoiding phase separation in the model system presented in this study, the effects of divalent cation binding on the entire bilayer structure can be assessed. Since large-scale phase separation is not conducive to maintaining a stable membrane structure, our results offer a perspective into a mechanism that would be consistent with localized cation-lipid binding events, perhaps regulated in vivo by the cellular protein machinery. In vivo, Ca2+, properly regulated, likely provides a more kinetically efficient response.
Simulations suggest that lipid bilayer fusion proceeds through the following pathway: contact (or stalk formation), formation of a hemifused system where the merging bilayers each contribute one leaflet though the contents remain separate, pore formation, followed by pore expansion that yields the fused state.14,15,49,63–66 The PS – divalent cation interaction, as evidenced by the results presented here, appears to play a critical role throughout this process. Contact appears to be achieved through an electrostatic interaction, as evidenced by aggregation, with fusion only being observed in lipid assemblies containing the negatively charged PS headgroup. Interestingly, once bound to PS, the divalent cation appears to bind the phosphate groups preferentially. The binding event is reported to release water molecules, previously coordinated to both the divalent cation and the phosphate moiety, thus increasing the general hydrophobic character of the region.54 An increasingly hydrophobic area would facilitate lipid rearrangements proceeding toward the formation of the hemifused state. Once in the hemifused state, the lateral tension induced by the PS domains (vida supra) result in the formation of a pore. The alignment of the lipids, reflected by the change in setting angle throughout the bilayer assembly, facilitates the expansion of the pore into the final fused state.
To our knowledge, this realignment of the acyl chains within lipid assemblies in the presence of a divalent cation has not been previously demonstrated. In the present study saturated lipid acyl chains were used to give rise to clear correlation field splitting components for determining microdomain sizes; however, for assessing the details of acyl chain microdomain behavior in the future, investigations could extend to model systems involving unsaturated acyl chains, polyvalent headgroups, and other lipids found in intact biological membranes. Similarly, future work examining the role of fusion proteins in combination with diverse lipids may aid in clarifying the complex lipid rearrangement mechanisms that lead to in vivo membrane fusion.
5. Conclusion
The data presented here provide additional insights regarding the bilayer interactions between DPPS and DPPC lipids in response to a membrane fusion catalyst. Our results indicate that single shell vesicles comprised of binary assemblies of DPPS and DPPC fuse in the presence of a divalent Mg2+ cation. Inspection of the acyl chain infrared spectroscopic melting curves indicates the existence of DPPS and DPPC microdomains distributed throughout the bilayer. In the presence of Mg2+, DPPS maintains a gel phase configuration above the phase transition. Analysis of the acyl chain methylene deformation modes demonstrate that interactions between DPPS head groups and a divalent cation, such as Mg2+, result in microdomain formation and a rearrangement tendency of the acyl chain planes within the bilayer cluster. DPPC does not exhibit a response to the Mg2+ cation; however the DPPC acyl chains rearrange in a manner consistent with the DPPS matrix. Large quantities of DPPC in the bilayer assembly inhibit fusion. In summary, we suggest that the lipid reorganizations within predominantly DPPS lipid microdomains are critical in order to mechanistically drive the bilayer to a fused membrane state.
Supplementary Material
Infrared vibrational spectra obtained of the phosphate antisymmetric stretching mode region are available for the pure DPPS and DPPC-d62 lipids along with all spectra obtained from the binary mixtures. The spectra are presented both with and without the addition of Mg2+.
Acknowledgement
The authors thank Dr. Shuko Yoshikami for assistance preparing vesicles containing fluorescence indicators. This work was funded through the NIDDK intramural research program.
References
- 1.Jacobson K, Mouritsen OG, Anderson RGW. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- 2.Brown DA. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology. 2006;21:430. doi: 10.1152/physiol.00032.2006. [DOI] [PubMed] [Google Scholar]
- 3.Lagerholm BC, Weinreb GE, Jacobson K, Thompson NL. Detecting Microdomains in Intact Cell Membranes. Annu. Rev. Phys. Chem. 2005;56:309. doi: 10.1146/annurev.physchem.56.092503.141211. [DOI] [PubMed] [Google Scholar]
- 4.Engelman DM. Membranes are more mosaic than fluid. Nature. 2005;438:578. doi: 10.1038/nature04394. [DOI] [PubMed] [Google Scholar]
- 5.Vereb G, Szollosi J, Matko J, Nagy P, Farkas T, Vigh L, Matyus L, Waldmann TA, Damjanovich S. Dynamic, yet structured: The cell membrane three decades after the Singer-Nicolson model. Proc. Nat. Acad. Sci. USA. 2003;100:8053. doi: 10.1073/pnas.1332550100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown DA, Rose JK. Sorting of Gpi-Anchored Proteins to Glycolipid-Enriched Membrane Subdomains During Transport to the Apical Cell-Surface. Cell. 1992;68:533. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 7.Chan Y-HM, Boxer SG. Model membrane systems and their applications. Curr. Opin. Chem. Biol. 2007;11:581. doi: 10.1016/j.cbpa.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.London E. How principles of domain formation in model membranes may explain ambiguities concerning lipid raft formation in cells. Biochim. Biophys. Acta. 2005;1746:203. doi: 10.1016/j.bbamcr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Edidin M. The state of lipid rafts: From model membranes to cells. Annu. Rev. Biophys. Biomol. Struct. 2003;32:257. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- 10.Edidin M. Lipids on the frontier: a century of cell-membrane bilayers. Nat. Rev. Mol. Cell Biol. 2003;4:414. doi: 10.1038/nrm1102. [DOI] [PubMed] [Google Scholar]
- 11.Feigenson GW, Buboltz JT. Ternary phase diagram of dipalmitoyl-PC/dilauroyl-PC/cholesterol: Nanoscopic domain formation driven by cholesterol. Biophys. J. 2001;80:2775. doi: 10.1016/S0006-3495(01)76245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radhakrishnan A, Anderson TG, McConnell HM. Condensed complexes, rafts, and the chemical activity of cholesterol in membranes. Proc. Natl. Acad. Sci. USA. 2000;97:12422. doi: 10.1073/pnas.220418097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao M, Mukherjee S, Maxfield FR. Cholesterol depletion induces large scale domain segregation in living cell membranes. Proc. Natl. Acad. Sci. USA. 2001;98:13072. doi: 10.1073/pnas.231377398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katsov K, Muller M, Schick M. Field Theoretic Study of Bilayer Membrane Fusion. I. Hemifusion Mechanism. Biophys. J. 2004;87:3277. doi: 10.1529/biophysj.103.038943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozlovsky Y, Kozlov MM. Stalk Model of Membrane Fusion: Solution of Energy Crisis. Biophys. J. 2002;82:882. doi: 10.1016/S0006-3495(02)75450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinreb G, Lentz BR. Analysis of Membrane Fusion as a Two-State Sequential Process: Evaluation of the Stalk Model. Biophys. J. 2007;92:4012. doi: 10.1529/biophysj.106.090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duzgunes N, Wilschut J, Fraley R, Papahadjopoulos D. Studies on the mechanism of membrane fusion. Role of head-group composition in calcium- and magnesium-induced fusion of mixed phospholipid vesicles. Biochim. Biophys. Acta. 1981;642:182. doi: 10.1016/0005-2736(81)90148-6. [DOI] [PubMed] [Google Scholar]
- 18.Pantazatos DP, MacDonald RC. Directly Observed Membrane Fusion Between Oppositely Charged Phospholipid Bilayers. J. Membr. Biol. 1999;170:27. doi: 10.1007/s002329900535. [DOI] [PubMed] [Google Scholar]
- 19.Silvius JR, Gagne J. Calcium-induced fusion and lateral phase separations in phosphatidylcholine-phosphatidylserine vesicles. Correlation by calorimetric and fusion measurements. Biochem. 1984;23:3241. [Google Scholar]
- 20.Sanchez-Migallon MP, Aranda FJ, Gomez-Fernandez JC. The dissimilar effect of diacylglycerols on Ca(2+)-induced phosphatidylserine vesicle fusion. Biophys. J. 1995;68:558. doi: 10.1016/S0006-3495(95)80217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massari S, Folena E, Ambrosin V, Schiavo G, Colonna R. pH-dependent lipid packing, membrane permeability and fusion in phosphatidylcholine vesicles. Biochim Biophys. Acta. 1991;1067:131. doi: 10.1016/0005-2736(91)90034-6. [DOI] [PubMed] [Google Scholar]
- 22.Ogawa Y, Kodaka M, Okuno H. Trigger lipids inducing pH-dependent liposome fusion. Chemistry and Physics of Lipids. 2002;119:51. doi: 10.1016/s0009-3084(02)00053-1. [DOI] [PubMed] [Google Scholar]
- 23.Higashino Y, Matsui A, Ohki K. Membrane fusion between liposomes composed of acidic phospholipids and neutral phospholipids induced by melittin: A differential scanning calorimetric study. J. Biochem., Tokyo. 2001;130:393. doi: 10.1093/oxfordjournals.jbchem.a002998. [DOI] [PubMed] [Google Scholar]
- 24.Burgess SW, McIntosh TJ, Lentz BR. Modulation of Poly(Ethylene Glycol)-Induced Fusion by Membrane Hydration - Importance of Interbilayer Separation. Biochem. 1992;31:2653. doi: 10.1021/bi00125a004. [DOI] [PubMed] [Google Scholar]
- 25.Johnsson M, Edwards K. Phase behavior and aggregate structure in mixtures of dioleoylphosphatidylethanolamine and poly(ethylene glycol)-lipids. Biophys. J. 2001;80:313. doi: 10.1016/S0006-3495(01)76016-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chanturiya A, Chernomordik LV, Zimmerberg J. Flickering fusion pores comparable with initial exocytotic pores occur in protein-free phospholipid bilayers. Proc. Natl. Acad. Sci. USA. 1997;94:14423. doi: 10.1073/pnas.94.26.14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dibble AR, Hinderliter AK, Sando JJ, Biltonen RL. Lipid lateral heterogeneity in phosphatidylcholine/phosphatidylserine/diacylglycerol vesicles and its influence on protein kinase C activation. Biophys. J. 1996;71:1877. doi: 10.1016/S0006-3495(96)79387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kraayenhof R, Sterk GJ, Wong Fong Sang HW, Krab K, Epand RM. Monovalent cations differentially affect membrane surface properties and membrane curvature, as revealed by fluorescent probes and dynamic light scattering. Biochim. Biophys. Acta. 1996;1282:293. doi: 10.1016/0005-2736(96)00069-7. [DOI] [PubMed] [Google Scholar]
- 29.Roldan-Vargas S, Martin-Molina A, Quesada-Perez M, Barnadas-Rodriguez R, Estelrich J, Callejas-Fernandez J. Aggregation of liposomes induced by calcium: A structural and kinetic study. Phys. Rev. E. 2007;75 doi: 10.1103/PhysRevE.75.021912. [DOI] [PubMed] [Google Scholar]
- 30.van Dijck PWM, de Kruijff B, Verkleij AJ, van Deenen LLM, de Gier J. Comparative studies on the effects of pH and Ca2+ on bilayers of various negatively charged phospholipids and their mixtures with phosphatidylcholine. Biochim. Biophys. Acta. 1978;512:84. doi: 10.1016/0005-2736(78)90219-5. [DOI] [PubMed] [Google Scholar]
- 31.Luna EJ, McConnell HM. Lateral phase separations in binary mixtures of phospholipids having different charges and different crystalline structures. Biochim. Biophys. Acta. 1977;470:303. doi: 10.1016/0005-2736(77)90108-0. [DOI] [PubMed] [Google Scholar]
- 32.Yang L, Huang HW. Observation of a Membrane Fusion Intermediate Structure. Science. 2002;297:1877. doi: 10.1126/science.1074354. [DOI] [PubMed] [Google Scholar]
- 33.Yang L, Ding L, Huang HW. New Phases of Phospholipids and Implications to the Membrane Fusion Problem. Biochem. 2003;42:6631. doi: 10.1021/bi0344836. [DOI] [PubMed] [Google Scholar]
- 34.Snyder RG, Liang GL, Strauss HL, Mendelsohn R. IR spectroscopic study of the structure and phase behavior of long-chain diacylphosphatidylcholines in the gel state. Biophys. J. 1996;71:3186. doi: 10.1016/S0006-3495(96)79512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hubner W, Mantsch HH, Paltauf F, Hauser H. Conformation of Phosphatidylserine in Bilayers as Studied by Fourier-Transform Infrared-Spectroscopy. Biochem. 1994;33:320. doi: 10.1021/bi00167a042. [DOI] [PubMed] [Google Scholar]
- 36.López-García F, Micol V, Villalaín J, Gómez-Fernández JC. Infrared spectroscopic study of the interaction of diacylglycerol with phosphatidylserine in the presence of calcium. Biochim Biophys. Acta. 1993;1169:264. doi: 10.1016/0005-2760(93)90250-d. [DOI] [PubMed] [Google Scholar]
- 37.Dluhy R, Cameron DG, Mantsch HH, Mendelsohn R. Fourier transform infrared spectroscopic studies of the effect of calcium ions on phosphatidylserine. Biochem. 1983;22:6318. [Google Scholar]
- 38.Schultz ZD, Levin IW. Lipid Microdomain formation: Characterization by infrared spectroscopy and ultrasonic velocimetry. Biophys. J. 2008;94:3104. doi: 10.1529/biophysj.107.119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirchhoff WH, Levin IW. Description of the Thermotropic Behavior of Membrane Bilayers in Terms of Raman Spectral Parameters - a 2-State Model. Journal of Research of the National Bureau of Standards. 1987;92:113. [Google Scholar]
- 40.Kaszuba M, Connah MT, McNeil-Watson FK, Nobbmann U. Resolving concentrated particle size mixtures using dynamic light scattering. Particle & Particle Systems Characterization. 2007;24:159. [Google Scholar]
- 41.Kodati VR, El-Jastimi R, Lafleur M. Contribution of the Intermolecular Coupling and Librotorsional Mobility in the Methylene Stretching Modes in the Infrared Spectra of Acyl Chains. J. Phys. Chem. 1994;98:12191. [Google Scholar]
- 42.Caffrey M. LIPIDAT: A Database of Thermodynamic Data and Associated Information on Lipid Mesomorphic and Polymorphic Transitions. Boca Raton: CRC Press, Inc.; 1993. Pages. [Google Scholar]
- 43.Hinderliter AK, Huang J, Feigenson GW. Detection of phase separation in fluid phosphatidylserine/phosphatidylcholine mixtures. Biophys. J. 1994;67:1906. doi: 10.1016/S0006-3495(94)80673-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang J, Swanson JE, Dibble AR, Hinderliter AK, Feigenson GW. Nonideal mixing of phosphatidylserine and phosphatidylcholine in the fluid lamellar phase. Biophys. J. 1993;64:413. doi: 10.1016/S0006-3495(93)81382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodriguez Y, Mezei M, Osman R. Association Free Energy of Dipalmitoylphosphatidylserines in a Mixed Dipalmitoylphosphatidylcholine Membrane. Biophys. J. 2007;92:3071. doi: 10.1529/biophysj.106.089078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis RNAH, McElhaney RN. Vibrational Spectroscopy of Lipids. In: Griffiths JMCaPR., editor. Handbook of Vibrational Spectroscopy: Applications in Life, Pharmaceutical and Natural Sciences. Vol. 5. Chichester: John Wiley & Sons Ltd; 2002. p. 3447. [Google Scholar]
- 47.Snyder RG, Goh MC, Srivatsavoy VJP, Strauss HL, Dorset DL. Measurement of the Growth-Kinetics of Microdomains in Binary N-Alkane Solid-Solutions by Infrared-Spectroscopy. J. Phys. Chem. 1992;96:10008. [Google Scholar]
- 48.Snyder RG. Vibrational Spectra of Crystalline N-Paraffins .2. Intermolecular Effects. J. Mol. Spectrosc. 1961;7:116. [Google Scholar]
- 49.Cohen FS, Melikyan GB. The Energetics of Membrane Fusion from Binding, through Hemifusion, Pore Formation, and Pore Enlargement. J. Membr. Biol. 2004;199:1. doi: 10.1007/s00232-004-0669-8. [DOI] [PubMed] [Google Scholar]
- 50.Duzgunes N, Nir S, Wilschut J, Bentz J, Newton C, Portis A, Papahadjopoulos D. Calcium- and magnesium-induced fusion of mixed phosphatidylserine/phosphatidylcholine vesicles: Effect of ion binding. 1981;59:115. doi: 10.1007/BF01875709. [DOI] [PubMed] [Google Scholar]
- 51.Hark S-K, Ho JT. Raman study of calcium-induced fusion and molecular segregation of phosphatidylserine/dimyristoyl phosphatidylcholine-d54 membranes. Biochim. Biophys. Acta. 1980;601:54. doi: 10.1016/0005-2736(80)90513-1. [DOI] [PubMed] [Google Scholar]
- 52.Sun ST, Hsang CC, Day EP, Ho JT. Fusion of phosphatidylserine and mixed phosphatidylserine-phosphatidylcholine vesicles Dependence on calcium concentration and temperature. Biochim. Biophys. Acta. 1979;557:45. doi: 10.1016/0005-2736(79)90088-9. [DOI] [PubMed] [Google Scholar]
- 53.Silvius JR. Calcium-induced lipid phase separations and interactions of phosphatidylcholine/anionic phospholipid vesicles. Fluorescence studies using carbazole-labeled and brominated phospholipids. Biochem. 1990;29:2930. doi: 10.1021/bi00464a007. [DOI] [PubMed] [Google Scholar]
- 54.Jena BP. Chapter 9 Understanding Membrane Fusion: Combining Experimental and Simulation Studies Methods in Cell Biology. In: Jena BP, editor. Methods in Nano Cell Biology. Volume 90. Academic Press; 2008. p. 183. [DOI] [PubMed] [Google Scholar]
- 55.Portis A, Newton C, Pangborn W, Papahadjopoulos D. Studies on the Mechanism of Membrane-Fusion - Evidence for an Inter-Membrane Ca2+-Phospholipid Complex, Synergism with Mg2+, and Inhibition by Spectrin. Biochem. 1979;18:780. doi: 10.1021/bi00572a007. [DOI] [PubMed] [Google Scholar]
- 56.Papahadjopoulos D, Vail WJ, Jacobson K, Poste G. Cochleate Lipid Cylinders - Formation by Fusion of Unilamellar Lipid Vesicles. Biochim. Biophys. Acta. 1975;394:483. doi: 10.1016/0005-2736(75)90299-0. [DOI] [PubMed] [Google Scholar]
- 57.Ohki SA. Mechanism of Divalent Ion-Induced Phosphatidylserine Membrane-Fusion. Biochim. Biophys. Acta. 1982;689:1. doi: 10.1016/0005-2736(82)90182-1. [DOI] [PubMed] [Google Scholar]
- 58.Jena BP. Chapter 8 Assembly and Disassembly of SNAREs in Membrane Fusion Methods in Cell Biology. In: Jena BP, editor. Methods in Nano Cell Biology. Volume 90. Academic Press; 2008. p. 157. [DOI] [PubMed] [Google Scholar]
- 59.Yoon T-Y, Lu X, Diao J, Lee S-M, Ha T, Shin Y-K. Complexin and Ca2+ stimulate SNARE-mediated membrane fusion. Nat Struct Mol Biol. 2008;15:707. doi: 10.1038/nsmb.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong JL, Koppel DE, Cowan AE, Wessel GM. Membrane Hemifusion Is a Stable Intermediate of Exocytosis. Dev. Cell. 2007;12:653. doi: 10.1016/j.devcel.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roux M, Bloom M. Calcium binding by phosphatidylserine headgroups. Deuterium NMR study. Biophys. J. 1991;60:38. doi: 10.1016/S0006-3495(91)82028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ross M, Steinem C, Galla H-J, Janshoff A. Visualization of Chemical and Physical Properties of Calcium-Induced Domains in DPPC/DPPS Langmuir-Blodgett Layers. Langmuir. 2001;17:2437. [Google Scholar]
- 63.Chernomordik LV, Zimmerberg J, Kozlov MM. Membranes of the world unite! J. Cell Biol. 2006;175:201. doi: 10.1083/jcb.200607083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chernomordik LV, Kozlov MM. Membrane Hemifusion: Crossing a Chasm in Two Leaps. Cell. 2005;123:375. doi: 10.1016/j.cell.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 65.Jahn R, Lang T, Südhof TC. Membrane Fusion. Cell. 2003;112:519. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 66.Kozlovsky Y, Chernomordik LV, Kozlov MM. Lipid Intermediates in Membrane Fusion: Formation, Structure, and Decay of Hemifusion Diaphragm. Biophys. J. 2002;83:2634. doi: 10.1016/S0006-3495(02)75274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Infrared vibrational spectra obtained of the phosphate antisymmetric stretching mode region are available for the pure DPPS and DPPC-d62 lipids along with all spectra obtained from the binary mixtures. The spectra are presented both with and without the addition of Mg2+.