Abstract
The NOS ouput state for NO production is a complex of the FMN-binding domain and heme domain, and thereby it faciltates the interdomain electron transfer from the FMN to the catalytic heme site. Emerging evidence suggests that interdomain FMN/heme interactions are important in formation of the output state by guiding the docking of the FMN domain to the heme domain. In this study, notable effects of mutations in the adjacent FMN domain on the heme structure in a human iNOS bi-domain oxygnease/FMN construct have been observed by using low-temperature MCD spectroscopy. The comparative MCD study of wild type and mutant proteins clearly indicate that a properly docked FMN domain contributes to the observed l-Arg-perturbation of heme MCD spectrum in the wild type protein, and that the conserved surface residues at the FMN domain (E546 and E603) play key roles in facilitating a productive alignment of the FMN and heme domains in iNOS.
In mammals, nitric oxide (NO) is synthesized by nitric oxide synthase (NOS), a homodimeric flavo-hemoprotein that catalyzes the oxidation of l-arginine (Arg) to NO and l-citrulline with NADPH and O2 as co-substrates.1 Each NOS subunit contains a C-terminal electron-supplying reductase unit with binding sites for NADPH (electron source), flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN), and an N-terminal catalytic heme-containing oxygenase domain. The FMN domain and oxygenase domain are linked by a calmodulin (CaM) binding region. Three NOS isoforms, iNOS, eNOS and nNOS, achieve their key biological functions via an intriguing regulation of interdomain electron transfer (IET) processes.1 It is of current interest to elucidate control mechanisms for the catalytically relevant IET processes in the NOS isoforms.2–5 In particular, the IET from FMN to heme is essential in the delivery of electrons required for O2 activation in the heme domain and the subsequent NO synthesis.6 Interdomain FMN–heme IET is facilitated by formation of the NOS output state, which is an electron-donating (output) state of the FMN domain. The structure of the functional output state for NO production has not yet been determined.
Bi-domain NOS oxyFMN constructs, in which only the oxygenase domain, the FMN domain and the CaM-binding region are expressed, were recently designed (Fig. 1).7 This is to preclude the FAD/FMN interactions, and favor interactions between the FMN-binding domain and the heme domain. Biochemical, spectroscopic and IET kinetic data have demonstrated that these homologous dimeric oxyFMN constructs are well-validated models of the NOS output state.7–9 Emerging evidence further indicates that CaM modulates the FMN–heme IET in the NOS oxyFMN constructs by facilitating the interdomain FMN/heme interactions.8,10 Therefore, experiments that focus on crucial interdomain interactions should provide key insight into regulating formation of the NOS output state. In this study, we use low-temperature magnetic circular dichroism (MCD) spectroscopy to probe how mutations in the adjacent FMN domain affect the heme center in a human iNOS oxyFMN construct.
Figure 1.
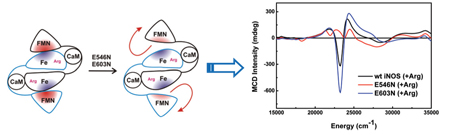
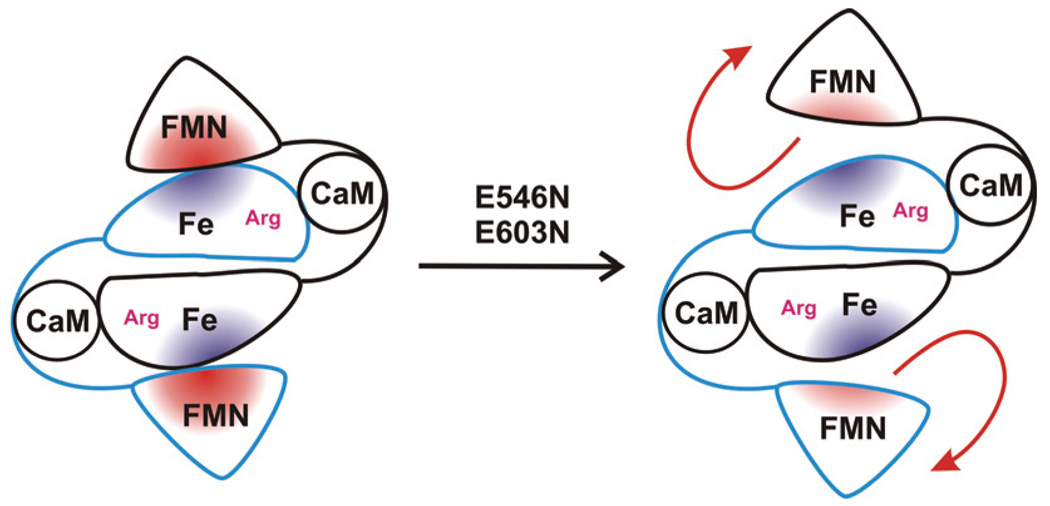
Schematic diagram of the truncated bi-domain oxyFMN construct of human iNOS (left). Charge neutralization mutation at E546 or E603 of the FMN domain diminishes l-Arg-perturbation of heme MCD spectrum, presumably by disrupting proper alignment of the FMN and heme domains (right).
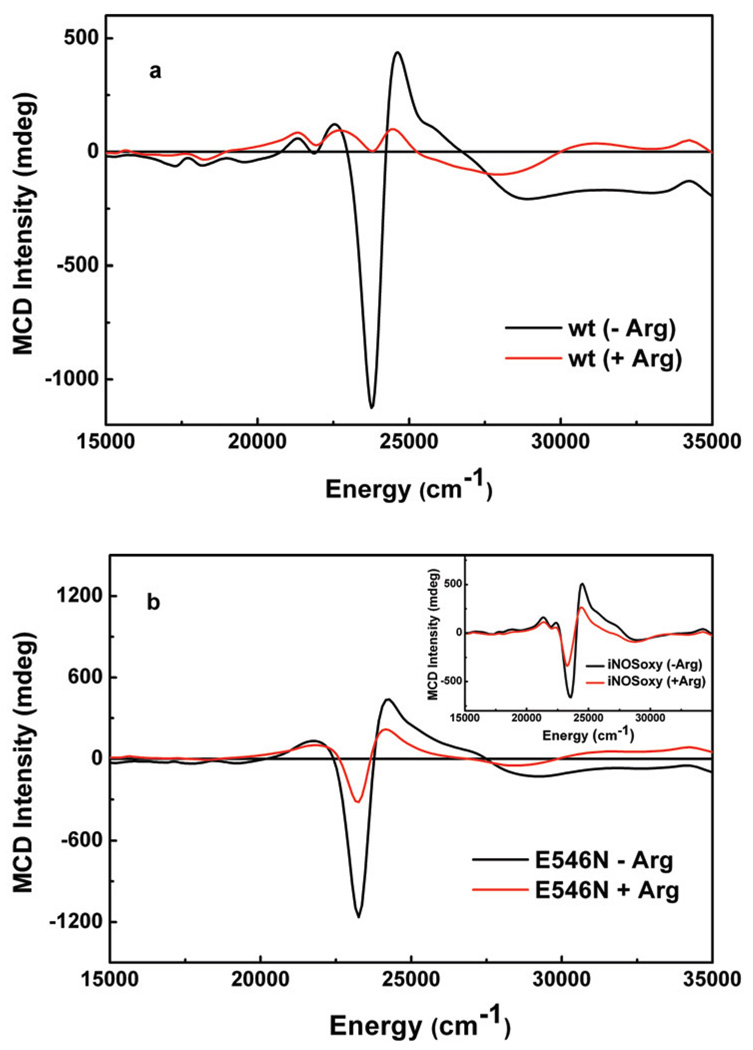
Wild type (wt) human iNOS oxyFMN construct was expressed and purified as described elsewhere.10 Located at the edge of the FMN domain of human iNOS, E546 and E603 are charged surface residues that are conserved in all NOS isoforms (Fig. S1, Supporting Information). In order to address the roles of these charged residues in interdomain FMN/heme interactions, E546N and E603N mutants were constructed, expressed, and purified (see Supporting Information). The MCD samples were prepared by using microvolumetric techniques, and temperature dependent MCD spectra, arising from the ferric heme site, were obtained at 5K, 10K and 20K in a 7T applied magnetic field (Fig. 2).11 Importantly, the MCD spectrum of the wt protein is noticeably perturbed upon incubation with the substrate l-Arg (Fig. 2a), whereas the E546N and E603N mutants have similar MCD spectra in both the presence and absence of l-Arg (Fig. 2b, and Fig. S5 in Supporting Information, respectively). Previous room temperature MCD studies on wt nNOS holoenzyme also suggested a perturbation of the heme signal upon l-Arg binding,12 consistent with our low-temperature observations for the wt iNOS oxyFMN construct, vide infra. This l-Arg perturbation in the nNOS holoenzyme has previously been attributed to the conversion of a mixed high-spin/low-spin state to an exclusively high-spin heme when l-Arg binds near the heme site.12 We have probed the ferric heme spin states in the wt and mutant iNOS oxyFMN constructs by variable-temperature variable-field (VTVH) MCD and EPR spectroscopies. Here we observe that binding of l-Arg near the wt construct heme center results in its conversion to a predominantly high-spin form (Fig. S6, Supporting Information). However, MCD and EPR spectra for the l-Arg-bound mutants indicate a greater percentage of low-spin heme present. Note that except for the l-Arg-bound wt oxyFMN (red trace in Fig. 2a), all other MCD spectra (Fig. 2) are reminiscent of low-spin ferric heme species.13 The direct implication of these results is that proper FMN docking at the heme domain is necessary for the formation of a high-spin ferric heme in the presence of l-Arg.
Figure 2.
MCD spectra recorded at 5K for ferric heme in (a) wt, and (b) E546N mutant of human iNOS oxyFMN construct without (black traces) or with (red traces) 20 mM l-Arg. Inset of panel b is of iNOS oxygenase (iNOSoxy) construct. The samples were made in a pH 7.6 buffer (50 mM Tris, 200 mM NaCl, 1 mM DTT, 4 µM H4B); 42% (v/v) ethylene glycol was added to the protein samples as a cryoprotectant and glassing agent.
Further support of this hypothesis is provided by the fact that the spectral perturbation induced by l-Arg binding in the wt protein (Fig. 2a) is completely absent in the iNOS oxygenase construct (inset of Fig. 2b), which only possesses a heme domain but no FMN domain. Thus, the oxygenase construct provides an important control to assess the effects of a properly aligned FMN domain in the wt oxyFMN construct. Taken together, these data strongly suggest a correlation between the l-Arg-perturbation of the heme MCD spectrum and the existence of a properly aligned FMN domain in the FMN/heme complex of the wt iNOS protein. Complementary evidence regarding the importance of these residues (E546, E603) in iNOS interdomain interactions derives from a recent mutational and kinetics study that indicates the involvement of the equivalent FMN sites (E762, E819) in rat nNOS FMN/FAD alignment.14
Due to the lack of a crystal structure for the NOS FMN/heme complex, there is a clear need to probe the effects of interdomain FMN/heme interactions at the molecular level. Although EPR studies have suggested the presence of magnetic interactions between the paramagnetic centers of the FMN semiquinone radical and heme iron in NOS holoenzymes,15–17 another EPR study on a nNOS holoenzyme argued that the flavin and heme centers are not magnetically coupled.18 The discrepancy in these EPR studies strongly indicate the need to develop new and complimentary spectroscopic approaches, such as low temperature and VTVH MCD spectroscopies, in order to determine the nature of interdomain FMN/heme interactions with molecular-level resolution.
In summary, this study provides the first direct paramagnetic spectroscopic evidence to indicate that the docked FMN domain affects the nature of interactions between the l-Arg substrate and the catalytic heme center located in an adjacent domain in iNOS. Specifically, we propose charge neutralization mutations at the E546 and E603 sites disrupt complementary electrostatic interdomain FMN/heme interactions, disturb the FMN/heme complex, and thereby diminish the l-Arg-perturbation. We have shown that a combination of low-temperature MCD and EPR spectroscopies provides a promising site-selective probe of key interdomain FMN/heme interactions that modulate the formation of the NOS output state. This comparative MCD study of wt and mutant proteins clearly indicate that a properly docked FMN domain contributes to the observed l-Arg-perturbation of heme MCD in the wt NOS protein, and that the conserved surface residues at the FMN domain play key roles in facilitating a productive alignment of the FMN and heme domains in iNOS.
Supplementary Material
Experimental procedures and Figs. S1–S6. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgement
This work was supported by grants from the National Institutes of Health (GM057378 to M.L.K.; GM081811 and HL091280 to C.F.) and NSERC (183521 to J.G.G.).
References
- 1.Roman LJ, Martasek P, Masters BSS. Chem. Rev. 2002;102:1179–1189. doi: 10.1021/cr000661e. [DOI] [PubMed] [Google Scholar]
- 2.Ilagan RP, Tiso M, Konas DW, Hemann C, Durra D, Hille R, Stuehr DJ. J. Biol. Chem. 2008;283:19603–19615. doi: 10.1074/jbc.M802914200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng CJ, Tollin G, Hazzard JT, Nahm NJ, Guillemette JG, Salerno JC, Ghosh DK. J. Am. Chem. Soc. 2007;129:5621–5629. doi: 10.1021/ja068685b. [DOI] [PubMed] [Google Scholar]
- 4.Haque MM, Panda K, Tejero J, Aulak KS, Fadlalla MA, Mustovich AT, Stuehr DJ. Proc Natl Acad Sci U S A. 2007;104:9254–9259. doi: 10.1073/pnas.0700332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jachymova M, Martasek P, Panda S, Roman LJ, Panda M, Shea TM, Ishimura Y, Kim JJP, Masters BSS. Proc Natl Acad Sci U S A. 2005;102:15833–15838. doi: 10.1073/pnas.0506522102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panda K, Ghosh S, Stuehr DJ. J. Biol. Chem. 2001;276:23349–23356. doi: 10.1074/jbc.M100687200. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh DK, Holliday MA, Thomas C, Weinberg JB, Smith SME, Salerno JC. J. Biol. Chem. 2006;281:14173–14183. doi: 10.1074/jbc.M509937200. [DOI] [PubMed] [Google Scholar]
- 8.Feng CJ, Tollin G, Holliday MA, Thomas C, Salerno JC, Enemark JH, Ghosh DK. Biochemistry. 2006;45:6354–6362. doi: 10.1021/bi060223n. [DOI] [PubMed] [Google Scholar]
- 9.Feng CJ, Thomas C, Holliday MA, Tollin G, Salerno JC, Ghosh DK, Enemark JH. J. Am. Chem. Soc. 2006;128:3808–3811. doi: 10.1021/ja0578606. [DOI] [PubMed] [Google Scholar]
- 10.Feng CJ, Dupont A, Nahm N, Spratt D, Hazzard JT, Weinberg J, Guillemette J, Tollin G, Ghosh DK. J. Biol. Inorg. Chem. 2009;14:133–142. doi: 10.1007/s00775-008-0431-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones RM, Inscore FE, Hille R, Kirk ML. Inorg. Chem. 1999;38:4963–4970. doi: 10.1021/ic990154j. [DOI] [PubMed] [Google Scholar]
- 12.Sono M, Stuehr DJ, Ikedasaito M, Dawson JH. J. Biol. Chem. 1995;270:19943–19948. doi: 10.1074/jbc.270.34.19943. [DOI] [PubMed] [Google Scholar]
- 13.Berka V, Palmer G, Chen P-F, Tsai A-L. Biochemistry. 1998;37:6136–6144. doi: 10.1021/bi980133l. [DOI] [PubMed] [Google Scholar]
- 14.Panda K, Haque MM, Garcin-Hosfield ED, Durra D, Getzoff ED, Stuehr DJ. J. Biol. Chem. 2006;281:36819–36827. doi: 10.1074/jbc.M606129200. [DOI] [PubMed] [Google Scholar]
- 15.Stuehr DJ, Ikedasaito M. J. Biol. Chem. 1992;267:20547–20550. [PubMed] [Google Scholar]
- 16.Galli C, MacArthur R, Abu-Soud HM, Clark P, Stuehr DJ, Brudvig GW. Biochemistry. 1996;35:2804–2810. doi: 10.1021/bi9520444. [DOI] [PubMed] [Google Scholar]
- 17.Tsai AL, Berka V, Chen PF, Palmer G. J. Biol. Chem. 1996;271:32563–32571. doi: 10.1074/jbc.271.51.32563. [DOI] [PubMed] [Google Scholar]
- 18.Perry JM, Moon N, Zhao Y, Dunham WR, Marletta MA. Chemistry & Biology. 1998;5:355–364. doi: 10.1016/s1074-5521(98)90069-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures and Figs. S1–S6. This material is available free of charge via the Internet at http://pubs.acs.org.